Abstract
Introduction:
Preterm birth is the most important cause of neonatal mortality and morbidity. It is a multifactorial disease with different etiologies, including genetic factors. Genetic variability is represented by single nucleotide polymorphisms (SNPs) in genes of proteins involved in the contractile activity. We determine the association between SNP 12109G> A in REN associated with preterm birth and premature rupture of membrane.
Materials and methods:
A study of cases (N=112, 22–36 weeks of gestation; mean: 31, 95% confidence interval 30.7–32.2) and controls (N=66; 38–40 weeks of gestation from the last menstrual period; mean: 39.8, 95% confidence interval 38.9–39.4) was performed. Genomic DNA was isolated in all patients from peripheral blood. The SNP 12109G> A (Mbo I) in REN was typified by PCR-restriction fragment length polymorphism.
Results:
A significant difference in the case group for the SNP 12109G>A was observed. The A allele was increased in women with preterm birth (81% cases vs. 15% control, p<0.0000004). There was also a significant difference between genotypes, mainly an excess of G/A heterozygotes in women with preterm birth (60% cases vs. 23% controls). The phenotype 12109G> A has odds ratio 6.62 (95% confidence interval 3.14–14.15), which means a high risk of preterm birth/premature rupture of membrane in presence of allele A, both in homozygotes and in heterozygotes.
Conclusion:
Allelic frequency of A of SNP 12109G>A was higher in women with preterm birth than in women with normal vaginal delivery and could be considered a risk factor.
Keywords: Preterm birth, REN gene, SNPs, PCR-RFLP, Mbo I
Introduction
Preterm birth (PTB) is considered a syndrome characterized by effacement and dilation of the cervix, or uterine irritability increased due to various factors. This varies according to the gestational age, the presence of intrauterine infection and systematic, utero-placental ischemia, excessive distention of the uterus and abnormal immune response of the fetus or the mother.1 PTB is the most important cause of neonatal mortality and morbidity, and it is responsible for almost 70% of neonatal deaths in Mexico with an incidence of 5% to 10%.2 PTB also a risk factor of cardiovascular diseases and diabetes of the offspring in later life.3 The pathologies most frequently associated with PTB are premature rupture of membranes (PROM), infectious complications that cause chorioamnionitis and neonatal sepsis.4 Some nutritional factors, such as maternal vitamin D deficiency and fetal5 and zinc deficiency6 are related to the PTB. Although scientific and technological advances in neonatal care have managed to increase survival in newborns, there has not been a positive impact on long-term morbidity.7
Epidemiological studies designed to recognize associated factors may explain below 40% of cases of PTB. Nevertheless, different genetic factors play an important role in its etiology.8 There are allelic variants or single nucleotide polymorphisms (SNPs) in genes involved in the contractile activity of the uterus. SNPs may predispose events of PTB or even alter the response to drugs used to treat it.
The renin–angiotensin system (RAS) includes a set of neurohumoral factors and mechanisms involved in the regulation of blood pressure. RAS is also involved in regulating the body’s sodium balance and urinary excretion of potassium.9 Renin (REN) is a member of the aspartyl proteinase enzyme family and it is synthesized as a pre-pro-protein. In humans, the gene encoding this protein is located on chromosome 1 (1q 32–1q 42) and it has sequences of steroid receptors on the 5′ promoter region. Transcription produces a 1.5 kb mRNA and the proenzyme has 340 amino acids, of which the first 43 are cleaved to produce for cAMP control and a number are an active enzyme.10 Different proteins may arise from the same REN gene by differential splicing; some polymorphisms in introns altered the alternative processing mechanisms in different kinds of cells.11
Several studies have proposed the involvement of RAS in different pathologies, such as hypertension and heart failure.12-15 Some of the SNPs of genes from this system have been studied in pregnancy complications; for example, preeclampsia. The attempt to determine the link between REN and high blood pressure (HBP) has been analyzed using affected siblings by determining haplotypes using diallelic polymorphic markers in the REN locus. Allelic variations were distinguishable by digestion with restriction enzymes Taq I, Hinf I, Hind III, Bgl I and Bgl II16 specific for populations. For example, the SNP characterized by Bgl I was significantly associated with HBP in American and Caucasian populations with hypercholesterolemia. This association was found in Chinese populations, but using Hind III enzyme.17,18 Some of the REN gene SNPs have been studied in some preterm delivery19 or birth complications such as preeclampsia,20 however, the polymorphism 12109G> A (Mbo I) has not been studied in this disease.
