Abstract
Mouse models of Rett syndrome, with targeted mutations in the Mecp2 gene, show a high degree of phenotypic consistency with the clinical syndrome. In addition to severe and age-specific regression in motor and cognitive abilities, a variety of studies have demonstrated that Mecp2 mutant mice display impaired social behavior. Conversely, other studies indicate complex enhancements of social behavior in Mecp2 mutant mice. Since social behavior is a complicated accumulation of constructs, we performed a series of classic and refined social behavior tasks and revealed a relatively consistent pattern of enhanced pro-social behavior in hypomorphic Mecp2308/Y mutant mice. Analyses of repetitive motor acts, and cognitive stereotypy did not reveal any profound differences due to genotype. Taken together, these results suggest that the mutations associated with Rett syndrome are not necessarily associated with autism-relevant social impairment in mice. However, this gene may be a valuable candidate for revealing basic mechanisms of affiliative behavior.
Keywords: Methyl-CpG Binding Protein 2, Rett Syndrome, Autism, Sociability, Mouse Model, Stereotypy
1.0 Introduction
Mutations of the MECP2 gene underlie the majority of cases of Rett syndrome (Amir et al. 1999; Bienvenu et al. 2000). Investigations are revealing the complex role this gene plays in intra- and inter-generational epigenetic regulation in non-disease states, and in somatic and psychiatric disorders. The gene’s protein product, methyl-CpG binding protein 2 (MeCP2), plays a prominent role in gene regulation due to its recognition of promoter-specific methylated CpG sites, and its subsequent interactions with co-repressors (Chahrour et al. 2008; Jørgensen and Bird, 2002; Lewis et al. 1992; Samaco et al. 2009), as well as its influences on alternative splicing of mRNA (Young et al. 2005) and microRNA (Wu et al. 2010). Females with MECP2 mutations display a wide range of symptom severity, due to patterns of X-chromosome inactivation (XCI), which in-turn leads to complications in the clinical characterization as well as the generation of informative mouse models. Mutations of MECP2 were previously considered embryonic lethal for hemizygous males, but recently, MECP2 mutations have been noted in human males and are associated with mental retardation, movement abnormalities, and other related symptoms (Clayton-Smith et al. 2000; Dotti et al. 2002; Kleefstra et al. 2002; Orrico et al. 2000). Evidence is accumulating that partial loss-of function mutations of MECP2 may contribute to neurodevelopmental and social disorders (Samaco et al. 2008).
Rett syndrome is characterized by normal early development, followed by a pronounced regression at about 6-18 months in age with loss of verbal functions, epilepsy, gastro-intestinal problems, stereotypies, and severe musculo-skeletal deformations (Hagberg et al. 1983). Rett syndrome is classified as a pervasive developmental disorder and is often considered an Autism Spectrum Disorder (ASD) due to impairments in social communication and presence of restricted-repetitive behavior (American Psychiatric Association, 2000). Although Rett syndrome is comparatively rare (approximately 1:10,000-15,000, Hagberg et al. 1985) compared to ASD (1:110, Centers for Disease Control and Prevention, 2009) there has been an upsurge of preclinical studies of single-gene and other ASD candidate mouse models under the ultimate impetus to reveal the neural genetics and molecular signature of developmental disorders affecting social behavior. These mouse models may play an important role in elucidating some of the biology underlying ASD.
A variety of mutant Mecp2 mice have been engineered to study the pathophysiology resulting from mutations in this gene (Ricceri et al. 2008). Newer cell and brain region-specific null mice are proving valuable in the characterization of pathways responsible for the phenotypic alterations associate with normal and perturbed Mecp2 expression. Global, whole gene null Mecp2 male mice exhibit severe symptoms such as seizures and reduced survivability (Chen et al. 2001; Guy et al. 2001; Pelka et al. 2006). Truncated and conditional knockouts show a range of symptom severity, age of onset, and mortality (see Calfa et al, 2011 for an exhaustive review).
Behavioral investigations have revealed an apparent decrease in social interactions indexed by impaired nest building and social avoidance of an unfamiliar stimulus mouse behind a partition within the home cage in Mecp2308/Y truncated protein mutants (Moretti et al. 2005). Gemelli et al. (2006) demonstrated that conditional postnatal knockout Mecp2 mice spent less time interacting with an unfamiliar mouse located behind a wire mesh enclosure in an unfamiliar arena, relative to wild-type littermates. Amygdala-specific siRNA knockdown of Mecp2 did not influence three-chamber social approach behaviors but impaired juvenile play behaviors in male rats (Kurian et al. 2008). With respect to social communication, Mecp2308/Y mutant mouse pups show impaired ultrasonic vocalization calls upon separation from their mother (De Filippis et al. 2010). These reductions in social behaviors in Mecp2 mutants have not been attributable to impaired social recognition abilities (Ricceri et al. 2008).
In contrast to these findings, a number of recent studies suggest that Mecp2 mutations in rodent models may be associated with increased sociability. Three-chamber social approach behavior was shown to be enhanced rather than decreased in the Mecp21lox mutant (Schaevitz et al. 2010). Similarly, Mecp2 conditional knockout mice (Sim1 –cre BAC transgenics) and their controls (Mecp2flox/+), who also show reduced Mecp2 expression, both show increased sociability as indexed by more proportional time in proximity to familiar and unfamiliar stimulus mice behind a wire-mesh enclosure (Fyffe et al. 2008; Samaco et al. 2008, 2009). A recent report has indicated that GABA-ergic neuron Mecp2 mutants show enhanced social interactions in the partition test and in a modified three-chamber task (Chao et al. 2010). Finally, Mecp2lox/Y hypomorphs have similar social approach as that of wild types, but show an apparent augmentation of social-novelty preference (Kerr et al. 2008). Table I provides an overview of studies revealing alterations in social behavior in targeted mutations of Mecp2 in rodents. Studies of Mecp2 mutant mice with no social behavior analysis were not included. These studies indicate that, at a minimum, Mecp2 may be an important gene for the investigation of mechanisms underlying social behaviors.
