Abstract
Introduction:
The purpose of this study was to determine whether macrophages migrated from the spleen are associated with angiotensin II-induced cardiac fibrosis and hypertension.
Methods:
Sprague-Dawley rats were subjected to angiotensin II infusion in vehicle (500 ng/kg/min) for up to four weeks. In splenectomy, the spleen was removed before angiotensin II infusion. In the angiotensin II AT1 receptor blockade, telmisartan was administered by gastric gavage (10 mg/kg/day) during angiotensin II infusion. The heart and aorta were isolated for Western blot analysis and immunohistochemistry.
Results:
Angiotensin II infusion caused a significant reduction in the number of monocytes in the spleen through the AT1 receptor-activated monocyte chemoattractant protein-1. Comparison of angiotensin II infusion, splenectomy and telmisartan comparatively reduced the recruitment of macrophages into the heart. Associated with this change, transforming growth factor β1 expression and myofibroblast proliferation were inhibited, and Smad2/3 and collagen I/III were downregulated. Furthermore, interstitial/perivascular fibrosis was attenuated. These modifications occurred in coincidence with reduced blood pressure. At week 4, invasion of macrophages and myofibroblasts in the thoracic aorta was attenuated and expression of endothelial nitric oxide synthase was upregulated, along with a reduction in aortic fibrosis.
Conclusions:
These results suggest that macrophages when recruited into the heart and aorta from the spleen potentially contribute to angiotensin II-induced cardiac fibrosis and hypertension.
Keywords: Angiotensin II AT1 receptor, collagen, hypertension, macrophages, myocardial fibrosis, splenectomy
Introduction
Accumulating evidence indicates an involvement of monocytes/macrophages in development of tissue injury and cardiac dysfunction after myocardial infarction. The spleen is the largest organ in the lymphatic system and forms a reservoir containing over half of the body’s monocytes besides the bone marrow.1 It has previously been reported that about 40–70% of monocytes are recruited to the infarcted myocardium from a splenic reservoir.2 Upon moving to injured tissue, these monocytes turn into macrophages to contribute to postischemic inflammatory response and injury. Animal studies have shown that the extent of myocardial infarction is positively correlated with the number of macrophages accumulated in the injured myocardium after coronary occlusion, where macrophages produce multiple cytokines such as tumor necrosis factor α, platelet derived endothelial cell growth factor, transforming growth factor β1 (TGFβ1) and interleukin-1 to initiate inflammation and progression of adverse cardiac remodeling.3,4 Clinical observations have also found that macrophage accumulation is closely associated with severe myocardial injury and poor functional outcome in patients with ST-segment elevation myocardial infarction.5–8
It is well known that angiotensin II (Ang II) has profound effects on cardiovascular diseases acting via its binding to two main cell surface receptors, AT1 and AT2. Ang II signaling has been associated with development of deleterious tissue repair after myocardial infarction through regulating monocyte motility and release from the splenic reservoir.2 In a mouse model of permanent coronary occlusion, enalapril, an angiotensin-converting enzyme (ACE) inhibitor, reduced the release of monocytes from the spleen and subsequently inhibited the recruitment of monocytes into the infarct site. This inhibition in macrophage accumulation in ischemic myocardium was reproduced by splenectomy.9 In this regard, we have recently reported that four weeks of continuous administration of Ang II to rats enhances macrophage accumulation and myofibroblast proliferation in the myocardium, which was significantly inhibited by an AT1 receptor blocker, telmisartan. Consequently, deposition of collagen in perivascular coronary vessels and interstitial myocardium was attenuated. In that study, we found that the extravasation and distribution of macrophages in the myocardium is the most important feature in activation of myofibroblast/TGFβ1/Smads-mediated fibrotic signaling.10
Although previous studies have identified the splenic reservoir monocytes as a major source of macrophages that accumulate in the infarcted myocardium,2,9 it is unknown whether splenic monocytes contribute to Ang II-induced macrophage accumulation in the myocardium and subsequent cardiac fibrosis and hypertension. Hence, the purpose of this study was designed to evaluate whether splenic release of monocytes participates in Ang II-elicited cardiac fibrosis and hypertension by modulating the population of macrophages in the myocardium and blood vessel in the in vivo rat model of Ang II infusion. Specifically, the effects of splenectomy and the AT1 receptor blockade on monocyte/macrophage recruitment, monocyte chemoattractant protein-1 (MCP-1), TGFβ1/Smads, collagens and endothelial nitric oxide synthase (eNOS) were examined as well as the interstitial/perivascular fibrosis and hypertension were assessed.
Materials and methods
Animals and noninvasive blood pressure measurement
The animal experimental procedures were approved by the Institutional Animal Care and Use Committee, Mercer University School of Medicine. Male Sprague-Dawley rats weighing 200±10 g obtained from the Harlan Laboratories, Indianapolis, Indiana, USA were kept individually in a 12-hour light/dark cycle, 60% humidity and temperature-controlled room with standard rat chow and water ad libitum. These procedures were in compliance with The Guide for the Care of Use of Laboratory Animals.11 The blood pressure was determined in conscious rats using a noninvasive blood pressure measuring system (PowerLab, ML125 AD Instruments NIBP controller, Colorado, USA). This system detects the signals via a pulse transducer during the periodic occlusion of blood flow in the tail.
Osmotic minipump implantation and splenectomy
The rats were anesthetized with an intraperitoneal injection of a mixture of ketamine (90 mg/kg) and zylaxine (10 mg/kg) and implanted subcutaneously with osmotic minipumps (Model 2004, ALZET Corp, California, USA) in the dorsal region to deliver Ang II. The pumps were preincubated in the tubes with 0.9% sterile saline only or saline containing Ang II at 37ºC overnight prior to implantation. In rats that received splenectomy, a midline skin incision was made after the skin was shaved and sterilized with povidone-iodine and alcohol. The splenic blood vessels were ligated after the abdominal cavity was made. The spleen was removed by transecting the vessels just distal to the ligature and the skin incision was then closed with sutures.
Experimental design and study group
Four weeks of experimental period was selected for all experimental groups. Five concurrent groups of rats were randomly assigned: (a) vehicle–infused rats (Sham, n=7): rats received saline minipump infusion; (b) Ang II-infused rats (Ang II, n=7): rats received subcutaneous Ang II infusion at a rate of 500 ng/kg/min (Sigma-Aldrich, Missouri, USA); (c) splenectomy+Ang II infusion rats (Splen, n=7): rats received Ang II infusion at day 2 after splenectomy; (d) telmisartan+Ang II (Telmi, n=7): telmisartan (Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, Connecticut, USA) was administered at a dose of 10 mg/kg/day by gastric gavage starting day 1 after Ang II minipump implantation; (e) splenectomy+telmisartan+Ang II infusion rats (S+T, n=6): rats received telmisartan by gastric gavage after splenectomy and during Ang II infusion. At the end of four weeks of the experimental period, the rats from different groups were euthanized. The hearts and proximal thoracic aortas were removed.