The discovery of a genetic etiological factor that may predispose PTB and some of its causes, such as PROM, should be a major link for future research in the biology, diagnosis and therapy of this syndrome. Because of this, we evaluated the association of the SNP 12109G> A (Mbo I) of the REN gene with PTB.
Materials and methods
Study population
A retrospective, descriptive, case–control study was performed. Samples were collected from a DNA biobank from 2000 to 2004. The samples came from pregnant Mestizo Mexican women who attended in the Obstetrics and Gynecology Department of the Obstetrics and Gynecology Hospital of the Western National Medical Center of the Mexican Institute of Social Security and General Hospital No. 45 (Ayala) in the metropolitan area of Guadalajara, Mexico. The case group was integrated with a total of 112 samples belonging to women with an age range of 16–40 years diagnosed with PROM ⩾ 24 h and PTB with pregnancy termination between 22 and 36 weeks of gestation from the date of the last menstrual period. General data were recorded for analysis (name, age, gynecological and obstetric history) in each case. The gestational age and the birth canal and the presence or absence of the variables were obtained from medical records, corroborating data with a personal interview with the patient. The variables considered for this study in the case group were: weeks of gestation, way of termination, history of preterm labor, PROM, infection at admission (genitourinary), diabetes mellitus, hypertensive disorders (hypertension in pregnancy or preeclampsia/eclampsia). The control group consisted of 66 women with an age range of 15–38 years, and at least one normal vaginal delivery (NVD) at term (38–40 weeks of gestation), and no history of PTB and PROM. Patients with drug treatment to prevent PTB and/or serious diseases (heart disease, cancer, HIV) and/or other obstetric complications (previous placenta, oligohydramnios, fetal death) were excluded.
Analysis of SNPs by PCR-restriction fragment length polymorphism
A venous blood sample was obtained in all patients by venipuncture. DNA was extracted from each sample using the methodology established by Miller.20 For SNP identification from the REN gene, a 250-bp segment was amplified using PCR, under the following conditions: 1 cycle of 94°C, 5 min; 28 cycles of 94°C, 1 min; 65°C, 1 min; 72°C, 2 min; and 1 cycle of 72°C. The concentrations of the reaction components were 1X buffer, MgCl2 1 mM, dNTPs 0.2 mm, Taq polymerase 0.03 U/µl, DNA 100 ng/µl, and 6 pM of each primer for the SNP 12109G>A. The sequences of the primers were 5′-TGAGGTTCGAGTCGGCCCCCT-3′ and 5′-TGCCCCAAACATGGCCACACAT-′3 for sense and antisense, respectively.
Subsequently, the amplified products were digested with the restriction enzyme MboI (↓GATC↑) using 10X buffer, 1 U of enzyme, 1μl of PCR product and completed with nuclease-free water. The mixture was incubated for 2 h at 37°C. The slices made it possible to differentiate between REN gene haplotypes. We observed in G/G homozygotes a 250 bp fragment (absence of the restriction site); the A/A homozygotes had two fragments of 169 and 81 bp each, and heterozygotes presented three fragments. The digested amplified products were visualized by electrophoresis in 6% polyacrylamide gel stained with silver nitrate (Figure 1).21
Figure 1.
