Abstract
Introduction:
We recently demonstrated that angiotensin II (Ang II) was involved in the etiology of Parkinson’s disease (PD) via induction of apoptosis of dopaminergic neurons, but the mechanisms are not completely elucidated. Here, we asked whether mitochondrial-dependent mechanisms contributed to the Ang II-induced dopaminergic neuronal apoptosis.
Materials and methods:
CATH.a cells were incubated with Ang II in combination with mitochondrial permeability transition pore (mPTP) inhibitors or angiotensin receptor antagonists, and apoptosis rate, caspase-3 activity, cytochrome c levels, and mPTP opening were assessed.
Results:
We showed that Ang II triggered apoptosis via a mitochondrial-dependent pathway, as elevated cytochrome c levels were observed in the cytosol. By employing cyclosporin A and sanglifehrin A, two specific mPTP inhibitors, we revealed that cytochrome c release from mitochondria into cytoplasm was ascribed to mPTP opening. Meanwhile, the aforementioned effects could be abrogated by an AT1 receptor antagonist losartan rather than an AT2 receptor antagonist PD123319.
Conclusion:
This study demonstrates that Ang II triggers mitochondrial-dependent apoptosis via facilitating mPTP opening through an AT1 receptor-mediated fashion in dopaminergic neurons. These findings give insight into the effect of Ang II in the etiology of PD, and reinforce the application of AT1 receptor antagonists for PD treatment.
Keywords: Parkinson’s disease, renin–angiotensin system, angiotensin II, apoptosis, mitochondrial permeability transition pore, cytochrome C
Introduction
As the most common type of neurodegenerative movement disorder, Parkinson’s disease (PD) is characterized by evident motor symptoms such as rigidity, bradykinesia, resting tremor, and postural instability.1 The reduction of dopamine levels within the striatum and substantia nigra is a well-known feature of PD, which is attributed to selective dopaminergic neuron loss within basal ganglia structures and is closely related to the progression of the aforementioned motor symptoms.2 To date, the molecular mechanisms underlying the loss of dopaminergic neurons are still unclear.
In the circulation system, the renin-angiotensin system (RAS) serves as a critical regulator in the homeostasis of water and sodium as well as blood pressure through its major effector, angiotensin II (Ang II). Recently, mounting evidence has suggested that an independent RAS exists in most parts of brain,3–5 and is closely related to the etiology of several neurodegenerative diseases, such as PD.6,7 An increased level of Ang II within the striatum and substantia nigra of PD rodent models has been noted, suggesting a hyperactivation of brain RAS in PD etiology.8 In addition, recent studies from our laboratory demonstrated that Ang II could directly cause dopaminergic neuron loss via triggering apoptosis,9,10 a specific type of programmed cell death. Nevertheless, the potential mechanisms which underlie Ang II-induced apoptosis in dopaminergic neurons remained largely unexplored.
Apoptosis is closely modulated by two different signaling pathways: the receptor-dependent apoptotic pathway and the mitochondrial-mediated apoptotic pathway.11 During the mitochondrial-dependent apoptotic process, the mitochondrial permeability transition pore (mPTP) was open in response to the stimulation of pro-apoptotic factors, and cytochrome c was released from the mitochondria into the cytosol, subsequently triggering the downstream apoptosis cascade.12 Interestingly, emerging evidence suggests that Ang II initiated apoptosis via a mitochondrial-dependent fashion in peripheral tissues including lung13 and heart.14 Based on this information, we hypothesized that Ang II may also trigger the apoptosis of dopaminergic neurons through a mitochondrial-dependent mechanism in the central nervous system. In the present study, we attempted to verify this hypothesis using a dopaminergic neuronal cell line (CATH.a cells).
