Abstract
Background:
The China STATUS II is a prospective, multicentre, open-label, post-marketing, observational study including Chinese adults (aged ⩾ 18 years) with essential hypertension who were prescribed once-daily valsartan/amlodipine (Val/Aml 80/5 mg) single-pill combination. In order to examine gender differences in treatment response to Val/Aml, we further analysed data from the China STATUS II study.
Methods:
A total of 11,312 patients (6456 (57%) men and 4856 (43%) women) received the Val/Aml treatment for 8 weeks. After the treatment, we compared the proportion of patients not achieving the target systolic blood pressure (SBP: < 140 mm Hg) or diastolic blood pressure (DBP: < 90 mm Hg) in different age groups (by Fisher exact probability test) and estimated the changes in blood pressure (BP) according to age and gender, using a mixed model.
Results:
At enrolment, mean SBP was higher in the female versus the male patients (160.0 ± 12.71 versus 159.3 ± 12.31 mm Hg; p = 0.003), whereas the mean DBP was higher in the male versus the female patients (96.4 ± 10.65 versus 94.5 ± 10.72 mm Hg; p < 0.001). The overall proportion of women not achieving the target BP was less than that of men (57.41% versus 59.59%; p < 0.05) at 4 weeks and (22.22% versus 23.78%; p < 0.05) at 8 weeks after the Val/Aml treatment. Among both men and women, the proportion of patients not achieving the target SBP increased with age; however, the proportion not achieving the target DBP decreased with age. The mixed-model analysis showed that the changes in SBP were closely related to gender, indicating that the SBP-lowering effect after Val/Aml treatment might be better in women. In addition, the changes in DBP were closely related to age.
Conclusions:
Gender might be a factor for consideration in the decision-making process of individualised antihypertensive therapy, in the future.
Keywords: Amlodipine, essential hypertension, gender differences, renin angiotensin aldosterone system, single-pill combination, STATUS II, valsartan
Introduction
Essential hypertension is a common risk factor for cardiovascular diseases (CVDs). Except for lifestyle modification, drug therapy is the main treatment strategy. A single-pill combination (SPC) of two drugs is recommended by several recent international guidelines for hypertension,1,2 including the Chinese guidelines of 20103 for the treatment of hypertension in high-risk patients who require marked blood pressure (BP) reductions. The China STATUS II (Survey of hyperTensive pAtienTs blood pressUre control rate in clinic Service) is a prospective, multicentre, open-label, post-marketing, observational study; including 11,312 Chinese adults (aged ⩾ 18 years) with essential hypertension, who were prescribed a once-daily valsartan/amlodipine (Val/Aml, 80/5 mg) SPC. China STATUS II is the first evidence-based, real-world data in Chinese hypertensive patients, which shows the efficacy and safety of the Val/Aml SPC.4
It is well known that young adult women have lower BP than age-matched men; but the prevalence of CVDs, including hypertension in women, increases rapidly after the onset of menopause. Recent studies show a few possible causes for gender-related differences in CVDs, such as: The change in female hormonal status and the loss of cardiovascular protection provided by oestrogen.5–7 Although numerous studies report gender-related differences in the regulation of arterial pressure and renal function by the renin-angiotensin system (RAS)8–10 and the response to RAS inhibition,11–13 there is no mention of the selection of antihypertensive drugs based on gender, except in pregnancy-related situations, in any of the guidelines worldwide. Moreover, insufficient gender-specific data has been provided by clinical trials,14 making it difficult to optimise drug selection for both genders. In order to understand gender differences in BP management, we examined the effect of gender on the efficacy of Val/Aml SPC in the China STATUS II study.
Methods
Study design and participants
The study design for China STATUS II has been reported elsewhere.4 To summarise, China STATUS II was a multicentre, post-marketing, prospective, observational study, which enrolled 11,312 adult Chinese patients with essential hypertension whose BP was not adequately controlled by monotherapy.