Western blot analysis of the AT1 receptor, MCP-1, TGFβ1, Smads and collagens in the spleen and myocardium
The freshly-frozen transmural tissue samples from the spleen and myocardium were homogenized in ice-cold lysis buffer. Total proteins (60 μg) were extracted, fractionated by 10% SDS-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membrane as described previously.10 The membrane was then incubated at 4°C overnight with the following antibodies: rabbit anti-AT1 (1:800) and AT2 (1:400) receptor polyclonal antibodies (Santa Cruz Biotech, Dallas, Texas, USA), a rabbit anti-MCP-1 polyclonal antibody and a mouse monoclonal anti-TGFβ1 antibody (1:1000, Abcam, Inc., Massachusetts, USA), rabbit anti-phospho-Smad2/3 monoclonal antibodies (1:1000, Cell Signaling, Massachusetts, USA), mouse anti-collagen type I and III monoclonal antibodies (1:5000, Sigma Chemical Co., Missouri, USA), respectively. Bound antibody was detected by horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG)). The membrane was incubated with chemiluminescence detection reagents and exposed to a X-ray film. The images were then imported into the ImageJ (National Institute of Health (NIH), Massachusetts, USA) and quantified as a ratio based on the density of the target protein band divided by actin to correct differences during protein loading and/or transfer.
Immunohistochemical staining of Ang II receptors, macrophages, myofibroblasts and eNOS in the spleen, myocardium, and thoracic aorta
All paraffin-embedded tissue sections (4 μm thick) were dewaxed in xylene, and rehydrated through graded alcohols as previously reported.10 Briefly, the sections were incubated overnight with primary antibodies including a rabbit anti-AT1 receptor polyclonal antibody (1:200, Santa Cruz Biotech, Dallas, Texas, USA), a mouse anti-rat monoclonal antibody against macrophages (CD68; 1:200, Millipore, California, USA), a monoclonal antibody against α-smooth muscle actin (SMA; 1:800, Sigma Chemical Co., Missouri, USA) and a rabbit polyclonal antibody against eNOS (1:200, Abcam, Inc., Massachusetts, USA), respectively. The slides were incubated with a biotinylated horse anti-rabbit IgG or an anti-mouse IgG (Vector Laboratories, California, USA), stained using the ABC-peroxidase kit or ABC-AR (alkaline phosphatase; Vector Laboratories, California, USA) and visualized with 3,3’-diaminobenzidine tetrahydrochloride or alkaline phosphatase substrate kit (Sigma, Missouri, USA). Quality of immunohistochemistry assay was controlled by either elimination of the primary antibody or incubation of the tissue with a non-immune IgG. Sections were counterstained with hematoxylin, dehydrated, and mounted by routine methods. The density in expression of the AT1 receptor and eNOS, the accumulation of macrophages and α-SMA–expressing myofibroblasts among groups were compared using computer-assisted morphometry (ImageJ, NIH). The final results were reported from the eight randomized high-powered fields.
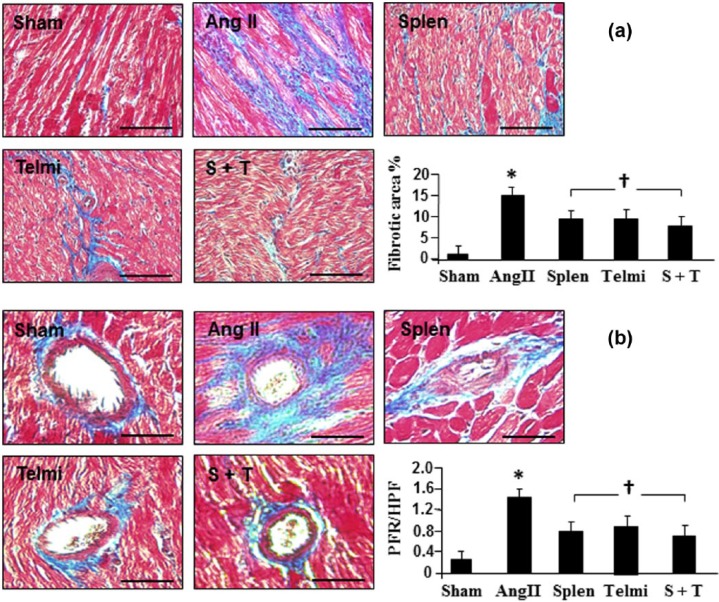
Masson’s trichrome staining of collagen deposition in the heart and thoracic aorta
The transversal myocardial blocks and proximal thoracic aorta were fixed in phosphate-buffered formalin solution and dehydrated in graded series of alcohol, and then embedded in paraffin as previously reported.10 Samples were sectioned to a thickness of 6 μm using a Microtome (Leica RM2135, Meyer Instruments, Texas, USA) and stained by Masson’s trichrome method producing collagen blue, nuclei black and muscle fiber red. Ten randomized high-powered fields (HPFs) per tissue section were evaluated to define the perivascular fibrosis, expressed as the area of perivascular fibrosis divided by the area of the vascular wall, i.e. perivascular fibrosis ratio (PFR).12 The interstitial fibrosis was quantitated as the positively stained collagen area (i.e. blue staining in the interstitial myocardium) and expressed as percentage fibrotic area per HPF. The fibrotic area of thoracic aorta was visualized by the fibrotic thickness in the adventitia and calculated as the micrometer per HPF. All histological evaluation was performed by a single well-trained observer using a digital image analyzer (ImageJ, NIH, Massachusetts, USA).
Statistical analysis
Data were reported as the mean±standard error. A one-way analysis of variance (ANOVA) followed by Student-Newman-Keul’s post-hoc test was used to analyze group differences in the intensity of AT1/AT2 receptors, the populations of macrophages and myofibroblasts, the expression of TGFβ1, collagens, and phospho-Smads using SigmaPlot (Systat Software Inc., California, USA). Statistical significance was set at a value of p<0.05.
