Abstract
Aim:
The purpose of this study was to explore whether mTOR/p70S6K1 signaling is activated in renal fibrosis of immunoglobulin A nephropathy.
Methods:
Seventy-two children with immunoglobulin A nephropathy were divided into three groups according to their clinical features and pathological grades. Six normal renal specimens were included in the control group. The expression levels of angiotensin II, mTOR, p70S6K1, E-cadherin, and α-smooth muscle actin in renal tissues were determined by immunohistochemistry method, the potential correlations of these indexes and relationship between these indexes and the clinicopathological indexes were analyzed.
Results:
Compared to the control group, the expression levels of angiotensin II, mTOR, p70S6K1, and α-smooth muscle actin were significantly higher and the expression levels of E-cadherin were lower both in glomeruli and tubulointerstitium of immunoglobulin A nephropathy children. And the most significant differences were found in the nephrotic syndrome group and pathological grade IV group. In immunoglobulin A nephropathy renal tissues, the expression levels of angiotensin II in glomeruli and tubulointerstitium were both positively correlated with the expression levels of mTOR and α- smooth muscle actin, and negatively correlated with the expression levels of E-cadherin.
Conclusion:
The mTOR/p70S6K1 signaling was activated in renal tissues of children with immunoglobulin A nephropathy, and future studies will need to address the mechanism of mTOR/p70S6K1 signaling in the progress of renal fibrosis in immunoglobulin A nephropathy.
Keywords: Angiotensin II, mTOR, p70S6K1, immunoglobulin A nephropathy, renal fibrosis, children
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis around the world, especially in the Asia-Pacific area. It accounts for 36.3% of the primary glomerulopathies in China. In children, there is almost 17.2% of IgAN found in primary glomerulopathies with renal biopsy.1 The progress of IgAN is repeated and persistent, and the prognosis is often not good. A study shows that approximately 30% of children with IgAN have progressed to end stage renal disease (ESRD) through 20 years follow-up.2 The common changes of pathological morphology in renal tissues of ESRD mainly present as glomerular sclerosis and renal interstitial fibrosis.
It has been confirmed that angiotensin II (Ang II) plays a key role in the progress of kidney fibrosis in IgAN, which may be related to the activation of renin-angiotensin system (RAS) by abnormal glycosylation of immunoglobulin A1 (IgA1) deposited in kidney, and initiation of a sequence of inflammation reaction and injury.3–5 Mammalian target of rapamycin (mTOR) is one of the atypical Ser/Thr protein kinases, which widely exist in many kinds of biological cells. It can control cell growth and proliferation by participating in the process of transcription, ribosome synthesis, translation, autophagy, and the structure of actin skeleton.6,7 It has shown in recent studies that the mTOR/p70S6K1 signaling pathway is widely involved in the renal fibrosis process in polycystic kidney disease, membranous nephropathy, unilateral ureteral obstruction (UUO) renal fibrosis, acute kidney injury, and diabetic nephropathy,8–15 but whether this signaling pathway has an important part in the renal fibrosis of IgAN is still unclear.
This study investigates the expression levels of AngⅡ, mTOR, p70S6K1, E-cadherin, and α-smooth muscle actin (α-SMA) in the renal tissues of children with IgAN, analyzes the potential correlations of these indexes as well as relationship between these indexes and the clinical pathology indexes, and try to explore whether the mTOR/p70S6K1 signaling pathway is activated in the progress of renal fibrosis of children with IgAN.
Materials and methods
Clinical data
From April 2007–April 2011, 78 children with primary IgAN were diagnosed in the Pediatric Nephrology Center of the Department of Pediatrics in the First Affiliated Hospital of Sun Yat-sen University, China. One child who coexisted with Crohn’s disease and five children who had received angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) treatments were excluded. Finally, 72 children were included in the study. Fifty-six were boys and 16 were girls, male: female ratio was 3.5:1. The median children age was 10.2, 3.0–14.0 years. The course of illness in the study, defined from the first time diagnosed with primary IgAN by renal biopsy, was from four days to nine years, and the median course of illness was 78 days. Six normal renal specimens were included in the control group, five of them were the kidney tissues adjacent to neoplastic lesions and the one sample was from a health donor who was not successfully matched before kidney transplant. There were four boys and two girls in the control group, and the median age was 7.8 years (from 4.2–13.8 years). This study was approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University, and was performed in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki. All of the children’s guardians had signed the consent form before the research.
Inclusion and exclusion criteria
Children having renal biopsy for the first time were included.
Children under 14 years old were included.
The renal samples of children had to correspond to the diagnosis standard of primary IgAN, the immunoglobulin, mainly IgA, deposited in the glomerularmesangial area and/or capillary loops.
Children with secondary IgAN, such as Henoch-Schönleinpurpura (HSP), systemic lupus erythematosus (SLE), and chronic liver diseases were excluded.
Children who had received glucocorticoids, immunosuppressants, ACEI and ARB treatments were excluded.