All enrolled patients had a mean sitting systolic BP (SBP) ⩾ 140 mm Hg (⩾ 130 mm Hg for diabetes or chronic kidney disease (CKD)) and/or a mean sitting diastolic BP (DBP) ⩾ 90 mm Hg (⩾ 80 mm Hg for diabetes or CKD), whose BP was inadequately controlled by monotherapy. Before enrolment, all patients provided written informed consent. The study complied with the International Conference on Harmonisation-Good Clinical Practice (ICH-GCP) and applicable local regulations in China, and was approved by the Ethical Review Committee of the First Hospital of Harbin Medical University. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki of 1975, as revised in 2000 and 2008.
The baseline characteristics of the randomised patients are described in the report of the China STATUS II study’s main findings.4
Eligible patients from 238 regional centres across 29 provinces of China were enrolled from 12 October 2010 to 20 February 2012. After enrolment, there was no dietary, lifestyle nor medication change in all subjects. The lifestyle questionnaires included questions on education and socioeconomic status, occupation, history of previous illness and disorders or surgical operations, lifetime history of consumption of tobacco and alcoholic beverages, and physical activity. All patients who had stopped taking other antihypertensive drugs received Val/Aml 80/5 mg SPC for 4 weeks, instead of their previous antihypertensive drugs. The initial BP target (< 140/90 mm Hg (or < 130/80 mm Hg for diabetes or CKD)) was unified to < 140/90 mm Hg, for easier management. The treatment was in accordance with the routine clinical outpatient practice in China. The study duration was 8 weeks, with a follow-up every 4 weeks. If a patient did not achieve BP control at the end of 4 weeks of the SPC, an additional antihypertensive agent could be added, according to the physician’s decision.
Statistical analysis
All statistical analyses were performed using SAS® software version 9.2 (SAS Institute Inc., Cary, NC, USA), with a 2-sided significance level (p) of < 0.05. Demographic and baseline variables were summarised using descriptive statistics, including the mean, SD, median, minimum and maximum values for numeric variables; and the count number and percentage for the categorical variables. We used the T test, chi-square test and Fisher accurate probability test.
We estimated the changes in SBP and DBP at 4 and 8 weeks for gender and age as fixed effects, by using SAS PROC MIXED (SAS Institute, Cary, NC, USA).15 For the analysis in Mixed Model 1, we calculated variance components for the baseline BP, their BMI, smoking, drinking, exercise and education level, using gender and age as fixed effects. The same analysis was then used in Mixed Model 2, adjusting for the baseline BP and statistically significant effects in Model 1.
Results
Demographic and baseline characteristics
The detailed demographic and baseline characteristics of the patients are presented in Table 1. We analysed a total of 11,312 patients (6456 (57%) men and 4856 (43%) women). At enrolment, the men were younger in age (p < 0.001) and had a higher BMI (p < 0.001) than women. Compared with the female patients, we observed a higher educational background, more physical activity, and more smoking and drinking in the male patients (p < 0.05). There were no significant differences in their present cardiovascular risk factors and medical history, and their previous use of antihypertensive drug classes, between these men and women (Table 1).
Table 1.
