Abstract
Introduction:
Angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) are widely used to block the renin-angiotensin system (RAS). Yet it remains uncertain whether these drugs are equally effective and safe.
Methods:
Systematic reviews and meta-analyses of ACEis/ARBs in diabetes and kidney disease published in PubMed, Chinese National Knowledge Infrastructure (CNKI) and Wanfang databases were searched for clinical outcomes including all-cause mortality, end-stage renal disease (ESRD), hyperkalemia and cough.
Results:
Eight meta-analyses included 2177–61,264 patients with follow-up of 6–108 months. RAS blockers reduced mortality (relative risk ratio (RR), 0.90, 95% confidence interval (CI), 0.86–0.95) without heterogeneity. The death protection was significant specifically with ACEis (RR, 0.85, 95% CI, 0.79–0.91), but not with ARBs. Protection against ESRD was homogenously evident by ARBs (RR, 0.79, 95% CI, 0.73–0.87), ACEis (RR, 0.79, 95% , 0.64–0.94), and both (RR, 0.79, 95% CI, 0.73–0.87). Significant side effects were hyperkalemia by ARBs (RR, 2.44, 95% CI, 1.13–5.26), and cough by ACEis (RR, 2.38, 95% CI, 1.75–3.22)
Conclusions:
In patients with diabetes and kidney disease, ACEis and ARBs are consistently protective for the development of ESRD. Use of ACEis alone additionally reduces deaths and increases the risk for cough. Use of ARBs alone increases the risk for hyperkalemia without additional benefit of death protection.
Keywords: Angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, renin-angiotensin system, meta-analysis, mortality, end-stage renal disease
Introduction
Diabetes mellitus (DM) and kidney disease are strong independent risk factor for death and renal failure.1,2 Moreover, DM and kidney disease hasten vascular complications.3–6 The combination of diabetes and kidney disease relates to a two- to four-fold increase in the risk of cardiovascular disease and death.7–11 The renin-angiotensin system (RAS) plays a major role in cardiovascular and renal dysfunction.12–15 Thus, RAS blockade by angiotensin-converting enzyme inhibitors (ACEis) and angiotensin-receptor blockers (ARBs) has been shown to reduce all-cause mortality in DM and kidney disease.16–18
Recently, a meta-analysis has shown that ACEis and ARBs may exert different effects on all-cause mortality in DM patients.19 Yet, it remains uncertain whether ACEis and ARBs have differential effects on all-cause mortality, end-stage renal disease (ESRD), and adverse reactions. The objective of this updated study is to perform a meta-analysis of meta-analyses to assess the comparative effects of ACEis and ARBs on all-cause mortality, ESRD, and adverse reactions in patients with DM and kidney disease.
Methods
Eligibility criteria
All studies that met the following criteria were included: (a) systematic reviews and meta-analyses; (b) patients with DM and kidney disease; (c) randomized controlled clinical trials of ACEis and ARBs (any dose or type); and (d) clinical outcomes including all-cause mortality, ESRD, and adverse events such as hyperkalemia (defined as plasma potassium in excess of 5.5 mmol /l), cough, and headache; (e) relative risk ratios (RRs) were calculated with corresponding 95% confidence intervals (CIs).
Exclusion criteria
Exclusion criteria included: (a) studies of patients with a diagnosis of acute kidney injury, as defined by an abrupt (within 48 h) reduction in renal function, manifested by an increase in serum creatinine level (≥0.3 mg/dl or ≥26.5 µmol/l) with or without reduced urine output (<400 ml/day ); (b) duplicated articles; and (c) meta-analysis not meeting the inclusion criteria. Eligibility assessment was performed independently by two investigators (JS, YMH), using pre-designed eligibility forms, with all questions resolved by consensus with other authors.