Cases groups
According to the standard developed by the Subspecialty Group of Nephrology, the Society of Pediatrics, Chinese Medical Association in 2001, 72 children with IgAN were divided into three groups based on the clinical features, 24 cases in the isolated hematuria (IH) group, 26 cases in the hematuria and proteinuria (HP) group and 22 cases in the nephrotic syndrome (NS) group.16,17 IH means the isolated microscopic hematuria and the isolated gross hematuria. HP is characterized by proteinuria and microscopic hematuria or gross hematuria, and 24 h urinary protein (Upro) less than 50 mg/kg. NS is defined as proteinuria, hypoalbuminemia with or without hypercholesterolemia and edema.
Based on the World Health Organization (WHO) pathological classification standard in 1982,18 children with IgAN were divided into three groups, 33 cases in the pathology II group, 25 cases in the pathology III group and 14 cases in the pathology IV group.
Research methods
The clinical baseline data of all included children in the study were collected, such as age, gender, course of illness, height, weight, and mean arterial pressure (MAP).
Fasting peripheral venous bloods (2 ml) were collected with separation gel tubes, after 30 min at room temperature environment, isolated serums were centrifuged under 10 min with 3500 r/min. Serum blood urea nitrogen (BUN) and serum creatinine (Scr) levels were detected by a 7170A automatic biochemical analyzer. Serum IgA level was tested by immunoturbidimetry.
Urine specimens (24 h) were collected from all IgAN children on the day before renal biopsy and the exact urine volumes were recorded with graduated cylinders. Fifteen ml of mixed urine samples were used for testing 24 h urinary protein by the sulfosalicylic acid method.
The renal tissues of children with IgAN were scored according to the Katafuchi semi-quantitative criteria.19 The IgA deposition intensity in glomeruli and tubulointerstitium was measured by direct immunofluorescence assay. The expression levels of Ang II, mTOR, p70S6K1, E-cadherin, and α-SMA in renal tissues were measured by immunohistochemistry method (two-step PowerVision). Kidney tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Consecutive sections of 4 μm were deparaffinized in xylene, antigen retrieval was performed with 1 mM EDTA, then incubated with the following antibodies. Primary antibodies included rabbit anti-human AngⅡantibody (Phoenix, USA), rabbit anti-human mTOR antibody (Cell Signal Technology, USA), rabbit anti-human p70S6K1 antibody (Millipor, USA), rabbit anti-human E-cadherin antibody and rabbit anti-humanα-SMA antibody (ABcam, UK). Secondary antibodies were mouse/rabbit two-step detection kit PV-7000 (Beijing Zhong Shan Jinqiao Biological Technology Co. Ltd, China). Sections were analyzed with the ZEISS Axio Imager Z1 microscope and the Image-Pro Plus 6.0 software (Media Cybernetics). The percentages of Ang II, mTOR, p70S6K1, E-cadherin, and α-SMA in the glomeruli and tubulointerstitium were calculated by dividing the positive staining area by the total area of the glomeruli and tubulointerstitium respectively.
Statistical analysis
Statistical analyses were conducted using SPSS (version 20.0). Data are shown as mean±standard deviation (SD), with statistical analyses performed using one-way analysis of variance. Pearson correlation analysis was used for correlation analysis between two groups. A value of p<0.05 was considered statistically significant.
Results
The expression levels and localizations of Ang II, mTOR, p70S6K1, E-cadherin, and α-SMA in the normal and IgAN kidneys
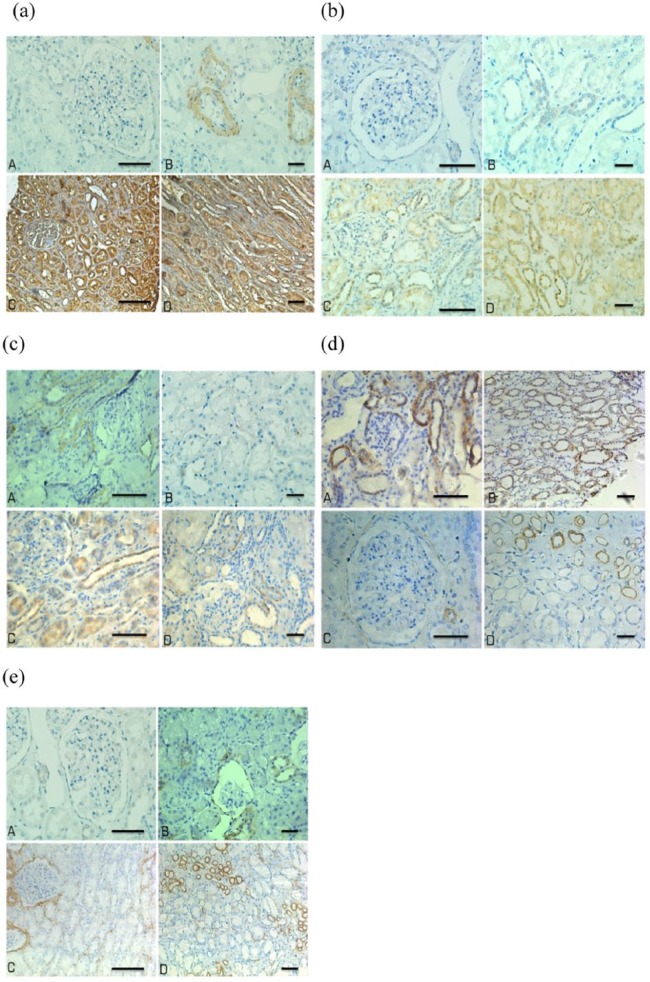
In children with IgAN, the expression levels of Ang II, mTOR, p70S6K1, and α-SMA were significantly higher and E-cadherin were significantly lower than the control group both in glomeruli and tubulointerstitium (p<0.05 or p<0.01) (Tables 1 and 2, Figure 1).