Materials and methods
Reagents and cell culture
Ang II, cyclosporin A (CsA, a specific mPTP inhibitor), sanglifehrin A (SfA, another specific mPTP inhibitor), losartan (an Ang II type 1 (AT1) receptor antagonist) and PD123319 (an Ang II type 2 (AT2) receptor antagonist) were purchased from Sigma-Aldrich Inc. Mouse CATH.a cells were provided by American Tissue Cell Collection, and maintained in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum, and 1% penicillin-streptomycin at 37°C with 5% CO2 according to the methods described previously.9,10 This dopaminergic neuronal cell line stably expresses AT1 and AT2 receptors, and the density ratio of AT1 to AT2 receptor is about 2.07:1.
Flow cytometry analysis
Quantitative evaluation of cell apoptosis was performed using a flow cytometer with a fluorescein isothiocyanate (FITC) Annexin V and propidium iodide (PI) Double Labeling Apoptosis Detection Kit (BD Biosciences) according to previously reported procedure.9,10,15 Briefly, cultured CATH.a cells were subjected to different treatments, and washed with phosphate-buffered saline (PBS). Trypsin was then utilized until the cells detached, and 4°C PBS was adopted to scatter the cells before centrifugation. Afterwards, cells were collected and stained with FITC Annexin V solution and/or PI solution at 37°C in the dark for 15 min. Following that step, the fluorescence of 10,000 cells was measured with a FACSCalibur System (BD Biosciences) within 1 h. Opening of mPTP in CATH.a cells was measured with flow cytometric analysis following the CoCl2-calcein fluorescence quenching assay.16 In brief, after co-incubation of cells with Calcein-AM (1 μM, Molecular Probes, Life Technologies) for 30 min at 37°C in the dark, CoCl2 (1 mM, Sigma) was added and cells incubated for additional 10 min. Afterwards, the fluorescence of 10,000 cells was evaluated with a flow cytometer (FACSCalibur) at the excitation wavelength of 530 nm. The data of above-mentioned two assay were processed with FSC express software (De Novo Software).
Colorimetric assay for caspase-3 activity
Colorimetric assay was employed to evaluate the caspase-3 activity in CATH.a cells as described elsewhere.17 Briefly, cultured CATH.a cells were subjected to indicated treatment. Then, cells were washed, harvested and lysed in extraction buffer. The caspase-3 activity was assessed according to the instructions of manufacturer for a colorimetric assay kit (Abcam).
Assessment of cytosolic cytochrome c levels
Cytochrome c levels in the cytosolic fractions were measured as described by Li and colleagues.18 In brief, cultured CATH.a cells were subjected to indicated treatment. Following that step, cells were collected and homogenized in a buffer containing 20 mM hydroxyethylpiperazine ethane sulfonic acid (HEPES)-KOH (pH 7.0), 250 mM sucrose, 10 mM KCl, 2 mM MgCl2, 1 mM ethylene glycol tetraacetic acid (EGTA), 1 mM ethylene diamine tetraacetic acid (EDTA), 1 mM dithiothreitol (DTT), 1 mM phenylmethyl sulphonyl fluoride (PMSF) and the protease inhibitors cocktail. Nuclei were removed through centrifugation at 1000× g for 10 min at 4°C. Post nuclear supernatant was centrifuged at 100,000× g for 1 h, and the supernatant (cytosolic fraction) were then collected. Cytochrome c levels were assessed with an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems).
CoCl2-calcein fluorescence quenching assay
The opening of mPTP was detected with a CoCl2-calcein fluorescence quenching assay.16 Under physiological conditions, calcein-acetomethoxy (AM) can freely cross cellular membranes, and the cytosolic esterase cleave the AM group to yield the fluorescent calcein. Co-incubation of cells with CoCl2 quenches the fluorescence in the cells, except in mitochondria, since CoCl2 cannot pass through mitochondrial membrane. However, calcein in mitochondria is also quenched by CoCl2 once the mPTP opening, leading to reduced fluorescence. Images of cells were taken at 488 nm excitation and 525 nm emissions according to manufacturer’s protocol.
