Abstract
It was shown recently that angiotensin-converting enzyme activity is limited by endogenous inhibition in vivo, highlighting the importance of angiotensin II (ACE2) elimination. The potential contribution of the ACE2 to cardiovascular disease progression was addressed. Serum ACE2 activities were measured in different clinical states (healthy, n=45; hypertensive, n=239; heart failure (HF) with reduced ejection fraction (HFrEF) n=141 and HF with preserved ejection fraction (HFpEF) n=47). ACE2 activity was significantly higher in hypertensive patients (24.8±0.8 U/ml) than that in healthy volunteers (16.2±0.8 U/ml, p=0.01). ACE2 activity further increased in HFrEF patients (43.9±2.1 U/ml, p=0.001) but not in HFpEF patients (24.6±1.9 U/ml) when compared with hypertensive patients. Serum ACE2 activity negatively correlated with left ventricular systolic function in HFrEF, but not in hypertensive, HFpEF or healthy populations. Serum ACE2 activity had a fair diagnostic value to differentiate HFpEF from HFrEF patients in this study. Serum ACE2 activity correlates with cardiovascular disease development: it increases when hypertension develops and further increases when the cardiovascular disease further progresses to systolic dysfunction, suggesting that ACE2 metabolism plays a role in these processes. In contrast, serum ACE2 activity does not change when hypertension progresses to HFpEF, suggesting a different pathomechanism for HFpEF, and proposing a biomarker-based identification of these HF forms.
Keywords: Angiotensin-converting enzyme 2, diastolic heart failure, systolic heart failure, hypertension, renin–angiotensin–aldosterone system, biomarker
Introduction
The cardiovascular continuum was first proposed by Dzau and Braunwald, giving a mechanistic view of the pathomechanism of heart failure (HF).1 Our current view of the cardiovascular continuum suggests that the steps of cardiovascular disease development are risk factors (including hypertension), vascular disease, tissue injury, pathological remodeling, target organ dysfunction, organ failure and death. In spite of much effort, the pathophysiological mechanism of the disease is still not identified in many cases. There is no known cause of hypertension in the majority of cases (primary or essential hypertension),2 and the exact molecular mechanisms of disease progression are unidentified. As a result, cardiovascular disease is still the leading cause of death in developed countries.3 The initial steps of preclinical cardiovascular disease are hypertension, dyslipidemia and diabetes.1 These underlying pathologies are all hazards for developing advanced coronary atherosclerosis and myocardial infarction. Despite aggressive coronary revascularization strategies, some patients with coronary heart disease display severe and progressive heart failure with reduced ejection fraction (HFrEF). From this aspect, HFrEF is principally the end stage of hypertensive, coronary and valvular cardiovascular disease. Epidemiological data from the Framingham study indicated that hypertension increased the age and risk factor-adjusted hazard of chronic HF twofold in men and threefold in women.4 Nevertheless, another distinct set of patients, with the same risk factor profile, displays heart failure with preserved ejection fraction (HFpEF). That crucial point in the chain of events of cardiovascular disease development where the pathophysiologies of the two kinds of heart failure diverge is still unclear.
Nonetheless, significant advances have been made in developing treatment options. One of the most successful was the introduction of inhibitors of the renin–angiotensin–aldosterone system (RAAS). One of the most important effector molecules of the RAAS is angiotensin II (AngII), which is produced from angiotensin I (AngI) by angiotensin-converting enzyme (ACE). Inhibitors of ACE are now primary drugs in the treatment of hypertension and HFrEF.5–8
It was therefore surprising that an endogenous regulation of ACE emerged recently,9–11 suggesting new perspectives in the RAAS. In particular, the high level of ACE inhibition by serum albumin suggested a prominent role for AngII-degrading enzymes, such as ACE2. In accordance, ACE2 has been related to hypertension and systolic heart function in human.12
An effort was made here to correlate serum ACE2 activities with the stages of the cardiovascular disease continuum. Serum ACE2 activity was determined in healthy individuals and in patients with hypertension (without HF), HFrEF and HFpEF. Serum ACE2 activity was also evaluated as a potential diagnostic tool in these diseases. Furthermore, serum ACE activities and ACE concentrations were also characterized in all study groups.
It was found that serum ACE2 activity correlates with the transition to hypertensive state and with the further progression to HFrEF. However, the occurrence of HFpEF in hypertensive patients was without change in serum ACE2 activity, suggesting different pathomechanisms in the development of HFrEF and HFpEF. Finally, serum ACE2 activity may be used as a biomarker to differentiate between HFrEF and HFpEF.
Methods
This study conforms with the principles outlined in the Declaration of Helsinki. The research has been approved by the Regional and Institutional Ethics Committee, University of Debrecen, (UD REC/IEC number: 3261-2010) and by the Medical Research Council of Hungary. Each patient provided written informed consent before enrolment.
Study population
Patients were recruited for clinical, echocardiographic and biomarker analyses as part of a single-centre, prospective study at the Institute of Cardiology University of Debrecen to identify the role of angiotensin-converting enzyme 2 (ACE2) in cardiovascular pathologies. During the 4-year inclusion period from the beginning of 2011 through to the end of 2014, 188 patients with HF were enrolled. Using a left ventricular ejection fraction (EF) cut-off of 50%, 47 HF patients showed evidence of left ventricular diastolic dysfunction with preserved systolic function (HFpEF),13 while the remaining 141 were adjudicated as HF patients with reduced ejection fraction (HFrEF).
A third study group was also established including 239 hypertensive patients (systolic blood pressure above 140 mmHg and/or diastolic blood pressure above 90 mmHg at the time of the diagnosis of the disease) without any sign and symptom of HF. This group was characterized by preserved EF (above 50%) and optimal antihypertensive therapy according to the European guidelines.14
In addition, 45 healthy individuals without any cardiovascular pathology or medication, with normal cardiac morphology and with left ventricular EF above 50% were enrolled. Clinical and biochemical data of the hypertensive and the healthy group have been partly published earlier.12 A flow diagram of subject selection is shown in Figure 1.