Table 1.
The expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, and α-smooth muscle actin (α-SMA) in glomeruli of the control and immunoglobulin A nephropathy (IgAN) kidneys ( ± s).
| Group | Cases | Glomeruli |
||||
|---|---|---|---|---|---|---|
| Ang II | mTOR | p70S6K1 | E-cadherin | α-SMA | ||
| Control group | 6 | 5.74 ± 1.82 | 4.37 ± 0.95 | 2.51 ± 0.64 | 9.75 ± 1.01 | 1.22 ± 0.35 |
| IgAN group | 72 | 20.70 ± 4.02a | 13.71 ± 1.58a | 7.10 ± 2.12b | 2.03 ± 1.24b | 9.73 ± 1.79a |
Compared with control group, p<0.01; bcompared with control group, p<0.05.
Table 2.
The expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, and α-smooth muscle actin (α-SMA) in tubulointerstitium of the control and immunoglobulin A nephropathy (IgAN) kidneys ( ± s).
| Group | Cases | Tubulointerstitium |
||||
|---|---|---|---|---|---|---|
| Ang II | mTOR | p70S6K1 | E-cadherin | α-SMA | ||
| Control group | 6 | 4.05 ± 1.58 | 5.51 ± 1.32 | 2.82 ± 0.86 | 16.09 ± 2.12 | 1.32 ± 0.25 |
| IgAN group | 72 | 19.53 ± 4.11a | 16.53 ± 3.25a | 10.02 ± 3.78a | 4.17 ± 1.31a | 12.38 ± 3.64a |
Compared with control group, p<0.01.
Figure 1.
The expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, and α-smooth muscle actin (α-SMA) in the glomeruli and tubulointerstitium of normal and immunoglobulin A nephropathy (IgAN) kidneys. (a) Ang II; (b) mTOR; (c) p70S6K1; (d) E-cadherin; (e) α-SMA. A and B are the normal renal tissues, C and D are the IgAN renal tissues. Bar denotes 40 μm.
The expression levels of Ang II, mTOR, p70S6K1, E-cadherin, and α-SMA in different clinical classifications
In glomeruli the highest expression levels of mTOR and p70S6K1 were found in the NS group (p<0.05 or p<0.01), while there were no significant differences on the expression levels of Ang II, E-cadherin, and α-SMA among different clinical groups (p>0.05) (Table 3).
Table 3.
The expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, and α-smooth muscle actin (α-SMA) in different clinical classifications in glomeruli ( ± s).
| Group | Cases | Glomeruli |
||||
|---|---|---|---|---|---|---|
| Ang II | mTOR | p70S6K1 | E-cadherin | α-SMA | ||
| Control group | 6 | 5.74 ± 1.82 | 4.37 ± 0.95 | 2.51 ± 0.64 | 9.75 ± 1.01 | 1.22 ± 0.35 |
| IH group | 24 | 20.53 ± 3.45a | 8.18 ± 0.72b | 6.27 ± 0.11a | 2.05 ± 0.21a | 8.90 ± 1.12a |
| HP group | 26 | 19.72 ± 1.23a | 9.22 ± 3.12a | 6.74 ± 0.21a | 2.13 ± 0.34a | 9.77 ± 0.15a |
| NS group | 22 | 21.60 ± 2.45a | 19.32 ± 2.33a,c,e | 10.11 ± 2.0a,d,e | 1.94 ± 0.89a | 10.74 ± 2.31a |
IH: isolated hematuria; HP: hematuria and proteinuria; NS: nephrotic syndrome.
Compared with control group, ap<0.01, bp<0.05; compared with IH group, cp<0.01, dp<0.05; compared with HP group, ep<0.05.
In tubulointerstitium the highest expression levels of mTOR, p70S6K1, α-SMA, and the lowest expression levels of E-cadherin were found in the NS group (p<0.05 or p<0.01), while there were no significant differences on the expression levels of Ang II among different clinical groups (p>0.05) (Table 4).
Table 4.
The expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, and α-smooth muscle actin (α-SMA) in different clinical classifications in tubulointerstitium ( ± s).
| Group | Cases | Tubulointerstitium |
||||
|---|---|---|---|---|---|---|
| Ang II | mTOR | p70S6K1 | E-cadherin | α-SMA | ||
| Control group | 6 | 4.05 ± 1.58 | 5.51 ± 1.32 | 2.82 ± 0.86 | 16.09 ± 2.12 | 1.32 ± 0.25 |
| IH group | 24 | 18.53 ± 5.21a | 15.18 ± 3.11a | 8.27 ± 1.31a | 4.15 ± 1.12a | 9.90 ± 2.93a |
| HP group | 26 | 18.92 ± 7.30a | 14.22 ± 1.66a | 10.24 ± 0.99a | 3.93 ± 0.35a | 11.77 ± 2.87a |
| NS group | 22 | 21.60 ± 3.11a | 21.32 ± 3.42a,b,c | 13.11 ± 3.12a,b,c | 1.94 ± 0.17a,b,c | 16.77 ± 2.11a,b,c |
Compared with control group, ap<0.01; compared with IH group, bp<0.05; compared with HP group, cp<0.05.
The expression levels of Ang II, mTOR, p70S6K1, E-cadherin, and α-SMA in different pathological grades
In glomeruli the expression levels of Ang II, mTOR, p70S6K1, and α-SMA were higher in group III and group IV than those in the grade II group and control group (p<0.05 or p<0.01), while there were no significant differences in the expression levels of E-cadherin between different pathological grade groups (p>0.05) (Table 5).
Table 5.
The expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, and α-smooth muscle actin (α-SMA) in different pathological grades in glomeruli ( ± s).
| Group | Cases | Glomeruli |
||||
|---|---|---|---|---|---|---|
| Ang II | mTOR | p70S6K1 | E-cadherin | α-SMA | ||
| Control group | 6 | 5.74 ± 1.82 | 4.37 ± 0.95 | 2.51 ± 0.64 | 9.75 ± 1.01 | 1.22 ± 0.35 |
| Group II | 33 | 12.19 ± 3.44a | 6.91 ± 1.42b | 3.95 ± 1.09b | 2.25 ± 0.42a | 4.02 ± 0.34a |
| Group III | 25 | 19.65 ± 5.11a,d | 16.55 ± 3.76a,d | 8.98 ± 4.11a,d | 1.73 ± 0.31a | 10.99 ± 4.11a,c |
| Group IV | 14 | 30.77 ± 7.68a,c,e | 17.32 ± 4.61a,d | 10.02 ± 2.62a,d | 2.24 ± 0.87a | 15.80 ± 7.11a,c,e |
Compared with control group, ap<0.01, bp<0.05; compared with group II, cp<0.01, dp<0.05; compared with group III, ep<0.01.
In the tubulointerstitium, the highest expression levels of Ang II, mTOR, and α-SMA, and the lowest expression levels of E-cadherin were found in the grade IV group (p<0.05 or p<0.01). The expression levels of p70S6K1 in the tubulointerstitium were higher in the grade III and IV group than those in the grade II group and control group (p<0.05) (Table 6).
Table 6.
The expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, and α-smooth muscle actin (α-SMA) in different pathological grades in tubulointerstitium ( ± s).
| Group | Cases | Tubulointerstitium |
||||
|---|---|---|---|---|---|---|
| Ang II | mTOR | p70S6K1 | E-cadherin | α-SMA | ||
| Control group | 6 | 4.05 ± 1.58 | 5.51 ± 1.32 | 2.82 ± 0.86 | 16.09 ± 2.12 | 1.32 ± 0.25 |
| Group II | 33 | 10.25 ± 3.33a | 12.91 ± 4.25a | 5.43 ± 1.11a | 6.55 ± 4.37a | 8.52 ± 1.07a |
| Group III | 25 | 21.83 ± 5.11a,b | 15.42 ± 5.12a,c, | 12.97 ± 0.33a,b | 4.99 ± 1.11a,b | 13.81 ± 4.22a,b |
| Group IV | 14 | 25.77 ± 3.52a,b,e | 22.32 ± 7.86a,b,d | 13.92 ± 2.99a,b | 2.96 ± 0.68a,b,d | 16.82 ± 5.11a,b,d |
Compared with control group, ap<0.01; compared with group II, bp<0.01, cp<0.05; compared with group III, dp<0.01, ep<0.05.
Correlation analyses
The correlations between the expression levels of Ang II, mTOR, p70S6K1, E-cadherin, and α-SMA in renal tissues of IgAN
The expression levels of Ang II were positively correlated with the expression levels of mTOR and α-SMA and negatively correlated with the expression levels of E-cadherin in the glomeruli and tubulointerstitium (p<0.05 or p<0.01). The expression levels of mTOR in the renal tissues were positively correlated with the expression levels of p70S6K1 and α-SMA (p<0.05 or p<0.01), and negatively correlated with the expression levels of E-cadherin in the tubulointerstitium (p<0.01). The expression levels of p70S6K1 were positively correlated with the expression levels of α-SMA in the glomeruli and tubulointerstitium (p<0.05) and negatively correlated with the expression levels of E-cadherin in the tubulointerstitium (p<0.01). The expression levels of E-cadherin in the tubulointerstitium were negatively correlated with the expression levels of α-SMA (p<0.01) (Tables 7 and 8).