Statistical analysis
Data were expressed as mean±standard deviation (SD) Comparisons among groups were carried out with one-way analysis of variance (ANOVA) followed by Turkey’s post-hoc test using SPSS software. Values of p<0.05 were considered to be statistically significant.
Results
Ang II induces CATH.a cells apoptosis via a mitochondria-dependent pathway
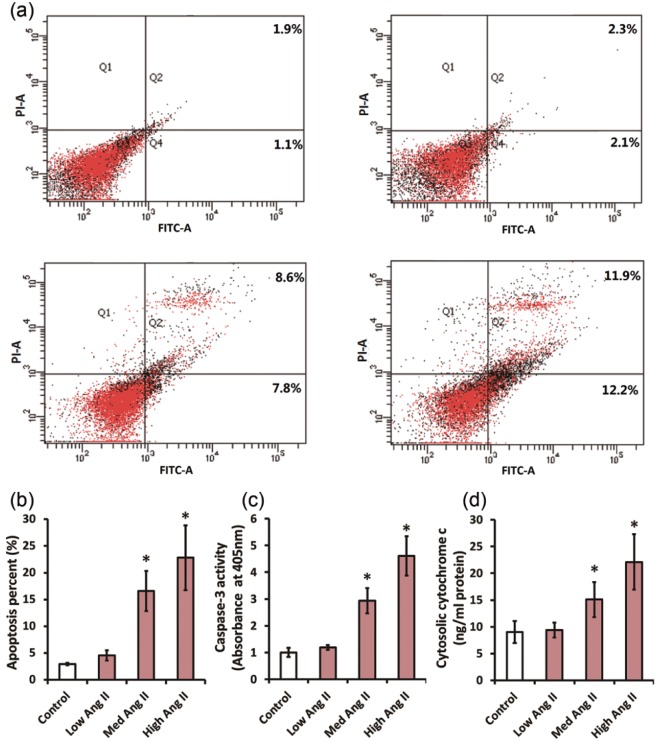
Firstly, we tested the actions of Ang II on CATH.a cell apoptosis. CATH.a cells were exposed to various concentrations of Ang II (5 nM, 50 nM and 500 nM) for 24 h. Afterwards, flow cytometry was applied to assess the percentage of apoptotic CATH.a cells. When compared with vehicle, 50 nM and 500 nM Ang II elevated the percentage of apoptotic cells from 3.0% to 16.6% and 22.8% (p<0.05), respectively (Figure 1(a) and (b)). To confirm the association of Ang II with apoptosis, we further detected activity of the caspase-3, the key apoptosis executioner,19 by means of colorimetric assay. Figure 1(c) shows that 50 nM and 500 nM Ang II markedly increased the caspase-3 activity by 2.9-fold and 4.6-fold (p<0.05), respectively. Mitochondrial release of apoptogenic cytochrome c is an indispensable step to initiate mitochondria-dependent apoptosis. To determine whether the mitochondria-dependent pathway participated in the apoptosis caused by Ang II, the cytosolic levels of cytochrome c was then detected using an ELISA kit. Figure 1(d) reveals that the cytochrome c levels in 50 nM and 500 nM Ang II-treated groups was higher than the vehicle-treated group by 1.7-fold and 2.5-fold (p<0.05), respectively. These findings indicated that Ang II induces CATH.a cells apoptosis via a mitochondria-dependent pathway.
Figure 1.
Angiotensin II (Ang II) induces apoptosis in CATH.a cells via a mitochondria-dependent pathway. CATH.a cells were treated with different concentrations of Ang II (5, 50, and 500 nM) for 24 h. In (a) and (b) cell apoptosis was measured by flow cytometry using a commercial apoptosis detection kit. The apoptosis rate=(annexin V+PI+ cells+annexin V+PI− cells)/total cells×100 %. (c) The activity of caspase-3 was directly evaluated using a colorimetric assay kit. (d) ELISA was performed with a commercial ELISA kit to valuate cytosolic cytochrome c. All figures are representative of three independent experiments, performed in triplicate. Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Columns represent mean±standard deviation (SD). *p<0.05 versus control group.
