Abstract
Introduction:
Adiponectin (ADIPOQ), involved in regulating glucose levels and fatty acid oxidation, plays key roles in metabolic derangements such as gestational diabetes mellitus (GDM). Previously, several studies have been conducted to assess the association between ADIPOQ +45 T/G polymorphism and risk of GDM. The results, however, are inconclusive. We aimed to evaluate the effect of the polymorphism on the risk of GDM using a meta-analysis.
Materials and methods:
After databases searching, eight records were identified. Pooled odds ratios (ORs) with their corresponding 95% confidence intervals (CIs) were used to evaluate the association between ADIPOQ +45 T/G polymorphism and risk of GDM.
Results:
No significant association was observed between the ADIPOQ +45 T/G polymorphism and the risk of GDM (heterozygote comparison: OR = 1.15, 95% CI, 0.70–1.89; homozygote comparison: OR = 1.21, 95% CI, 0.48–3.03; dominant model: OR = 0.86, 95% CI, 0.50–1.48, recessive model: OR = 1.21, 95% CI, 0.62–2.33, and allele comparison: OR = 1.17, 95% CI, 0.79–1.76, respectively). Apparent heterogeneity was detected. However, no evidence of publication bias was found.
Conclusions:
This meta-analysis provides evidence that the ADIPOQ +45 T/G polymorphism was not related to the risk of GDM. Further multicenter, prospective studies with larger sample size would be valuable to confirm the result.
Keywords: Gestational diabetes mellitus, adiponectin, polymorphism, meta-analysis, susceptibility
Introduction
Gestational diabetes mellitus (GDM), characterized as glucose intolerance firstly occurring or recognized during pregnancy, is an important public health problem worldwide.1,2 In China, the incidence increased during 1999–2013, from 2.4% to 12.1%.3,4 Women with GDM possess high risks of adverse pregnancy outcomes and several maternal and perinatal complications, including neonatal hypoglycemia and adiposity, metabolic syndrome and future diabetes mellitus.5–9 Regarding risk factors for GDM, several environmental triggers have been identified so far, such as obesity, poor diet and physical inactivity.10–12 In addition, genetic components have also been reported to contribute to the development of GDM.13–15
Adiponectin (ADIPOQ) is a protein hormone involved in regulating glucose levels and fatty acid oxidation.16,17 The hormone plays a key role in the metabolic derangements that may lead to type 2 diabetes, atherosclerosis, obesity, metabolic syndrome and insulin resistance.16,18–20 The ADIPOQ gene, located on chromosome 3q27 in humans, contains three exons. A single nucleotide polymorphism (SNP) in exon 2 of ADIPOQ (+45 T/G, rs2241766) may influence the protein expression by affecting RNA splicing and stability.21 The SNP has been shown to be related to type 2 diabetes and insulin resistance.22–24
Given the potential role of the ADIPOQ +45 T/G polymorphism in diabetes, several association studies have been conducted to assess the association between the polymorphism and risk of GDM.25–32 The results, however, are inconsistent and inconclusive. For example, Takhshid et al. reported that the TG/GG genotypes had a 2.55-fold increased risk of GDM compared with the TT genotype,31 whereas Beltcheva et al. reported that no association with GDM was found for the polymorphism.25 One reason for the conflicting results is small samples in a single study. Meta-analysis is a useful tool to increase power by pooling all the published data together. In this study, we performed a meta-analysis to clarify whether the ADIPOQ +45 T/G polymorphism is associated with the risk of GDM.
Materials and methods
Records selection
PubMed, China National Knowledge Infrastructure, Weipu and Wangfang databases were searched using the following terms: “ADIPOQ or adiponectin” and “polymorphism* or variant” and “gestational diabetes mellitus” or “Gestational diabetes” (last search update October 30, 2015). Forty-one articles were identified after database searching. We selected publications using the following criteria: (a) studies evaluating the association between ADIPOQ +45 T/G polymorphism and the risk of GDM; (b) original genotyping data to compute pooled odds ratios (ORs) and 95% confidence interval (CI); (c) language was not restricted. Among them, 18 articles were removed for duplicate publication. After full-text assessment, 12 articles were excluded for these reasons: no ADIPOQ +45 T/G polymorphism (n = 8), reviews (n = 3), and not a study of GDM (n = 1). During data collection, we excluded three articles for repeated data. Finally, eight studies were included in this meta-analysis. A flow diagram of studies selection is presented in Figure 1.