Search strategy and databases
This updated systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. We selected relevant studies published between 1 January 1980–15 June 2015, by searching PubMed, Medline, Chinese National Knowledge Infrastructure (CNKI), Chinese Science and Technology Periodical Database, and Wanfang databases. All potentially relevant articles including reference lists of retrieved papers were investigated as full text in English. For ambiguous or missing information, we contacted the authors where possible. We used the following combined text and MeSH terms: “Angiotensin converting enzyme inhibitors,” “Angiotensin II receptor blockers,” “Renin-angiotensin system,” “Meta analysis,” “Diabetes mellitus,” and “Kidney disease”. We also did a manual search, using the reference lists of published articles. In the next step, the title and then the abstract of papers were examined. Duplicated articles were excluded, and then potentially eligible studies were retrieved for perusal in full text. The extracted data included first author name or study title, year of publication date, country of origin, number characteristics of participants, details of intervention, outcome measures, intervention durations, adverse events, and relative risk ratio (RR) with the corresponding 95% confidence interval (CI). All articles were read by two independent reviewers (XNS, XZH), who archived data from the articles according to a standardized data extraction form. Disagreements were resolved in all cases by discussion among our team members.
Quality assessment
The methodological quality of systematic reviews and meta-analyses was assessed by the Assessment of Multiple Systematic Reviews (AMSTAR) guidelines.20,21 This form of assessment has good inter-rater reliability, validity, and responsibility, and has been widely applied for measuring the methodological quality of systematic reviews.22 Based on the recommendations of the Canadian Agency for Drugs and Technologies in Health (CADTH), those with score <4 were considered as low quality, 5–8 were considered as moderate, and 9–11 as high quality.23 Two independent reviewers (JS, YMH) appraised the quality of the included reviews using AMSTAR scores. Each article was given an AMSTAR total score, based on the number of AMSTAR criteria that were fulfilled.24,25 A third reviewer (HLZ) served to resolve disputes.
Outcome measures
Outcomes were risks of all-cause mortality, ESRD, hyperkalemia (defined as plasma potassium in excess of 5.5 mmol/l), cough and headache.
Synthesis of data
Dichotomous outcome data from individual trials were analyzed by using RR measure and corresponding 95% CI. Data were pooled using the random-effects model when the heterogeneity was significant and the fixed effect model when the included studies showed homogeneity. We assessed the p value of the Chi-square test to determine heterogeneity and I2 to measure for inconsistency. Heterogeneity was assessed using the Chi-square test, with values greater than 50% regarded as being indicative of moderate-to-high heterogeneity and were calculated by using a random-effects meta-analysis model;26 otherwise, we used the fixed-effects meta-analysis model.27 The possibility of publication bias was quantified using the Begg’s and Egger’s test.28,29 A two-tailed p>0.05 was considered to show no bias. This was followed by confirmation with performing a visual inspection of Begg funnel plots in which RRs were plotted against their standard errors (SEs).
Results
Description of the included studies
The study selection process that resulted from our meta-analysis is shown in Supplementary Material, Figure 1. A total of 295 articles were initially identified. Eventually, eight meta-analyses met our inclusion criteria, enrolling 2177–61,264 (median, 21,871) patients.30–37 All the eight meta-analyses were published between 2005–2015 (Table 1). Two studies were conducted in China, three in Australia, one in England, one in New Zealand, and one in Thailand. Diabetes including diabetic kidney disease was found in seven studies and five studies concerned kidney disease including diabetic kidney disease. Four studies compared ARBs with placebo, and seven studies compared ACEis with control using other anti-hypertensive agents. The age of the participants ranged from 18–80 years. The duration of studies ranged from six months to nine years. Table 1 shows the characteristics of the meta-analyses included in the analysis.
Table 1.
Characteristics of the eight included meta-analyses.