Table 7.
The correlations between the expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, and α-smooth muscle actin (α-SMA) in glomeruli of immunoglobulin A nephropathy (IgAN).
| r | Glomeruli |
||||
|---|---|---|---|---|---|
| Ang II | mTOR | p70S6K1 | E-cadherin | α-SMA | |
| Ang II | – | 0.450a | 0.123 | −0.663a | 0.667b |
| mTOR | 0.450a | – | 0.654a | 0.204 | 0.708a |
| p70S6K1 | 0.123 | 0.654a | – | 0.221 | 0.574b |
| E-cadherin | −0.663a | 0.204 | 0.221 | – | 0.132 |
| α-SMA | 0.667b | 0.708a | 0.574b | 0.132 | – |
p<0.01, bp<0.05.
Table 8.
The correlations between the expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, and α-smooth muscle actin (α-SMA) in tubulointerstitium of immunoglobulin A nephropathy (IgAN).
| r | Tubulointerstitium |
||||
|---|---|---|---|---|---|
| Ang II | mTOR | p70S6K1 | E-cadherin | α-SMA | |
| Ang II | – | 0.677a | 0.102 | −0.727a | 0.703a |
| mTOR | 0.677a | – | 0.658b | −0.476a | 0.737a |
| p70S6K1 | 0.102 | 0.658b | – | −0.432a | 0.376b |
| E-cadherin | −0.727a | −0.476a | −0.432a | – | −0.798b |
| α-SMA | 0.703a | 0.737a | 0.376b | −0.798b | – |
p<0.01, bp<0.05.
The correlations between the expression levels of Ang II, mTOR, p70S6K1, E-cadherin, α-SMA, and the course of illness, the 24 h urinary protein quantitation in IgAN
There were no significant correlation between the expression levels of Ang II, mTOR, p70S6K1, E-cadherin, α-SMA in IgAN renal tissues and the course of illness (from the onset to final diagnosis by renal biopsies) (p>0.05).
The 24 h urinary protein quantitation was positively correlated with expression levels of Ang II, mTOR, and α-SMA, and negatively correlated with the expression levels of E-cadherin in the glomeruli and tubulointerstitium (p<0.05 or p<0.01) (Table 9 and 10).
Table 9.
The correlations between the expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, α-smooth muscle actin (α-SMA), and the clinicopathological indexes in glomeruli of immunoglobulin A nephropathy (IgAN).
| r/rs | Glomeruli |
||||
|---|---|---|---|---|---|
| Ang II | mTOR | p70S6K1 | E-cadherin | α-SMA | |
| Courses of illness | −0.112 | −0.143 | −0.028 | 0.014 | −0.127 |
| 24h urinary protein | 0.434a | 0.327a | −0.198 | −0.275b | 0.368a |
| Serum IgA | −0.423a | −0.358a | 0.188 | 0.214b | −0.435a |
| Intensity of IgA deposition | −0.045 | −0.102 | −0.098 | −0.115 | −0.201 |
p<0.01, bp<0.05.
Table 10.
The correlations between the expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, α-smooth muscle actin (α-SMA), and the clinicopathologicalal indexes in tubulointerstitium of immunoglobulin A nephropathy (IgAN).
| r/rs | Tubulointerstitium |
||||
|---|---|---|---|---|---|
| Ang II | mTOR | p70S6K1 | E-cadherin | α-SMA | |
| Courses of illness | −0.151 | −0.144 | −0.772 | 0.123 | −0.159 |
| 24h urinary protein | 0.512a | 0.455a | 0.181 | −0.442a | 0.545a |
| Serum IgA | −0.339a | −0.264b | 0.012 | 0.379a | −0.448a |
| Intensity of IgA deposition | 0.072 | −0.111 | −0.102 | −0.223 | −0.071 |
p<0.01, bp<0.05.
The correlations between the expression levels of Ang II, mTOR, p70S6K1, E-cadherin, α-SMA, and the serum IgA levels, the intensity of IgA deposition in IgAN
The serum IgA levels in children with IgAN were positively correlated with the expression levels of Ang II, mTOR, and α-SMA, and negatively correlated with the expression levels of E-cadherin in the glomeruli and tubulointerstitium (p<0.05 or p<0.01). There was no significant correlation between the expression levels of Ang II, mTOR, p70S6K1, E-cadherin, α-SMA, and the intensity of IgA deposition in IgAN renal tissues (p>0.05) (Tables 9 and 10).
The correlations between the expression levels of Ang II, mTOR, p70S6K1, E-cadherin, α-SMA, and the glomerular lesion and renal tubulointerstitial lesion scores in IgAN
The glomerular lesion scores were positively correlated with the expression levels of Ang II, mTOR, and p70S6K1, and negatively correlated with the expression levels of E-cadherin in the glomeruli (p<0.01). The renal tubulointerstitial lesion scores were positively correlated with the expression levels of Ang II, mTOR, p70S6K1, and α-SMA, and negatively correlated with the expression levels of E-cadherin in the tubulointerstitium (p<0.01) (Table 11).