Table 1.
Review of Mecp2 in social behaviour
|
|
||||
|---|---|---|---|---|
| Mutant | Region/Cell | Background | Reduced/Impaired | Reference |
| MeCP2308/y | Constitutive Truncation | 129SvEv or 129SvEv × C57BL/6J | Tube Test Avoidance | Shahbazian et al. 2002 |
| MeCP2308/y | Constitutive Truncation | 129/SvEv | Tube Test Avoidance; Nest Building; Divided Homecage Proximity; Interaction with Unfamiliar Juvenile | Moretti et al. 2005 |
| MeCP2CKO | Forebrain Conditional | B6 | Divided Homecage Proximity | Gemelli et al. 2006 |
| Rat siRNA | Amygdala | Sprague Dawley | Juvenile Play | Kurian et al. 2008 |
| Mecp2Flox/y | CNS-Conditional Flanked Hypomorph | 129S6/B6 | Nest Building | Samaco et al. 2008 |
| MeCP2308/y | Constitutive Truncation | B6 | USVs Maternal Separation | De Fillipis et al. 2010 |
| MeCP2CKO | GABA Neurons | 129;FVB | Nest Building | Chao et al. 2010 |
|
|
||||
| Mutant | Region/Cell | Background | Increased/Enhanced | Reference |
|
| ||||
| MeCP2lox/y | Constitutive Flanked Hypomorph | 129S1/SvImJ;B6/CBA | Three Chamber Social Preference and Novelty Preference | Kerr et al. 2008 |
| Mecp2Flox/y | CNS-Conditional Flanked Hypomorph | 129S6/B6 | Proximity in Partition Test | Samaco et al. 2008 |
| MeCP2flox/+, and MeCP2CKO | Flanked WT, and Hypothalamus | 129SvEV/B6 | Proximity in Partition Test# | Fyffe et al. 2008 |
| MeCP2flox/y, and MeCP2CKO | Flanked WT, DA/NE- and 5-HT-Neurons | 129S6SvEV (flox), 129;FVB (DA/NE), 129;B6 (5-HT) | Proximity in Partition Test# | Samaco et al. 2009 |
| MeCP2flox, and MeCP2CKO | Flanked WT, GABA-, and FB GABA Neurons | 129S6SvEV (flox), 129;FVB (GABA) | Proximity in Partition Test; Three-Chamber Social Preference | Chao et al. 2010 |
| MeCP21lox | CNS-Conditional | B6 | Three-Chamber Social Preference and Social Novelty Preference | Schaevitz et al. 2010 |
Signifies accompanying aggression in mutant
The purpose of the current study was to perform analyses of social behavior with attempts to differentiate pro-social from aggressive or sexual motivations in social interactions. We undertook a battery of behavioral tests in male Mecp2 wild type and their hemizygous mutant littermates. The Mecp2308/Y mouse exhibits the same Rett syndrome-like abnormalities as the whole gene knockouts, but with improved background strain survivability and a later age of symptom onset (Shahbazian et al. 2002). This permitted the assessment of adult mice prior to the onset of severe sleep, neurological, and musculo-skeletal deficits. The tests used were designed to reveal subtle and overt distinctions in social interactions and repetitive behavior, which have proven to be relevant variables for mouse models of neurodevelopmental disorders (Arakawa et al. 2007; Defensor et al. 2011; Moy et al. 2004; Pearson et al. 2010, 2011; Pobbe et al. 2010).
2.0 Materials and Methods
2.1 Experimental Subjects
Experimental animals were bred from C57BL/6J-backcrossed stock obtained from The Jackson Laboratory (B6.129S-Mecp2tm1Hzo/J, stock # 005439) and bred from heterozygous mutant dams and hemizygous father sibling pairs. Stimulus mice used for social behavior tests were adult CD-1 mice bred in-house from stock obtained from Charles River Labs, and C57BL/6J (B6) mice bred from stock obtained from The Jackson Laboratory. Mutant mouse genotype was determined according to the PCR parameters obtained from The Jackson Laboratory with purified DNA collected from tail biopsy after weaning at post-natal day 25. Since Mecp2 is an X-linked gene, only wild type (y/+) and hemizygous (y/−) males were obtained and compared in behavioral testing. Mice were housed with up to five same-sex littermates under a 12-h light/dark schedule with lights on at 0600h. Mice had ad libitum access to tap water and lab rodent diet. Mice were 10-13 weeks old at the beginning of behavioral studies. All procedures were performed according to protocols approved by the University of Hawaii Laboratory Animal Service Institutional Animal Care and Use Committee.
2.2 Experimental Design
In total, nine mice per genotype were used as subjects. Mice were subjected to a series of behavioral tests in a sequence intended to prioritize both the accumulating effects of multiple testing and the influence of housing and experience on the measures. All mice were weighed prior to the first behavioral test, and weighed again after testing for urinary scent marking to determine baseline weight differences and weight gain trajectories. One mutant animal showing degenerative features, severe weight loss and seizures and was euthanized after the scent marking test; its data were excluded from scent marking scores. No other animals showed complications during testing. One mutant Visible Burrow System (VBS) colony (n=3) was not included in analyses due to a recorder error. Animals were moved to the behavioral testing room at least 30 minutes prior to testing and all arenas and equipment were cleaned with 70% ethanol and dried completely between mice. Unless otherwise noted, testing was performed under ambient fluorescent lighting between 0900 and 1800 hours. Temperature (22±1°C) and humidity (50-70%) were controlled in the experimental room.