Demographic and baseline characteristics of the study patients (n = 11,312).a
| Men (n = 6456) | Women (n = 4856) | p-value | |
|---|---|---|---|
| Mean age (SD) | 57.4 (14.27) | 59.8 (13.09) | < 0.001 |
| Patients in each group, n (%) | < 0.0001 | ||
| < 55 years old | 2913 (45.12%) | 1798 (37.03%) | |
| 55–64 years old | 1641 (25.42%) | 1351 (27.82%) | |
| 65–74 years old | 1019 (15.78%) | 1015 (20.90%) | |
| ⩾ 75 years old | 883 (13.68%) | 692 (14.25%) | |
| BMI (kg/m2) | 25.0 (2.95) | 24.3 (3.34) | < 0.001 |
| BMI ⩾ 25 kg/m2, n (%) | 3088 (47.9%) | 1824 (37.6%) | < 0.0001 |
| Ethnicity, n (%) | 0.3074 | ||
| Han | 6262 (96.9%) | 4727 (97.3%) | |
| Hui | 98 (1.51%) | 78 (1.60%) | |
| Manchu | 38 (0.58%) | 22 (0.45%) | |
| Mongolian | 18 (0.27%) | 7 (0.14%) | |
| other | 40 (0.61%) | 22 (0.45%) | |
| SBP (SD), mm Hg | 159.3 (12.31) | 160.0 (12.71) | 0.003 |
| DBP (SD), mm Hg | 96.4 (10.65) | 94.5 (10.72) | < 0.001 |
| Heart rate (SD), bpm | 75.6 ( 7.74) | 75.2 (8.00) | 0.003 |
| Current smoker, n (%) | 2090 (32.3%) | 94 (1.93%) | < 0.0001 |
| Current alcohol drinker, n (%) | 1446 (22.3%) | 50 (1.02%) | < 0.0001 |
| College degree or above, n (%) | 1615 (25.0%) | 630 (12.9%) | < 0.0001 |
| Appropriate physical activityb, n (%) | 4908 (76.0%) | 3607 (74.2%) | 0.0334 |
| Present cardiovascular risk factors or medical history, n (%) | |||
| Dyslipidemia | 1588 (24.5%) | 1114 (22.9%) | 0.1136 |
| Diabetes | 1038 (16.0%) | 827 (17.0%) | 0.3029 |
| CHD | 957 (14.8%) | 796 (16.3%) | 0.0726 |
| Heart failure | 104 (1.61%) | 85 (1.75%) | 0.6971 |
| Kidney disease | 206 (3.19%) | 149 (3.06%) | 0.9317 |
| Previous antihypertensive drug classes, n (%) | 0.9889 | ||
| β-Blockers | 518 (8.02%) | 385 (7.92%) | |
| CCBs | 3083 (47.7%) | 2330 (47.9%) | |
| ACEIs | 994 (15.3%) | 734 (15.1%) | |
| Diuretics | 181 (2.80%) | 140 (2.88%) | |
| ARB | 1630(25.2%) | 1223(25.1%) | |
| Others | 44 (0.68%) | 38 (0.78%) | |
| Unknown | 6 (0.09%) | 6 (0.12%) |
We used the T test for mean age, mean height, mean weight, BMI, SBP, DBP and heart rate; Chi square test for patients in each group: BMI ⩾ 25, ethnicity, current smoker, current alcohol drinker, college degree or above, appropriate physical activity, present risk factors of medical history related to cardiovascular disease, and previous anti-hypertensive drug classes used.
ACEI: ACE inhibitor; ARB: angiotensin II receptor blocker; BMI: body mass index; BPM: beats per minute; CCB: calcium channel blocker; DBP:diastolic blood pressure; mm Hg: millimetres of mercury as units of pressure; SBP: systolic blood pressure.
Appropriate physical activity (if the subject participates in any regular physical activities, such as walking, swimming, running, etc.).
Blood pressure during follow-up
SBP was higher in the female patients versus the male patients (160.0 ± 12.71 versus 159.3 ± 12.31 mm Hg; p = 0.003); whereas DBP was higher in male patients versus female patients, at enrolment (96.4 ± 10.65 versus 94.5 ± 10.72 mm Hg; p < 0.001).
