Bruton’s tyrosine kinase (BTK) is highly expressed and activated in more than 90% of acute myeloid leukemia (AML) cases.1, 2 An important contribution of BTK has been reported in the context of FLT3-ITD-positive AML2 and is involved in signaling pathways of Toll-like receptors,3 nuclear factor κB (NFκB)2 and chemokine receptor CXCR4.4 Expression and activation of BTK are associated with poor prognosis of AML patients.2, 5 The mixed-lineage leukemia (MLL) gene is frequently disrupted by chromosomal rearrangements and is associated with distinct clinical features and poor prognosis. Human cord blood hematopoietic CD34+ progenitor cells transduced with MLL-AF9 (MA9) maintain a myelomonocytic AML-like phenotype in cytokine-supplemented medium.6 The cells induce leukemia in non-obese diabetic/severe combined immunodeficiency mice representing a human model of MA9-positive leukemia.6 The specific contribution of BTK in MLL-rearranged AML (MLL-AML) is not known. Because an essential role of NFκB signaling by MLL oncoproteins in the maintenance of leukemic stem cells was reported,7 we investigated the contribution of BTK and p65 in MLL-rearranged leukemia.
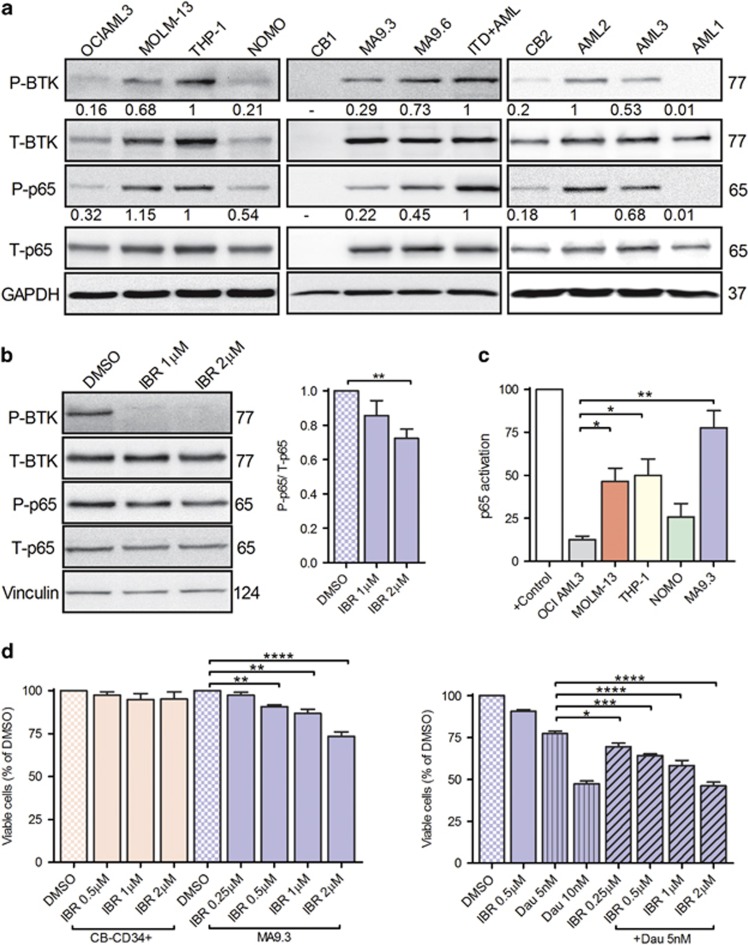
Whole cell lysates from AML cell lines (Figure 1a, left panel), cord blood CD34+ cells (CB1 and 2), MA9 clones 3 and 6 (MA9.3 and 9.6; Figure 1a, middle panel) and leukemic blasts from MLL-AML patients and CB2 (Figure 1a, right panel; Supplementary Table 1) were probed for the expression and phosphorylation of p65 (pS536) and BTK (pY223). Interestingly, AML cell lines with MLL rearrangements (MOLM-13 and THP-1), both MA9 clones and AML patients (AML 2 and 3) indicated constitutive phosphorylation of both p65 and BTK, albeit to different degrees. Importantly, short-term treatment of MA9.3 and MOLM-13 cells with BTK-specific inhibitor, ibrutinib, inhibited the phosphorylation of BTK and p65 in a dose-dependent manner (Figure 1b; Supplementary Figure 1), positioning BTK upstream of NFκB. NFκB transactivation assay demonstrated that p65 is transcriptionally active in MOLM-13, THP-1, NOMO and MA9.3 cells in comparison to OCI AML3 (Figure 1c). Activated p65 is reported to induce the expression of BTK in bortezomib-resistant multiple myeloma cell lines and primary malignant plasma cells.8 That study indicated the presence of two p65-binding sites in BTK promoter region. These observations led us to investigate the involvement of activated p65 in regulating the expression of BTK. We performed luciferase-based BTK promoter assays with empty (pGL4) or test construct (pGL4-BTK). MOLM-13 and MA9.3 cells electroporated with pGL4-BTK vector revealed substantially elevated luciferase activity demonstrating the transcriptional activity of p65 (Supplementary Figure 2A, left panel). Interestingly, ibrutinib treatment suppressed BTK promoter activity in these cell lines (Supplementary Figure 2A, right panel). OCI AML3 cells with a lower level of activated p65 indicated minimal luciferase activity (Supplementary Figure 2A, left panel). In addition, MA9.3 cells treated with a proteasomal inhibitor (MG-132) or NFκB inhibitor (BAY 11-7082) reduced BTK expression in a dose-dependent manner, again indicating the involvement of activated p65 in regulation of BTK expression (Supplementary Figure 2B). Taken together, our data identify BTK as one of the kinase’s involved in the phosphorylation of p65 subsequently regulating its expression. Given the promising results associated