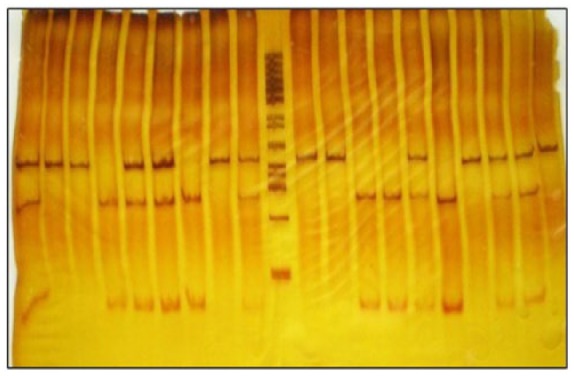
Characterization of polyacrylamide gel for SNP 12109G> A (Mbo I) by PCR restriction fragment length SNP. Lines are numbered from left to right. Homozygous G/G (Lanes 2, 3, 8, 11, 12, 17 and 20), homozygous A/A (lanes 4, 7, 13 and 14) and heterozygous G/A (Lanes 1, 5, 6, 15, 18 and 19) are observed. Molecular weight marker 50 bp (Lane 10).
Statistical analysis
Comparisons between observed and expected genotypic proportions were performed by Hardy–Weinberg equilibrium, using a Finetti generator.22 SPSS version 20.0 was used for data analysis and a p⩽0.05 value was considered a statistically significant difference. Chi-square analysis was applied to contrast differences in the distribution of genotypes, alleles and phenotypes23 and multivariate analysis and to detect the existence of any confounding variable.24 Relative risk of the SNP was estimated and the odds ratio (OR) was calculated by Epi Info v.7.1.
Results
A total of 112 patients diagnosed with PTB were analyzed for this study; genotypic, phenotypic and allelic frequencies are shown in Table 1. A significant difference between genotypes was found, mainly an excess of GA heterozygotes in women with PTB (60% of cases vs. 23% of controls). The comparison of cases and controls showed a significant difference for the polymorphic marker REN 12109G>A, where an increase in the A allele in the PTB group was found (41% of cases with allele A vs. 15% in controls; p<0.0000004). The difference for phenotype A (genotypes AA + AG) was significant; this phenotype was increased in patients with PTB (66% of cases vs. 23% in controls; p <0.0000001). The OR for the phenotype 12109G> A (Mbo I) was 6.62 (95% confidence interval (CI) 3.14–14.15). Our results indicate that the A allele was associated with a high risk of PTB in homozygotes as well as heterozygotes. No interaction among variables and no confounding variables were found in the cases group.
Table 1.
Distribution of genotypes, alleles and phenotypes in the case and control groups. The presence of genotypes with alleles in the case group has a highly significant relationship with PTB events.
| Study group | Genotype, n (%) |
Alleles, n (%) |
Phenotypes, n (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G/G | G/A* | A/A | G | A** | G | A*** | ||||||||
| Control, NVD=66 | 51 | (77) | 15 | (23) | 0 | (0) | 117 | (87) | 15 | (11) | 51 | (77) | 15 | (23) |
| Case, PTB=112 | 38 | (34) | 67 | (60) | 7 | (6) | 143 | (59) | 81 | (41) | 38 | (34) | 74 | (66) |
NVD: normal vaginal delivery; PTB: preterm birth; *p<0.0001; **p<0.0001; ***p<0.0001.
Discussion
The study of gene SNPs of RAS for several years has focused on hypertension due to its direct involvement in blood pressure.25,26 However, the system has been implicated in various tissues, having various effects for different diseases, as in the pregnancy case.26 The counterbalance changes of RAS during the normal pregnancy are rigid. There is marked activation of the RAS with increased production of renin angiotensinogen, angiotensin I and angiotensin II, whose primary effect is the release of aldosterone, which contributes to the retention of sodium and water in the proximal tubule.27 Initially in pregnancy, there is decreased systemic vascular tone and vasodilation mediated by progesterone. In addition, sustained volume expansion associated with peripheral vasodilatation, renal vasodilation and RAS stimulation occurs. If a genetic change occurs that modifies the proteins of RAS, there is support for a pathophysiology.28 However, the etiology of this dysfunction requires further exploration, to the level of the genes involved in the RAS. The components of the RAS have been found both in maternal decidua and in fetal placental tissues.29 In this report we found a SNP of a RAS component that could change the normal mechanisms in pregnancy, culminating in a PTB risk.