| Summary of the characteristics of the included meta-analyses | |||||||
|---|---|---|---|---|---|---|---|
| First author, year | Country | Disease | Age range (year) | Trials/ participants | ACEis/ARBs vs placebo | Outcome | Follow up (month) |
| Palmer et al., 201530 | New Zealand | Type 2 diabetes mellitus, chronic kidney disease | 40–64 | 157/43,256 | ACEis, ARBs, placebo | Mortality, end-stage kidney disease, hyperkalemia, cough | >24 |
| Cheng et al., 201432 | China | Diabetes mellitus | 30-76 | 35/56,444 | ACEis, placebo | Mortality | 12–108 |
| Hao et al., 201431 | China | Type 2 diabetes mellitus | 25–70 | 10/21,871 | ACEis, ARBs, placebo | Mortality | 30–108 |
| Lv et al., 201234 | Australia | Diabetic kidney disease | >18 | 26/61,264 | ACEis, ARBs, placebo | Mortality, end-stage kidney disease, hyperkalemia, cough, headache | 6–72 |
| Vejakama et al., 201233 | Thailand | Type 2 diabetes mellitus | 44–65 | 28/12,728 | ACEis, ARBs, placebo | End-stage kidney disease | 6–101 |
| Sharma et al., 201135 | England | Chronic kidney disease | 18–70 | 4/2177 | ACEis, placebo | Mortality | 36–79 |
| Strippoli et al., 200636 | Australia | Diabetic kidney disease | 18–80 | 49/12,067 | ACEis, ARBs, placebo | Mortality, end-stage kidney disease, hyperkalemia, cough, headache | 12-65 |
| Strippoli et al., 200537 | Australia | Diabetic kidney disease | 20–70 | 16/7603 | ACEis, placebo | Mortality, hyperkalemia, cough, headache | 6–72 |
ACEis: Angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers.
Risk of bias within studies
As shown in Supplementary Material, Table 1, the range in the total AMSTAR score for the eight meta-analyses was 7–10 (theoretical range 0–11) and the mean (standard deviation (SD)) was 8.5 (0.77). Study quality in general was good: five of eight of studies had an AMSTAR score of ≥9, and the other three had an AMSTAR score of 7–8. Based on the recommendations of the CADTH, five studies were considered high quality, and three considered moderate quality.
Publication bias was quantified using the Begg’s and Egger’s test, p>0.05 was considered to be no bias. The p-values were 0.13 and 0.12 for all-cause mortality, 1 and 0.65 for ESRD, 1 and 0.79 for hyperkalemia, 0.73 and 0.45 for cough, and 0.30 and 0.28 for headache, indicating no evidence for publication bias.
Primary outcomes
All-cause mortality
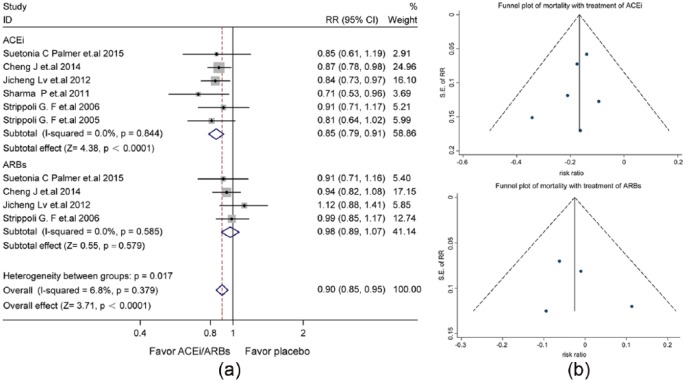
Seven studies reported all-cause mortality (Supplementary Material, Table 2). Treatment with ACEis/ARBs significantly reduced all-cause mortality (RR: 0.90, 95% CI: 0.86–0.95), with homogeneity and consistency of the study results (I2=0.0%, p=0.618). Therefore, the fixed-effects model was used for statistical analysis (Supplementary Material, Figure 2(a)), the symmetric funnel plot suggested no significant publication bias (Supplementary Material, Figure 2(b)). Specifically, the level of death protection was significant by ACEis (RR: 0.85, 95% CI: 0.79–0.91, p<0.0001), but not ARBs (RR: 0.98, 95% CI: 0.89–1.07, p=0.579) (Figure 1(a)). No significant publication bias was found in the symmetric funnel plot (Figure 1(b)).
Figure 1.
The differential effect of angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) on the risk all-cause mortality in patients with diabetes and kidney disease. (a) Forest plot; (b) Funnel plot. CI: confidence interval; RR: relative risk ratio; SE: standard error.