Results
Identification of monocytes and expression of the AT1 receptor in the red pulp of the spleen
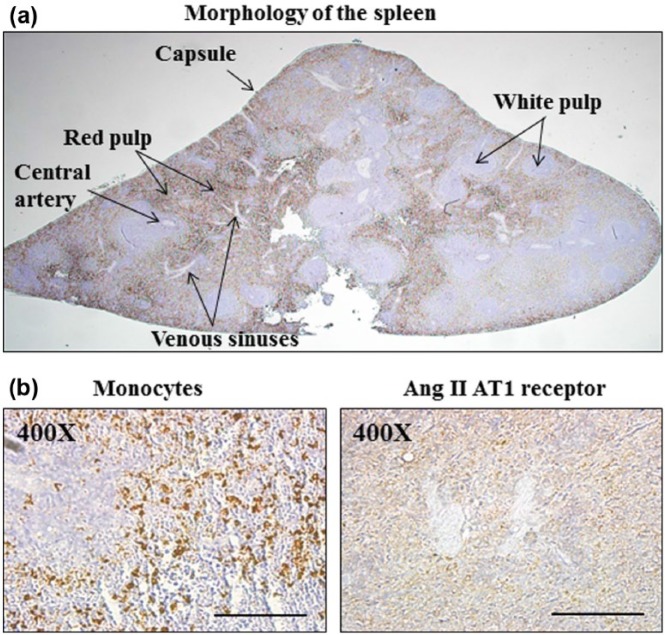
Histological features of the spleen in rat are shown in Figure 1(a). The spleen is composed of two functionally and morphologically distinct tissues, the white pulp and the red pulp. The white pulp regions of the spleen consist of lymphatic tissue containing T lymphocytes and B lymphocytes that are responsible for destroying pathogens in the blood and producing antibodies. The red pulp makes up 75% of the volume of the spleen and serves as an important reservoir for large quantities of phagocytic white blood cells called monocytes. To further demonstrate the distribution of monocytes and the expression of the AT1 receptor, we stained the tissue sections of the spleen using immunohistochemistry. As shown in Figure 1(b), monocytes and the AT1 receptor were predominately localized and expressed in the red pulp.
Figure 1.
Detection of monocytes and expression of the AT1 receptor from paraffin-embedded transverse sections in normal rat spleen. The low-power view in (a) shows the average amount of two types of tissue: the red pulp and white pulp in the parenchyma of the spleen. The red pulp makes up the majority of the spleen and serves as an important reservoir for large quantities of phagocytic white blood cells called monocytes. White pulp is lymphatic tissue that mainly consists of lymphocytes called B-lymphocytes and T-lymphocytes that surround arteries. Venous sinuses are essentially cavities filled with blood. The presence of macrophages and expression of the AT1 receptor were confirmed by immunohistochemistry (b). In a higher magnification of the cross-section, it was found that monocytes are predominantly located within the red pulp, where the positively stained AT1 receptor shown as brown granular pigment was identified. Magnification×400; scale bars: 100 µm (n=6).
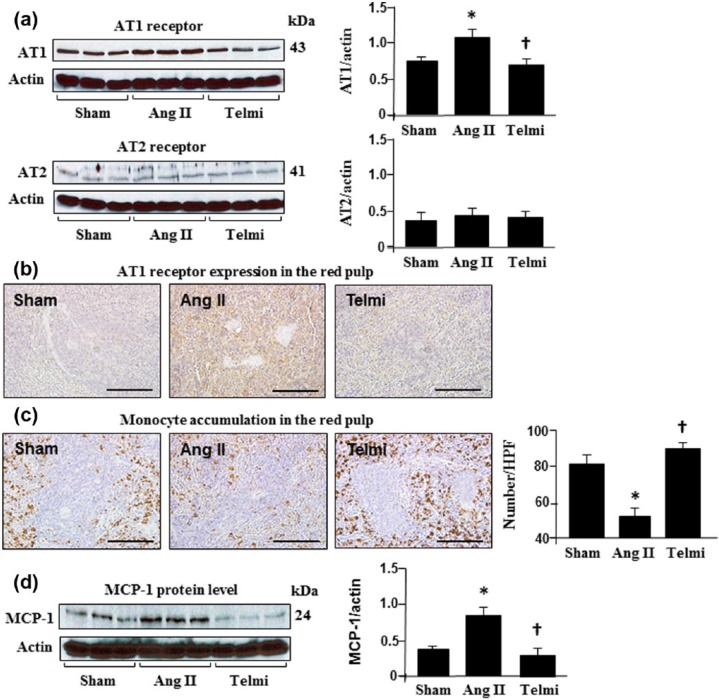
Infusion of Ang II increased the protein level and expression of the AT1 receptor and reduced the monocyte population in the red pulp of the spleen
The protein level of the AT1 receptor and its expression in the red pulp were analyzed using Western blot assay and immunohistochemistry. As shown in Figure 2(a), infusion of Ang II for four weeks significantly increased the protein level of the AT1 receptor relative to the sham group. Positive immunohistostaining for the AT1 receptor was found in the red pulp during the course of the experiment in the sham group but the immunohistostaining intensity of the AT1 receptor was markedly enhanced with Ang II infusion at week 4 (Figure 2(b)). Consistent with changes in the AT1 receptor protein and expression, the population of monocytes in the red pulp was significantly reduced with Ang II infusion relative to the sham control (Figure 2(c)). Treatment with telmisartan over four weeks abrogated the upregulated AT1 receptor. The protein level and expression of the AT1 receptor were significantly attenuated, coinciding with preserved numbers of monocytes in the red pulp (Figure 2(c)), suggesting a role of Ang II in the release of monocytes in the spleen via stimulating the AT1 receptor. The AT2 receptor was constitutively expressed in the spleen. Relative to the sham group, the infusion of Ang II and administration of the AT1 receptor blocker, telmisartan, did not alter expression of the AT2 receptor (Figure 2(a)).
Figure 2.
Angiotensin II (Ang II) receptor expression, macrophage accumulation and monocyte chemoattractant protein-1 (MCP-1) level in the spleen during Ang II infusion. Protein levels of the AT1/AT2 receptors (a) and MCP-1 (d) were analyzed using Western blot and all bands were normalized by actin. Expression of the AT1 receptor (b) and macrophage accumulation (c) were detected with immunohistochemistry. Macrophage localization was determined by the number of positively-stained cells (brown granular pigment) in each high-powered field (HPF; original magnification: ×400; scale bars: 100 µm). Sham: rats were infused with a saline pump for four weeks; Ang II: rats received Ang II subcutaneous infusion at a rate of 500 ng/kg/min; Telmi: telmisartan plus Ang II. Telmisartan was administered by gastric gavage at a dose of 10 mg/kg/day during Ang II infusion. Values are means±standard error of the mean (SEM) (n=7/each group). *p<0.05 Ang II vs Sham; †p<0.05 Telmi vs Ang II.