with ibrutinib treatment in the context of FLT3-ITD cell lines and AML patient material,2, 5, 9 we sought to determine the cytotoxic effect of BTK inhibition in MLL-rearranged leukemia. Control (CB-CD34+) and MA9.3 cells were treated with increasing doses of ibrutinib (0.25, 0.5, 1.0 and 2 μm), and cell viability was analyzed (Figure 1d, left panel). Ibrutinib treatment (⩾0.5 μm) significantly reduced the viability of MA9.3 cells as compared to dimethylsulfoxide (DMSO)-treated cells. Ibrutinib treatment affected MA9.3 cell metabolism (ability to reduce tetrazolium dye) (Supplementary Figure 3A), induced cell cycle arrest (Supplementary Figure 3B) and suppressed colony formation (Supplementary Figure 3C) in a dose-dependent manner. Of note, ibrutinib treatment had no effect on cell cycle distribution (Supplementary Figure 3D) and failed to induce cell death (Supplementary Figure 3E) in the non-MLL-rearranged AML cell line, OCI AML3. Daunorubicin (a standard chemotherapy drug in AML) in combination with ibrutinib further sensitized the cells and induced a strong effect on cell viability (Figure 1d, right panel). The nature of drug interaction (ibrutinib: daunorubicin) was analyzed by calculating the coefficient of drug interaction (CDI) value.10 CDI values <1.0 indicated that daunorubicin synergistically increased the cytotoxic efficacy of ibrutinib (Supplementary Table 2). Control cells treated under identical conditions were nonresponsive to ibrutinib treatment. Collectively, the data indicate a pivotal role for activated BTK in MLL-rearranged leukemic cells and inhibition of BTK activity affected cell viability in MA9.3 cells.
Figure 1.
BTK and p65 are activated in MLL-rearranged cells and pharmacological inhibition of BTK induced cell death. (a) AML cell lines OCI AML3, MOLM-13, THP-1, NOMO (left), cord blood CD34+ cells (CB1), MA9 clones (MA9.3 and MA9.6), FLT3-ITD+AML (ITD+AML) patient leukemic blasts (middle) and CB2, MLL+AML patient leukemic blasts (AML1, 2 and 3; right) were lysed and immunoblotted for phospho-p65 (pS536) and -BTK (pY223) and total (T) proteins. GAPDH is internal loading control. ITD+AML cell lysates served as a positive control for the detection of phosphorylated p65 and BTK. Densitometric analysis of phosphorylated BTK and p65 relative to total proteins is indicated under the blots. (b) MA9.3 cells were serum-starved for 3 h and subsequently treated with 1 and 2 μm ibrutinib (IBR) for additional 2 h. Whole cell lysates were analyzed for the activation of p65 and BTK proteins. Vinculin is internal loading control (left). Densitometric analysis of P-p65 relative to T-p65 from three independent experiments is shown (right). (c) OCI AML3, MOLM-13, THP-1, NOMO and MA9.3 cells were lysed and transcriptionally active p65 is detected via NFκB activation assay following the manufacturer’s instructions. Nuclear extracts from Jurkat cells stimulated with calcium ionophore and tissue plasminogen activator served as positive (+) control. p65 activation from three independent experiments (in duplicates) is plotted. (d) Control CB-CD34+ cells and MA9.3 cells were treated with DMSO or different doses of ibrutinib (IBR) (left) or daunorubicin (DAU) alone or in combination (right) as indicated for 48 h and assayed for cell viability. Percentage of viable cells (Annexin V and sytox blue negative) from four independent experiments is shown. Error bars indicate s.e.m. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.