End-stage renal disease
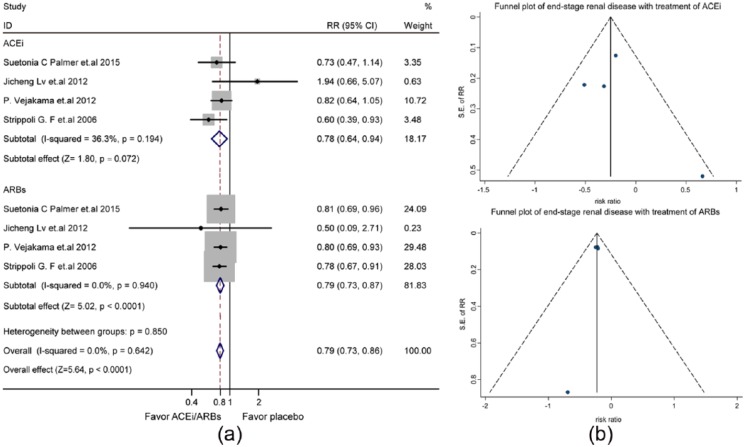
ESRD was the outcome in four studies (Supplementary Material, Table 3). Use of ACEis/ARBs reduced the risk of ESRD (RR: 0.79, 95% CI: 0.73–0.87, p<0.0001) (Supplementary Material, Figure 3(a)) in fixed-effects model analysis (I2=0.0%, p=0.814). The symmetry of the funnel plot revealed little publication bias (Supplementary Material, Figure 3(b)). As shown in Figure 2(a), the protection against ESRD was similar for ACEis (RR: 0.78, 95% CI, 0.64–0.94) and ARBs (RR, 0.79, 95% CI, 0.73–0.87). The funnel plot demonstrated publication bias unlikely (Figure 2(b)).
Figure 2.
The differential effect of angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) on the risk of end-stage renal disease in patients with diabetes and kidney disease: (a) forest plot; (b) funnel plot. CI: confidence interval; RR: relative risk ratio; SE: standard error.
Hyperkalemia
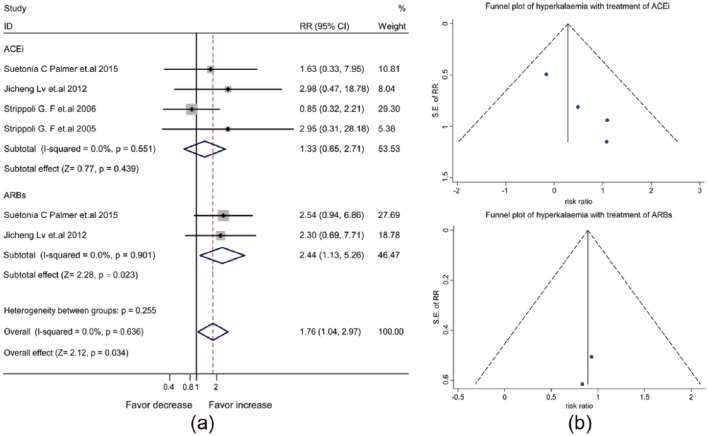
The pooled data of four meta-analyses (Supplementary Material, Table 4) showed an increased risk of hyperkalemia with the use of ACEis/ARBs (RR, 1.76, 95% CI, 1.04–2.97) (Supplementary Material, Figure 4(a)). The symmetric funnel plot suggested no publication bias (Supplementary Material, Figure 4(b)). In fixed-effects model analysis shown in Figure 3(a), the increased risk of hyperkalemia was attributed to ARBs (RR, 2.44, 95% CI, 1.13–5.26), but not ACEis (RR, 1.33, 95% CI, 0.65–2.71). Consistently, little publication bias was evident by the symmetric funnel plot (Figure 3(b)).
Figure 3.
The differential effect of angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) on the risk of hyperkalemia in patients with diabetes and kidney disease: (a) forest plot; (b) funnel plot. CI: confidence interval; RR: relative risk ratio; SE: standard error.