Table 11.
The correlations between the expression levels of angiotensin II (Ang II), mammalian target of rapamycin (mTOR), p70S6K1, E-cadherin, α-smooth muscle actin (α-SMA), and the glomerular lesion and renal tubulointerstitial lesion scores in immunoglobulin A nephropathy (IgAN).
| r/rs | Ang II | mTOR | p70S6K1 | E-cadherin | α-SMA |
|---|---|---|---|---|---|
| Glomerular lesion scores | 0.732a | 0.613a | 0.598a | −0.116 | 0.032 |
| Tubulointerstitium lesion scores | 0.841a | 0.795a | 0.617a | −0.886a | 0.769a |
p<0.01.
Discussion
The mTOR/p70S6K1 signaling pathway is activated in IgAN
Previous studies have shown that many important signaling pathways in the process of kidney injury are usually found in the process of kidney development, such as the mTOR/p70S6K1 signaling pathway.9,20 In our study the expression of Ang II, mTOR, p70S6K1, and α-SMA was also found in the control group, which may mainly be involved in the control of kidney development. But compared to the control group, the expression levels of these indexes were markedly higher and the expression levels of E-cadherin were obvious lower in the children with IgAN, which may be related to the kidney injury and suggest that the mTOR/p70S6K1 signaling pathway is activated in the children with IgAN.
The mTOR signaling plays diverse roles in different types of renal cells. The mTOR inhibitor can reduce cyst growth in polycystic kidney disease by significantly decreasing the proliferation and apoptosis of renal tubular epithelial cells.21,22 In endotoxemia-induced chronic kidney injury and subsequent fibrosis in the male C57BL/6 mice model, the mTOR signaling of kidney macrophages was activated, and rapamycin can markedly ameliorate kidney pathological changes.23 It was reported that a low dose of the mTOR inhibitor rapamycin can inhibit mesangial cell proliferation and slow the progression of renal fibrosis of IgAN by modulating the cell cycle regulatory proteins and blocking the G1-to-S transition.24 Tamouza et al. found that the MAPK/ERK pathway was activated in mesangial areas of IgAN patients with proteinuria (>1 g/day) and elevated blood pressure, which could alter the cross-talk between mesangial cells and podocytes and thus generate renal dysfunction in IgAN. What is more, as IgA1-dependent MAPK/ERK activation requires RAS activity, using RAS blocker therapy can more effectively mitigate proteinuria in IgAN patients with a high mesangial score for MAPK/ERK activation.25
Our study showed that the highest expression levels of mTOR, p70S6K1, and α-SMA were found in the NS group, and the expression levels of Ang II, mTOR, p70S6K1, and α-SMA were significantly higher in the renal pathological groups III and IV than that in group II, while the lowest expression levels of E-cadherin were found in group IV. It is suggested that the expression levels of Ang II, mTOR, p70S6K1, α-SMA, and E-cadherin were closely related to the clinical classifications and renal pathological grades, the more serious of the clinical features and pathological changes, the more distinct of the activation of the mTOR/p70S6K1 signaling pathway and renal fibrosis.
The activation of mTOR/p70S6K1 signaling pathway may be related to the renal fibrosis in IgAN induced by Ang II
Many studies have shown that Ang II can induce renal fibrosis through different transcellular pathways.26–29 Whaley-Connell et al.30 found that Ang II could be involved in the process of renal fibrosis through the mTOR/p70S6K1 signaling pathway in the rat models of hypertensive nephropathy.30 The possible mechanism is that the environment of inflammation induced by Ang II can make mTOR out of control in the kidney, and the activation of mTOR signaling pathway and the decrease of cadherin can jointly promote the process of epithelial-mesenchymal transition (EMT) in renal proximal tubules. The overproduction and excessive accumulation of the extracellular matrix (ECM) induced by EMT can expedite the progress of renal fibrosis. And the significant symbols of EMT are the low expression level of E-cadherin and the high expression level of α-SMA.31
In our study the correlations between the expression levels of Ang II, mTOR, p70S6K1, E-cadherin, and α-SMA in renal tissues of IgAN further suggested that the renal fibrosis induced by Ang II may relate to the mTOR signaling pathway. The possible reason is that the expression level of Ang II increases in the kidney tissue of IgAN, which can activate the mTOR/p70S6K1 signaling pathway, and promote the process of glomerular sclerosis and renal interstitial fibrosis. Thus, it may become a significant target to block the renal fibrosis in IgAN induced by Ang II through the control of mTOR/p70S6K1 signaling pathway.
The results show that, using the Katafuchi scoring, the glomerular lesion scores were positively correlated with the expression levels of Ang II, mTOR, and p70S6K1, and negatively correlated with the expression levels of E-cadherin in glomeruli. The renal tubulointerstitial lesion scores were positively correlated with the expression levels of Ang II, mTOR, p70S6K1, and α-SMA, and negatively correlated with the expression levels of E-cadherin in tubulointerstitium. They further suggest that Ang II and the mTOR/p70S6K1 signaling pathway might play an important role in glomerular sclerosis, renal tubular injury and renal interstitial fibrosis.