Blockade of the AT1 receptor reduced the protein level of MCP-1 in the spleen and macrophage accumulation in the heart: comparison to the splenectomy
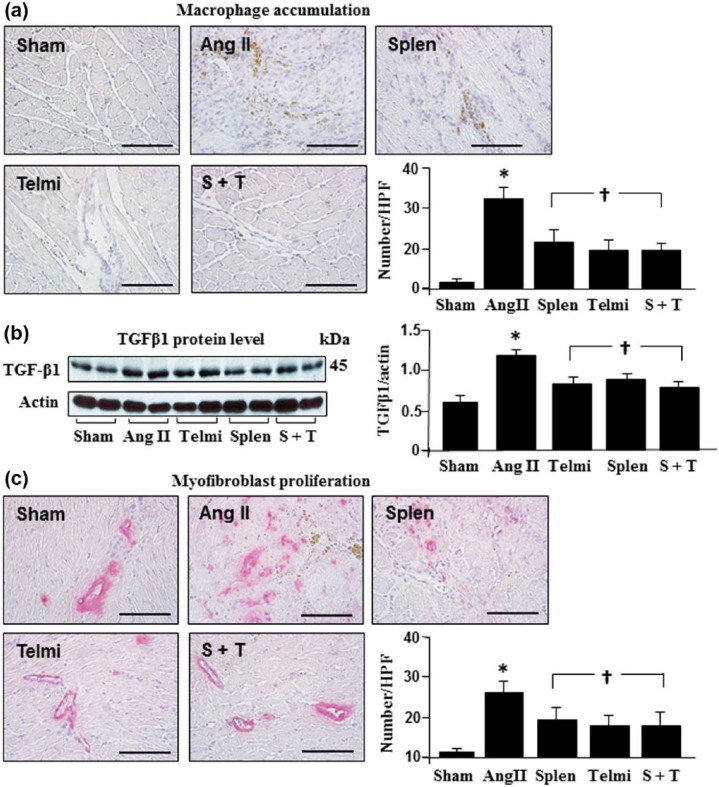
MCP-1 is a potent chemoattractant specific for monocytes and known to be associated with monocyte migration. As shown in Figure 2(d), MCP-1 was constitutively expressed in the spleen and significantly increased with Ang II infusion at week 4, which was consistent with reduced monocyte populations in the spleen (Figure 2(c)). To further demonstrate the receptor mechanism underlying how Ang II promoted monocyte migration from the spleen, we treated the animals using telmisartan, and found that along with an inhibition of MCP-1 expression in the spleen, the splenetic monocyte population was also increased (Figure 2(c)), indicating a role of MCP-1 in migrating monocytes via the AT1 receptor. To associate the release of monocytes from the spleen with the macrophage accumulation in the heart, the distribution of macrophages in the heart after splenectomy and the AT1 blockade was compared. As shown in Figure 3(a), the number of macrophages was significantly reduced after splenectomy to a comparable level shown as the number of macrophages in the heart after the AT1 receptor inhibition, suggesting that the spleen is the source of macrophage accumulation in the heart via the AT1 receptor-mediated pathway. Treatment with splenectomy plus telmisartan did not provide further inhibition of macrophage accumulation in the heart (Figure 3(a)).
Figure 3.
Accumulation of macrophages, protein level of transforming growth factor β1 (TGFβ1) and proliferation of myofibroblasts in the heart during angiotensin II (Ang II) infusion. The presence of macrophages (a) and myofibroblasts (c) and expression of TGFβ1 (b) were detected using immunohistochemistry and Western blot. The number of macrophages and myofibroblast was determined by positively-stained cells (brown and red granular pigments) in each high-powered field (HPF; original magnification: ×400; scale bars: 100 µm). Sham: rats were infused with a saline pump for four weeks; Ang II: rats received Ang II subcutaneous infusion at a rate of 500 ng/kg/min; Splen: splenectomy was performed before Ang II infusion; Telmi: telmisartan was administered orally during Ang II infusion; S+T: splenectomy was performed and telmisartan was administered during Ang II infusion. Values are means±standard error of the mean (SEM) (n=7/each group). *p<0.05 Ang II vs Sham; †p<0.05 Telmi vs Ang II.
Splenectomy and the AT1 receptor antagonism reduced TGFβ1 protein level and inhibited myofibroblast proliferation in the heart
It is known that the release of TGFβ1 from macrophages is capable of stimulating myofibroblast proliferation. To address whether this is a potential signaling mechanism involved in inhibition of myofibroblast proliferation in the heart after splenectomy and the AT1 receptor antagonism, TGFβ1 expression and myofibroblast proliferation were detected using Western blot assay and immunohistochemistry. As shown in Figure 3(b), Ang II infusion caused a significant increase in TGFβ1 protein level at week 4. Along with an augmentation in expression of TGFβ1 protein, the number of positively-stained α-SMA-expressing myofibroblasts was increased, suggesting a role of TGFβ1 in myofibroblast proliferation (Figure 3(c)). Splenectomy before Ang II infusion or treatment with telmisartan over four weeks reduced the protein level of TGFβ1 and myofibroblast proliferation. No further inhibition in macrophage accumulation, TGFβ1 expression, and myofibroblast proliferation was observed when treated with splenectomy and telmisartan (Figure 3).
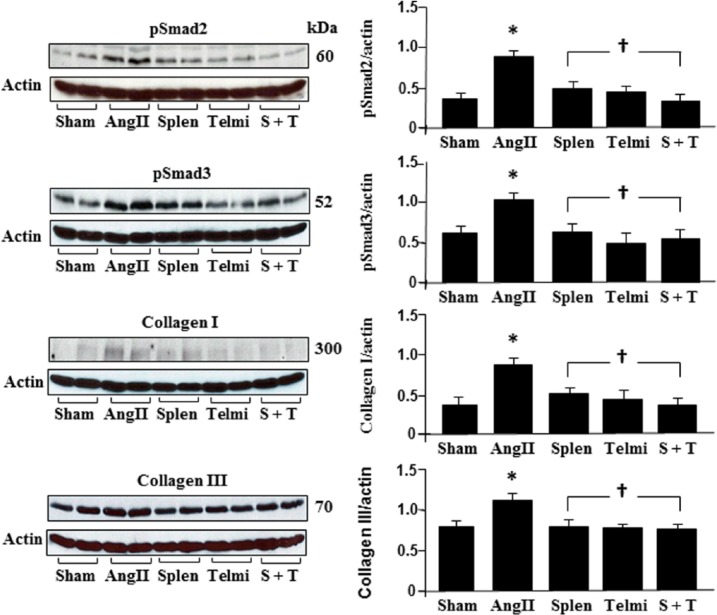
Splenectomy and the AT1 receptor antagonism reduced production of phosph-Smad2/3 and collagen I/III in the heart
Phosphorylation of Smads stimulates the synthesis of collagens following myofibroblast proliferation. We selected Smad2 and Smad3 as signaling targets to detect collagen synthesis using Western blot assay. As shown in Figure 4, Smad2 and Smad3 were significantly phosphorylated at week 4 after Ang II infusion, coinciding with enhanced synthesis of collagen I and III. Splenectomy before Ang II infusion or treatment with telmisartan over four weeks reduced phosphorylated levels of Smad2 and Smad3, which was in compliance with an inhibition of protein levels of collagen I and III. Splenectomy plus administration of telmisartan during Ang II infusion did not further affect the protein levels of Smad2/3 and collagen I/III throughout the experiment (Figure 4).
Figure 4.
Protein levels of pSmad2, pSmad3, collagen I and III in the heart during angiotensin II (Ang II) infusion. Expression of Smads and collagens were analyzed using Western blot. All bands were normalized by actin as illustrated among groups. Sham: rats were infused with a saline pump for four weeks; Ang II: rats received Ang II subcutaneous infusion at a rate of 500 ng/kg/min; Splen: splenectomy was performed before Ang II infusion; Telmi: telmisartan was administered orally during Ang II infusion; S+T: splenectomy was performed and telmisartan was administered during Ang II infusion. Values are means±standard error of the mean (SEM) (n=7/each group). *p<0.05 Ang II vs Sham; †p<0.05 Telmi vs Ang II.