Cough
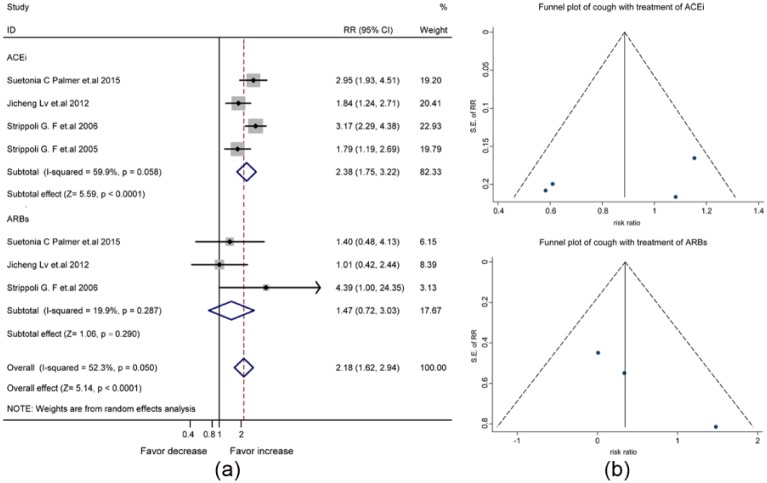
The synthesized data of four studies (Supplementary Material, Table 5) suggested an increased risk of cough by the treatment with ACEis/ARBs (RR, 2.27, 95% CI, 1.64-3.15) (Supplementary Material, Figure 5(a)). Concerning the significant heterogeneity between the four studies (I2=68.5%, p=0.023), we used the random-effects model for statistical analysis. Publication bias was not significant in the funnel plot (Supplementary Material, Figure 5(b)). In the random-effects model analysis shown in Figure 4(a), the increased risk of cough was attributed to the use of ACEis (RR, 2.38, 95% CI, 1.75–3.22) rather than ARBs (RR, 1.47, 95% CI, 0.72–3.03). The symmetric funnel plot suggested no significant publication bias (Figure 4(b)).
Figure 4.
The differential effect of angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) on the risk of cough in patients with diabetes and kidney disease: (a) forest plot; (b) funnel plot. CI: confidence interval; RR: relative risk ratio; SE: standard error.
Headache
The pooled data of three studies (Supplementary Material, Table 6) revealed that the risk of headache was not increased by the treatment with ACEis/ARBs (RR, 0.81, 95% CI, 0.61–1.08) (Figure 5(a)) in the fixed-effects model analysis (I2=0.0%). Consistently, the symmetric funnel plot suggested no significant publication bias (Figure 5(b)).
Figure 5.
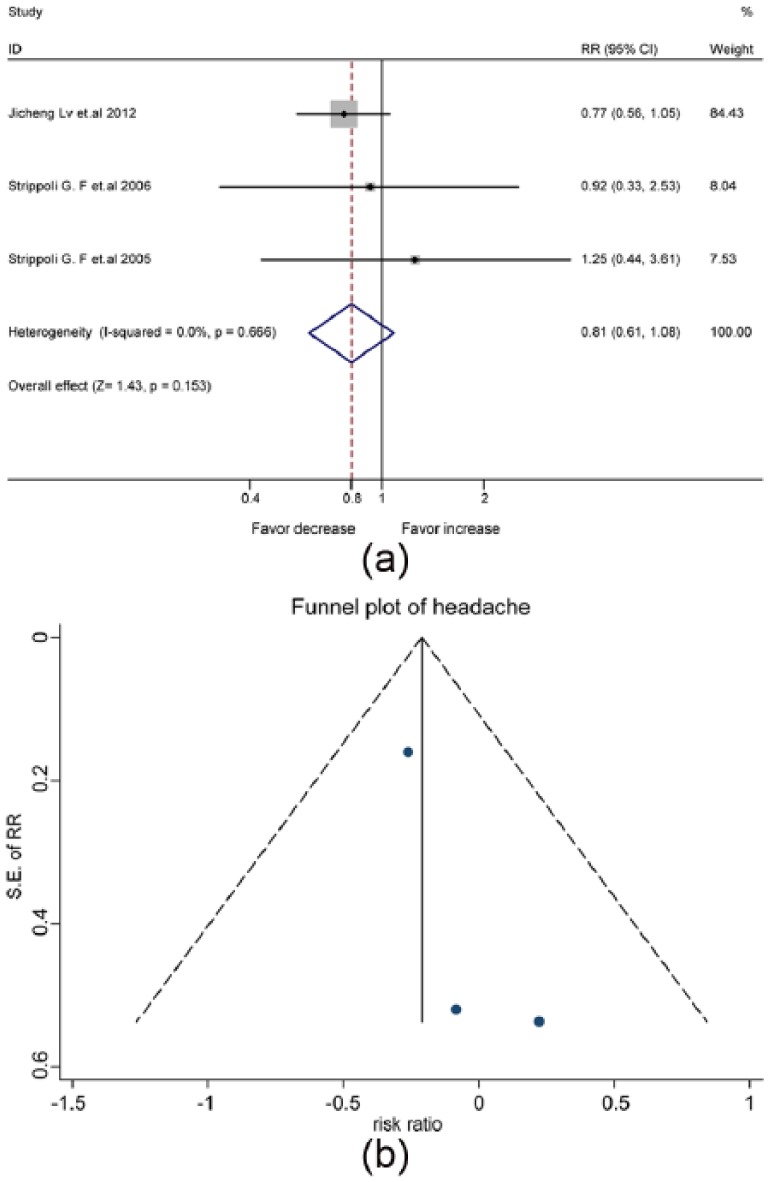
The mixed effect of angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) on the risk of headache in patients with diabetes and kidney disease: (a) forest plot; (b) funnel plot. CI: confidence interval; RR: relative risk ratio; SE: standard error.
Discussion
In this updated meta-analysis of meta-analyses with moderate to high methodological quality, ACEis and ARBs are consistently protective for the development of ESRD in patients with diabetes and kidney disease, Use of ACEis alone additionally reduces the risk of death and increases the risk for cough. In contrast, use of ARBs alone increases the risk for hyperkalemia without additional benefit of death protection.