Figure 1.
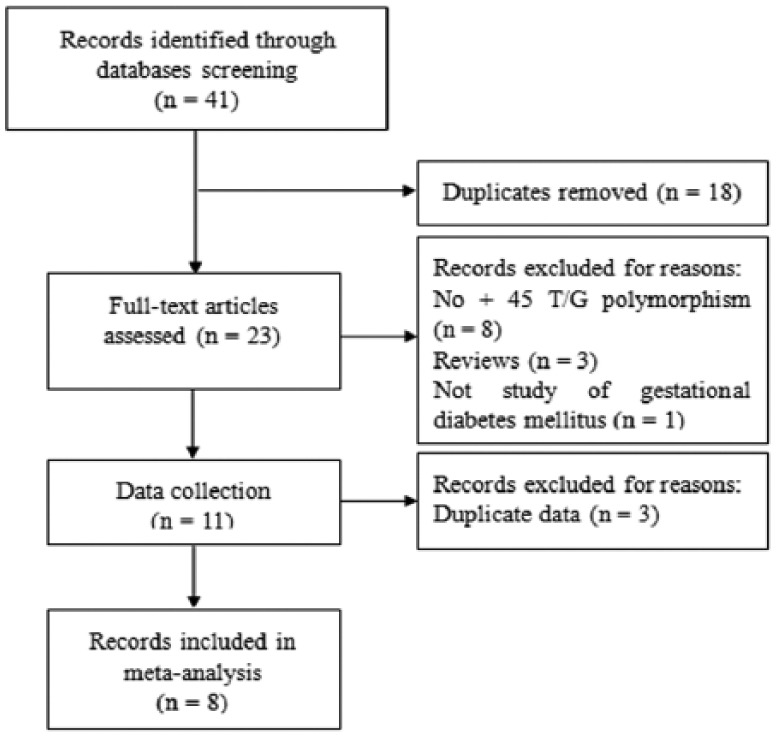
Flow diagram of studies selection.
Data extraction
The following information was extracted by two researchers independently: author, year of publication, country (origin), ethnicity, study design, number of cases and controls, matching criteria, genotyping method, and minor allele frequency in controls. The information was checked by a third researcher to confirm the accuracy.
Statistical analysis
The association between the ADIPOQ +45 T/G polymorphism and the risk of GDM was evaluated by pooled ORs and their 95% CI under a heterozygote comparison, homozygote comparison, dominant model, recessive model, and allele comparison. A fixed-effects model was used to estimate summary ORs because p values for heterogeneity test were ⩽ 0.10 in all comparison analyses.33 To identify the sources of heterogeneity, subgroup analyses were performed based on the Hardy-Weinberg equilibrium (HWE) and sample size (>100 vs. ⩽100). Logistic meta-regression was also performed according to ethnicity, genotyping methods, HWE, and sample size. Begg’s funnel plot was used to test publication bias.34 All analyses were conducted using STATA software (version 10.0, STATA Corporation, College Station, TX, USA).
Results
Characteristics of studies
The characteristics of the included studies are shown in Table 1. In total, eight studies were included in this meta-analysis with 875 cases and 884 controls. Among them, seven studies were of Asian descendants26–32 and one study was of European descendants.25 The sample sizes were relatively small in all the studies, with the least having 79 participants30 and the most having 436 participants.28 Most of the studies (62.5%) reported a matched frequency of controls to cases.25,27,29,31,32 Several genotyping methods were used, including TaqMan, polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), and DNA sequencing. However, only two studies reported quality control to ensure the reproducibility of the genotyping assay.25,31 Most of the studies (75.0%) were consistent with HWE except for one study departing from HWE27 and one study without available data to assess HWE.25 Minor allele frequency in controls varied greatly, ranging from 0.10 to 0.49.