ELISA: enzyme-linked immunosorbent assay; FITC: fluorescein isothiocyanate; PI: propidium iodide.
Opening of mPTP is involved in the mitochondria-dependent apoptosis induced by Ang II in CATH.a cells
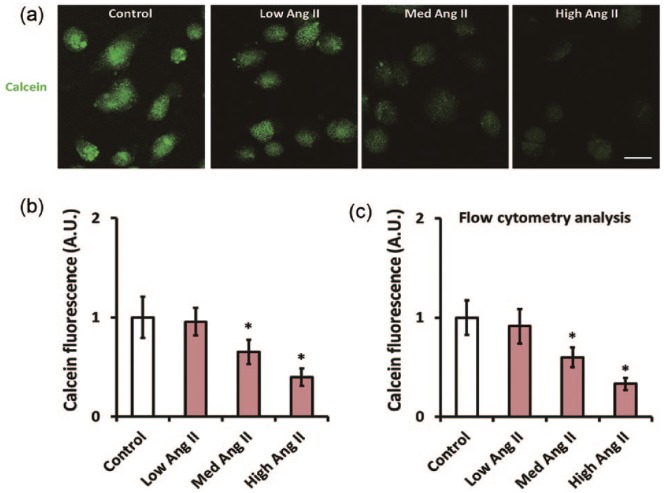
To investigate possible mechanisms underlying the mitochondria-dependent apoptosis triggered by Ang II, we then focused on the mPTP opening. The mPTP opening in CATH.a cells after Ang II treatment was measured with a CoCl2-calcein fluorescence quenching assay. As shown by Figure 2(a) and (b), 50 nM and 500 nM Ang II significantly reduced the signal of calcein fluorescence in CATH.a cells by 34.9% and 60.4% (p<0.05), respectively, indicating an increase in mPTP opening. This result was further confirmed using flow cytometry (Figure 2(c)).
Figure 2.
Angiotensin II (Ang II) facilitates mitochondrial permeability transition pore (mPTP) opening in CATH.a cells. CATH.a cells were treated with different concentration of Ang II (5, 50, and 500 nM) for 24 h, and (a) and (b) illustrate the CoCl2-calcein fluorescence quenching assay which was employed to evaluate mPTP opening. Note that the calcein fluorescence was decreased after incubation with Ang II. (c) The calcein fluorescence was further evaluated by flow cytometric analysis. All figures are representative of three independent experiments, performed in triplicate. Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Columns represent mean±standard deviation (SD). *p<0.05 versus control group.
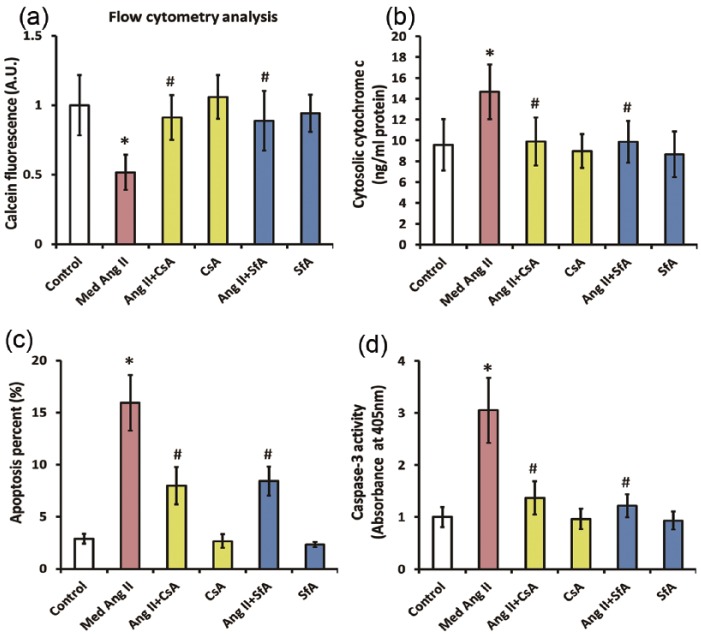
During mitochondrial apoptotic cascade, the opening of mPTP is believed to cause apoptosis via facilitating cytochrome c release. To determine whether the opening of mPTP led to the release of cytochrome c and subsequent cell apoptosis in this scenario, we co-incubated CATH.a cells with Ang II (50 nM) and mPTP inhibitor CsA (20 µM) or SfA (1 µM), for 24 h. As shown by Figure 3(a), the reduction of calcein fluorescence signal caused by Ang II in CATH.a cells was fully reversed by CsA or SfA, indicating that the mPTP opening was markedly inhibited. Meanwhile, the increase in cytochrome c levels induced by Ang II was abolished by CsA or SfA, suggesting that mitochondrial release of cytochrome c was also attenuated by inhibition of mPTP opening (Figure 3(b)). In addition, inhibiting mPTP rescued the Ang II-induced apoptosis in CATH.a cells, as the elevation in apoptosis rate and caspase-3 activity were remarkably attenuated by CsA or SfA (Figure 3(c) and (d)). Taken together, these results suggested that opening of mPTP participated in the mitochondria-dependent apoptosis triggered by Ang II in CATH.a cells.
Figure 3.