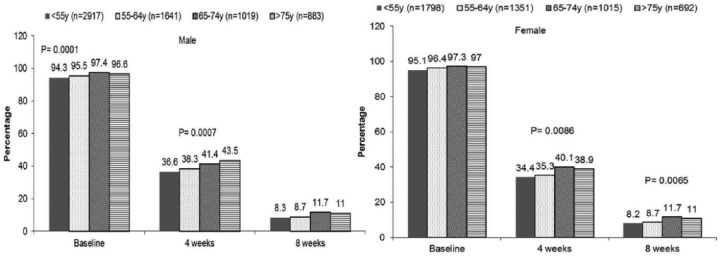
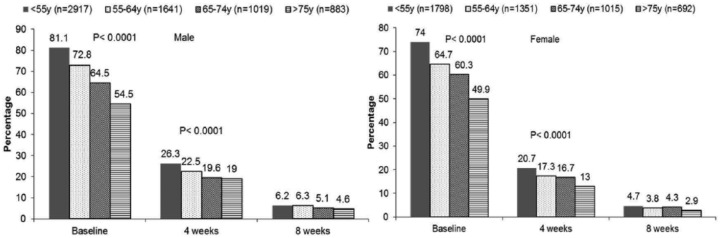
After the treatment, a significantly lower proportion of women did not achieve the target BP, compared with men (57.41% versus 59.59%, p < 0.05 at 4 weeks; and 22.22% versus 23.78%, p < 0.05, at 8 weeks). In order to examine the effect of age on drug efficacy, both genders were divided into four different age groups: < 55, 55–64, 65–75 and > 75 years. As a result, for all enrolled patients, the proportion of patients not achieving the target SBP increased with age; however, the proportion of patients not achieving the target DBP was the opposite (Figure 1).
Figure 1(a).
The proportion of patients not achieving the target SBP of < 140 mm Hg in different age groups of men and women, respectively; at baseline, 4 weeks and 8 weeks after their treatment with the Val/Aml combination therapy.
Figure 1(b).
The proportion of patients not achieving the target diastolic blood pressure (DBP) of < 90 mmHg in different age groups of men and women, respectively: at baseline, 4 weeks and 8 weeks after their treatment with the Val/Aml combination therapy.
Aml: amlodipine; DBP: diastolic blood pressure; mmHg: millimetres of mercury; SBP: systolic blood pressure; Val: valsartan.
The next assessment included the changes in SBP and DBP at 4 and 8 weeks after treatment, according to gender and age, by using SAS PROC MIXED. The p values obtained from the Mixed Model were as follows: Changes in SBP at 4 weeks, p < 0.0001 for gender and p = 0.3832 for age; changes in SBP at 8 weeks, p = 0.0026 for gender and p = 0.0225 for age; changes in DBP at 4 weeks, p = 0.0602 for gender and p = 0.0219 for age; changes in DBP at 8 weeks, p = 0.1035 for gender and p = 0.0057 for age. In the Mixed Model 1 analysis, age and gender were fixed effects and the baseline BP, BMI, smoking, drinking, exercise and education level were random effects. There was no significant correlation between the patients’ age, gender, BMI, smoking, drinking, exercise and education level.
The Mixed Model 2 was adjusted for the baseline BP and statistically significant effects in the Model 1. That mixed model showed that the changes in SBP were closely related to gender. Given that the proportion of the women who did not achieve the target BP was lower as compared with the men in this study, the SBP-lowering effect of the Val/Aml treatment might be better in women. In addition, the changes in DBP were closely related to age. These findings were consistent in both Mixed Model 1 and Mixed Model 2 (Table 2).
Table 2.
Changes in SBP and DBP at 4 weeks and 8 weeks, according to gender and age, using SAS PROC MIXED analysis using the mixed model, with the age group and gender as fixed factors and the baseline BP at 4 weeks and 8 weeks as a covariate.