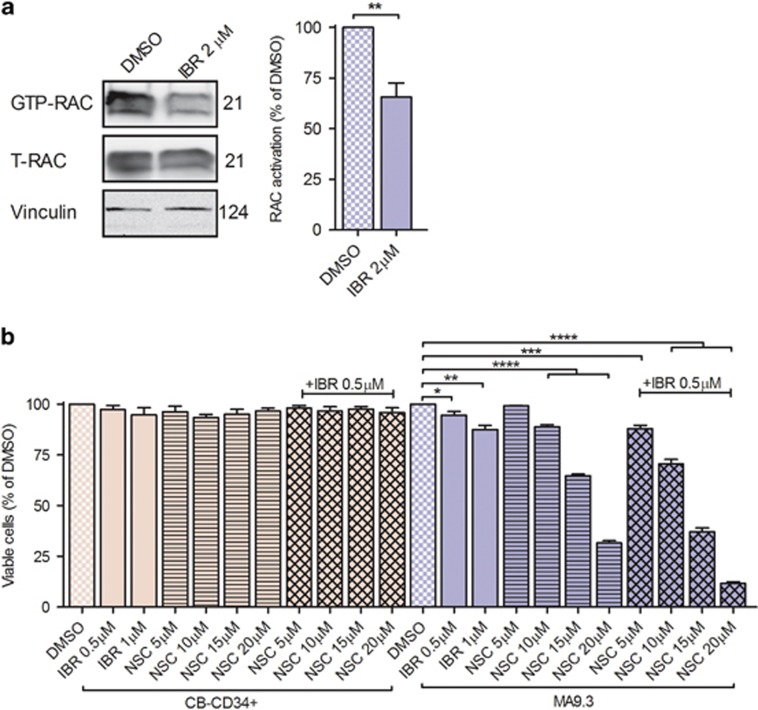
RAC GTPases (RAC1, RAC2 and RAC3) act as a molecular switch in multiple cellular processes that affect cancer progression, cell cycle progression, cell survival and including cytoskeletal dynamics.11 Previous studies have established a positive relationship between small Rho GTPases RAC1, 2 and MA9 rearrangements.6, 12 BTK has an important role in the migration of mouse bone marrow-derived mast cells in response to chemo-attractants via RAC activation.13 It is therefore tempting to speculate on a role for BTK in the activation of RAC1. RAC1-GTPase activation assays were performed in MA9.3 cells treated with DMSO and ibrutinib (Figure 2a). In line with observations by others,6, 12 we identified RAC1-GTP in DMSO-treated cells. Interestingly, ibrutinib treatment significantly reduced RAC1 activation. Similar data were observed in MOLM-13 cells but not in non-MLL-rearranged cell line OCI AML3 (Supplementary Figures 4A and B). The findings thus underscore the contribution of activated BTK in the activation of RAC1-GTPase. RAC inhibitor treatment, NSC23766, affected MA9.3 cell viability.6, 12 As BTK lies upstream of RAC1 and is playing a functional role in cell survival (Figures 1b and d), we hypothesized that ibrutinib treatment might increase the efficacy of RAC inhibition. MA9.3 cells were treated with various doses of NSC23766 (5, 10, 15 and 20 μm) alone or in combination with clinically relevant doses of ibrutinib (0.5 μm; Figure 2b). The drugs were more potent in combination and CDI values indicated synergism between NSC23766 and ibrutinib (CDI<1.0; Supplementary Table 3). Again, control cells (CB-CD34+) were inert to the inhibitor treatment. Of note, short-term NSC23766 treatment did not affect p65 phosphorylation, suggesting RAC1 inhibition-induced cell death is independent of changes in p65 phosphorylation (Supplementary Figure 5). These observations collectively highlight the dependency of the MA9.3 cells on BTK activation and its downstream signaling for cell survival. Bone marrow stroma and stromal cell-derived soluble factors have been implicated in the cytoprotection of AML cells.2, 14 So we further investigated in this direction. Short-term treatment of serum-starved MA9.3 with HS5 conditioned medium resulted in the phosphorylation of BTK and p65 (Supplementary Figures 6A–C). Gαq protein directly stimulated the activity of purified BTK.15 Furthermore, an important role for CXCR4 and Notch signaling in the hematopoietic stem cell microenvironment has also been described.16, 17 Therefore, we used pharmacological inhibitors to disrupt signaling at the receptor level (CXCR4 antagonist AMD3100, gamma-secretase inhibitor XII (GSI XII) to block Notch activation) and at the level of heterotrimeric G protein function (FR900359 and BIM46187).18, 19 MA9.3 cells were pre-treated with various inhibitors and analyzed for activation of BTK and p65 (Supplementary Figures 6D and E). Interestingly, pan G protein inhibitor BIM46187 induced phosphorylation of BTK and p65, whereas no such effect was noted