2.2.1 Visible Burrow System (VBS)
Three days prior to behavioral testing, mice were anesthetized with an IP injection of 12.5mg/ml of Avertin (2,2,2-Tribromoethanol, Sigma-Aldrich, St. Louis, MO) at a 336 mg/kg dose. Once mice were unresponsive, peroxide based hair bleach was applied to the dorsal coat of each mouse so they were distinguishable in videotape analysis. Mice were allowed to recover two days prior to being placed in the VBS according to procedures outlined previously (Pobbe et al. 2010). Briefly, each colony was housed in a rectangular, galvanized metal bin, 86 × 61 × 26 cm (H). The colony arena consisted of three chambers, each 12 × 7 × 6 cm (H), which were positioned behind a barrier wall extending across a short width (61 cm) of the bin, 30 cm from the end wall. This wall separated an open surface area (30 × 61 × 26cm [H]) from the chambers in the other compartment. These chambers were connected to the wall via clear Plexiglas tubes 5 cm in diameter. Two of the three chambers, each connected to the surface area via a Z shaped tube, were connected to each other via a straight clear Plexiglas tube. The third chamber was connected only to the surface via a straight tube. The animals could pass freely between each chamber and the surface area, or between the two connected chambers, by these tubes. The experimental room was maintained on a 12-h light/dark cycle (lights on at 0600h), being illuminated by fluorescent lamps during the light period and by infrared light during the dark phase. An assistant who was blind to genotype scored VBS videotapes by sub-sampling 30 second intervals every 10 minutes for the first 4 hours of the dark and light phases. The observer manually recorded all occurrences of Approach Front, Approach Rear, Flight, Chase/Follow, Vigorous behavior, Autogroom, Allogroom, and Huddle. Approach [to the] Front/Back of another animal was defined in terms of a line bisecting the approached mouse, perpendicular to the long axis of its body. Flight was defined as rapid locomotion away from an approaching animal; Chase/Follow was rapid locomotion toward another animal, or a slow approach toward an animal that was moving away. Vigorous behavior was high-intensity chase, bite, or attack. Autogrooming was lick or rub self while Allogrooming is lick or rub with paws of another animal. Huddle was characterized by a mouse lying in contact with another animal for more than 10 seconds of the 30-second time sample. Data were summarized separately for the dark and light phases, and the mean frequency of each behavior for each genotype was compared across the three days of testing.
2.2.2 Three-Chamber Social Approach
Twenty-four hours after removal from the VBS, mice were tested for social approach behavior in the three chamber apparatus, which was constructed according to published studies (Moy et al. 2004). Initially, mice are placed into the center of the divided 41 × 70 × 28 cm (H) apparatus, which contained two empty, inverted wire cups (Galaxy Pencil/Utility Cup, Spectrum Diversified Designs, Inc., Streetsboro, OH). Empty glass jars of the same diameter were placed on top of the base of the wire cup to prevent movement of the enclosures, or escape by stimulus mice. For the habituation phase, the sliding doors were elevated and the mouse was permitted ten minutes to explore the three chambers. At the end of the ten minute habituation session, mice were placed back into the middle of the apparatus, the sliding doors were lowered, and an unfamiliar adult (57-83 day old) male CD-1 mouse was placed into one of two outer wire cups, and the doors were again lifted and the mouse was permitted to explore the entire apparatus for ten minutes; this constituted the sociability phase. The time spent in each compartment during both sessions was collected in real-time with two stopwatches by a single observer who was blind to the genotype of the subject. During both the habituation and sociability phases, cameras were mounted in front of both outer compartments and connected to a DVD recorder. The frequency and duration of Rear, Autogroom, Contact (with the stimulus cup), Sniff, Stretch-Attend, Quick-Withdraw, and Nose-to-Nose were scored off-line using Noldus Observer software (Noldus Information Technology, Wageningen, The Netherlands) for each of the two outer compartments during the sociability phase for each subject.
2.2.3 Autogrooming
Detailed grooming analyses were performed as previously described (Pearson et al. 2011). Mice were placed in a 14 × 7 × 30 (H) cm Plexiglas chamber for 20 minutes under normal fluorescent lighting. An aluminum lid that permitted air circulation, but prevented escape, was placed over the top. Two digital cameras were used to collect video from the front and side aspect so that the mouse’s grooming behavior was always visible. Videotapes were scored using Noldus software for the frequency and duration of Paw Licking, Head Washing, Body Grooming, Leg Licking, and Tail/Genital grooming. In addition to the collection of frequency and duration of body directed grooming, the following variables were determined according to Kalueff et al. (2007). A Bout was defined as at least one episode of any category of grooming, or an uninterrupted sequence of grooming types. Bouts were divided by at least 6 seconds of inactivity or by an activity other than grooming. An Interrupted Bout was defined as a grooming bout that is interrupted by less than 6 seconds; the proportion of bouts that were interrupted was calculated as (interrupted bouts/total bouts)*100. Transitions were transfers between regional grooming subtypes. Incorrect transitions were transfers, which did not follow the cephalo-caudal progression (0-No Grooming, 1-Paw Licking, 2-Head Wash, 3-Body Groom, 4-Leg Licking, 5-Tail/Genital). Proportion of incorrect transitions was calculated as (incorrect transitions/total transitions)*100.