| < 55 years of age | 55 years to < 64 years | 65 years to < 75 years | > 75 years | p value (age) | ||
|---|---|---|---|---|---|---|
| n (men and women study subjects) | 2917/1798 | 1641/1351 | 1019/1015 | 883/692 | ||
| SBP (mm Hg) | ||||||
| Baseline | men | 158.2 ± 12.40 | 159.3 ± 11.92 | 160.6 ± 12.04 | 161.3 ± 12.67 | < 0.0001 |
| women | 158.4 ± 12.07 | 160.1 ± 12.54 | 161.6 ± 13.34 | 161.6 ± 13.23 | ||
| p value (gender) | 0.0041 | |||||
| Differences at 4 weeks | men | − 19.1 ± 11.10 | − 19.7 ± 10.94 |
− 20.2 ± 11.07 |
− 20.4 ± 11.12 | 0.3832 |
| women | − 19.6 ± 11.29 |
− 21.1 ± 12.28 |
− 21.6 ± 12.54 |
− 21.6 ± 11.97 | 0.7305a
0.6889b |
|
| p value (gender) | < 0.0001 < 0.0001a < 0.0001b |
|||||
| Differences at 8 weeks | men | 26.1 ± 11.97 | 26.9 ± 11.75 | −27.3 ± 11.86 |
− 27.9 ± 12.10 | 0.0225 |
| women | 26.6 ± 12.07 | 27.8 ± 12.64 | 28.7 ± 13.19 | − 28.6 ± 12.51 | 0.0858a
0.0225b |
|
| p value (gender) | 0.0026 0.0023a 0.0026b |
|||||
| DBP (mm Hg) | ||||||
| Baseline | men | 98.6 ± 9.97 | 96.2 ± 9.78 | 94.6 ± 10.53 | 91.4 ± 12.27 | < 0.0001 |
| women | 96.7 ± 9.71 | 94.6 ± 10.31 | 93.0 ± 11.29 | 90.4 ± 11.64 | ||
| p value (gender) | < 0.0001 | |||||
| Differences at 4 weeks | men | 12.0 ± 8.54 | 10.7 ± 7.66 | 10.1 ± 8.36 | − 8.7 ± 8.13 | 0.0219 |
| women | 10.7 ± 8.01 | 10.8 ± 8.61 | 9.6 ± 8.60 | − 8.6 ± 8.90 | 0.0206a
0.0166b |
|
| p value (gender) | 0.0602 0.0206a 0.0403b |
|||||
| Differences at 8 weeks | men | 17.0 ± 9.37 | 15.5 ± 8.56 | 14.4 ± 9.20 | − 12.8 ± 9.22 | 0.0057 |
| women | 15.7 ± 9.03 | 14.9 ± 9.40 | 13.6 ± 9.51 | − 12.5 ± 9.87 | 0.0601a
0.0023b |
|
| p value (gender) | 0.1035 0.8275a 0.0771b |
|||||
Analysis using the Mixed Model 1, age and gender as a fixed effects, adjusted for the baseline of BP, BMI, smoking, drinking, exercise and education level.
Analysis using the Mixed Model 2, adjusted for the baseline BP and statistically significant effects in Model 1. BP changes at 4 weeks and 8 weeks, after being adjusted for baseline BP and exercise.
BMI: body mass index; BP: blood pressure; DBP: diastolic blood pressure; mm Hg: millimetres of mercury as units of pressure; SBP: systolic blood pressure.
Discussion
One major finding of our study was that a gender difference existed in the treatment response to Val/Aml among Chinese hypertension patients, indicating that this therapy might show better SBP-lowering effects in women.