Opening of mitochondrial permeability transition pore (mPTP) contributes to the mitochondrial-dependent apoptosis induced by angiotensin II (Ang II) in CATH.a cells. CATH.a cells were co-incubated with Ang II (50 nM), a specific mPTP inhibitor cyclosporin A (CsA) (20 µM), and another specific mPTP inhibitor sanglifehrin A (SfA) (1 µM) for 24 h, and (a) the calcein fluorescence was evaluated by flow cytometric analysis. (b) ELISA was performed with a commercial kit to valuate cytosolic cytochrome c. (c) Cell apoptosis was measured by flow cytometry using a commercial apoptosis detection kit. (d) The activity of caspase-3 was measured by a commercial detection kit. All figures are representative of three independent experiments, performed in triplicate. Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Columns represent mean±standard deviation (SD). *p<0.05 versus control group, #p<0.05 versus Ang II-treated group.
AT1 receptor is implicated in the mPTP opening and mitochondria-dependent apoptosis caused by Ang II
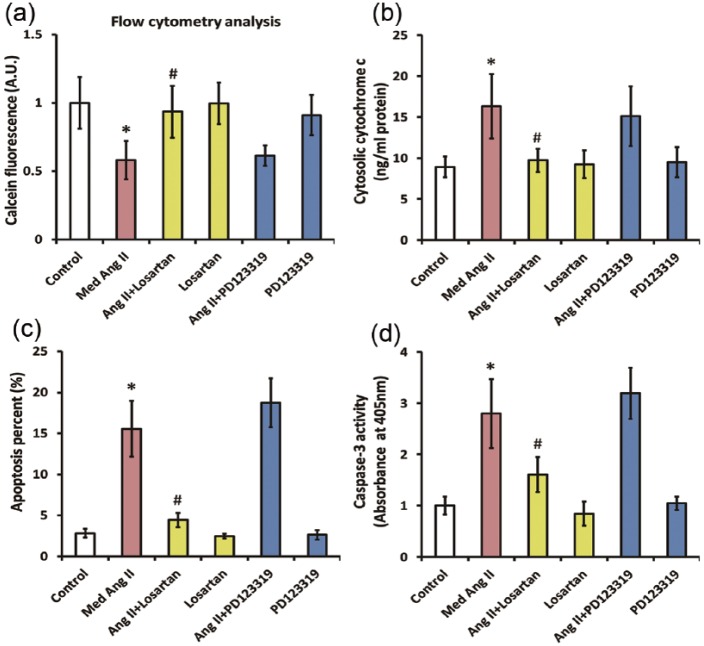
To ascertain which type of receptors mediated the aforementioned actions of Ang II, we co-treated CATH.a cells with 50 nM Ang II and 1 µM losartan or 1 µM PD123319 for 24 h. The dose for PD123319 (1 µM) was selected according our previous findings that PD123319 at this dose could effectively block most of the effects mediated by the AT2 receptor in CATH.a cells.15 Figure 4(a) reveals that co-incubation with losartan completely reversed the Ang II-induced decline of calcein fluorescence signal in CATH.a cells, indicating that the mPTP opening was significantly inhibited. Meanwhile, the increase in cytosolic cytochrome c levels induced by Ang II was blunted by losartan, suggesting that mitochondrial release of cytochrome c was also ameliorated (Figure 4(b)). Moreover, losartan rescued the Ang II-induced apoptosis in CATH.a cells, since the elevation in apoptosis rate and caspase-3 activity were remarkably attenuated (Figure 4(c) and (d)). As opposed to losartan, no significant effect of PD123319 on Ang II-induced increase in mPTP opening and the subsequent release of cytochrome c was noted, since the calcein fluorescence signal as well as the cytosolic cytochrome c levels in CATH.a cells was unaffected by PD123319 co-treatment. In addition, PD123319 cannot ameliorate the apoptosis caused by Ang II, since the increase in apoptosis rate as well as the caspase-3 activity stayed unchanged following PD123319 co-treatment in CATH.a cells. Taken together, these results highlighted that AT1 receptor is closely relevant to the mPTP opening and mitochondria-dependent apoptosis caused by Ang II.
Figure 4.
The mitochondrial-dependent apoptosis induced by angiotensin II (Ang II) is mediated by AT1 receptor instead of AT2 receptor in CATH.a cells. CATH.a cells were co-incubated with Ang II (50 nM), an AT1 receptor antagonist losartan (1 µM), and an AT2 receptor antagonist PD123319 (1 µM) for 24 h, and (a) the calcein fluorescence was evaluated by flow cytometric analysis. (b) ELISA was performed using a commercial ELISA kit to valuate cytosolic cytochrome c in CATH.a cells. (c) Cell apoptosis was measured by flow cytometry using a commercial apoptosis detection kit. (d) The activity of caspase-3 was measured by a commercial detection kit. All figures are representative of three independent experiments, performed in triplicate. Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Columns represent mean±standard deviation (SD). *p<0.05 versus control group, #p<0.05 versus Ang II-treated group. AT1: Ang II type 1; AT2: Ang II type 2; ELISA: enzyme-linked immunosorbent assay.