Various factors related to PTB; one limitation of the study is that there was not stratified in the different variables for studying REN 12109G> A (Mbo I) SNP (gestational age, diabetes mellitus, infections, hypertensive disorders) that showed patients with PTB, since subgroups of each variable would be too small to have representative statistical power. The presence of hypertensive disorders in all women with PTB (hypertension/preeclampsia/eclampsia) was 12%; slightly greater than the prevalence among pregnant women with these abnormalities, which is considered to be 8% to 10%. It has been observed that levels of RAS components in plasma increase normally during an uncomplicated pregnancy;30 however, the levels of angiotensinogen are diminished in pregnancy with preeclampsia or hypertension, and strongly associated with the risk of a PROM and PTB.31 However, it is unknown whether this suppression of RAS components is linked to hypertension in pregnancy or secondary to another process.9 In contrast, plasma renin levels increase in preeclampsia.32 The mechanisms that contribute to decrease or increase in plasma levels of RAS components in the pathogenesis of PTB or complications in pregnancy are not yet defined. The action of angiotensin II is mediated by binding two receptors (AT1 and AT2). AT1 receptors are especially involved in controlling blood pressure and AT2 are primarily expressed in the fetal period,33 causing apoptosis and fetal growth restriction.34 These factors have a high association with PROM. The genes coding for proteins of the RAS have been studied as angiotensin-converting enzyme (ACE). The presence of the D allele of the ACE gene was related to increased risk of preeclampsia. Other RAS gene polymorphisms, such as AT1 A1166C, AGT Met235Thr, AGT Thr174Met and 83A/G-REN, seem related to preeclampsia (reviewed in Yang et al.35). Similarly, some REN polymorphisms were studied (rs5705, rs1464818, and rs3795575), the finding being that there is no associated risk of preeclampsia with these polymorphisms.29
It has been established that SNPs of the REN gene that are characterized by the digesting of Bgl I and Mbo I enzymes are associated with essential hypertension in United States’ populations. Both SNPs of Bgl I and Mbo I are in the first and ninth intron of the REN gene, respectively. Also, while the introns are not translated into protein, changes in structure can modulate gene expression. In this genetic influence, which seems to show a recessive mode of inheritance, both increased systolic and diastolic pressure could be involved.36 Regarding the SNP determined by Mbo I, the most frequent genotype was GG and the most common allele was G (Table 2). The observed distribution of G and A alleles for this study was 73.3 and 26.7%, respectively. The frequency of allele A is observed in 24% of a Caucasian population from the USA,36 similar to that observed in this study (26.7%). This SNP has been determined in different races, including Quechua, which showed a high frequency of G allele.37 The frequency of the G allele in Mestizo populations is consistent with that found in American Caucasians and in Mayan populations (Table 2). It has been considered that genetic changes, including those located in an intron, may influence the enzymatic activity of renin.34 The SNP of Mbo I in the REN gene was significantly associated with a family history of hypertension in a population of Gulf Arabs from the United Arab Emirates.37 However, none of the SNPs have been linked directly with PTB.
Table 2.
Frequency of genotypes and alleles of SNP Mbo I in the REN gene in different populations. The genotype and allele frequencies determined in this study (*) are within the mean, compared with other populations and races.
| Population |
Genotype, % |
Allele, % |
|||
|---|---|---|---|---|---|
| SNP Mbo I | AA | AG | GG | A | G |
| Mexico (Mestizos)* | 5.5 | 42.5 | 52.0 | 26.7 | 73.3 |
| US Caucasians | 7.1 | 34 | 58.9 | 24 | 76 |
| Quechua | 2.1 | 31.6 | 66.3 | 18 | 82 |
| Mayan | 3.9 | 41.2 | 54.9 | 25 | 75 |
| United Arab Emirates | 14.0 | 44.9 | 41.0 | 36.5 | 63.5 |
SNP: single nucleotide polymorphism.
The association of PTB and infection (genitourinary) was reported in 38% of our study group, similar to the 30% reported in the literature.38,39 Decreased renal blood flow increases the production of REN/AT2, which in turn gives rise to fetoplacental vasoconstriction through the AT1 receptor. This causes decreased placental and renal blood flow and also fetal growth retardation, oliguria and oligohydramnios. Similarly, but through the AT2 receptor, there is vasodilation which causes PTB; in this case, metalloproteinases act by degrading membranes prematurely.40-43 They have been described as risk factors for vascular disease and diabetes mellitus44-47 as well as for PTB.48 The frequency of diabetes mellitus in our study group was 3%. There are no reports that have studied the Mbo I SNP of REN gene with diabetes mellitus.