Major gender differences exist in the development and progression of hypertension and CVD. Before menopause, women usually have lower BP and less hypertension and CVD, relative to age-matched men16,17; however, this cardiovascular protection in women is lost after menopause.18 Several studies confirm that gender differences exist in terms of the pharmacokinetic and pharmacodynamic characteristics of drugs.19–21 Wing et al.22 shows that treatment with angiotensin-converting enzyme (ACE) inhibitors correlates with a better outcome, compared with treatment with diuretics; whereas another study concludes that the response to specific treatment agents appears to differ between men and women.23 These findings also suggested that antihypertensive regimens need to be tailored according to gender. Some might argue that female patients are always more aware of the need for treatment and show better compliance; thus, could get a better BP-lowering effect. We also evaluated both the physician and patients’ self-compliance assessments, and found that there were no significant differences between the male and female genders (p = 0.7517 and p = 0.0702, respectively). Also, given that the longitudinal BP response was appropriate in both men and women and that the study length was short, we did not consider male patients lacking compliance in our study. Although the cause for these gender-related differences in response to therapy is not certain, these gender differences are associated with sexual dimorphism in the physiological mechanisms that regulate arterial pressure, which can impact the male and female patients’ responses to different therapeutic approaches.24
Notably, gender-related differences also exist in the RAS, due to differential modulation by sex hormones.25,26 In our study, most patients were aged > 55 years (6601/11,312 (58%)); and of these, 46% (3058/6601) of them were women. Given that the mean post-menopausal age is 56 years in China,27 quite a few women in our study were in the post-menopausal period. Oestrogen regulates all components of the RAS, increasing the synthesis of angiotensinogen, while decreasing the synthesis and activity of renin and ACE. Oestrogen decreases the expression of the angiotensin Type 1 receptor (AT1R) in target tissue, but increases the expression of Type 2 receptor AT2R.28,29 Studies in animal models show that oestrogen and RAS blockade may act synergistically to downregulate the AT1R.30,31 Since valsartan is an angiotensin II receptor blocker (ARB), the Val/Aml combination therapy might inhibit RAS activation and provide a better antihypertensive effect for post-menopausal women. A small study among 51 post-menopausal hypertensive women showed that the BP-lowering effect of the ARB irbesartan is augmented by co-administration with 17-estradiol, suggesting there are potentiating effects of the two different antihypertensive mechanisms.32 Of course, further study into the mechanisms of the gender differences in hypertension treatment is needed.
Another finding of our study was that for all of the enrolled patients, the proportion of patients not achieving the target SBP (⩽ 140 mm Hg) increased with age; however, the proportion of patients not achieving the target DBP (⩽ 90 mm Hg) was the opposite.
Elevated BP is an important cardiovascular risk factor. Although the target DBP and SBP are defined by guidelines, DBP has historically taken precedence in hypertension management; however, there is strong evidence that SBP is superior to DBP as a predictor of cardiovascular events, particularly in the elderly.33 Stamler et al.34 found that SBP has a stronger association with cardiovascular risk, as compared with DBP, in middle-aged and elderly individuals. Moreover, the superior predictive ability of SBP was also confirmed in a meta-analysis of 61 prospective observational studies that recorded BP and cause-specific mortality.35 With an increasing proportion of aging population, the prevalence of hypertension and related cardiovascular morbidity in Asian patients continues to rise, placing a substantial and escalating social and economic burden on this region.36 The prevalence of hypertension in the Chinese population is 39% overall, 59.4% in those aged > 60 years and 72.8% in those aged > 75 years.37 The age-specific prevalence of hypertension increased with age, throughout the age range. In our study, the mean age of the male patients was 57.4 ± 14.27 years and that of female patients was 59.8 ± 13.09 years. The proportion of patients who did not achieve the target SBP increased with age. An elevated SBP increases the risk of CVD, mortality and renal function decline, and that risk may increase at lower SBP levels in the Asian than in the Western population. Hence, reducing SBP should be the primary goal in the management of hypertension, particularly as the patients age.38
There were several limitations in our study. First, the focus was only on short-term BP lowering. The long-term clinical outcomes might be more important in elucidating gender differences and cardiovascular events. Besides, 686 (6.06%) of the subjects of this study had to take additional antihypertensive agents to control their BP. The most used combined antihypertensive agents were metoprolol (1.67%) and hydrochlorothiazide (1.45%). Because the majority (10,626 out of 11,312 (93.94%)) of the subjects in this study received only Val/Aml SPC for BP control, we consider that the potential impact of additional antihypertensive treatment on the BP outcome is limited. We will avoid this kind of possible bias in future studies. Moreover, we did not control or monitor the patients’ sodium or protein intake, although that could be extremely difficult to implement in such a large-scale observational study.
Conclusions
For all the patients enrolled in our study, the proportion of patients not achieving the target SBP increased with age; however, the proportion not achieving the target DBP was the opposite. Although the mechanisms responsible for gender differences in the treatment response to Val/Aml among Chinese patients were not addressed, our findings indicated that women might have a better SBP-lowering effect with such therapy. Gender might be a factor for consideration in the decision-making process of individualised antihypertensive therapy, in the future.