Table 1.
Characteristics of studies included in this meta-analysis.
| First authorreference | Year of publication | Country (region) | Ethnicity | Study design | Number of cases | Controls | Matching criteria | Genotyping method | MAF in controls |
|---|---|---|---|---|---|---|---|---|---|
| Beltcheva25 | 2014 | Bulgaria (Sofia) | European | Case-control | 130 | 130 | Age and degree of obesity | TaqMan | 0.10 |
| Han26 | 2015 | China (Nantong) | Asian | Cross-section | 128 | 140 | – | PCR-RFLP | 0.26 |
| Hua27 | 2014 | China (Wuxi) | Asian | Case-control | 112 | 150 | Age and gestational week | PCR-RFLP | 0.49 |
| Li28 | 2013 | China (Beijing) | Asian | Case-control | 264 | 172 | – | Sequencing | 0.24 |
| Liu29 | 2006 | China (Wuxi) | Asian | Case-control | 52 | 34 | Age and gestational week | PCR-RFLP | 0.47 |
| Low30 | 2011 | Malaysia (Selangor) | Asian | Case-control | 26 | 53 | – | PCR-RFLP | 0.18 |
| Takhshid31 | 2015 | Iran (Shiraz) | Asian | Case-control | 65 | 70 | Age | PCR-RFLP | 0.11 |
| Zhang32 | 2014 | China (Nanning) | Asian | Case-control | 98 | 135 | Age, BMI and gestational week | PCR-RFLP | 0.27 |
MAF: minor allele frequency; PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphism; BMI: body mass index.
Meta-analysis
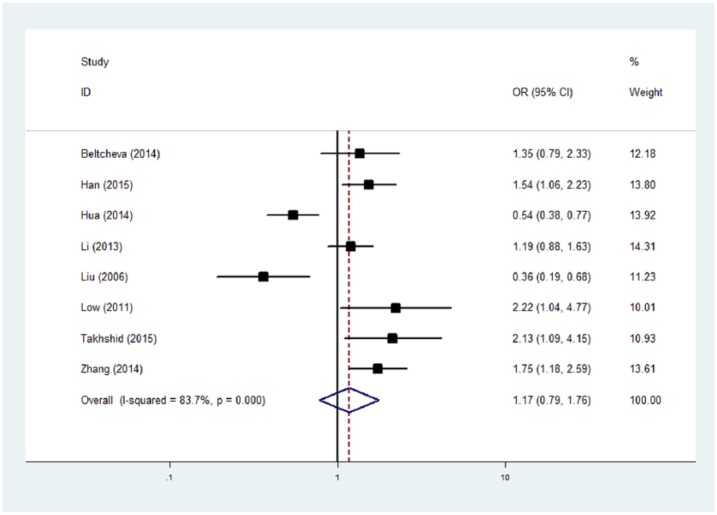
As shown in Table 2, no significant association was observed between the ADIPOQ +45 T/G polymorphism and the risk of GDM (heterozygote comparison: OR = 1.15, 95% CI, 0.70–1.89; homozygote comparison: OR = 1.21, 95% CI, 0.48–3.03; dominant model: OR = 0.86, 95% CI, 0.50–1.48, recessive model: OR = 1.21, 95% CI, 0.62–2.33, and allele comparison: OR = 1.17, 95% CI, 0.79–1.76, respectively) (Figure 2). After excluding two studies with Hardy-Weinberg disequilibrium, no significant association was found. Additionally, we performed subgroup analysis according to sample size (> 100 and ⩽100); negative results were also observed in both subgroups.
Table 2.