In the marker 12109G> A (Mbo I) evaluated in this work between study groups, allele A was more prevalent in women with PTB compared with those with NVD. Although it is well known that both gestational diabetes and preeclampsia increase the risk of PTB, the A allele was present in women who did not have any of these risk factors, according to their medical history. PTB presents as a result of a complex interaction between individual genetic background and environmental factors.8
This study represents the first report that determines the relationship between the SNP 12109G> A of the REN gene in PTB. Based on our results, the allele, genotype and phenotype frequencies for this SNP were obtained for both cases and controls. The A allele frequency was higher in women with PTB than in those with NVD. Thus it can be suggested that the SNP 12109G> A (Mbo I) is a possible risk factor for PTB. However, future studies are necessary to consider other known risk factors for PTB such as socioeconomic status, gynecological clinical history and habits to obtain a complete a multiple regression model to confirm this association. Thus it can be suggested that the SNP 12109G> A (Mbo I) is a possible risk factor for PTB.
Acknowledgments
The authors gratefully acknowledge the critical reading of the manuscript by Sergio Lozano-Rodriguez, MD, and Alejandro Quiroga-Garza, MD, as well as graduate student Yusvi E Farias.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by PROMEP (grant number PTC-120) and CONACYT (grant number 226165).
References
- 1. Saling E. Basic aspects of prematurity prevention and results achieved by a suitable, simple program. J Perinat Med 1998; 26: 466–468. [DOI] [PubMed] [Google Scholar]
- 2. Challis JRG. Mechanism of parturition and preterm labor. Obstet Gynecol Surv 2005; 55: 650–660. [DOI] [PubMed] [Google Scholar]
- 3. Reichetzeder C, Dwi Putra SE, Li J, et al. Developmental origins of disease. Crisis precipitates change. Cell Physiol Biochem 2016; 39: 919–938. [DOI] [PubMed] [Google Scholar]
- 4. Mancilla J. Ruptura prematura de membranas y parto pretérmino I. Mediadores inflamatorios en la ruptura prematura de membranas. Gaceta Medica Mexicana 1998; 134: 423–426. [PubMed] [Google Scholar]
- 5. Rchetzeder C, Chen H, Föller M, et al. Maternal vitamin D deficiency and fetal programming – lessons learned from humans and mice. Kidney Blood Press Res 2014; 39: 315–329. [DOI] [PubMed] [Google Scholar]
- 6. Terrin G, Berni Canani R, Di Chiara M, et al. Zinc in early life: A key element in the fetus and preterm neonate. Nutrients 2015; 7: 10427–10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steer P. The epidemiology of preterm labor-a global perspective. J Perinat Med 2005; 33: 273–276. [DOI] [PubMed] [Google Scholar]
- 8. Hoffman JD, Ward K. Genetic factors in preterm delivery. Obstet Gynecol Surv 1999; 54: 203–210. [DOI] [PubMed] [Google Scholar]
- 9. Malacra JM, García-Víveros M, Valverde-Rodríguez C. Fundamentos de ENDOCRINOLOGIA clínica. Editorial; La Prensa Médica Mexicana. 3rd ed. México: DF, 1979, pp.162–170. [Google Scholar]
- 10. Dluhy R, Williams G. Endocrice hypertension. In: Wilson JD, Kroneberg HM and Larsen PR (eds) Textbook of Endorinology. 9th ed W.B. Sunders Company, 1998, pp.285–928. [Google Scholar]
- 11. Morris BJ. New possibilities for intracellular renin and inactive renin now that the structure of the human renin gene has been elucidated. Clin Sci 1986; 71: 345–355. [DOI] [PubMed] [Google Scholar]