Figure 1.
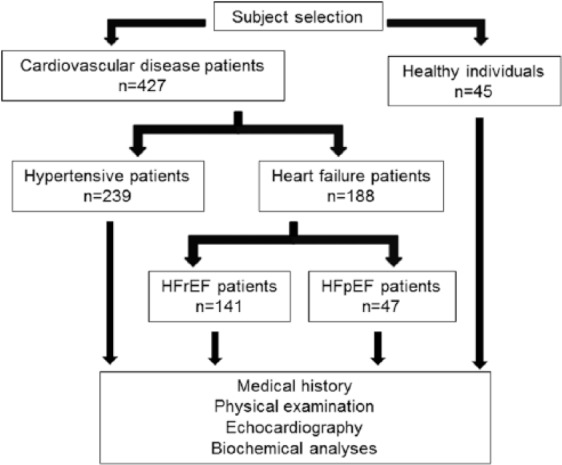
Flow diagram of subject selection.
Beside a control group of healthy individuals without any cardiovascular pathology (n=45) hypertensive patients (n=239), patients with heart failure with reduced ejection fraction (HFrEF, n=141) and patients with heart failure with preserved ejection fraction (HFpEF, n=47) were enrolled for biochemical and echocardiographic analyses.
Clinical assessment comprised age, sex, blood pressure, presence of hypercholesterolemia, diabetes mellitus and atrial fibrillation, besides to other minor parameters. Each visit included echocardiographic measurements and blood sample collection for biochemical measurements. Biochemical analyses comprised serum ACE activity and concentration, serum ACE2 activity, amino-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration measurements and in HFrEF and HFpEF groups determination of glomerular filtration rate (GFR) and C-reactive protein (CRP) values. Medical reports and medication history were obtained from all patients. Examinations were performed at the enrolment (healthy group) and at regular visits at the outpatient ward of the Department of Cardiology, University of Debrecen (hypertensive, HFrEF, HFpEF groups).
Echocardiographic measurements
Transthoracic echocardiography was performed using Accuson Sequoia (Siemens AG, Germany) or Vivid E9 (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) ultrasound machines.
Cardiologists with specialized echocardiography training (blinded to the biomarker analyses) interpreted the echocardiograms. Left ventricular EF was measured using Simpson’s biplane method of disks. Cut-off for normal (preserved) EF was >50%.3,13 In the case of patients with signs or symptoms of HF and with preserved EF, complementary echocardiographic studies for assessment of diastolic dysfunction consisted of blood flow and tissue Doppler measurements.13
Blood sample collection
Standard aseptic technique was used for blood sample collection. Native blood samples were incubated for 60 minutes at room temperature. Serum fractions were separated by centrifugation (1500g, 15 min) and kept in a freezer (–20°C) until the measurements were performed.
Measurement of serum ACE2 activity
ACE2 activity measurement was performed using a specific quenched fluorescent substrate as previously described, with some modifications.12,15–17 The reaction mixture (200 µl) contained 20 µl serum, 80 µl buffer and 100 µl (50 µM) ACE2-specific fluorescent substrate (7-methoxycoumarin-4-yl)acetyl-Ala-Pro-Lys(2,4-dinitrophenyl)-OH [Mca-APK(Dnp)] (EZ Biolab, Carmel, USA). ACE2 activity was assessed by the change in fluorescence intensity upon the enzymatic cleavage of K(Dnp) from the Mca-APK(Dnp).
The reaction buffer contained protease inhibitors (10 µM Bestatin-hidrochloride, 10 µM Z-prolyl-prolinal, (Enzo Life Science, Exeter, UK), 5 µM Amastatin-hidrochloride, 10 µM Captopril in a buffer of 500 mM NaCI, 100 µM ZnCI2, 75 mM TRIS HCI, pH 6.5). All chemicals were from Sigma (St. Louis, MO, USA) if not stated otherwise.
The reaction was performed in black 96-well microtiter plates (Greiner Bio-One, Frickenhauser, Germany). The assay was monitored continuously by measuring the increase in fluorescence (excitation wavelength: 320 nm, emission wavelength: 405 nm) upon substrate hydrolysis using a fluorescence microplate reader (NOVOstar; BMG Labtech GmbH, Offenburg, Germany). Initial enzyme activities were determined from the linear rate of fluorescence increase over the 0–120 min time course. The increase in fluorescence was plotted as a function of reaction time and fitted with a linear regression.
ACE2 activity was calculated by the equation: ACE2 activity=(S/k)*D, where S is the rate of increase in fluorescence intensity, k is the change in fluorescence intensity upon the complete cleavage of 0.1 nmol of Mca-APK(Dnp), and D is the dilution of the serum. One unit (U) corresponds to 0.1 nmol Mca-APK(Dnp) cleavage in 1 hour at 37°C. The specificity of the ACE2 enzyme activity assay was tested by the specific human ACE2 inhibitor DX600, which resulted in a complete inhibition of Mca-APK(Dnp) cleavage. Fits were accepted when r >0.95.
Measurement of serum ACE activity
Assessment of ACE activity was based on the spectrophotometric measurement of FAPGG (N-[3-(2-Furyl)acryloyl]-L-phenylalanyl-glycyl-glycine) (Sigma, St. Louis, MO, USA) substrate hydrolysis as detailed elsewhere.12
Measurement of serum ACE concentration
ACE concentration was determined using a human ACE enzyme-linked immunosorbent assay (ELISA) (Development System catalog No. DY929; R&D System, Inc, Minneapolis, USA) according to the manufacturer’s instruction, with minor modifications.12
Amino-terminal NT-proBNP measurements
NT-proBNP levels were measured in serum using a commercially available kit (Elecsys proBNPII., Roche Ltd., Mannheim, Germany) according to the manufacturer’s instructions.