Meta-analysis of the ADIPOQ +45 T/G polymorphism with the risk of gestational diabetes mellitus.
| n | Cases/controls | TG vs. TT |
GG vs. TT |
Dominant |
Recessive |
G vs. T |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI)a | p b | OR (95% CI)a | p b | OR (95% CI)a | p b | OR (95% CI)a | p b | OR (95% CI)a | p b | |||
| Total | 8 | 875/884 | 1.15 (0.70–1.89) | <0.001 | 1.21 (0.48–3.03) | <0.001 | 0.86 (0.50–1.48) | <0.001 | 1.21 (0.62–2.33) | 0.01 | 1.17 (0.79–1.76) | <0.001 |
| HWE | 6 | 763/734 | 1.35 (0.84–2.17) | 0.006 | 1.54 (0.56–4.11) | 0.004 | 0.72 (0.43–1.19) | 0.001 | 1.49 (0.77v2.88) | 0.10 | 1.33 (0.93–1.90) | 0.001 |
| Sample size | ||||||||||||
| >100 | 6 | 797/797 | 1.29 (0.83–2.01) | 0.01 | 1.46 (0.55–3.83) | 0.001 | 0.76 (0.45–1.28) | <0.001 | 1.34 (0.64v2.82) | 0.01 | 1.27 (0.86–1.89) | <0.001 |
| ⩽100 | 2 | 78/87 | 0.73 (0.07–7.88) | 0.001 | 0.93 (0.03–29.22) | 0.01 | 1.35 (0.11–16.57) | <0.001 | 1.05 (0.12–9.44) | 0.09 | 0.88 (0.15–5.26) | <0.001 |
ADIPOQ: adiponectin; OR: odds ratio; CI: confidence interval; HWE: Hardy-Weinberg equilibrium.
Random-effects model was used.
P value for heterogeneity test.
Figure 2.
Meta-analysis of the ADIPOQ +45 T/G polymorphism with the risk of gestational diabetes mellitus (G vs. T). The random-effects model was used.
Heterogeneity analysis and publication bias
In overall and subgroup analyses, we detected apparent heterogeneity (p ⩽ 0.10). Logistic meta-regression was used to explore the sources of heterogeneity. We took the common variables influencing heterogeneity into consideration: ethnicity, genotyping methods, HWE, and sample size. However, none of these factors affected the heterogeneity among studies.
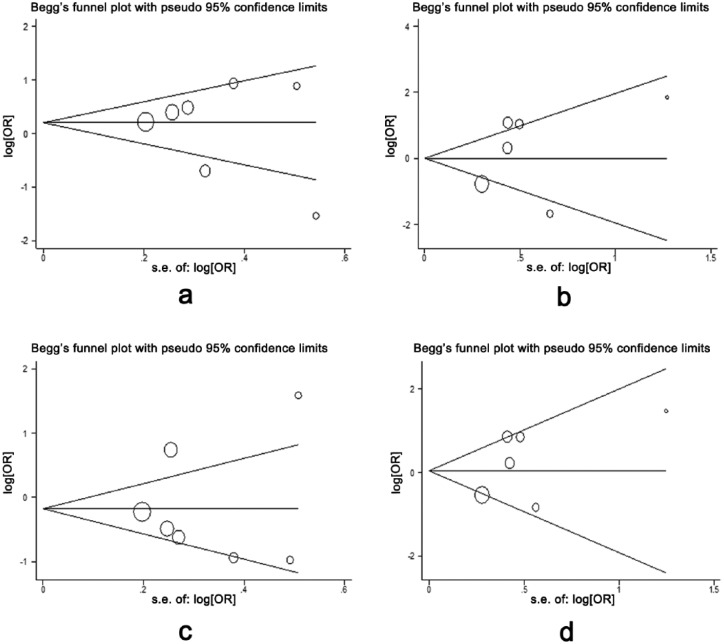
Begg’s funnel plot was used to test publication bias, and no evidence of publication bias was found (p > 0.05) in the meta-analysis (Figure 3).
Figure 3.