Patients with diabetes38,39 or kidney disease40–42 are at increased risk of death and renal failure. Prevalent in patients with diabetes or kidney disease is comorbidity with hypertension. ACEis and ARBs have been recommended for clinical treatment.43,44 Previous studies have demonstrated that ACEis and ARBs protect against the progression to renal failure in patients with kidney disease.44–46 In this updated meta-analysis, both ACEis and ARBs are protective for the development of ESRD in people with diabetes and kidney disease. Furthermore, ACEis are generally superior to ARBs in minimizing the risks of all-cause mortality and hyperkalemia. A major concern for the use of ACEis relate to the occurrence of cough. A possible rationale for the additional benefit of ACEis in contrast to ARBs on death protection may relate to angiotensin-(1-7).47,48 Moreover, ACEis also increase bradykinin levels to stimulate glucose metabolism49 and trigger cough. In cases where the cough is intolerable, ARBs fit patients at risk of renal failure.
There are several potential limitations of this study. First, our results are subject to limitations inherent to any meta-analysis based on pooling data from different meta-analyses. Meta-analyses of ACEis and ARBs were not equivalent. Second, there is the possibility of overlap trials between the included meta-analyses. Third, substantial differences exist in the doses of the used drugs, duration of intervention, period of follow-up, and study population. It is hard to ensure quality control. Fourth, patients included in the reported meta-analyses have uneven baseline data, other concomitant conditions, and background therapies. These potential factors might have impacts on the interpretation of our findings.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows: JS, YMH, and HLZ designed the study; JS collected and analyzed the data and wrote the manuscript; JS, MW, and XZH contributed to the collection and assembly of the data; WM, XZH, WL, and XXZ conducted the statistical analyses; JS, YNH, XZH, and HLZ interpreted the data; HLZ revised the manuscript and approved the submission. All authors agreed on the final version of the manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Guilin Medical University (KY2011002), Program for Innovative Research Team of Guilin Medical University (PIRTGMU), National Natural Science Foundation of China (81270934, 81471054) and Innovation Project of Guangxi Graduate Education (YCSZ2015213).