2.2.4 Repetitive Novel Object Contact Task/Locomotion
On the day following the autogrooming analyses, mice were relocated to the behavior room at least 30 minutes before habituation and testing for the repetitive novel object contact task (Pearson et al. 2011). The habituation session consisted of placing a mouse in a clean standard mouse cage containing 1cm of bedding. No lid, water bottle, or food hopper was present. A micro-isolator lid was modified by removing the filter element and frame, and thick gauge wire bisecting both horizontal planes of the lid were added which divided the overhead image into four equal sized compartments. The number of transitions between quadrants and amount of time spent within each quadrant was scored for a 10-minute habituation session under normal fluorescent illumination using Noldus Observer software. This permitted assessment of any baseline differences in motor activation or exploration tendencies of the genotypes.
On the following day, and at the same time of day as the prior habituation session, mice were placed into the same apparatus which contained four small plastic objects (a multicolored 3 cm long arrangement of Lego blocks, a green 4 cm long jacks piece, a 1.5 cm3 multicolored die, and a 3.5 cm long white and red bowling pin) arranged 2 cm from the four corners. Video was collected for ten minutes and all instances of investigation of the objects, and the order in which they were investigated, were scored offline. Investigation was defined as clear facial or vibrissae contact with or burying of the novel objects; merely passing or pausing by an object was insufficient for investigation. The occurrence of repetitive contact with three and four toys and the frequency of times that the mice buried each object were counted. Total frequency of contact with each of the four toys, and the total number of burying episodes were also calculated. In order to determine if there was a strain effect on the tendency to display preferences for particular toys, the frequencies of contact with each object were ranked in decreasing order from maximum to minimum preference (contact) values for each subject, and the frequencies were averaged by genotype and compared.
2.2.5 Social Proximity
Social proximity testing was conducted in a clear rectangular chamber (7 ×14 × 30 cm H) constructed of acrylic plastic according to previously reported parameters (Defensor et al. 2011). The dimensions of this arena were identical to that used for analyses of individual autogrooming behavior. For testing, the subject mouse and an unfamiliar 5-7 month old male B6 mouse were placed into the chamber simultaneously and an aluminum lid was placed over the top to prevent escape. Video from two cameras providing front and side views was transferred to a video merge processor, which combined both channels into a single side-by-side output. The availability of both views aided in the discrimination of behaviors by reducing occlusion of one animal from view by the other. The output from the video processor displaying both the front and side view was transmitted to a DVD recorder for storage and subsequent analysis. The frequencies of the following behaviors were manually quantified by an observer blind to the subject’s genotype:
Nose-to-Nose- subject’s nose tip and/or vibrissae contact the nose tip and/or vibrissae of the other mouse.
Nose-to-Face- subject’s nose tip contacts the head of the stimulus mouse.
Nose-to-Anus- subject’s nose or vibrissae contacts the base of the tail or anogenital region of the other mouse.
Crawl Over- subject’s forelimbs cross the midline of the dorsal surface of the other mouse.
Crawl Under- subject’s head goes under the ventral surface of the other mouse to a depth of at least the ears of the subject animal crossing the midline of the other mouse’s body.
Upright- subject displays a reared posture oriented towards the other mouse with head and/or vibrissae contact.
2.2.6 Urinary Scent Marking
Baseline scent marking in a divided arena was characterized on the first day of testing, after 7 days of single housing. Previous studies have demonstrated that single housing is critical to establishing motivation to engage in detectable scent marking (Arakawa et al. 2009). The scent-marking arena was an inverted rat cage with a steel mesh divider wall installed to bisect the arena. This apparatus was placed on top of a 30 × 45 cm section of drawing paper, and the subject mouse was placed on one side for a 20-minute baseline session. Twenty-four hours later, the mouse was tested for urinary scent marking to an unfamiliar CD-1 (91-103 days old) mouse stimulus placed immediately before in the opposite half of the arena. At the end of the baseline and social marking sessions the mice were removed and the number of fecal boli counted. The placement of the compartments was marked and the paper was allowed to dry overnight. The paper was then fixed and stained with a 6% solution of ninhydrin (Fisher) in methanol and dried. To quantify the amount of urinary scent marking, a 1×1 cm printed transparency grid was placed over the paper and the number of squares containing a stained mark was counted manually by an assistant blind to the genotype of the subject.
2.2.7 Resident Intruder
An unfamiliar, ten month old intruder B6 mouse was introduced into a resident Mecp2 mutant or wild type home cage, and the interactions between those animals were recorded for five minutes. The latency to and duration of agonistic attack by the resident was scored. Additionally, the frequency and duration of the following behaviors was quantified: Face Sniff, Body Sniff, Anogenital Sniff, Tail Sniff, Vigorous Allogroom, and Following. Mice analyzed in this test, having already been assessed in all of the previous behavioral tasks were then euthanized by CO2 inhalation.
2.3 Statistical Analyses
Two-way repeated-measures analyses of variance (ANOVA) were performed to compare body weights. Genotype was the between-subjects factor and the day of weight measurement was the within-subjects repeated-measures factor. Two-way repeated-measures ANOVAs were performed for data obtained from the VBS, with genotype as a between-subjects factor, and day as the repeated-measures, within-subjects factor. Separate analyses were performed across means from the dark and light phases. Unpaired t-tests were performed to compare the mean time spent in the empty and stimulus chambers of the three chamber task, and to compare the number of entries. Two-way repeated measures ANOVAs were applied to assess any differences in the amount of time spent in each of the four compartments during the habituation phase of the repetitive novel object investigation task and to compare the number of scent marks during both scent marking conditions. For all ANOVA tests Bonferroni post-hoc analyses were conducted to reveal any significant effects of genotype across the within-subjects factor when significant main effects or interactions resulted. Unpaired t-tests were performed to compare average frequencies and durations of each behavior category in the autogrooming analysis, the social proximity task, the resident-intruder test, and the number of lines crossed during the habituation phase of the repetitive novel object contact task. Similarly, for the Three-Chamber Social Approach Task, the mean frequency and duration of Stretch-Attend, Quick Withdraw, and Nose-to-Nose for each genotype was compared with unpaired t-tests. When assumptions were violated for these comparisons (non-normal distributions or unequal variances), nonparametric Mann-Whitney U tests were performed instead. Statistica (v.6) and GraphPad PRISM (v.4) software programs were used for statistical analyses and figures.