GFR measurements
GFR values were measured in serum by a kinetic colorimetric assay on a Roche cobas c system (Roche Ltd., Mannheim, Germany) according to the manufacturer’s instructions.
CRP measurements
CRP levels were measured in serum by a turbidimetric immunodiagnostic assay on a Roche cobas c system (Roche Ltd., Mannheim, Germany) according to the manufacturer’s instructions.
Statistical analysis
Results are expressed as mean ± SEM for all groups. Patients’ characteristics were tested by one-way analysis of variance (ANOVA) (Table 1). Most of the groups did not pass the D’Agostino and Pearson omnibus normality test when tested for serum ACE2 activity or NT-proBNP concentration and therefore nonparametric evaluation was performed. Statistical difference in these cases was tested by one-way ANOVA on ranks (Kruskal-Wallis test, Figure 2, Figure 3, Figure 4 and Figure 8). Linear regression analysis was performed to correlate serum ACE2 activity with echocardiographic parameters (Figures 5 and 6) and correlation was considered to be significant when r2>0.1 and p<0.05. Receiver operating characteristic (ROC) curves were generated to test the diagnostic value of serum ACE2 activity and NT-proBNP concentration (Figure 7). To predict the relationship between ACE2 activities and different biomedical factors such as gender, obesity, cardiovascular comorbidities (diabetes mellitus, dyslipidemia, atrial fibrillation) and cardiovascular medications, logistic regression analyses were performed. Parameters with a p-value of <0.05 were considered to be meaningful predictors of changes in ACE2 activities (Figure 9). All statistical analyses were performed by GraphPad Prism, version 6.0 (GraphPad Software, Inc., San Diego, CA, USA).
Table 1.
General characteristics of enrolled patients. Patients were enrolled in four groups: healthy, hypertensive, heart failure with reduced (HFrEF) or with preserved ejection fraction (HFpEF). The general clinical characteristics of the patients are shown. Statistical differences were addressed by one-way analysis of difference (ANOVA) and shown by asterisks (*, different from healthy) of hashtags (#, different from hypertensive in the HF population). In the HFrEF group in the case of CRP 126 patients, in the case of GFR 132 patients and in the case of BMI 64 patients, data were available/applicable.
| Variables | Healthy individuals n=45 | Hypertensive patients n=239 | HFrEF patients n=141 | HFpEF patients n=47 |
|---|---|---|---|---|
| Age, years (mean±SD) | 30.2 ± 8.7 | 62.3 ± 9.6* | 63 ± 10.8* # | 70.1 ± 8.8* # |
| Cardiovascular comorbidities, % | ||||
| Hypertension | 0 | 100 | 73 | 100 |
| Diabetes | 0 | 23 | 30 | 36 |
| Dyslipidemia | 0 | 70 | 72 | 75 |
| Atrial fibrillation | 0 | 7 | 21 | 34 |
| CRP | – | – | 6.2 ± 7.6 | 3.6 ± 2.6 |
| Renal function | – | – | ||
| GFR>90 ml/min/1.73m2 (%) | – | – | 33.3 | 25.7 |
| GFR: 60–89 ml/min/1.73m2 (%) | – | – | 40.2 | 41.8 |
| GFR: 30–59 ml/min/1.73m2 (%) | – | – | 25 | 30.2 |
| GFR<30 ml/min/1.73m2 (%) | – | – | 1.5 | 2.3 |
| BMI | 24.9 ± 4.0 | 29.9 ± 5.5* | 27.7 ± 5.8* # | 31.9 ± 5.4* # |
| Echocardiographic parameters (mean±SD) | ||||
| LVEF, % | 62.0 ± 4.2 | 56.6 ± 4.6* | 28.6 ± 5.7* # | 60.9 ± 6.9# |
| LVEDD, mm | 48.6 ± 3.9 | 51.7 ± 5.7* | 67.2 ± 9.5* # | 51.1 ± 6.7 |
| LVESD, mm | 29.9 ± 3.6 | 33.4 ± 5.4* | 56.3 ± 9.9* # | 32.3 ± 6.2 |
| Medication at enrolment, % | ||||
| ACE inhibitor | 0 | 83 | 91 | 57 |
| ARB | 0 | 15 | 9 | 39 |
| β-blocker | 0 | 79 | 100 | 96 |
| Aldosterone antagonist | 0 | 6 | 100 | 8.2 |
| Diuretics | 0 | 53 | 92 | 96 |
CRP: C-reactive protein; GFR: glomerular filtration rate; BMI: body mass index; LVEF: left ventricular ejection fraction; LVEDD: left ventricular end diastolic diameter; LVESD: left ventricular end systolic diameter; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blockers.
: healthy vs. others.
: hypertensive vs. others.
Figure 2.
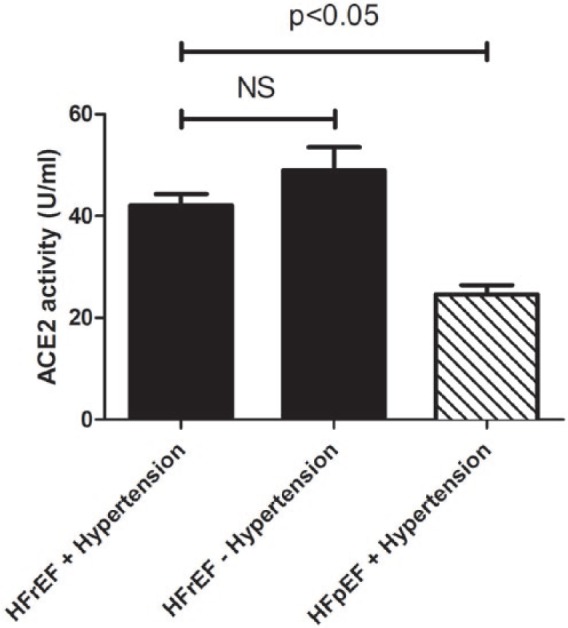
Serum ACE2 activity parallels cardiovascular disease development.