References
- 1. Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: Heart disease and stroke statistics–2010 update: A report from the American Heart Association. Circulation 2010; 121: 948–954. [DOI] [PubMed] [Google Scholar]
- 2. Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 1999; 341: 1127–1133. [DOI] [PubMed] [Google Scholar]
- 3. van de Woestijne AP, van der Graaf Y, Westerink J, et al. Effect of statin therapy on incident type 2 diabetes mellitus in patients with clinically manifest vascular disease. Am J Cardiol 2015; 115: 441–446. [DOI] [PubMed] [Google Scholar]
- 4. Morita T, Morimoto S, Nakano C, et al. Erratum for renal and vascular protective effects of ezetimibe in chronic kidney disease. Int Med 2015; 54: 1683. [DOI] [PubMed] [Google Scholar]
- 5. Lewis JR. Early kidney disease, an important factor in cognitive decline or merely a harbinger of early vascular brain injury? Am J Nephrol 2015; 41: 303–304. [DOI] [PubMed] [Google Scholar]
- 6. Fadini GP, Menegazzo L, Bonora BM, et al. Effects of age, diabetes, and vascular disease on growth differentiation factor 11: First-in-human study. Diabetes Care 2015; 38: e118-119. [DOI] [PubMed] [Google Scholar]
- 7. Peralta CA, Shlipak MG, Fan D, et al. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol 2006; 17: 2892–2899. [DOI] [PubMed] [Google Scholar]
- 8. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003; 108: 2154–2169. [DOI] [PubMed] [Google Scholar]
- 9. Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: A meta-analysis of randomised controlled trials. Lancet 2009; 373: 1765–1772. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura K, Okamura T, Hayakawa T, et al. Chronic kidney disease is a risk factor for cardiovascular death in a community-based population in Japan: NIPPON DATA90. Circ J 2006; 70: 954–959. [DOI] [PubMed] [Google Scholar]
- 11. Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16: 489–495. [DOI] [PubMed] [Google Scholar]
- 12. Cao Z, Cooper ME. Efficacy of renin-angiotensin system (RAS) blockers on cardiovascular and renal outcomes in patients with type 2 diabetes. Acta Diabetol 2012; 49: 243–254. [DOI] [PubMed] [Google Scholar]
- 13. Mohamed RH, Abdel-Aziz HR, Abd El, Motteleb DM, et al. Effect of RAS inhibition on TGF-beta, renal function and structure in experimentally induced diabetic hypertensive nephropathy rats. Biomed Pharmacother 2013; 67: 209–214. [DOI] [PubMed] [Google Scholar]
- 14. Ando K, Nitta K, Rakugi H, et al. Comparison of the antialbuminuric effects of benidipine and hydrochlorothiazide in Renin-Angiotensin System (RAS) inhibitor-treated hypertensive patients with albuminuria: The COSMO-CKD (COmbination Strategy on Renal Function of Benidipine or Diuretics TreatMent with RAS inhibitOrs in a Chronic Kidney Disease Hypertensive Population) study. Int J Med Sci 2014; 11: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin AE, Alexander ME, Colan SD, et al. Clinical, pathological, and molecular analyses of cardiovascular abnormalities in Costello syndrome: A Ras/MAPK pathway syndrome. Am J Med Genet A 2011; 155A: 486–507. [DOI] [PubMed] [Google Scholar]
- 16. Strippoli GF, Craig M, Deeks JJ, et al. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: Systematic review. Br Med J 2004; 329: 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruggenenti P, Perna A, Remuzzi G. on behalf of Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). ACE inhibitors to prevent end-stage renal disease: When to start and why possibly never to stop: A post hoc analysis of the REIN trial results. Ramipril Efficacy in Nephropathy. J Am Soc Nephrol 2001; 12: 2832–2837. [DOI] [PubMed] [Google Scholar]
- 18. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860. [DOI] [PubMed] [Google Scholar]
- 19. Cheng J, Zhang W, Zhang XH, et al. ACEI/ARB therapy for IgA nephropathy: A meta analysis of randomised controlled trials. Int J Clin Pract 2009; 63: 880–888. [DOI] [PubMed] [Google Scholar]
- 20. Burda BU, Norris SL, Holmer HK, et al. Quality varies across clinical practice guidelines for mammography screening in women aged 40–49 years as assessed by AGREE and AMSTAR instruments. J Clin Epidemiol 2011; 64: 968–976. [DOI] [PubMed] [Google Scholar]
- 21. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009; 62: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 23. Canadian Agency for Drugs and Technologies in Health. Interventions directed to consumers, https://www.cadth.ca/interventions-directed-consumers (2011, accessed 12 October 2015).