3.0 Results
3.1 Body Weight
All mice were weighed before anesthetization for fur bleaching and on the second day of scent marking. Mean ±1 S.E.M. body weights before the VBS are as follows: wild-type mice weighed 23.216 ± 0.553 g while mutant mice weighed 25.953 ± 0.691 g. After the second scent marking session, wild-type mice weighed 22.722 ± 0.496 g and hemizygous mice weighed 23.611 ± 1.013 g. A two-way repeated measures ANOVA revealed no significant main effect of Genotype [F(1,16)=3.635, p=0.075], but a significant main effect for Day [F(1,16)=16.21, p=0.001] and a significant Genotype × Day interaction [F(1,16)=6.892, p=0.0184, data not shown]. Post-hoc tests failed to indicate any significant differences between genotypes within each day of weight measurement. The significant interaction indicates that hemizygous mice weighed more at the beginning of experimentation, but no significant weight differences were noted towards the end of behavioral assessments.
3.2 VBS
Table II provides statistics for two-way repeated measures ANOVA for VBS colonies for each behavior during light and dark phases. Figure 1 displays Mean ± 1 S.E.M frequencies of all behaviors in the VBS across the dark (Figure 1a,b) and light (Figure 1c,d) phases for hemizygous and wild-type Mecp2 mice. Significant genotype differences were of particular note. During the light phase, mutant mice engaged in higher rates of frontal-oriented approaches and they also made fewer rear-oriented approaches during the dark period. Hemizygous mice had lower frequencies of autogrooming during the light phase, with significantly more allogrooming in the dark phase. Finally, hemizygous males showed increased rates of huddling in the dark phase.
Table 2.
Mecp2 VBS
| Behavior | Main Effects | Interaction | ||
|---|---|---|---|---|
|
| ||||
| Phase | Genotype | Day | Genotype × Day | |
| Approach Front | Dark | F(1,26)=0.84, p=0.376 n.s. | F(2,26)=11.58, p=0.003** | F(2,26)=0.52, p=0.603 n.s. |
| Light | F(1,32)=13.88, p=0.0018** | F(2,32)=1.43, p=0.254 n.s. | F(2,32)=1.43, p=0.254 n.s. | |
| Approach Rear | Dark | F(1,26)=5.47, p=0.036* | F(2,26)= 0.41, p=0.669 n.s. | F(2,26)=0.76, p=0.478 n.s. |
| Light | F(1,32)=2.84, p=0.112 n.s. | F(2,32)=3.58, p=0.040* | F(2,32)=2.07, p=0.143 n.s. | |
| Flight | Dark | F(1,26)=1.30, p=0.276 n.s. | F(2,26)=1.49, p=0.243 n.s. | F(2,26)=2.05, p=0.149 n.s. |
| Light | F(1,32)=0.55, p=0.468 n.s. | F(2,32)=1.14, p=0.332 n.s. | F(2,32)=1.14, p=0.332 n.s. | |
| Chase/Follow | Dark | F(1,26)=1.50, p=0.243 n.s. | F(2,26)=1.68, p=0.205 n.s. | F(2,26)=0.57, p=0.570 n.s. |
| Light | F(1,32)=0.08, p=0.780 n.s. | F(2,32)=3.22, p=0.053 n.s. | F(2,32)=1.50, p=0.239 n.s. | |
| Autogroom | Dark | F(1,26)=0.17, p=0.687 n.s. | F(2,26)= 3.94, p=0.032* | F(2,26)=0.02, p=0.976 n.s. |
| Light | F(1,32)=6.34, p=0.023* | F(2,32)=4.31, p=0.022* | F(2,32)=0.72, p=0.496 n.s. | |
| Allogroom | Dark | F(1,26)=9.74, p=0.008** | F(2,26)= 2.20, p=0.131 n.s. | F(2,26)=1.59, p=0.224 n.s. |
| Light | F(1,32)=2.42, p=0.140 n.s. | F(2,32)=1.99, p=0.154 n.s. | F(2,32)= 0.97, p=0.389 n.s. | |
| Huddle | Dark | F(1,26)=36.77, p<0.0001*** | F(2,26)= 45.20, p<0.0001*** | F(2,26)=4.21, p=0.026* |
| Light | F(1,32)=0.47, p=0.505 n.s. | F(2,32)=1.92, 0.163 n.s. | F(2,32)=1.24, p=0.303 n.s. | |
| Vigorous | Dark | F(1,26)=0.65, p=0.435 n.s. | F(2,26)=0.65, p=0.530 n.s. | F(2,26)=0.65, p=0.530 n.s. |
| Light | – | – | – | |
Figure 1. Mecp2 mutant mice show increased affiliation in the VBS.