Begg’s funnel plot for testing publication bias. Each circle indicates a separate study. (a) TG vs. TT; (b) GG vs. TT; (c) dominant model; (d) recessive model. OR: odds ratio.
Discussion
To the best of our knowledge, this is the first meta-analysis to investigate the relationship of the ADIPOQ +45 T/G polymorphism with GDM. We failed to find any association between the SNP and the risk of GDM, suggesting that the ADIPOQ +45 T/G polymorphism may not be a risk factor for the etiology of GDM.
Previously, several SNPs have been performed to evaluate the association with GDM, such as rs2241766 (Gly15Gly, +45 T/G) in exon 2, rs266729 (−11377 C/G) and rs17300539 (−11391G/A) in the promoter region, and rs1501299 (+276 G/T) in intron 2.25–32 Among them, the ADIPOQ +45 T/G polymorphism is the most frequently investigated. Controversial results, however, have been obtained. In this study, we collected all previous publications and performed a meta-analysis to assess the association of the ADIPOQ +45 T/G polymorphism with the risk of GDM. Our meta-analysis provided evidence that the polymorphism was not a risk factor for the development of GDM although 75% (six of eight) studies reported a positive result.26,27,29–32 After subgroup analysis according to HWE and sample size, null results were also observed.
Obvious heterogeneity was detected among studies. We used logistic meta-regression to examine the sources of heterogeneity based on ethnicity, genotyping methods, HWE, and sample size. None of these parameters could explain the heterogeneity, indicating that other unknown reasons may influence the heterogeneity. As most of the studies previously reported a significant association between the ADIPOQ +45 T/G polymorphism and susceptibility for GDM, in this meta-analysis we used Begg’s funnel plot to test if there is a tendency of prior publication positive results. However, no evidence of publication bias was found.
These are some limitations in this study. Firstly, sample size is very small in each single study as well as in this meta-analysis, which may result in false-negative result due to insufficient power. Secondly, very limited data in the literature reported the association between the ADIPOQ +45 T/G polymorphism and serum adiponectin level, which prevented further genotype-phenotype analysis. Thirdly, nearly all the studies included in this meta-analysis were of Asian descendants, and thus the results cannot be directly extended to other ethnicities. Further evaluation is warranted, especially in multi-ethnic populations.
In conclusion, this meta-analysis provides evidence that the ADIPOQ +45 T/G polymorphism was not related to the risk of GDM. Further multicenter, prospective studies with larger sample size would be of great value to confirm the result. Additionally, gene-gene and genotype-phenotype analysis may help to comprehensively understand the role of the SNP in the etiology of GDM.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Natural Science Foundation of China (grant number 81520108013).
References
- 1. Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009; 361: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reece EA, Leguizamon G, Wiznitzer A. Gestational diabetes: The need for a common ground. Lancet 2009; 373: 1789–1797. [DOI] [PubMed] [Google Scholar]
- 3. Zhang F, Dong L, Zhang CP, et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabet Med 2011; 28: 652–657. [DOI] [PubMed] [Google Scholar]
- 4. Zhu WW, Fan L, Yang HX, et al. Fasting plasma glucose at 24–28 weeks to screen for gestational diabetes mellitus: New evidence from China. Diabetes Care 2013; 36: 2038–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wendland EM, Torloni MR, Falavigna M, et al. Gestational diabetes and pregnancy outcomes—a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012; 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Retnakaran R, Qi Y, Sermer M, et al. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care 2008; 31: 2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 8. Kubo A, Ferrara A, Windham GC, et al. Maternal hyperglycemia during pregnancy predicts adiposity of the offspring. Diabetes Care 2014; 37: 2996–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009; 373: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 10. Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med 2004; 21: 103–113. [DOI] [PubMed] [Google Scholar]