- 24. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: A systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med 2014; 160: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shea BJ, Bouter LM, Peterson J, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PloS One 2007; 2: e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Oxford: Wiley, 2008. [Google Scholar]
- 27. Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med 1991; 10: 1665–1677. [DOI] [PubMed] [Google Scholar]
- 28. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 30. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: A network meta-analysis. Lancet 2015; 385: 2047–2056. [DOI] [PubMed] [Google Scholar]
- 31. Hao G, Wang Z, Guo R, et al. Effects of ACEI/ARB in hypertensive patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled studies. BMC Cardiovasc Disord 2014; 14: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng J, Zhang W, Zhang X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: A meta-analysis. JAMA Intern Med 2014; 174: 773–785. [DOI] [PubMed] [Google Scholar]
- 33. Vejakama P, Thakkinstian A, Lertrattananon D, et al. Reno-protective effects of renin-angiotensin system blockade in type 2 diabetic patients: A systematic review and network meta-analysis. Diabetologia 2012; 55: 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lv J, Perkovic V, Foote CV, et al. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev 2012; 12: CD004136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharma P, Blackburn RC, Parke CL, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease. Cochrane Database Syst Rev 2011: CD007751. [DOI] [PubMed] [Google Scholar]
- 36. Strippoli GF, Bonifati C, Craig M, et al. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev 2006: CD006257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strippoli GF, Craig M, Craig JC. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev 2005: CD004136. [DOI] [PubMed] [Google Scholar]
- 38. Hirst JA, Taylor KS, Stevens RJ, et al. The impact of renin-angiotensin-aldosterone system inhibitors on Type 1 and Type 2 diabetic patients with and without early diabetic nephropathy. Kidney Int 2012; 81: 674–683. [DOI] [PubMed] [Google Scholar]
- 39. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580–591. [DOI] [PubMed] [Google Scholar]
- 40. Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 2006; 17: 2034–2047. [DOI] [PubMed] [Google Scholar]
- 41. Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164: 659–663. [DOI] [PubMed] [Google Scholar]
- 42. Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137: 479–486. [DOI] [PubMed] [Google Scholar]
- 43. American Diabetes Association. Standards of medical care in diabetes–2015: Summary of revisions. Diabetes Care 2015; 38: S4-S4. [DOI] [PubMed] [Google Scholar]
- 44. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869. [DOI] [PubMed] [Google Scholar]
- 45. Rodby RA, Rohde RD, Clarke WR, et al. The Irbesartan type II diabetic nephropathy trial: Study design and baseline patient characteristics. For the Collaborative Study Group. Nephrol Dial Transplant 2000; 15: 487–497. [DOI] [PubMed] [Google Scholar]
- 46. Ruggenenti P, Perna A, Gherardi G, et al. Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet 1998; 352: 1252–1256. [DOI] [PubMed] [Google Scholar]
- 47. Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol 2006; 17: 2985–2991. [DOI] [PubMed] [Google Scholar]
- 48. Kon V, Fogo A, Ichikawa I. Bradykinin causes selective efferent arteriolar dilation during angiotensin I converting enzyme inhibition. Kidney Int 1993; 44: 545–550. [DOI] [PubMed] [Google Scholar]
- 49. Shiuchi T, Cui TX, Wu L, et al. ACE inhibitor improves insulin resistance in diabetic mouse via bradykinin and NO. Hypertension 2002; 40: 329–334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.