- 12. Van Dijk MA, Kroon I, Kamper AM, et al. The angiotensin-converting enzyme gene polymorphism and responses to angiotensins and bradykinin in the human forearm. J Cardiovasc Pharmacol 2000; 35: 484–490. [DOI] [PubMed] [Google Scholar]
- 13. Agachan B, Isbir T, Yilmaz H, et al. Angiotensin converting enzyme I/D, angiotensinogen T174M-M235T and angiotensin II type 1 receptor A1166C gene polymorphisms in Turkish hypertensive patients. Exp Mol Med 2003; 6: 545–549. [DOI] [PubMed] [Google Scholar]
- 14. Siani A, Russo P, Paolo Cappuccio F, et al. Combination of renin–angiotensin system polymorphisms is associated with altered renal sodium handling and hypertension. Hypertension 2004. 3: 598–602. [DOI] [PubMed] [Google Scholar]
- 15. Mondry A, Loh M, Liu P, et al. Polymorphisms of the insertion/deletion ACE and M235T AGT genes and hypertension: Surprising new findings and meta-analysis of data. BMC Nephrol 2005; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fornage M, Amos Cl, Kardia S, et al. Variation in the region of the angiotensin-converting enzyme gene influences interindividual differences in blood pressure levels in young white males. Circulation 1998; 97: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 17. Chiang FT, Hsu KL, Tseng CD, et al. Association of the renin gene polymorphism with essential hypertension in a Chinese population. Clin Genet 1997; 51: 370–374. [DOI] [PubMed] [Google Scholar]
- 18. Frossard PM, Lestringant GG, Malloy MJ, et al. Human renin gene BgII dimorphism associated with hypertension in two independent populations. Clin Genet 1999; 56: 428–433. [DOI] [PubMed] [Google Scholar]
- 19. Valdez-Velazquez LL, Quintero-Ramos A, Perez SA, et al. Genetic polymorphisms of the renin-angiotensin system in preterm delivery and premature rupture of membranes. J Renin Angiotensin Aldosterone Syst 2007; 8: 160–168. [DOI] [PubMed] [Google Scholar]
- 20. Vefring HK, Wee L, Jugessur A, et al. Maternal angiotensinogen (AGT) haplotypes, fetal renin (REN) haplotypes and risk of preeclampsia; estimation of gene-gene interaction from family-triad data. BMC Med Genet 2010; 11: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Division of Health Informatics & Surveillance (DHIS), Center for Surveillance, Epidemiology & Laboratory Services (CSELS). Epi Info™ v. 7.1, 2014. [Google Scholar]
- 23. DeFinetti program. Available at: https://ihg.gsf.de/cgi-bin/hw/hwa1.pl (accessed February 2016).
- 24. Sasieni PD. From genotypes to genes: Doubling the sample size. Biometrics 1997; 53: 1253–1261. [PubMed] [Google Scholar]
- 25. Tényi I, Németh M, Nemes J, et al. Study of the renin–angiotensin system in essential hypertension. Acta Med Acad Sci Hung 1979; 36: 91–104. [PubMed] [Google Scholar]
- 26. Yang YL, Mo YP, He YS, et al. Correlation between renin-angiotensin system gene polymorphisms and essential hypertension in the Chinese Yi ethnic group. J Renin Angiotensin Aldosterone Syst 2015; 16: 975–981. [DOI] [PubMed] [Google Scholar]
- 27. Dandrea J, Cooper S, Ramsay MM, et al. The effects of pregnancy and maternal nutrition on the maternal renin–angiotensin system in sheep. Exp Physiol 2002; 87: 353–359. [DOI] [PubMed] [Google Scholar]
- 28. Procopciue G, Garacostea M, Puscas G, et al. The T344C-CYP11B2 polymorphism as a risk for preeclampsia in Romanian pregnant women association with M235T(AGT) and I/D(ACE) polymorphisms. J Hypertens 2010; 28A: e392. [Google Scholar]
- 29. Shaw KJ, Do YS, Kjos S, et al. Human decidua is a major source of renin. J Clin Invest 1989; 83: 2085–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karlberg BE, Rydén G, Wichman K. Changes in the renin–angiotensin–aldosterone and kallikrein–kinin systems during normal and hypertensive pregnancy. Acta Obstet Gynecol Scand Suppl 1984; 118: 17–24. [DOI] [PubMed] [Google Scholar]