- 11. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: Review of epidemiologic evidence. Am J Clin Nutr 2011; 94 (6 Suppl): 1975S-1979S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007; 30: 2070–2076. [DOI] [PubMed] [Google Scholar]
- 13. Zhang C, Bao W, Rong Y, et al. Genetic variants and the risk of gestational diabetes mellitus: A systematic review. Hum Reprod Update 2013; 19: 376–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lambrinoudaki I, Vlachou SA, Creatsas G. Genetics in gestational diabetes mellitus: Association with incidence, severity, pregnancy outcome and response to treatment. Curr Diabetes Rev 2010; 6: 393–399. [DOI] [PubMed] [Google Scholar]
- 15. Shaat N, Groop L. Genetics of gestational diabetes mellitus. Curr Med Chem 2007; 14: 569–583. [DOI] [PubMed] [Google Scholar]
- 16. Diez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 2003; 148: 293–300. [DOI] [PubMed] [Google Scholar]
- 17. Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A 2001; 98: 2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Renaldi O, Pramono B, Sinorita H, et al. Hypoadiponectinemia: A risk factor for metabolic syndrome. Acta Med Indones 2009; 41: 20–24. [PubMed] [Google Scholar]
- 19. Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7: 941–946. [DOI] [PubMed] [Google Scholar]
- 20. Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006; 116: 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang WS, Tsou PL, Lee WJ, et al. Allele-specific differential expression of a common adiponectin gene polymorphism related to obesity. J Mol Med (Berl) 2003; 81: 428–434. [DOI] [PubMed] [Google Scholar]
- 22. Hara K, Boutin P, Mori Y, et al. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes 2002; 51: 536–540. [DOI] [PubMed] [Google Scholar]
- 23. Menzaghi C, Ercolino T, Di Paola R, et al. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes 2002; 51: 2306–2312. [DOI] [PubMed] [Google Scholar]
- 24. Li LL, Kang XL, Ran XJ, et al. Associations between 45T/G polymorphism of the adiponectin gene and plasma adiponectin levels with type 2 diabetes. Clin Exp Pharmacol Physiol 2007; 34: 1287–1290. [DOI] [PubMed] [Google Scholar]
- 25. Beltcheva O, Boyadzhieva M, Angelova O, et al. The rs266729 single-nucleotide polymorphism in the adiponectin gene shows association with gestational diabetes. Arch Gynecol Obstet 2014; 289: 743–748. [DOI] [PubMed] [Google Scholar]
- 26. Han Y, Zheng YL, Fan YP, et al. Association of adiponectin gene polymorphism 45TG with gestational diabetes mellitus diagnosed on the new IADPSG criteria, plasma adiponectin levels and adverse pregnancy outcomes. Clin Exp Med 2015; 15: 47–53. [DOI] [PubMed] [Google Scholar]
- 27. Hua P. Association of adiponectin polymorphism with the risk of gestational diabetes mellitus [article in Chinese]. Maternal Child Health Care China 2014; 29: 1742–1744. [Google Scholar]
- 28. Li GH, Kong LJ, Zhang L, et al. Association of adiponectin gene polymorphisms +45T/G with gestational diabetes mellitus and neonate birth weight [article in Chinese]. Zhonghua Yi Xue Za Zhi 2013; 93: 3770–3772. [PubMed] [Google Scholar]
- 29. Liu L, Sun L, Xu J. Association between adiponectin polymorphism and gestational diabetes mellitus [article in Chinese]. Guangdong Med J 2006; 27: 1822–1824. [Google Scholar]
- 30. Low CF, Mohd Tohit ER, Chong PP, et al. Adiponectin SNP45TG is associated with gestational diabetes mellitus. Arch Gynecol Obstet 2011; 283: 1255–1260. [DOI] [PubMed] [Google Scholar]
- 31. Takhshid MA, Haem Z, Aboualizadeh F. The association of circulating adiponectin and + 45 T/G polymorphism of adiponectin gene with gestational diabetes mellitus in Iranian population. J Diabetes Metab Disord 2015; 14: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang C, Liang X. The relationship between human adiponectin gene polymorphism and gestational diabetes mellitus in Guangxi Zhuang [article in Chinese]. J Chin Physician 2014; 16: 1221–1223. [Google Scholar]
- 33. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 34. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]