It was shown in this study that there was no significant correlation between the expression levels of Ang II, mTOR, p70S6K1, E-cadherin, α-SMA, and the intensity of IgA deposition in IgAN. The findings indirectly support the view that once the abnormal glycosylated IgA1 deposited in kidney and initiated the subsequent pathophysiological process, the result seemed have little relationship to the original cause, and also suggest that the injury process in the later stage of inflammation played a critical role in the development of renal fibrosis.32–35
It is thought that proteinuria is one of the independent risk factors of IgAN.36,37 Our study showed that the 24 h urinary protein quantitation were positively correlated with expression levels of Ang II, mTOR, and α-SMA, and negatively correlated with the expression levels of E-cadherin in glomeruli and tubulointerstitium. It is suggested that the activation of the mTOR/p70S6K1 signaling pathway in IgAN might relate to the level of proteinuria, and the specific mechanism is worthy of being further studied.
Conclusion
The expression levels of Ang II, mTOR, p70S6K1, and α-SMA in renal tissues of children with IgAN are significantly higher and the expression levels of E-cadherin are significantly lower, which is closely related to the clinical classifications, renal pathological grades and lesion scores. It implies that the activation of mTOR/p70S6K1 signaling pathway may play an important role in the progress of glomerular sclerosis, renal tubular injury, and renal interstitial fibrosis in children with IgAN, and future studies will need to address the mechanism of mTOR/p70S6K1 signaling in the progress of renal fibrosis in IgAN.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (No 81670648); the Science and Technology Program of Guangdong Province, China (No 2016A020215033); the Science and Technology Program of Guangzhou, China (No 201607010284) and the Natural Science Foundation of Guangdong Province, China (No 2015A030313045).
References
- 1. Xu X, Wang G, Chen N, et al. Long-Term exposure to air pollution and increased risk of membranous nephropathy in china. J Am Soc Nephrol 2016; 27: 3739–3746. DOI: 10.1681/ASN.2016010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wyatt RJ, Kritchevsky SB, Woodford SY, et al. IgA nephropathy: Long-term prognosis for pediatric patients. J Pediatr 1995; 127: 913–919. [DOI] [PubMed] [Google Scholar]
- 3. Lai KN, Tang SC, Guh JY, et al. Polymeric IgA1 from patients with IgA nephropathy upregulates transforming growth factor-beta synthesis and signal transduction in human mesangial cells via the renin-angiotensin system. J Am Soc Nephrol 2003; 14: 3127–3137. [DOI] [PubMed] [Google Scholar]
- 4. Lai KN, Chan LY, Tang SC, et al. Mesangial expression of angiotensin II receptor in IgA nephropathy and its regulation by polymeric IgA1. Kidney Int 2004; 66: 1403–1416. [DOI] [PubMed] [Google Scholar]
- 5. Wang C, Liu X, Tang Y, et al. Medium from mesangial cells incubated with aggregated IgA1 from IgA nephropathy patients reduces podocyte adhesion through activation of the renin angiotensin system. Swiss Med Wkly 2011; 141: w13304. [DOI] [PubMed] [Google Scholar]
- 6. Laplante M, Sabatini DM. MTOR signaling in growth control and disease. Cell 2012; 149: 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol 2014; 36: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Novalic Z, van der Wal AM, Leonhard WN, et al. Dose-dependent effects of sirolimus on mTOR signaling and polycystic kidney disease. J Am Soc Nephrol 2012; 23: 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huber TB, Walz G, Kuehn EW. MTOR and rapamycin in the kidney: Signaling and therapeutic implications beyond immunosuppression. Kidney Int 2011; 79: 502–511. [DOI] [PubMed] [Google Scholar]
- 10. Wu L, Feng Z, Cui S, et al. Rapamycin upregulates autophagy by inhibiting the mTOR-ULK1 pathway, resulting in reduced podocyte injury. PLoS One 2013; 8: e63799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cybulsky AV. Membranous nephropathy. Contrib Nephrol 2011; 169: 107–125. [DOI] [PubMed] [Google Scholar]
- 12. Ma SK, Joo SY, Kim CS, et al. Increased phosphorylation of PI3K/Akt/mTOR in the obstructed kidney of rats with unilateral ureteral obstruction. Chonnam Med J 2013; 49: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim WY, Nam SA, Song HC, et al. The role of autophagy in unilateral ureteral obstruction rat model. Nephrology (Carlton) 2012; 17: 148–159. [DOI] [PubMed] [Google Scholar]
- 14. Nakagawa S, Nishihara K, Inui K, et al. Involvement of autophagy in the pharmacological effects of the mTOR inhibitor everolimus in acute kidney injury. Eur J Pharmacol 2012; 696: 143–154. [DOI] [PubMed] [Google Scholar]