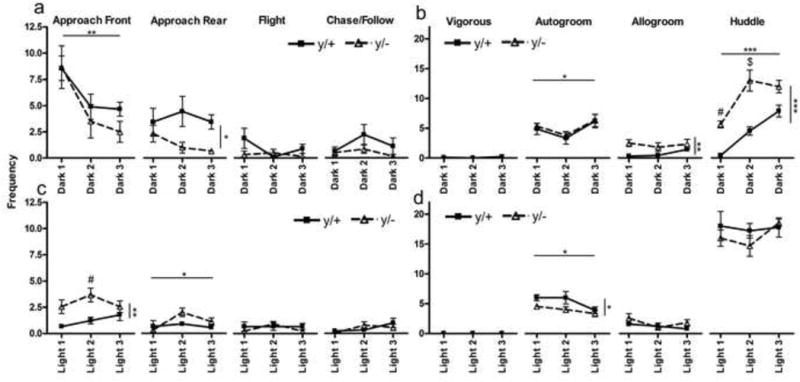
(a) and (b) display the frequencies of each of the eight behaviors scored during 24 scans across the first four hours of three consecutive dark phases and (c) and (d) display the same behaviors across three consecutive light phases. Horizontal lines indicate a significant main effect of Day, and vertical lines indicate a significant Genotype main effect. y/+ denotes wild-type and y/− are hemizygous mutant mice. * p<0.05; ** p<0.01, *** p<0.0001. Symbols above individual means indicate a significant post-hoc effect: # p<0.05; $ p<0.001. Wild-type (y/+) N=9, mutant (y/−) N=6.
3.3.1 Three-Chamber Social Approach: Chamber Durations
Mice displayed no significant side preferences in the habituation phase [WT, t=1.529, p=0.146; KO, t=−0.910, p=0.376, data not shown]. Figure 2a displays the amount of time spent in each compartment of the three-chamber social approach arena. Duration of time spent in the center compartment is displayed for reference but was not included in analyses. Unpaired t-tests indicated that both wild type and mutant males spent a greater amount of time in the chamber associated with the unfamiliar mouse (t= 2.217, p=0.042; t=4.346, p=0.0005, respectively) in the sociability phase. There were no significant genotype differences in the number of entries into the chambers (Figure 2b).
Figure 2. Three-Chamber Sociability in Mecp2 mutant (y/−) and wild-type (y/+) littermates.
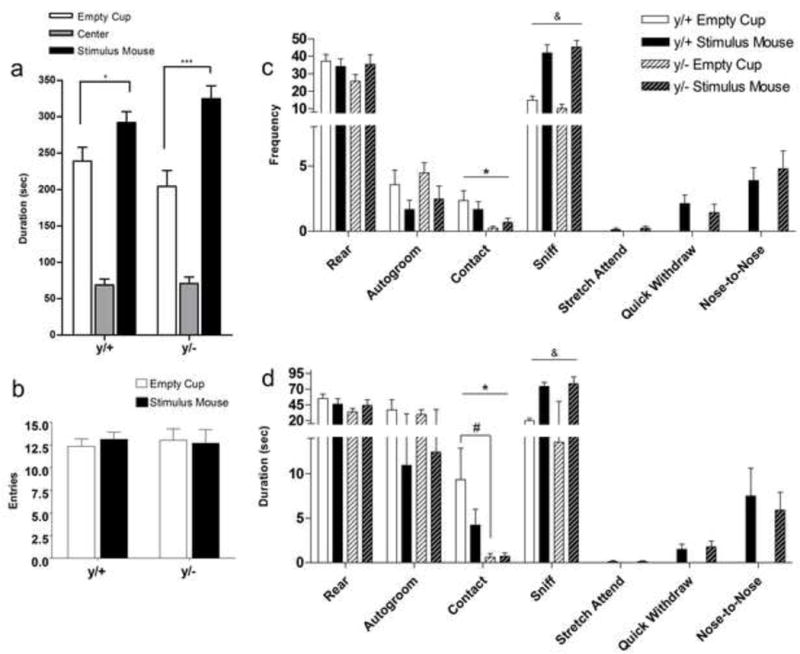
Both wild-type (y/+) and mutant mice (y/−) display increased duration of time in the chamber containing an unfamiliar mouse compared to an empty cup (a). Wild type and mutant mice did not differ in the number of entries into the outer compartments of the sociability phase of the three chamber task (b). Frequency (c) and duration (d) of behaviors in each of the two outer stimulus compartments indicate that mutant mice have lower durations of contact with the cup in either chamber. Horizontal lines above columns indicate significant main effects for Genotype or Stimulus. Asterisks (*) indicate significant Genotype main effects. Stimulus main effects are denoted by an ampersand (&). * p<0.05, *** p<0.001, & p<0.001. Post hoc tests: # p<0.05. N=9/group.
3.3.2 Three-Chamber Social Approach: Behavioral Analysis
Figure 2 presents the frequency (Figure 2c) and duration (Figure 2d) of behaviors scored during the sociability phase of the three-chamber task. Significant main effects for Genotype for frequency and duration of contact [frequency: F(1,16)=6.52, p=0.021; duration: F(1,16)=6.768, p=0.019] were found. Bonferroni post-hoc tests indicated a significant genotype difference in the duration of contact with the empty cup (t=2.626, p<0.05); mutant males displayed a reduced duration. There was also a significant Stimulus main effect for the frequency and duration of sniffing [frequency: F(1,16)=69.32, p<0.0001; duration: F(1,16)=60.05, p<0.0001]. No other statistically significant main differences were found.
3.4 Autogrooming
Hemizygous mice showed a significantly increased frequency of tail/genital grooming [t(16)=−2.7, p=0.016] and increased duration of paw licking [t(16)=−4.074, p=0.0009] relative to wild-type littermates (Figure 3a,b). No statistically significant differences in the frequency or duration of other autogrooming sub-types were found. Similarly, no significant differences in the number of bouts, interrupted bouts, proportion of interrupted bouts, transitions between grooming stages, incorrect transitions, and proportion of incorrect transitions were found (Figure 3c).