Acknowledgments
The contributors to the investigation of ‘Gender difference in response to valsartan/amlodipine single-pill combination in essential hypertension’ using China STATUS II data are gratefully acknowledged.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). J Am Med Ass 2014; 311: 507–520. [DOI] [PubMed] [Google Scholar]
- 2. Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1925–1938. [DOI] [PubMed] [Google Scholar]
- 3. Writing Group of 2010 Chinese Guidelines for the Management of Hypertension. 2010 Chinese guidelines for the management of hypertension (in Chinese). Chin J Cardiol 2011; 39(7): 579–616. [PubMed] [Google Scholar]
- 4. Hu D, Liu L, Li W. Efficacy and safety of valsartan/amlodipine single-pill combination in 11,422 Chinese patients with hypertension: An observational study. Adv Ther 2014; 31: 762–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Regitz-Zagrosek V, Dworatzek E, Kintscher U, et al. Sex and sex hormone-dependent cardiovascular stress responses. Hypertension 2013; 61: 270–277. [DOI] [PubMed] [Google Scholar]
- 6. Sampson AK, Jennings GL, Chin-Dusting JP. Y are males so difficult to understand? A case where X does not mark the spot. Hypertension 2012; 59: 525–531. [DOI] [PubMed] [Google Scholar]
- 7. Hilliard LM, Sampson AK, Brown RD, et al. The “his and hers” of the renin-angiotensin system. Curr Hypertens Rep 2013; 15: 71–79. [DOI] [PubMed] [Google Scholar]
- 8. Miller JA, Cherney DZ, Duncan JA, et al. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol 2006; 17: 2554–2560. [DOI] [PubMed] [Google Scholar]
- 9. Cherney DZ, Lai V, Miller JA, et al. The angiotensin II receptor Type 2 polymorphism influences haemodynamic function and circulating RAS mediators in normotensive humans. Nephrol Dial Transplant 2010; 25: 4093–4096. [DOI] [PubMed] [Google Scholar]
- 10. Chidambaram M, Duncan JA, Lai VS, et al. Variation in the renin angiotensin system throughout the normal menstrual cycle. J Am Soc Nephrol 2002; 13: 446–452. [DOI] [PubMed] [Google Scholar]
- 11. Safar ME, Myers MG, Leenen F, et al. Gender influence on the dose-ranging of a low-dose perindopril-indapamide combination in hypertension: Effect on systolic and pulse pressure. J Hypertens 2002; 20: 1653–1661. [DOI] [PubMed] [Google Scholar]
- 12. Sullivan JC. Sex and the renin-angiotensin system: Inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 2008; 294: R1220–1226. [DOI] [PubMed] [Google Scholar]
- 13. Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- and aldosterone-induced hypertension: The central protective effects of estrogen. Am J Physiol Regul Integr Comp Physiol 2013; 305: R459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blauwet LA, Hayes SN, McManus D, et al. Low rate of sex-specific result reporting in cardiovascular trials. Mayo Clin Proc 2007; 82: 166–170. [DOI] [PubMed] [Google Scholar]
- 15. SAS Institute Inc. SAS/STAT®9.2 Cary, NC: SA, 2008. [Google Scholar]
- 16. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the third national health and nutrition examination survey, 1988–1991. Hypertension 1995; 25: 305–313. [DOI] [PubMed] [Google Scholar]
- 17. Wiinberg N, Hoegholm A, Christensen HR, et al. Twenty-four hour ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens 1995; 8: 978–986. [DOI] [PubMed] [Google Scholar]
- 18. Kannel WB. The Framingham study: Historical insight on the impact of cardiovascular risk factors in men versus women. J Gend Specif Med 2002; 5: 27–37. [PubMed] [Google Scholar]
- 19. Franconi F, Brunelleschi S, Steardo L, et al. Gender differences in drug responses. Pharmacol Res 2007; 55: 81–95. [DOI] [PubMed] [Google Scholar]