Splenectomy and the AT1 receptor antagonism inhibited intermyocardial and perivascular fibrosis in the heart
The scale of intermyocardial fibrosis and the ratio of perivascular fibrosis were examined using Masson’s trichrome method. Consistent with increased synthesis of collagens, the production of fibrotic tissue in the intermyocardium and perivascular region was augmented with Ang II infusion. At week 4, percent fibrotic area in the myocardium (Figure 5(a)) and fibrotic ratio in the perivascular area (Figure 5(b)) were significantly increased at week 4. Splenectomy before Ang II infusion or treatment with telmisartan over four weeks significantly reduced the fibrotic tissue formation induced by Ang II infusion. Combination of splenectomy plus administration of telmisartan during Ang II infusion provided a comparable inhibition in fibrosis formation in the myocardium and perivascular region when compared with each individual intervention (Figure 5).
Figure 5.
Representative photomicrographs of myocardial cross sections stained with Masson’s trichrome method. Interstitial fibrosis (a) and perivascular fibrosis (b) at week 4 of angiotensin II (Ang II) infusion were identified by blue staining. Interstitial and perivascular fibrosis were calculated as percent fibrotic area and fibrosis ratio (PFR) in each high-powered field (HPF; original magnification: ×400; scale bars: 100 µm). Sham: rats were infused with a saline pump for four weeks; Ang II: rats received Ang II subcutaneous infusion at a rate of 500 ng/kg/min; Splen: splenectomy was performed before Ang II infusion; Telmi: telmisartan was administered orally during Ang II infusion; S+T: splenectomy was performed and telmisartan was administered during Ang II infusion. Values are means±standard error of the mean (SEM) (n=7/each group). *p<0.05 Ang II vs Sham; †p<0.05 Telmi vs Ang II.
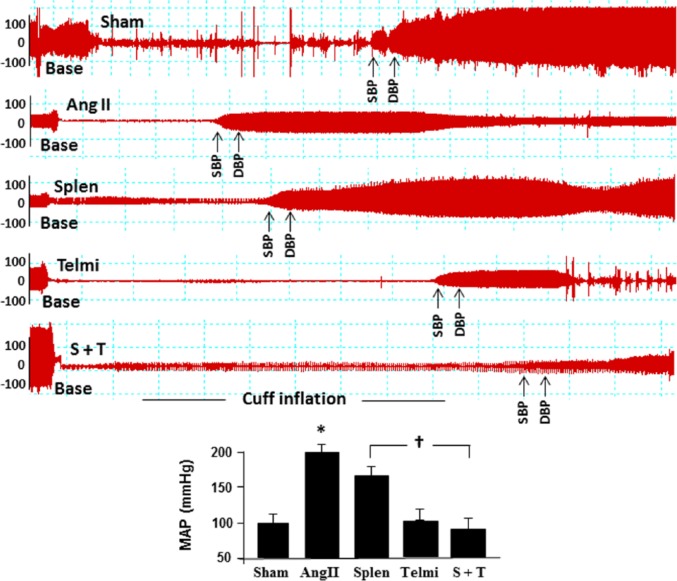
Splenectomy and the AT1 receptor antagonism reduced blood pressure, attenuated macrophage invasion and myofibroblast proliferation as well as augmented eNOS expression in the proximal thoracic aorta
Formation of perivascular and interstitial fibrosis is associated with an increase in blood pressure. As shown in Figure 6, subcutaneous infusion of Ang II significantly increased mean arterial pressure (MAP) calculated from systolic/diastolic pressure at week 4 relative to the sham group (202±8.9 mm Hg vs 98±3.8 mm Hg in the sham group, p=0.03). Splenectomy before Ang II infusion significantly reduced the MAP compared with the Ang II group (176±12 mm Hg vs 202±8.9 mm Hg in the Ang II group, p=0.039). The maximal reduction in MAP was observed throughout the experiment to a comparable level in the sham group when treated with telmisartan (97±14 mm Hg vs 98±3.8 mm Hg). There was no statistical difference in reduction of MAP between splenectomy plus telmisartan group and telmisartan alone at week 4 (91±18 mm Hg vs 97±14 mm Hg).
Figure 6.
Change in blood pressure during angiotensin II (Ang II) infusion. Blood pressure was measured by inflating a tail cuff with simultaneous monitoring of the cuff pressure. The arrows indicate systolic blood pressure (SBP) and diastolic blood pressure (DBP). Change in blood pressure among groups can be identified by the distance during cuff inflation between the baseline (Base) and the beginning of SBP tracing. Sham: rats were infused with a saline pump for four weeks; Ang II: rats received Ang II subcutaneous infusion at a rate of 500 ng/kg/min; Splen: splenectomy was performed before Ang II infusion; Telmi: telmisartan was administered orally during Ang II infusion; S+T: splenectomy was performed and telmisartan was administered during Ang II infusion. Values are means± standard error of the mean (SEM) (n=7/each group). *p<0.05 Ang II vs Sham; †p<0.05 Telmi vs Ang II.