ACE2 activity was measured in the sera of hypertensive patients with heart failure with reduced ejection fraction (HFrEF, n=102), hypertensive patients with heart failure with preserved ejection fraction (HFpEF, n=47) and patients with HFrEF without hypertension (n=39). ACE2 activity is expressed as U/ml of serum, where 1 unit (U) corresponds to 0.1 nmol Mca-APK(Dnp) cleavage in 1 hour at 37°C. Bars represent the mean and SEM. Statistical significance was tested by the nonparametric Kruskal–Wallis test among the groups. Correlation was considered to be significant when p<0.05. There is no statistical difference between values for HFrEF patients with or without hypertension, which is shown by NS.
Figure 3.
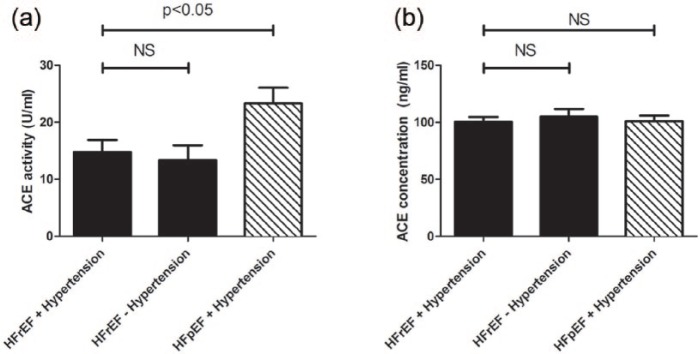
Comparisons of serum ACE activities and serum ACE concentrations between heart failure cohorts.
Serum ACE activity (panel (a)) and ACE concentration (panel (b)) were measured in hypertensive patients with heart failure with reduced ejection fraction (HFrEF, n=102), hypertensive patients with heart failure with preserved ejection fraction (HFpEF, n=47) and patients with HFrEF without hypertension (n=39). Statistical analyses of biochemical measurements were performed by one-way analysis of variance (ANOVA) followed by nonparametric Kruskal–Wallis test among groups. Bars represent mean ± SEM. Correlation was considered to be significant when p<0.05. Lack of statistical difference is labelled by NS.
Figure 4.
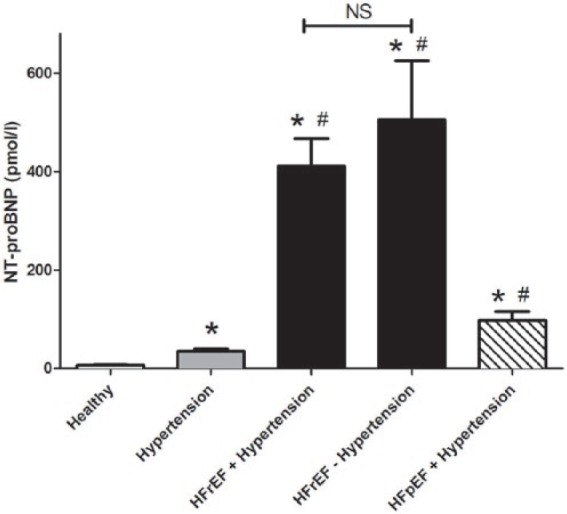
Serum amino-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration parallels cardiovascular disease development.
NT-proBNP concentration was measured in the sera of healthy (n=45) individuals and hypertensive patients without signs of heart failure (n=239), hypertensive patients with heart failure with reduced ejection fraction (HFrEF, n=102), hypertensive patients with heart failure with preserved ejection fraction (HFpEF, n=47) and patients with HFrEF without hypertension (n=39). NT-proBNP concentration is expressed as pmol/l. Bars represent the mean and SEM. Statistical significance was tested by the nonparametric Kruskal–Wallis test among the groups. Asterisks show statistical difference from the healthy and hash tags show statistical difference from the hypertensive group. There is no statistical difference between values for HFrEF patients with or without hypertension, which is shown by NS.
Figure 8.
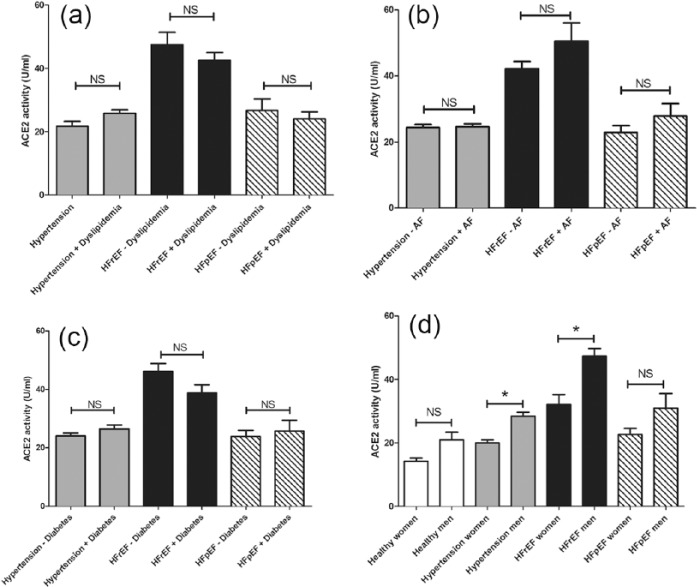
Effects of comorbidities on serum ACE2 activity in cardiovascular patients.
The effects of comorbidities such as dyslipidemia (panel (a)), atrial fibrillation (AF, panel (b)), diabetes (panel (c)) and gender (panel (d)) are shown in cardiovascular patients (healthy, hypertensive without heart failure, heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF)). Statistical differences between patient groups with and without of the defined comorbidity are indicated: asterisks represent statistically significant differences, NS represents no significant statistical difference. Statistical analysis was made by a nonparametric test (Kruskal–Wallis).