- 31. Chen YP, Lu YP, Li J, et al. Fetal and maternal angiotensin (1-7) are associated with preterm birth. J Hypertens 2014; 32: 1833–1841. [DOI] [PubMed] [Google Scholar]
- 32. Nartita T, Ichihara A, Matsuoka K, et al. Placental (pro)renin receptor expression and plasma soluble (pro)renin receptor levels in preeclampsia. Placenta 2016; 37: 72–78. [DOI] [PubMed] [Google Scholar]
- 33. Sanguinetti CJ, Dias Neto E, Simpson AJ. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques 1994; 5: 914–921. [PubMed] [Google Scholar]
- 34. Ahmad U, Saleheen D, Bokhari A, et al. Strong association of a renin intronic dimorphism with essential hypertension. Hypertens Res 2005; 28: 339–344. [DOI] [PubMed] [Google Scholar]
- 35. Yang HY, Lu KC, Fang WH, et al. Impact of interaction of cigarette smoking with angiotensin-converting enzyme polymorphisms on end-stage renal disease risk in a Han Chinese population. J Renin Angiotensin Aldosterone Syst 2015; 16: 203–210. [DOI] [PubMed] [Google Scholar]
- 36. Frossard PM, Malloy MJ, Lestringant GG, et al. Haplotypes of the human renin gene associated with essential hypertension and stroke. J Hum Hypertens 2001; 15: 49–56. [DOI] [PubMed] [Google Scholar]
- 37. Rupert JL, Kidd KK, et al. Genetic polymorphisms in the renin–angiotensin system in high-altitude and low-altitude native American populations. Ann Hum Genet 2003; 67: 17–25. [DOI] [PubMed] [Google Scholar]
- 38. Von Bohlen und Halbach O, Albrecht D. The CNS renin-angiotensin system. Cell Tissue Res 2006; 326: 599–616. [DOI] [PubMed] [Google Scholar]
- 39. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. New Eng J Med 2000; 342, 1500–1507. [DOI] [PubMed] [Google Scholar]
- 40. Sibai BM, Caritis S, Hauth J, et al. Risks of preeclampsia and adverse neonatal outcomes among women with pregestational diabetes mellitus. Am J Obstet Gynecol 2000; 182: 364–369. [DOI] [PubMed] [Google Scholar]
- 41. Saydah SH, Chandra A, Eberhardt MS. Pregnancy experience among women with and without gestational diabetes in the US, 1995 National Survey of Family Growth. Diabetes Care 2005; 28: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 42. Challis JR, Matthews SG, Gibb W, et al. Endocrine and paracrine regulation of birth at term and preterm 1. Endocr Rev 2000; 21: 514–550. [DOI] [PubMed] [Google Scholar]
- 43. González-Merlo J, Jm Laillla Vicens, Fabre-González E, et al. Obstetricia. Masson Ed. 5th ed Barcelona: 2005; pp 508–510. [Google Scholar]
- 44. Romero R, Rolansky P, Wan M, et al. Infection and labor: Endotoxin concentration in amniotic fluid of women in preterm labor. Am J Obst Gynecol 1988; 158: 1044. [DOI] [PubMed] [Google Scholar]
- 45. Aggarwal B, Pocsik E. Cytoquines: From clone to clinic. Arch Biochem Biophys 1992; 292: 335–359. [DOI] [PubMed] [Google Scholar]
- 46. Romero R, Emanian M, Wan M, et al. Prostaglandin concentration in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol 1987; 157: 1461. [DOI] [PubMed] [Google Scholar]
- 47. Valdez LL, Quintero A, Garcia E, et al. Thrombophilic polymorphisms in preterm delivery. Blood Cells Mol Dis 2004; 1: 51–56. [DOI] [PubMed] [Google Scholar]
- 48. Leslie JC, Galvin SL, Diehl SJ, et al. Infant mortality, low birth weight, and prematurity among Hispanic, white, and African American women in North Carolina. Am J Obstet Gynecol 2003; 188: 1238–1240. [DOI] [PubMed] [Google Scholar]