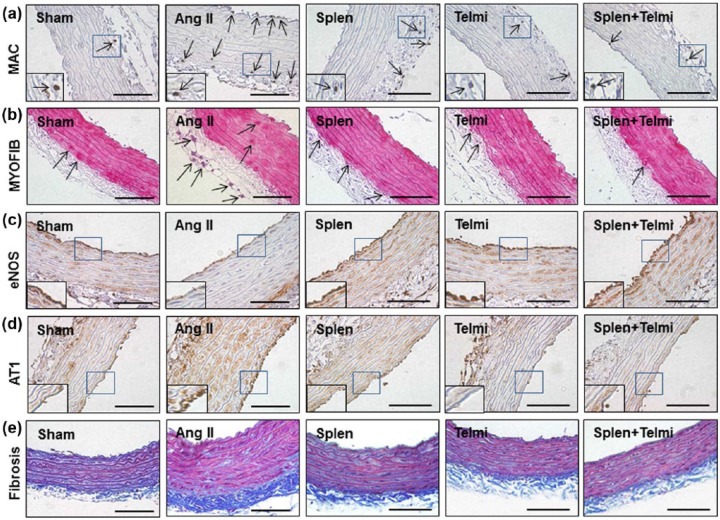
Aortic hypertrophy is associated with an elevation in blood pressure after continuous infusion of Ang II. To further explore whether macrophage recruitment to the aorta is involved in initiation of this process, the proximal thoracic aorta was stained using immunohistochemistry to detect the infiltration of macrophages, proliferation of myofibroblasts, expression of eNOS and the AT1 receptor. As shown in Figure 7(a), there were a significantly increased number of positively stained macrophages in the adventitia and tunica intima (endothelium) at week 4 relative to the sham control (7.1±2.3 vs 2.0±0.6/HPF, p=0.032). Consistent with the presence of macrophages after Ang II infusion, immunohistostaining confirmed proliferation of myofibroblasts at the perivascular region relative to the sham control (6.9±1.7 vs 1.7±0.9/HPF, p=0.028, Figure 7(b)). eNOS expression was constitutively expressed in the endothelium and tunica media of thoracic aorta in the sham control, but its expression was markedly downregulated by Ang II infusion at week 4 (Figure 7(c)). As shown in Figure 7(a)–(d), splenectomy or dietary treatment with telmisartan reduced the numbers of recruited macrophages (4.0±0.1 and 3.0±0.7 vs 7.1±2.3/HPF in the Ang II group, p=0.038) and proliferated myofibroblasts (2.8±0.9 and 2.0±1.0 vs 6.9±1.7/HPF in the Ang II group, p=0.036). In coincidence with these modifications, eNOS expression on the endothelium and tunica media was upregulated and the AT1 receptor was downregulated by splenectomy or telmisartan. Furthermore, the fibrotic thickness in the adventitia, as identified by Masson’s trichrome staining, was reduced relative to the Ang II group with these interventions (0.7±0.1 µm/HPF in the splenectomy group and 0.65±0.2 µm/HPF in the telmisartan group vs 1.6±0.2 µm/HPF in the Ang II group, p=0.042, Figure 7(e)). With these improvements, the aortic wall thickness was reduced in both splenectomy and telmisartan groups (312±36 and 303±46 vs 537±36 µm/HPF in the Ang II group, p=0.04), indicating less smooth muscle proliferation and medial hypertrophy. These data suggest that Ang II-induced vascular remolding is associated with invasion of macrophages into the aortic walls by an AT1 receptor-mediated process. No further modulation in infiltration of macrophages, proliferation of myofibroblasts, expression of eNOS and the AT1 receptor as well as adventitial fibrotic area was observed when splenectomy and telmisartan treatment were combined relative to each intervention alone (Figure 7).
Figure 7.
Representative photomicrographs of aortic cross sections stained with immunohistochemistry and Masson’s trichrome method. In each high-powered field (HPF; original magnification: ×400; scale bars: 100 µm), the recruitment of macrophages (MAC; (a)) and myofibroblasts (MYOFIB, (b)) in aortic intima and adventitia was determined by the number of positively-stained cells (brown and red granular pigments as indicated by arrows). Expression of eNOS (c) and the AT1 receptor (d) was identified by brown staining in the aortic intima and media. Collagen deposition in aorta (e) was analyzed by the thickness of adventitial fibrotic area shown as blue staining. Sham: rats were infused with a saline pump for four weeks; Ang II: rats received Ang II subcutaneous infusion at a rate of 500 ng/kg/min; Splen: splenectomy was performed before Ang II infusion; Telmi: telmisartan was administered orally during Ang II infusion; Splen+Telmi: splenectomy was performed and telmisartan was administered during Ang II infusion; n=6/each group.
Discussion
Recent studies highlight the role of splenic reservoir-derived monocytes/macrophages in the development of myocardial infarction and heart failure. In the mouse model of permanent coronary occlusion, it has been shown that monocytes clustered in the spleen express the AT1 receptor.2 Monocytes mobilized from the splenic reservoir into the infarcted myocardium after coronary ligation impaired the infarct healing and provoked pathological ventricular remodeling by eliciting potent inflammation.3,4 In this regard, we have recently reported that infusion of Ang II causes significant tissue fibrosis by producing macrophage accumulation via stimulating the AT1 receptor in the heart.12 However, we do not know the mechanisms involved in the recruitment of macrophages. This is the first study to illustrate that the release of monocytes from their splenic reservoir to the heart plays an important role in development of Ang II-induced cardiac fibrosis and hypertension. We demonstrate that, in the normal condition, monocytes presented in the red pulp of the spleen, an area extremely rich in macrophages, primarily express the AT1 receptor. Ang II infusion caused a significant accumulation of macrophages in the heart after the AT1 receptor is activated in the spleen, potentially through activating MCP-1. Splenectomy or blockade of the AT1 receptor reduced the recruitment of macrophages and the proliferation of myofibroblasts, followed by an inhibition of Smads-mediated collagen production and fibrotic tissue formation. In the proximal thoracic aortic wall, splenectomy or the AT1 receptor blockade inhibited invasion of macrophages and myofibroblasts, and enhanced eNOS expression, indicating a role of macrophages in Ang II-elevated blood pressure.
Infusion of Ang II using an osmotic pump builds a constant plasma concentration of Ang II and allows us to observe the direct effect of Ang II on tissue injury. We chose this model of Ang II infusion because it likely reproduces innate pathological processes during the development of cardiac fibrosis resulting from myocardial infarction, hypertension, and heart failure in humans.13,14 In these patients, elevated concentration of Ang II in plasma and heart has been recognized as a main trigger of pathogenesis.15 Of note, in the present study, the substantial reduction in accumulation of macrophages in the heart by splenectomy, which was simulated through inhibiting the AT1 receptor-mediated process, suggests that there is a parallel relationship of reduced level of splenic monocytes and enhanced accumulation of macrophages in the heart. Splenectomy plus administration of the AT1 receptor blocker did not show further attenuation in macrophage accumulation and tissue fibrosis in the heart relative to each intervention alone, indicating that the maximal protection through inhibiting macrophage migration can be achieved either by splenectomy or the AT1 receptor antagonism. In this regard, other factors such as production of reactive oxidant species and inflammatory cytokines in the heart in addition to recruitment of macrophages may also participate in Ang II-induced cardiac fibrosis.16,17
We have previously shown that blockade of the AT1 receptor has inhibitory effects on myocardial fibrosis and hypertension through activating the AT2 receptor.10 To ensure the relative contribution of splenic monocytes to cardiac fibrosis via the AT1 receptor antagonism, we also measured expression of the AT2 receptor in the spleen. As shown in Figure 2, the AT2 receptor is normally expressed in the spleen, but its expression was not altered by Ang II infusion and telmisartan, excluding a role of the AT2 receptor in mobilization of splenic monocytes. MCP-1, a potent mononuclear cell chemoattractant, is reported to be produced by macrophages, endothelial cells, and myocardium to promote the migration of inflammatory cells. The available evidence suggests that the induction of MCP-1 in response to Ang II is associated with mobilization of macrophages through the production of inflammatory cytokines.18 In the present study, following the AT1 receptor antagonism, expression of MCP-1 in the spleen was downregulated and the population of macrophages was preserved, evidenced by less accumulation of macrophages in the myocardium. These results suggested that local production of MCP-1 in the spleen can be considered as a potential mechanism by which macrophages are mobilized to the heart. This mobilization of monocytes/macrophages from the spleen to the heart is activated by the AT1 receptor.