- 15. Mavroeidi V, Petrakis I, Stylianou K, et al. Losartan affects glomerular AKT and mTOR phosphorylation in an experimental model of type 1 diabetic nephropathy. J Histochem Cytochem 2013; 61: 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The Subspecialty Group of Nephrology, Society of Pediatrics, Chinese Medical Association. Evidence-based guidelines on diagnosis and treatment of childhood common renal disease (IV): IgA nephropathy. Chin J Ped 2010; 48: 355–357. [PubMed] [Google Scholar]
- 17. The Subspecialty Group of Nephrology, Society of Pediatrics, Chinese Medical Association. Clinical classification, diagnosis and treatment of glomerular diseases in children. Chin J Pediatrics 2001; 39: 746–749. [Google Scholar]
- 18. Lee SM, Rao VM, Franklin WA, et al. IgA nephropathy: Morphologic predictors of progressive renal disease. Hum Pathol 1982; 13: 314–322. [DOI] [PubMed] [Google Scholar]
- 19. Katafuchi R, Kiyoshi Y, Oh Y, et al. Glomerular score as a prognosticator in IgA nephropathy: Its usefulness and limitation. Clin Nephrol 1998; 49: 1–8. [PubMed] [Google Scholar]
- 20. Grahammer F, Wanner N, Huber TB. MTOR controls kidney epithelia in health and disease. Nephrol Dial Transplant 2014; 29: i9–i18. [DOI] [PubMed] [Google Scholar]
- 21. Ravichandran K, Zafar I, He Z, et al. An mTOR anti-sense oligonucleotide decreases polycystic kidney disease in mice with a targeted mutation in Pkd2. Hum Mol Genet 2014; 23: 4919–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ravichandran K, Zafar I, Ozkok A, et al. An mTOR kinase inhibitor slows disease progression in a rat model of polycystic kidney disease. Nephrol Dial Transplant 2015; 30: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen H, Zhu J, Liu Y, et al. Lipopolysaccharide induces chronic kidney injury and fibrosis through activation of mTOR signaling in macrophages. Am J Nephrol 2015; 42: 305–317. [DOI] [PubMed] [Google Scholar]
- 24. Tian J, Wang Y, Liu X, et al. Rapamycin ameliorates IgA nephropathy via cell cycle-dependent mechanisms. Exp Biol Med (Maywood) 2015; 240: 936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tamouza H, Chemouny JM, Raskova KL, et al. The IgA1 immune complex-mediated activation of the MAPK/ERK kinase pathway in mesangial cells is associated with glomerular damage in IgA nephropathy. Kidney Int 2012; 82: 1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu GX, Li YQ, Huang XR, et al. Disruption of Smad7 promotes ANG II-mediated renal inflammation and fibrosis via Sp1-TGF-beta/Smad3-NF.kappaB-dependent mechanisms in mice. PLoS One 2013; 8: e53573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim H, Moon SY, Kim JS, et al. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am J Physiol Renal Physiol 2015; 308: F226–F236. [DOI] [PubMed] [Google Scholar]
- 28. Li RX, Yiu WH, Tang SC. Role of bone morphogenetic protein-7 in renal fibrosis. Front Physiol 2015; 6: 114 DOI: 10.3389/fphys.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farris AB, Colvin RB. Renal interstitial fibrosis: Mechanisms and evaluation. Curr Opin Nephrol Hypertens 2012; 21: 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whaley-Connell A, Habibi J, Panfili Z, et al. Angiotensin II activation of mTOR results in tubulointerstitial fibrosis through loss of N-cadherin. Am J Nephrol 2011; 34: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gong Q, Hou F. Silencing of angiotensin II type-1 receptor inhibits high glucose-induced epithelial-mesenchymal transition in human renal proximal tubular epithelial cells via inactivation of mTOR/p70S6K signaling pathway. Biochem Biophys Res Commun 2016; 469: 183–188. [DOI] [PubMed] [Google Scholar]
- 32. Moldoveanu Z, Wyatt RJ, Lee JY, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 2007; 71: 1148–1154. [DOI] [PubMed] [Google Scholar]
- 33. Novak J, Julian BA, Mestecky J, et al. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin Immunopathol 2012; 34: 365–382. [DOI] [PubMed] [Google Scholar]
- 34. Lai KN. Pathogenesis of IgA nephropathy. Nat Rev Nephrol 2012; 8: 275–283. [DOI] [PubMed] [Google Scholar]
- 35. Barratt J, Feehally J. Primary IgA nephropathy: New insights into pathogenesis. Semin Nephrol 2011; 31: 349–360. [DOI] [PubMed] [Google Scholar]
- 36. Tan M, Li W, Zou G, et al. Clinicopathological features and outcomes of IgA nephropathy with hematuria and/or minimal proteinuria. Kidney Blood Press Res 2015; 40: 200–206. [DOI] [PubMed] [Google Scholar]
- 37. Kamei K, Harada R, Hamada R, et al. Proteinuria during follow-up period and long-term renal survival of childhood IgA nephropathy. PLoS One 2016; 11: e150885. [DOI] [PMC free article] [PubMed] [Google Scholar]