Figure 5.
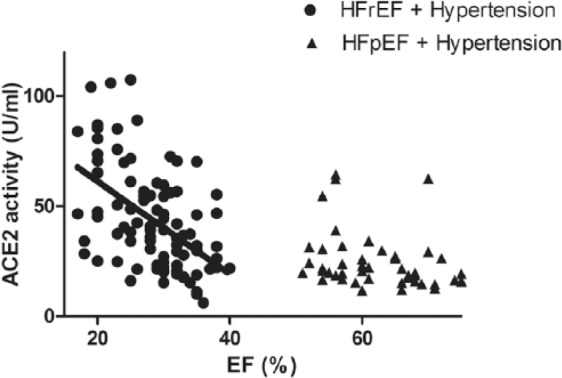
Serum ACE2 activity correlates with the severity of systolic dysfunction.
Serum ACE2 activity (expressed as U/ml of serum) of hypertensive patients with heart failure with reduced EF (HFrEF, n=141) and of hypertensive patients with heart failure with preserved EF (HFpEF, n=47) is shown as a function of the ejection fraction (EF). Serum ACE2 activity and EF negatively correlated (r2=0.26 and p<0.001, slope −2.1±0.36) in the HFrEF group. There was no correlation (defined as r2>0.1 and p<0.05) among these parameters in the healthy, hypertensive and HFpEF groups. Data of the healthy and hypertensive groups have been published earlier.12
Figure 6.
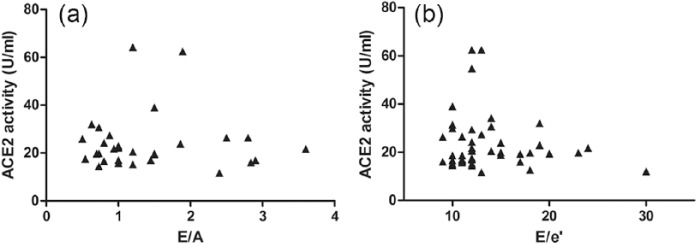
There is no correlation between serum ACE2 activity and the severity of diastolic dysfunction.
There was no correlation between serum ACE2 activity (expressed as U/ml serum) and left ventricular diastolic parameters (E/A, n=30, panel (a) and E/e’, n=43, panel (b)) of patients with heart failure with preserved ejection fraction (HFpEF). E/A values were determined in HFpEF patients without atrial fibrillation. E/e’ values were determined in patients with sufficient acoustic window for accurate echocardiographic measurement. The threshold for correlation was defined as r2>0.1 and p<0.05.
Figure 7.
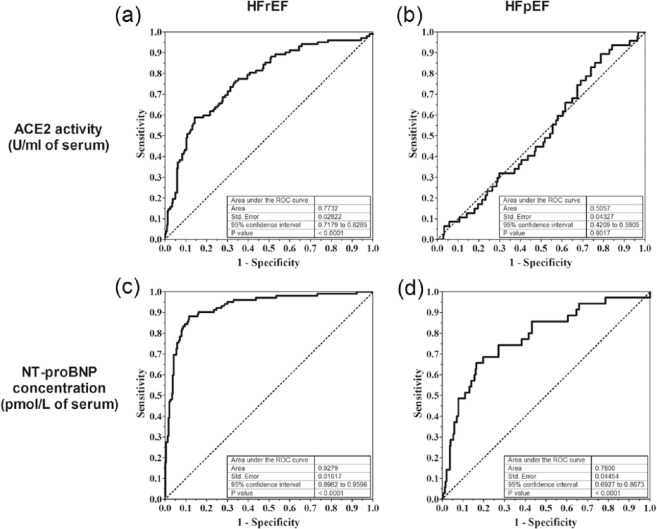
Comparison of the prognostic value for serum ACE2 activity and amino-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration to differentiate HFrEF and HFpEF from hypertension.
Receiver operating characteristic (ROC) curves were generated to test the diagnostic value of serum ACE2 activity (panels (a) and (b)) or NT-proBNP (panels (c) and (d)) to differentiate between patients with heart failure and hypertension without heart failure. Results for HFrEF vs. hypertensive are shown on panels (a) and (c), results for HFpEF vs. hypertensive are shown on panels (b) and (d). Parameters of the ROC analysis are shown as inserts in the plots.
Figure 9.
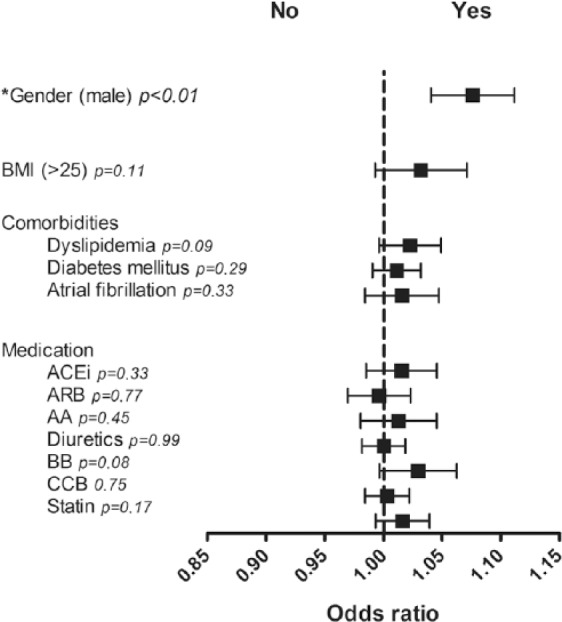
Logistic regression analyses for confounding variables such as gender, elevated BMI values, cardiovascular comorbidities and cardiovascular drug therapy in hypertensive patients.