Discussion
Herein, we demonstrated that Ang II triggered CATH.a cells apoptosis, as flow cytometry analysis revealed that the cell apoptosis rate was significantly elevated after Ang II incubation. Moreover, this was further supported by an increased caspase-3 activity, the key apoptosis executioner,19 in the cytoplasm of Ang II-treated CATH.a cells. These results were consistent with our recent observations that Ang II caused apoptosis in dopaminergic neurons.9,10
There has been wide agreement that apoptosis is closely regulated by two different signaling pathways: the receptor-dependent apoptotic pathway and the mitochondrial-mediated apoptotic pathway.11 During the mitochondrial-dependent apoptotic process, cytochrome c is released from mitochondria into cytosol in response to the stimulation of pro-apoptotic factors, subsequently triggering the downstream apoptosis cascade.20 In this study, we showed that the level of cytochrome c in cytosol of CATH.a cells was significantly elevated after Ang II administration, suggesting that Ang II initiated apoptosis via a mitochondrial-mediated fashion. This was in according with prior findings revealing that Ang II facilitated cytochrome c release and thus triggered mitochondrial-dependent apoptosis in primary lung endothelial cells,13 atrial myocytes21 as well as mouse calvaria osteoblast.18
mPTP is comprised of the matrix protein cyclophilin D (CypD), the inner mitochondrial membrane protein adenine nucleotide translocator, and the outer mitochondrial membrane protein voltage dependent anion channel.22 During the process of mitochondria-dependent apoptosis, the opening of mPTP resulted in the release of several pro-apoptotic factors, such as cytochrome c, from the mitochondria into the cytosol.12 Based on this information, we hypothesized that the cytochrome c release in this scenario might be ascribed to the opening of mPTP caused by Ang II. To test this hypothesis, a specific mPTP inhibitor CsA was used in our experiment. As expected, inhibition of mPTP by CsA attenuated cytochrome c release and ameliorated cell apoptosis caused by Ang II. Meanwhile, this result was confirmed by SfA, another specific mPTP inhibitor, further indicating that the opening of mPTP was involved in the cytochrome c release as well as the subsequent apoptosis caused by Ang II. This was compatible with previous findings from Ricci and colleagues, which revealed that Ang II facilitated the release of cytochrome c via opening of mPTP and subsequently triggered apoptosis in isolated neonatal cardiomyocytes.23