Figure 3. Slight alterations in patterns of autogrooming in Mecp2 mutant mice.
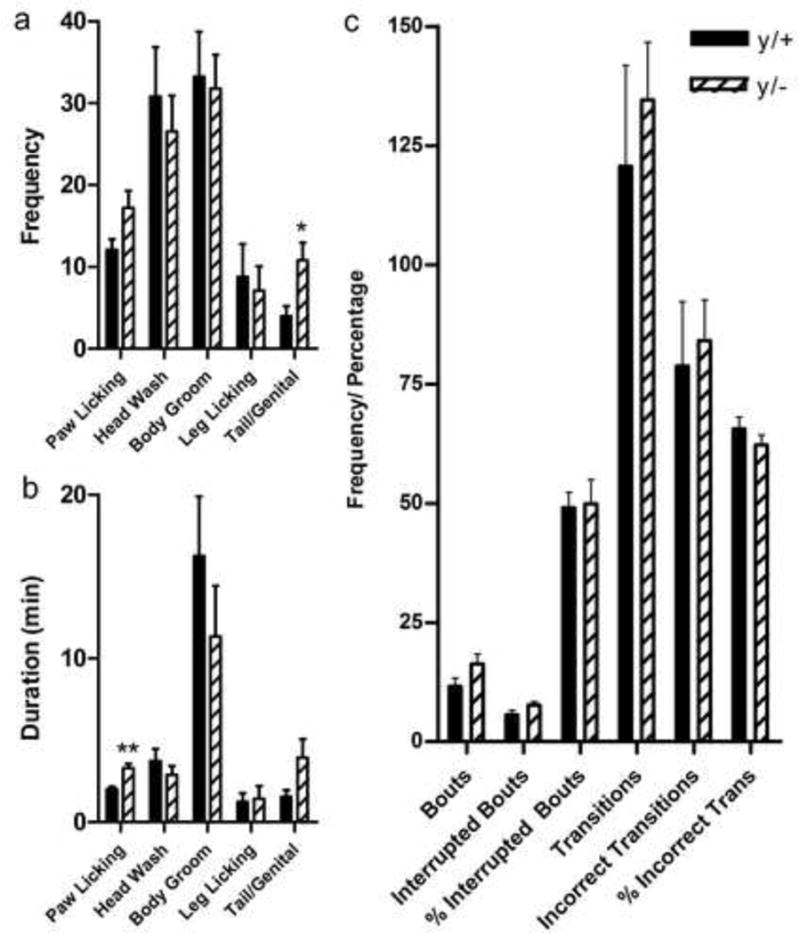
Frequency (a) and duration (b) of body site-specific patterns of grooming. MeCp2 mutant mice (y/−) show increased frequency of tail/genital grooming and an increased duration of paw licking compared to wild-type littermate controls (y/+). No significant differences were found in syntactical parameters of grooming (c). *p<0.05; **p<0.01. N=9/group.
3.5 Repetitive Novel Object Contact Task/Locomotion
During the habituation phase, no significant differences were noted in the number of lines crossed [t(16)=−0.598, p=0.558, data not shown] nor in the duration of time spent within each quadrant [F(1,16)=0.827, p=0.377, data not shown]. Wild-type and hemizygous mice investigated unfamiliar novel objects at a similar rate [F(1,64)=0, p=1] (Figure 4a). Similarly, when proportional preferences for each toy were ranked and averaged for each genotype, no significant difference in object preferences was found [F(1,32)=0.803, p=0.377] (Figure 4b). Finally, when the number of identical three- and four-object sequences was compared between genotypes, no differences were observed [F(1,64)=4E−12, p=1] (Figure 4c). Taken together, Mecp2 mutants and wild-type mice are indistinguishable in cognitive, object-investigation-based measures of stereotypy or restricted interest.
Figure 4. Repetitive novel object contact task.
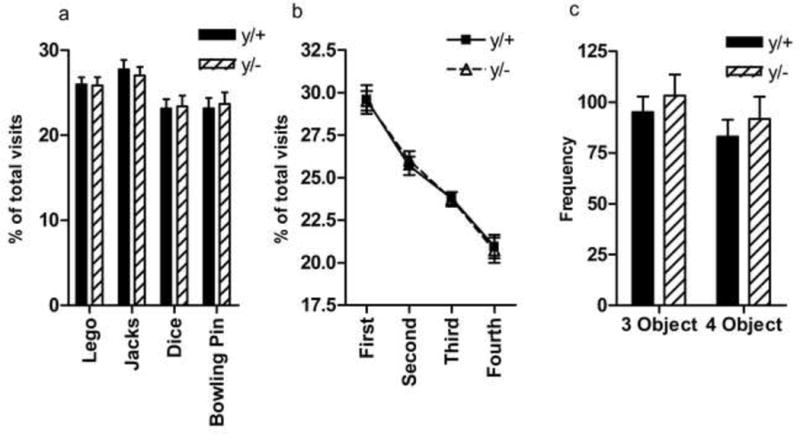
Wild-type and mutant Mecp2 mice show similar spontaneous investigation of novel toys (a,b). Similarly, wild-type (y/+) and mutant Mecp2 mice (y/−) show no differences in the number of sequenced visits to the unfamiliar objects (c). N=9/group.
3.6 Social Proximity
Figure 5a,b displays the frequency and duration of social behaviors of Mecp2 wild-type and mutant mice to unfamiliar B6 mice. Mann-Whitney U-tests indicated a significant increase in the frequency of Crawl Under behavior in hemizygous mutants [p=0.006]. They also showed significant elevations in the duration of Crawl-Over [p=0.026] and Crawl-Under [p=0.010] behavior relative to wild-type littermates. Finally, Mecp2 hemizygous males showed an increased duration of Nose-to-Face behavior [p=0.040]. No other statistically-significant effects were noted in the social proximity chamber between hemizygous and wild-type Mecp2 mice.