TGF-β has been thought to be a key factor in the progression of myocardial lesions following Ang II stimulation.19,20 TGFβ1 produced from activated macrophages contributes to tissue fibrosis by stimulating Smads signaling and releasing extracellular matrix, primarily collagens from myocardial myofibroblasts.10,21 In the present study, differentiation of fibroblasts to myofibroblasts was identified by the number of α-SMA-expressing positive cells in the heart. We found that, along with a reduction in migration of monocytes from the spleen and macrophage accumulation in the heart by telmisartan, expression of TGFβ1 and Smad2//3 was inhibited to a comparable level as seen after splenectomy. Immunohistochemical assessment of splenectomy/telmisartan-treated rats during Ang II infusion showed a significant resolution of normalizing the fibrotic parameters such as the number of α-SMA-expressing myofibroblasts and protein levels of collagens in the heart. Histological data presented at week 4 also showed attenuation in tissue fibrosis as measured by a lower extent of collagen deposition. It is known that the spleen may have some beneficial effects on wound healing and health in general.22 However, we found that at week 4 after Ang II infusion, the scale of fibrotic tissue in the heart was reduced to a compatible level by splenectomy as seen in the telmisartan-treated animals, suggesting a predominant role of the splenic monocytes in the development of cardiac fibrosis via the AT1 receptor. However, we found that the fibrotic myocardium is still detected in the splenectomy/telmisartan-treated animals. Therefore, we cannot exclude the possibility that progenitor macrophages released from the bone marrow or resident macrophages in the heart may also participate in the Ang II-stimulated cardiac fibrosis,23,24 which may not be altered by splenectomy/telmisartan. Furthermore, using flow cytometry may help to quantitatively distinguish subtypes of monocytes in the spleen and demonstrate the surface marker expression of monocytes after the AT1 receptor stimulation.2 Subendothelial infiltration of monocyte/macrophages has previously been reported to be associated with endothelial dysfunction and spontaneous hypertension.25 Treatment with an ACE inhibitor lowed blood pressure by reducing subendothelial accumulation of monocyte/macrophages.26 A recent study has also shown that depletion of macrophages using liposome-encapsulated clodronate reduces blood pressure and vascular free radicals.27 In the present study, we found that Ang II, through the AT1 receptor, significantly increases blood pressure by provoking adverse vascular remodeling. Immunohistochemistry revealed that reduction in invasion of macrophages and myofibroblasts in the aortic endothelium and adventitia by splenectomy to a comparable level as seen in the AT1 receptor antagonism suggests a role of monocytes/macrophages mobilized from the splenic reservoir in the formation of fibrotic thoracic aorta.
It is well known that downregulated eNOS from the endothelium is related to impairment of endothelium-dependent relaxation and production of vascular proliferation/hypertrophy during hypertension.28–30 We found that, in absence of the AT1 receptor stimulation by Ang II in the sham condition, eNOS is constitutively expressed in the endothelium and tunica media. We and others have previously shown that activation of the AT1 receptor enhances expression of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), facilitates migration of inflammatory cells such as neutrophils and macrophages, and reduces eNOS expression.31–33 In these studies, downregulation of eNOS by TNFα released from macrophages was identified. Our data were consistent with these findings. In the present study, telmisartan blocked the AT1 receptor, inhibited macrophage migration, and upregulated eNOS. Enhancement in eNOS expression by splenectomy further supported that macrophages recruited in the aorta from the spleen reservoir may participate in vascular remodeling by downregulating eNOS expression in Ang II-receiving rats. In this regard, vascular/myocardial fibrosis in response to Ang II stimulation has been associated with elevated blood pressure.10,34–36 As shown in Figure 7(e), along with a reduction in the perivascular fibrosis and aortic hypertrophy by splenectomy and telmisartan, as identified by a reduction in intimal/medial thickness, the blood pressure was significantly reduced, indicating a role of macrophages in induction of high blood pressure.
Limitations of this study are expressed as follows. First, although we found that macrophage migration from the spleen is mediated by MCP-1, also known as CCL2 (CC chemokine ligand 2), after activating the AT1 receptor, the direct cause-effect relationship between MCP-1 and macrophage migration needs to be further explored using CCL2 knockout mice in the presence of Ang II. Second, data from our laboratory10 and others37,38 have shown the alternative inhibitory role after the AT1 receptor blockade in Ang II–mediated cardiac and vascular effects, i.e. activation of angiotensin-converting enzyme 2 (ACE2)-Mas receptor, we do not know whether this pathway is also associated with an inhibition of macrophage migration from the spleen. Therefore, studies are needed to compare the potency of splenectomy and ACE2 analog in modulation of macrophages in animals receiving Ang II. Finally, we showed that splenectomy or telmisartan reduces blood pressure. However, we cannot exclude the possibility that reduction in blood pressure by these interventions is directly linked to an attenuation of Ang II-driven tissue fibrosis. Therefore, using a model of transverse aorta constriction might allow us to differentiate the blood pressure-dependent and/or -independent effects of these interventions on myocardial/vascular fibrosis.10,16 Furthermore, it remains to be determined whether acute infusion of Ang II after splenectomy affects the blood count of monocytes and blood pressure.
Conclusions
Although signaling in tissue fibrosis and hypertension is a highly complex process involving many cell types and signaling molecules, we demonstrate novel mechanistic insights involved in Ang II-induced perivascular/interstitial fibrosis and hypertension through migrating monocytes/macrophages from the spleen reservoir via the AT1 receptor. Macrophages mobilized from the spleen to the heart trigger proliferation of myofibroblasts through TGFβ1, and activate Smads-mediated collagen synthesis and myocardial fibrosis. On the other hand, stimulation of the AT1 receptor also causes invasion of macrophages into the thoracic aorta and induces a fibrotic response, and thereby promotes vascular hypertrophy/stiffness and hypertension. However, the favorable and unfavorable effects after splenectomy have also been identified clinically.39,40 We cannot comment on the clinical application of splenectomy in patients based on the current data, but, our findings might be useful in exploring how therapeutics already established, for example, ACE inhibitors or angiotensin receptor blockers, affect macrophage biology. Therefore, the development of macrophage-targeted new therapeutic interventions may further prevent cardiac fibrosis and vascular hypertrophy during hypertension.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by grants from the Mercer University School of Medicine, the Medcen Community Health Foundation, Georgia, USA and the National Natural Science Foundation of China (81170145, 81470436).