- 20. Franconi F, Carru C, Spoletini I, et al. A GENS-based approach to cardiovascular pharmacology: Impact on metabolism, pharmacokinetics and pharmacodynamics. Ther Deliv 2011; 2: 1437–1453. [DOI] [PubMed] [Google Scholar]
- 21. Oertelt-Prigione S, Regitz-Zagrosek V. Gender aspects in cardiovascular pharmacology. J Cardiovasc Transl Res 2009; 2: 258–266. [DOI] [PubMed] [Google Scholar]
- 22. Wing LM, Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin-converting-enzyme inhibitors and diuretics for hypertension in the elderly. New Engl J Med 2003; 348: 583–592. [DOI] [PubMed] [Google Scholar]
- 23. Safar ME, Smulyan H. Hypertension in women. Am J Hypertens 2004; 17: 82–87. [DOI] [PubMed] [Google Scholar]
- 24. Hilliard LM, Mirabito KM, Denton KM. Unmasking the potential of the AT(2)R as a therapeutic target in hypertension in men and women: What we know and what we still need to find out. Clin Exp Pharmacol Physiol 2013; 40: 542–550. [DOI] [PubMed] [Google Scholar]
- 25. Denton KM, Hilliard LM, Tare M. Sex-related differences in hypertension: Seek and ye shall find. Hypertension 2013; 62: 674–677. [DOI] [PubMed] [Google Scholar]
- 26. Cohall DH, Scantlebury-Manning T, James S, et al. Renin-angiotensin-aldosterone system gender differences in an Afro-Caribbean population. J Renin Angiotensin Aldosterone Syst 2015; 16: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Y, Wang D, Yang X, et al. Effect of menopausal status on carotid intima-media thickness and presence of carotid plaque in Chinese women generation population. Sci Rep 2015; 5: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hilliard LM, Sampson AK, Brown RD, et al. The “his and hers” of the renin-angiotensin system. Curr Hypertens Rep 2013; 15: 71–79. [DOI] [PubMed] [Google Scholar]
- 29. Yanes LL, Romero DG, Iliescu R, et al. Postmenopausal hypertension: Role of the renin-angiotensin system. Hypertension 2010; 56: 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsuda M, Iwai M, Li JM, et al. Inhibitory effects of AT1 receptor blocker, olmesartan and estrogen on atherosclerosis via anti-oxidative stress. Hypertension 2005; 45: 545–551. [DOI] [PubMed] [Google Scholar]
- 31. Liu H-W, Iwai M, Takeda-Matsubara Y, et al. Effect of estrogen and AT1 receptor blocker on neointima formation. Hypertension 2002; 40: 450–457. [DOI] [PubMed] [Google Scholar]
- 32. Mirza FS, Ong P, Collins P, et al. Effects of estradiol and the angiotensin II receptor blocker irbesartan on vascular function in postmenopausal women. Menopause 2008; 15: 44–50. [DOI] [PubMed] [Google Scholar]
- 33. Franklin SS, Jacobs MJ, Wong ND, et al. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: Analysis based on the National Health and Nutrition Examination Survey III (NHANES III). Hypertension 2001; 37: 869–874. [DOI] [PubMed] [Google Scholar]
- 34. Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med 1993; 153: 598–615. [DOI] [PubMed] [Google Scholar]
- 35. Lewington S, Clarke R, Qizilbash N, et al. Prospective studies collaboration age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for 1 million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913. [DOI] [PubMed] [Google Scholar]
- 36. Nakamura K, Barzi F, Lam TH, et al. Cigarette smoking, systolic blood pressure and cardiovascular diseases in the Asia-Pacific region. Stroke 2008; 39: 1694–1702. [DOI] [PubMed] [Google Scholar]
- 37. Yun Gao, Gang Chen, Haoming Tian, et al. Prevalence of hypertension in China: A cross-sectional study. PLoS One 2013; 8: e65938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park JB, Kario K, Wang JG. Systolic hypertension: An increasing clinical challenge in Asia. Hypertens Res 2015; 38: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]