Male gender has a positive predictive value for elevation of ACE2 activities (p<0.01), no other clinical parameter (BMI>25, presence of diabetes mellitus, dyslipidemia or atrial fibrillation) and no type of the investigated cardiovascular medication (angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), beta blockers (BB), aldosterone antagonists (AA), diuretics, calcium-channel blockers (CCB), statins) have a predictive value for changes of ACE2 activities in hypertensive patients.
Results
Baseline characteristics of study groups
Clinical characteristics of the study groups are shown in Table 1. The healthy group was characterized by normal heart performance and function, without any signs or symptoms of HF or any cardiovascular disorder, and were not treated with any pharmaceutics. The patient groups were chosen to represent the major steps in the cardiovascular continuum. Among the cardiovascular comorbidities in the HF group hypertension was the most common. The EF was severely reduced in the HFrEF group with enlarged end-systolic (ESD) and end-diastolic (EDD) diameters. The hypertensive and the HFpEF groups displayed normal left ventricular dimensions with a normal (preserved) EF (EF>50%). Patients were treated according to national guidelines.
Serum ACE2 activity was the lowest in the healthy group (16.2±0.8 U/ml,12) which was significantly increased in hypertensive patients (24.8±0.8 U/ml,12) and further increased when hypertension was accompanied by HFrEF, representing progression of cardiovascular disease toward systolic dysfunction (42.1±2.2 U/ml, Figure 2). HFrEF patients without hypertension also had higher serum ACE2 activities (49.0±4.5 U/ml, n=39, Figure 2). In contrast, patients with hypertension and HF with preserved systolic function (HFpEF) had similar serum ACE2 activities (24.6±1.9 U/ml, Figure 2) to that of hypertensive patients without HF.
On the other hand, activity of the classic ACE enzyme was highest in the healthy group (33.6± 1.7 U/ml,12) and was significantly lower in hypertensive patients (14.5±0.9 U/ml,12). HFrEF patients with or without hypertension had similarly low ACE activity (HFrEF + Hypertension: 14.7±2.2 U/ml; HFrEF – Hypertension: 13.3±2.6 U/ml, Figure 3(a)) and these values are most probably due to effective ACE inhibitory therapy. Since only 57% of the patients in the HFpEF group used ACE inhibitor drug as an antihypertensive agent, this section represented an intermediate level ACE activity between healthy and successfully inhibited hypertensive and HFrEF patients’ values (23.3±2.8 U/ml, Figure 3(a)).
Patients with heart failure – regardless of the type – represented lower ACE concentrations (HFrEF + Hypertension: 100.5±4.1 ng/ml; HFrEF – Hypertension: 105±6.8 ng/ml; HFpEF + Hypertension: 100.9±5.1 ng/ml, Figure 3(b)) than subjects without heart failure (healthy: 159.0±9.3 ng/ml, hypertensive: 149.6±6.9 ng/ml,12).
The most often used biochemical marker for cardiovascular disease progression is the amino-terminal pro-B-type natriuretic peptide (NT-proBNP). NT-proBNP levels were increased in hypertension (healthy: 6.5±4.8 pmol/l vs. hypertensive: 32.5±69.4 pmol/l, Figure 4) and further increased in HF, irrespective of the form of HF (HFrEF with hypertension: 411±56 pmol/l, HFrEF without hypertension: 505±119 pmol/l and HFpEF with hypertension: 98±18 pmol/l, Figure 4).
The emerging hypothesis that serum ACE2 activity correlates with systolic HF was tested next. Serum ACE2 activities were plotted as a function of the EF in hypertensive HFpEF and hypertensive HFrEF patients (Figure 5). There was no correlation between serum ACE2 activity and systolic HF in patient populations with normal (preserved) EF. However, serum ACE2 activity correlated negatively with the EF in the HFrEF group (p<0.001, r2=0.21). In contrast to this, there was no correlation with left ventricular diastolic parameters (E/A, Figure 6(a) and E/e’, Figure 6(b)) in HFpEF patients.
The clinical applicability of serum ACE2 activity as a biomarker for left ventricular systolic dysfunction in human HF was also addressed. A ROC curve was generated, in which the clinical applicability of serum ACE2 activity was tested to discriminate HF patients from hypertensive patients. Area under the curves was 0.77 for HFrEF (Figure 7(a)) and 0.51 for HFpEF patients (Figure 7(b)). Similar values for NT-proBNP were 0.93 for HFrEF (Figure 7(c)) and 0.78 for HFpEF (Figure 7(d)) patients.
Effects of comorbidities, such as dyslipidemia (Figure 8(a)), atrial fibrillation (Figure 8(b)), diabetes (Figure 8(c)) and gender (Figure 8(d)) were tested on serum ACE2 activities in the different cardiovascular patient populations. There was a general tendency toward males having higher serum ACE2 activities, which was statistically significant among hypertensive and HFrEF patients (Figure 8(d)). None of the other comorbidities had any effects on serum ACE2 activity. Regression analyses of ACE2 and CRP data revealed no correlation in the HFrEF (p=0.65, r2=0.001, n=126) as well as in the HFpEF group (p=0.33, r2=0.02, n=47). In the same way, in neither the HFrEF (p=0.504, r2=0.003, n=132) nor the HFpEF group (p=0.27, r2=0.03, n=47) was correlation between ACE2 and GFR values observed (figure not shown).
This observation was confirmed by logistic regression analyses. Serum activities of ACE2 differ according to gender in hypertensive patients (p<0.01); however, in assessing confounding variables such as high body mass index (BMI) (>25), cardiovascular drug therapy and cardiovascular comorbidities, ACE2 activities proved to have no predictive value (p=0.11 for high BMI, p=0.33 for ACEi, p=0.77 for angiotensin receptor blockers (ARB), p=0.45 for aldosterone antagonists (AA), p=0.99 for diuretics, p=0.08 for beta blockers (BB), p=0.75 for calcium-channel blockers (CCB), p=0.17 for statins, p=0.09 for dyslipidemia, p=0.29 for diabetes mellitus and p=0.33 for atrial fibrillation) (Figure 9).