using Gαq/11-specific inhibitor FR900359, AMD3100 or GSI XII. The inhibitors also failed to block phosphorylation on BTK and p65, indicating the Gαq/11 family of G proteins, CXCR4 and Notch signaling is not involved in activation of BTK and p65. We next used human bone marrow stromal cells (HS5) and primary stromal cells and investigated whether BTK-RAC1 inhibition would block the protective effects of stromal cells. Compared to MA9.3 cells without stromal cell support, MA9.3 cells cocultured with HS5 (Supplementary Figure 7A) or primary bone marrow stromal cells (Supplementary Figure 7B) were resistant to the cytotoxic action of ibrutinib and showed reduced cytotoxicity to NSC23766. However, in comparison to single drug treatment, a combination of ibrutinib and NSC23766 demonstrated considerable induction of cell death (Supplementary Figures 7A and B).
Figure 2.
Ibrutinib inhibits RAC1 activation and sensitized cells for NSC23766 treatment in MA9.3 cells. (a) MA9.3 cells were treated with ibrutinib (IBR) (2 μm) for 14 h. GTP-RAC1 was enriched and detected using active GTP-RAC1 activation assay. Bound RAC1 and total RAC1 (upper and lower blots) were detected using RAC1 antibody (left). RAC1-GTP protein was densitometrically quantified. Percentage of RAC1 activation from four independent experiments is shown (right). (b) Control and MA9.3 cells were treated with DMSO or NSC23766 (NSC) or ibrutinib (IBR) as indicated for 48 h and assayed for cell viability. Percentage of viable cells from four independent experiments is shown. Error bars indicate s.e.m. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.
These findings expand our current understanding of the multiple mechanisms by which MLL rearrangements regulate cellular viability. Specifically, we demonstrate the contribution of aberrantly active BTK and p65 in cells with MLL rearrangements. The effect of ibrutinib on MA9.3 cells indicates specific vulnerability of MLL-rearranged leukemia to BTK inhibition. Our results may provide the basis for future clinical trials with ibrutinib used as a single agent or in combination with daunorubicin or RAC1 inhibition. This strategy might benefit MLL-rearranged AML where there is a high risk of relapse and resistance to conventional chemotherapy.
Acknowledgments
This project was funded by BMBF (eBIO AML-031A304 to TF). We thank Professor James C Mulloy and Mark Wunderlich (Experimental Hematology and Cancer Biology, Cincinnati Children's Hospital Medical Center, Cincinnati, USA) for providing MA9.3 and 9.6 cells, and Dr Dimitrios Mougiakakos (Department of Internal Medicine 5, Hematology and Oncology, University of Erlangen-Nuremberg, Germany) for HS5 cells. We acknowledge Corinna Fahldieck and Uta Schönborn for their excellent technical support and Peter Müller for critical reading.
Author contributions
SCN and TF planned the study; SCN, SF, BE and CH performed the experiments; GMK and EK provided FR900359; SCN and TF wrote the manuscript.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
The authors declare no conflict of interest.