References
- 1. Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol 2006; 34: 455–465. [DOI] [PubMed] [Google Scholar]
- 2. Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325: 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischemic heart disease. Cardiovasc Res 2014; 102: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ismahil MA, Hamid T, Bansal SS, et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: Critical importance of the cardiosplenic axis. Circ Res 2014; 114: 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Laan AM, Hirsch A, Robbers LF, et al. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: Monocytes and myocardial infarction. Am Heart J 2012; 163: 57–65. [DOI] [PubMed] [Google Scholar]
- 6. Tsujioka H, Imanishi T, Ikejima H, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol 2009; 54: 130–138. [DOI] [PubMed] [Google Scholar]
- 7. Maekawa Y, Anzai T, Yoshikawa T, et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction: A possible role for left ventricular remodeling. J Am Coll Cardiol 2002; 39: 241–246. [DOI] [PubMed] [Google Scholar]
- 8. Van derLaan AM, Ter Horst EN, Delewi R, et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J 2014; 35: 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leuschner F, Panizzi P, Chico-Calero I, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res 2010; 107: 1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bai F, Pang XF, Zhang LH, et al. Angiotensin II AT1 receptor alters ACE2 activity, eNOS expression and CD44-hyaluronan interaction in rats with hypertension and myocardial fibrosis. Life Sci 2016; 153: 141–152. [DOI] [PubMed] [Google Scholar]
- 11. US National Institute of Health. The guide for the care of use of laboratory animals. 8th edition, revised US National Institute of Health, 2011. [Google Scholar]
- 12. Dai Z, Aoki T, Fukumoto Y, et al. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J Cardiol 2012; 60: 416–421. [DOI] [PubMed] [Google Scholar]
- 13. Tsukamoto Y, Mano T, Sakata Y, et al. A novel heart failure mice model of hypertensive heart disease by angiotensin II infusion, nephrectomy, and salt loading. Am J Physiol Heart Circ Physiol 2013; 305: H1658–H1667. [DOI] [PubMed] [Google Scholar]
- 14. Masuzawa T, Fukui Y, Smith NT. Cardiovascular simulation using a multiple modeling method on a digital computer–simulation of interaction between the cardiovascular system and angiotensin II. J Clin Monit 1992; 8: 50–58. [DOI] [PubMed] [Google Scholar]
- 15. Serneri GG, Boddi M, Cecioni I, et al. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res 2001; 88: 961–968. [DOI] [PubMed] [Google Scholar]
- 16. Gomolak JR, Didion SP. Angiotensin II-induced endothelial dysfunction is temporally linked with increases in interleukin-6 and vascular macrophage accumulation. Front Physiol 2014; 5: e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muñoz-Durango N, Fuentes CA, Castillo AE, et al. Role of the renin-angiotensin-aldosterone system beyond blood pressure regulation: Molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int J Mol Sci 2016; 17: e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han YL, Li YL, Jia LX, et al. Reciprocal interaction between macrophages and T cells stimulates IFN-γ and MCP-1 production in Ang II-induced cardiac inflammation and fibrosis. PLoS One 2012; 7: e35506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sui X, Wei H, Wang D. Novel mechanism of cardiac protection by valsartan: Synergetic roles of TGF-β1 and HIF-1α in Ang II-mediated fibrosis after myocardial infarction. J Cell Mol Med 2015; 19: 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jugdutt BI, Idikio H, Uwiera RR. Angiotensin receptor blockade and angiotensin-converting-enzyme inhibition limit adverse remodeling of infarct zone collagens and global diastolic dysfunction during healing after reperfused ST-elevation myocardial infarction. Mol Cell Biochem 2007; 303: 27–38. [DOI] [PubMed] [Google Scholar]
- 21. Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res 2013; 112: 1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol 2015; 35: 1066–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barrett JP, Minogue AM, Falvey A, et al. Involvement of IGF-1 and Akt in M1/M2 activation state in bone marrow-derived macrophages. Exp Cell Res 2015; 335: 258–268. [DOI] [PubMed] [Google Scholar]
- 24. Sepúlveda DE, Cabeza Meckert P, Locatelli P, et al. Activated macrophages as a feeder layer for growth of resident cardiac progenitor cells. Cytotechnology 2016; 68: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ligresti G, Aplin AC, Zorzi P, et al. Macrophage-derived tumor necrosis factor-alpha is an early component of the molecular cascade leading to angiogenesis in response to aortic injury. Arterioscler Thromb Vasc Biol 2011; 31: 1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clozel M, Kuhn H, Hefti F, et al. Endothelial dysfunction and subendothelial monocyte macrophages in hypertension. Effect of angiotensin converting enzyme inhibition. Hypertension 1991; 18: 132–141. [DOI] [PubMed] [Google Scholar]
- 27. Thang LV, Demel SL, Crawford R, et al. Macrophage depletion lowers blood pressure and restores sympathetic nerve α2-adrenergic receptor function in mesenteric arteries of DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 2015; 309: H1186–H1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Filho AG, Kinote A, Pereira DJ, et al. Infliximab prevents increased systolic blood pressure and upregulates the AKT/eNOS pathway in the aorta of spontaneously hypertensive rats. Eur J Pharmacol 2013; 700: 201–209. [DOI] [PubMed] [Google Scholar]
- 29. Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease. Circulation 2006; 113: 1708–1714. [DOI] [PubMed] [Google Scholar]
- 30. Buchwalow IB, Podzuweit T, Bocker W, et al. Vascular smooth muscle and nitric oxide synthase. FASEB J 2002; 16: 500–508. [DOI] [PubMed] [Google Scholar]
- 31. Ando H, Zhou J, Macova M, et al. Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke 2004; 35: 1726–1731. [DOI] [PubMed] [Google Scholar]
- 32. Arriero MM, Rodríguez-Feo JA, Celdrán A, et al. Expression of endothelial nitric oxide synthase in human peritoneal tissue: Regulation by Escherichia coli lipopolysaccharide. J Am Soc Nephrol 2000; 11: 1848–1856. [DOI] [PubMed] [Google Scholar]
- 33. Valerio A, Cardile A, Cozzi V, et al. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest 2006; 116: 2791–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ali A, Alghasham A, Ismail H, et al. ACE I/D and eNOS E298D gene polymorphisms in Saudi subjects with hypertension. J Renin Angiotensin Aldosterone Syst 2013; 14: 348–353. [DOI] [PubMed] [Google Scholar]
- 35. Zhang LH, Pang XF, Bai F, et al. Preservation of glucagon-like peptide-1 level attenuates angiotensin II-induced tissue fibrosis by altering AT1/AT2 receptor expression and angiotensin-converting enzyme 2 activity in rat heart. Cardiovasc Drugs Ther 2015; 29: 243–255. [DOI] [PubMed] [Google Scholar]
- 36. Drapala A, Sikora M, Ufnal M. Statins, the renin-angiotensin-aldosterone system and hypertension – a tale of another beneficial effect of statins. J Renin Angiotensin Aldosterone Syst 2014; 15: 250–258. [DOI] [PubMed] [Google Scholar]
- 37. Simões E, Silva AC, Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1–7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res 2016; 107: 154–162. [DOI] [PubMed] [Google Scholar]
- 38. Mercure C, Yogi A, Callera GE, et al. Angiotensin(1–7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res 2008; 103: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 39. Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood 2009; 114: 2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel NY, Chilsen AM, Mathiason MA, et al. Outcomes and complications after splenectomy for hematologic disorders. Am J Surg 2012; 204: 1014–1019. [DOI] [PubMed] [Google Scholar]