Discussion
Cardiovascular disease is the leading cause of death in most developed countries.18 Cardiovascular disease usually starts with hypertension, dyslipidemia and diabetes.1 These may progress to HF, which has two distinct forms: HFrEF is characterized by systolic dysfunction, while HFpEF is characterized by diastolic dysfunction.13
We have extended the view that serum ACE2 activity correlates with the worsening of HF,16,19 earlier showing its correlation with the improvement of EF upon biventricular pacing in the case of long QRS morphology.12 Moreover, serum ACE2 activity was found to be elevated in hypertensive patients, opening up new perspectives in the field.12 Here we continued these efforts. Serum ACE2 activity was measured in various stages of the cardiovascular continuum, with particular attention to the transition of hypertension to HF. The most important finding was that serum ACE2 activity does not change upon progression of hypertension to HFpEF, contrasting with progression of hypertension to HFrEF (twofold increase). These findings suggested that serum ACE2 activity is a selective biomarker of systolic dysfunction. This idea was tested in detail. ROC analysis confirmed that serum ACE2 activity has no predictive value for HFpEF among hypertensive patients, while it was fairly predictive in identifying hypertensive patients with HFrEF. Amino-terminal pro-B-type natriuretic peptide (NT-proBNP, a widely used biomarker for HF) levels were sixfold increased in hypertensive patients, and further increased in HFpEF (threefold) and in HFrEF (12-fold), suggesting that natriuretic peptide release is being activated in both HFrEF and HFpEF. Although NT-proBNP was found to be a superior biomarker identifying HFrEF in hypertensive patients when compared with serum ACE2 activity, it was inferior in selectivity, since it was also predictive for HFpEF among the same patients. It appears therefore that NT-proBNP is a rather general biomarker for HF, irrespectively of the form of HF, while serum ACE2 activity is selective for HFrEF.
One of the limitations of clinical studies dealing with biomarkers is that their level is influenced by various comorbidities. For example, serum ACE2 activity appears to be gender dependent, a factor which is usually overlooked in clinical studies.20 Here we made an effort to specifically test the effects of comorbidities. There was no effect of diabetes, atrial fibrillation and dyslipidemia on serum ACE2 activity in the different cardiovascular populations. No correlation existed between ACE2 activities and GFR as well as CRP values and in these cohorts of HF patients. In contrast, males had significantly higher serum ACE2 activity than females, confirming earlier reports.17,21 It was most significant in the HFrEF group, where serum ACE2 activity was about 50% higher in male subjects. Nonetheless, increasing serum ACE2 activities in both genders paralleled the progression of cardiovascular disease to HFrEF, and therefore differences cannot be explained by the different gender ratios (such as male dominance in HFrEF and female dominance in HFpEF, Table 1). In a recent study it was shown that ACE2 activity directly correlated with male gender, diabetes and older age as the classical cardiovascular risk factors in chronic kidney disease patients at different clinical stages.22 That patient population with a high cardiovascular risk, namely diabetic chronic kidney disease patients, represented also an increased circulating ACE2 activity and elevation of ACE2 activity correlated with disease progression, further supporting the role of ACE2 as a potential cardiovascular biomarker.
Our data showed highly elevated serum ACE2 activities in HFrEF and moderate elevation in hypertensive patients (when compared with healthy individuals). This suggests that ACE2 expression is either increased as a counter-regulatory mechanism to the dysregulation of the RAAS,16 or on the contrary, tissue ACE2 is being released into the circulation (a process called ACE2 shedding), providing a significant step in the pathomechanism of the disease.12 According to this latter hypothesis, ACE2 shedding plays an important role in the development of HF: release of ACE2 into the circulation limits the availability of ACE2 and promotes angiotensin II accumulation in the tissues. This is supported by the recognition of endogenous ACE inhibitors,9 in particular by serum albumin in human.10 Based on these data we proposed that angiotensin II formation may be a rate-limiting step and local angiotensin II levels are determined by its elimination.12 This is strongly supported by the observation of a positive feedback in the RAAS in mice whereby activation of angiotensin II type 1 receptor (AT1R) increases ADAM17 (an enzyme responsible for the cleavage of ACE2 into the circulation) expression, resulting in shedding of ACE2.23,24 A similar but circulating leukocyte (i.e. monocyte)-related regulatory mechanism was proposed recently in chronic kidney disease patients with high cardiovascular risk. In this human study an adverse relation of monocytic ACE and ACE2 induced a severe pro-atherogenic condition.25
Here we extended the potential role of ACE2 shedding in cardiovascular disease. We showed that serum ACE2 activity is already increased at the initiation phase of the cardiovascular continuum (hypertension), and then further increases when hypertension progresses toward systolic dysfunction, but not when diastolic dysfunction develops with maintained systolic function. Moreover, we found similar ACE concentrations in the case of both kinds of HF, while the activity of ACE changed according to the rate of ACE inhibitory drug use in the different study groups. It is important to note that these observations are in accordance with the clinical success of RAAS inhibition. RAAS inhibition is one of the primary treatment options to reduce blood pressure26–29 or to treat HFrEF30–34 according to recent clinical guidelines. On the other hand, there is no elevated ACE2 shedding in HFpEF patients (over hypertensive patients). In accordance, RAAS inhibition is not particularly effective in HFpEF5,6,35–37 over the antihypertensive effects, especially when compared with HFrEF.
In summary, here we have shown that serum ACE2 activity increases in parallel with the progression of cardiovascular disease. It is elevated in hypertension, then further elevates when systolic dysfunction develops. However, it is not being affected by the development of diastolic HF in hypertensive patients. All of these observations suggest that (i) changes in serum ACE2 activity may be related to the pathomechanism of cardiovascular disease progression; (ii) different therapeutic responses to RAAS inhibition in HFrEF and HFpEF are related to ACE2 dysregulation; (iii) serum ACE2 activity is a biomarker which can be used to differentiate between HFrEF and HFpEF patients.