Moreover, we tried to investigate which type of receptors mediated the aforementioned effects of Ang II. We showed that the opening of mPTP, release of cytochrome c and subsequent apoptosis in Ang II-treated CATH.a cells were completely abolished by losartan, an AT1 receptor blocker. On the contrary, an AT2 receptor blocker PD123319 had no impact on these actions triggered by Ang II. Therefore, we ascertained that Ang II induced mitochondrial-mediated apoptosis was dependent on AT1 receptor in this scenario. Our findings were consistent with a previous study from Zhao and colleagues, which showed that Ang II triggered mitochondrial-dependent apoptosis in cardiomyocytes by interacting with the AT1 receptor.14 However, in contrast to our findings, Lv and colleagues reported that Ang II induced apoptosis in cardiomyocytes through the AT2 receptor rather than the AT1 receptor.24 This discrepancy needs to be investigated in future studies.
Conclusion
Herein, we reveal that Ang II facilitates the release of cytochrome c by opening of mPTP and thus triggers mitochondria-dependent apoptosis in CATH.a cells. We also show that the aforementioned impacts of Ang II on CATH.a cells are dependent on AT1 receptors. These results give more insight into the effects of Ang II in the etiology of PD, and reinforce the application of AT1 receptor blockers for the therapies of this neurodegenerative disease. In future, animal models of PD should be employed to validate these findings, and further studies are warranted to investigate other potential mechanisms involved in Ang II-induced apoptosis of dopaminergic neurons.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (81271418) and Natural Science Foundation of Jiangsu Province (BK20151084).
References
- 1. Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord 2011; 26(6): 1049–1055. [DOI] [PubMed] [Google Scholar]
- 2. Schapira AH. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol Sci 2009; 30(1): 41–47. [DOI] [PubMed] [Google Scholar]
- 3. Stragier B, De Bundel D, Sarre S, et al. Involvement of insulin-regulated aminopeptidase in the effects of the renin–angiotensin fragment angiotensin IV: A review. Heart Fail Rev 2008; 13(3): 321–337. [DOI] [PubMed] [Google Scholar]
- 4. Wright JW, Harding JW. Brain renin–angiotensin–a new look at an old system. Prog Neurobiol 2011; 95(1): 49–67. [DOI] [PubMed] [Google Scholar]
- 5. Jiang T, Gao L, Lu J, et al. ACE2-Ang-(1–7)-Mas axis in brain: A potential target for prevention and treatment of ischemic stroke. Curr Neuropharmacol 2013; 11(2): 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mertens B, Vanderheyden P, Michotte Y, et al. The role of the central renin–angiotensin system in Parkinson’s disease. J Renin Angiotensin Aldosterone Syst 2010; 11(1): 49–56. [DOI] [PubMed] [Google Scholar]
- 7. Wright JW, Kawas LH, Harding JW. A role for the brain RAS in Alzheimer’s and Parkinson’s diseases. Front Endocrinol (Lausanne) 2013; 4: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Labandeira-García JL, Garrido-Gil P, Rodriguez-Pallares J, et al. Brain renin–angiotensin system and dopaminergic cell vulnerability. Front Neuroanat 2014; 8: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao Q, Jiang T, Zhao HR, et al. Activation of autophagy contributes to the angiotensin ii-triggered apoptosis in a dopaminergic neuronal cell line. Mol Neurobiol 2016; 53(5): 2911–2919. [DOI] [PubMed] [Google Scholar]