Supplementary Material
References
- Gu TL, Nardone J, Wang Y, Loriaux M, Villen J, Beausoleil S et al. Survey of activated FLT3 signaling in leukemia. PLos One 2011; 6: e19169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth SA, Murray MY, Zaitseva L, Bowles KM, MacEwan DJ. Identification of Bruton's tyrosine kinase as a therapeutic target in acute myeloid leukemia. Blood 2014; 123: 1229–1238. [DOI] [PubMed] [Google Scholar]
- Oellerich T, Mohr S, Corso J, Beck J, Dobele C, Braun H et al. FLT3-ITD and TLR9 use Bruton tyrosine kinase to activate distinct transcriptional programs mediating AML cell survival and proliferation. Blood 2015; 125: 1936–1947. [DOI] [PubMed] [Google Scholar]
- Zaitseva L, Murray MY, Shafat MS, Lawes MJ, MacEwan DJ, Bowles KM et al. Ibrutinib inhibits SDF1/CXCR4 mediated migration in AML. Oncotarget 2014; 5: 9930–9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger G, Abdul-Aziz A, Zaitseva L, Lawes M, MacEwan DJ, Bowles KM et al. Targeting BTK for the treatment of FLT3-ITD mutated acute myeloid leukemia. Sci Rep 2015; 5: 12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell 2008; 13: 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HP, Wang Z, Lee DF, Iwasaki M, Duque-Afonso J, Wong SHK et al. Epigenetic roles of MLL oncoproteins are dependent on NF-kappa B. Cancer Cell 2013; 24: 423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MY, Zaitseva L, Auger MJ, Craig JIO, MacEwan DJ, Rushworth SA et al. Ibrutinib inhibits BTK-driven NF-kappa B p65 activity to overcome bortezomib-resistance in multiple myeloma. Cell Cycle 2015; 14: 2367–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Hu C, Wang A, Weisberg EL, Wang W, Chen C et al. Ibrutinib selectively targets FLT3-ITD in mutant FLT3-positive AML. Leukemia 2016; 30: 754–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SP, Sun GP, Shen YX, Peng WR, Wang H, Wei W. Synergistic effect of combining paeonol and cisplatin on apoptotic induction of human hepatoma cell lines. Acta Pharmacol Sin 2007; 28: 869–878. [DOI] [PubMed] [Google Scholar]
- Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett 2008; 582: 2093–2101. [DOI] [PubMed] [Google Scholar]
- Mizukawa B, Wei JP, Shrestha M, Wunderlich M, Chou FS, Griesinger A et al. Inhibition of Rac GTPase signaling and downstream prosurvival Bcl-2 proteins as combination targeted therapy in MLL-AF9 leukemia. Blood 2011; 118: 5235–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn HS, Radinger M, Brown JM, Ali K, Vanhaesebroeck B, Beaven MA et al. Btk-dependent Rac activation and actin rearrangement following Fc epsilon RI aggregation promotes enhanced chemotactic responses of mast cells. J Cell Sci 2010; 123: 2576–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia 2002; 16: 1713–1724. [DOI] [PubMed] [Google Scholar]
- Bence K, Ma W, Kozasa T, Huang XY. Direct stimulation of Bruton's tyrosine kinase by G(q)-protein alpha-subunit. Nature 1997; 389: 296–299. [DOI] [PubMed] [Google Scholar]
- Tavor S, Eisenbach M, Jacob-Hirsch J, Golan T, Petit I, BenZion K et al. The CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiation. Leukemia 2008; 22: 2151–2158. [DOI] [PubMed] [Google Scholar]
- Evans AG, Calvi LM. Notch signaling in the malignant bone marrow microenvironment: implications for a niche-based model of oncogenesis. Ann N Y Acad Sci 2015; 1335: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub MA, Damian M, Gespach C, Ferrandis E, Lavergne O, De Wever O et al. Inhibition of heterotrimeric G protein signaling by a small molecule acting on G alpha subunit. J Biol Chem 2009; 284: 29136–29145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage R, Schmitz AL, Gaffal E, Annala S, Kehraus S, Wenzel D et al. The experimental power of FR900359 to study Gq-regulated biological processes. Nat Commun 2015; 6: 10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.