Footnotes
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from the National Research, Development and Innovation Office – NKFIH, K 116940 (to AT) and PD 116212 (to MF); the Hungarian Scientific Research Fund (OTKA: K 109083 to ZP), and by the European Union Project FP7-HEALTH-2010: “MEDIA-Metabolic Road to Diastolic Heart Failure” MEDIA-261409 (to WJP and ZP).
References
- 1. Dzau V, Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: A workshop consensus statement. Am Heart J 1991; 121: 1244–1263. [DOI] [PubMed] [Google Scholar]
- 2. Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet 2007; 370: 591–603. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 4. Ho KK, Pinsky JL, Kannel WB, et al. The epidemiology of heart failure: The Framingham Study. J Am Coll Cardiol 1993; 22: 6A–13A. [DOI] [PubMed] [Google Scholar]
- 5. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 6. Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006; 27: 2338–2345. [DOI] [PubMed] [Google Scholar]
- 7. Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: An inconvenient truth! J Am Coll Cardiol 2010; 55: 526–537. [DOI] [PubMed] [Google Scholar]
- 8. Shahin Y, Khan JA, Samuel N, et al. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: A meta-analysis of randomised controlled trials. Atherosclerosis 2011; 216: 7–16. [DOI] [PubMed] [Google Scholar]
- 9. Fagyas M, Uri K, Siket IM, et al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) I: Endogenous angiotensin converting enzyme (ACE) inhibition. PLoS One 2014; 9: e87843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fagyas M, Uri K, Siket IM, et al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) II: Albumin suppresses angiotensin converting enzyme (ACE) activity in human. PLoS One 2014; 9: e87844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fagyas M, Uri K, Siket IM, et al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) III: Endogenous inhibition of angiotensin converting enzyme (ACE) provides protection against cardiovascular diseases. PLoS One 2014; 9: e93719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uri K, Fagyas M, Manyine Siket I, et al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) IV: Circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PLoS One 2014; 9: e87845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 14. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281–1357. [DOI] [PubMed] [Google Scholar]
- 15. Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 2002; 277: 14838–14843. [DOI] [PubMed] [Google Scholar]
- 16. Epelman S, Tang WH, Chen SY, et al. Detection of soluble angiotensin-converting enzyme 2 in heart failure: Insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol 2008; 52: 750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel SK, Velkoska E, Burrell LM. Emerging markers in cardiovascular disease: Where does angiotensin-converting enzyme 2 fit in? Clin Exp Pharmacol Physiol 2013; 40: 551–559. [DOI] [PubMed] [Google Scholar]
- 18. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2015 update: A report from the American Heart Association. Circulation 2015; 131: e29–e322. [DOI] [PubMed] [Google Scholar]
- 19. Epelman S, Shrestha K, Troughton RW, et al. Soluble angiotensin-converting enzyme 2 in human heart failure: Relation with myocardial function and clinical outcomes. J Card Fail 2009; 15: 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel SK, Velkoska E, Freeman M, et al. From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol 2014; 5: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng JM, Akkerhuis KM, Battes LC, et al. Biomarkers of heart failure with normal ejection fraction: A systematic review. Eur J Heart Fail 2013; 15: 1350–1362. [DOI] [PubMed] [Google Scholar]
- 22. Anguiano L, Riera M, Pascual J, et al. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol Dial Transplant 2015; 30: 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang J, Li N, Gao F, et al. Balance between angiotensin converting enzyme and angiotensin converting enzyme 2 in patients with chronic heart failure. J Renin Angiotensin Aldosterone Syst 2015; 16: 553–558. [DOI] [PubMed] [Google Scholar]
- 24. Patel VB, Clarke N, Wang Z, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: A positive feedback mechanism in the RAS. J Mol Cell Cardiol 2014; 66: 167–176. [DOI] [PubMed] [Google Scholar]
- 25. Trojanowicz B, Ulrich C, Kohler F, et al. Monocytic angiotensin-converting enzyme 2 relates to atherosclerosis in patients with chronic kidney disease. Nephrol Dial Transplant. Epub ahead of print 24 May 2016. DOI: 10.1093/ndt/gfw206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006; 354: 1685–1697. [DOI] [PubMed] [Google Scholar]
- 27. Braunwald E, Domanski MJ, Fowler SE, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med 2004; 351: 2058–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simoons ML, Vos J, de Feyter PJ, et al. EUROPA substudies, confirmation of pathophysiological concepts. European trial on reduction of cardiac events with perindopril in stable coronary artery disease. Eur Heart J 1998; 19(Suppl J): J56–J60. [PubMed] [Google Scholar]
- 29. PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 30. Cowley AJ, Stainer K, Wynne RD, et al. Comparison of the effects of captopril and enoximone in patients with severe heart failure: A placebo controlled double-blind study. Int J Cardiol 1989; 24: 311–316. [DOI] [PubMed] [Google Scholar]
- 31. The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 32. The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325: 293–302. [DOI] [PubMed] [Google Scholar]
- 33. Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA 1995; 273: 1450–1456. [PubMed] [Google Scholar]
- 34. Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation 1999; 100: 2312–2318. [DOI] [PubMed] [Google Scholar]
- 35. Yamamoto K. beta-Blocker therapy in heart failure with preserved ejection fraction: Importance of dose and duration. J Cardiol 2015; 66: 189–194. [DOI] [PubMed] [Google Scholar]
- 36. Senni M, Paulus WJ, Gavazzi A, et al. New strategies for heart failure with preserved ejection fraction: The importance of targeted therapies for heart failure phenotypes. Eur Heart J 2014; 35: 2797–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Preserved Trial. Lancet 2003; 362: 777–781. [DOI] [PubMed] [Google Scholar]