- 10. Zhao HR, Jiang T, Tian YY, et al. Angiotensin II triggers apoptosis via enhancement of NADPH oxidase-dependent oxidative stress in a dopaminergic neuronal cell line. Neurochem Res 2015; 40(4): 854–863. [DOI] [PubMed] [Google Scholar]
- 11. Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol 2015; 7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 2007; 12(5): 815–833. [DOI] [PubMed] [Google Scholar]
- 13. Lee YH, Mungunsukh O, Tutino RL, et al. Angiotensin-II-induced apoptosis requires regulation of nucleolin and Bcl-xL by SHP-2 in primary lung endothelial cells. J Cell Sci 2010; 123(Pt 10): 1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao H, Qi G, Han Y, et al. 20-Hydroxyeicosatetraenoic acid is a key mediator of angiotensin II-induced apoptosis in cardiac myocytes. J Cardiovasc Pharmacol 2015; 66(1): 86–95. [DOI] [PubMed] [Google Scholar]
- 15. Lu J, Wu L, Jiang T, et al. Angiotensin AT2 receptor stimulation inhibits activation of NADPH oxidase and ameliorates oxidative stress in rotenone model of Parkinson’s disease in CATH.a cells. Neurotoxicol Teratol 2015; 47: 16–24. [DOI] [PubMed] [Google Scholar]
- 16. Gautier CA, Giaime E, Caballero E, et al. Regulation of mitochondrial permeability transition pore by PINK1. Mol Neurodegener 2012; 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang T, Yu JT, Zhu XC, et al. Temsirolimus promotes autophagic clearance of amyloid-beta and provides protective effects in cellular and animal models of Alzheimer’s disease. Pharmacol Res 2014; 81: 54–63. [DOI] [PubMed] [Google Scholar]
- 18. Li G, Wang M, Hao L, et al. Angiotensin II induces mitochondrial dysfunction and promotes apoptosis via JNK signalling pathway in primary mouse calvaria osteoblast. Arch Oral Biol 2014; 59(5): 513–523. [DOI] [PubMed] [Google Scholar]
- 19. Creagh EM. Caspase crosstalk: Integration of apoptotic and innate immune signalling pathways. Trends Immunol 2014; 35(12): 631–640. [DOI] [PubMed] [Google Scholar]
- 20. Hüttemann M, Pecina P, Rainbolt M, et al. The multiple functions of cytochrome C and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion 2011; 11(3): 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng LY, Zhang MH, Xue JH, et al. Effect of angiotensin II on STAT3 mediated atrial structural remodeling. Eur Rev Med Pharmacol Sci 2014; 18(16): 2365–2377. [PubMed] [Google Scholar]
- 22. Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 2009; 46(6): 821–831. [DOI] [PubMed] [Google Scholar]
- 23. Ricci C, Pastukh V, Schaffer SW. Involvement of the mitochondrial permeability transition pore in angiotensin II-mediated apoptosis. Exp Clin Cardiol 2005; 10(3): 160–164. [PMC free article] [PubMed] [Google Scholar]
- 24. Lv J, Zhang P, Zhang Y, et al. Maternal high-salt intake during pregnancy reprogrammed renin–angiotensin system-mediated cardiomyocyte apoptosis in the adult offspring heart. Reprod Sci 2014; 21(1): 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]