Figure 5. Patterns of social interaction in the social proximity task.
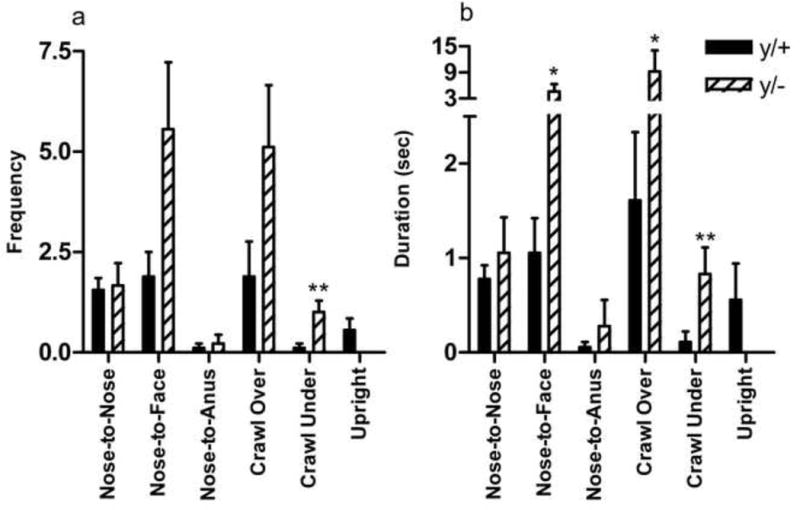
Mecp2 mutant mice (y/−) show increased frequency of crawl under (a). Additionally, they show higher duration of nose-to-face interactions, as well as increased durations of crawl over and crawl under behavior (b) compared to wild-type littermates (y/+). *p<0.05; **p<0.01. N=9/group.
3.7 Urinary Scent Marking
Figure 6 presents the average number of 1 × 1cm squares containing scent marks in the baseline and social scent marking conditions. Two-way repeated measures ANOVA revealed a significant main effect of Genotype [F1,32)=6.15, p=0.0195]. Neither the main effect for scent marking Condition [F(1,32)=0.024, p=0.878] nor the Genotype × Condition interaction were statistically significant [F(1,32)=0.280, p=0.601]. No significant post-hoc differences were noted between Genotypes in either Condition. Therefore, mutant Mecp2 mice show elevated scent marking across social and non-social contexts.
Figure 6. Elevated scent marking in a nonsocial and social context in Mecp2 mutant mice.
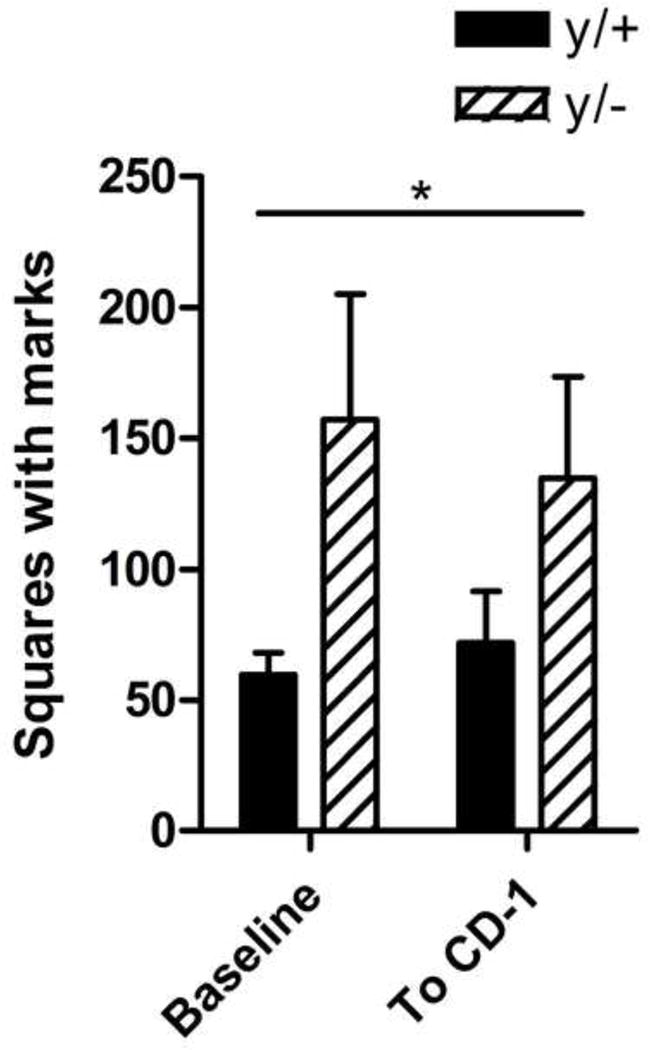
Mutant mice (y/−) scent mark more to an empty chamber and when an unfamiliar adult CD-1 is behind a divider compared to wild-type littermates (y/+). Wild-type (y/+) N=9, mutant (y/−) N=8. Genotype main effect * p<0.05.
3.8 Resident Intruder
No attack behavior was noted in any of the Mecp2 wild-type or mutant mice; therefore, no attack variables are reported. Resident male Mecp2 hemizygous mutants demonstrated lower average durations of following behavior than wild-type littermates [t(15)=2.273, p=0.038]. No other significant differences were noted (Figure 7a,b).
Figure 7. Comparison of behavior in the resident-intruder task.
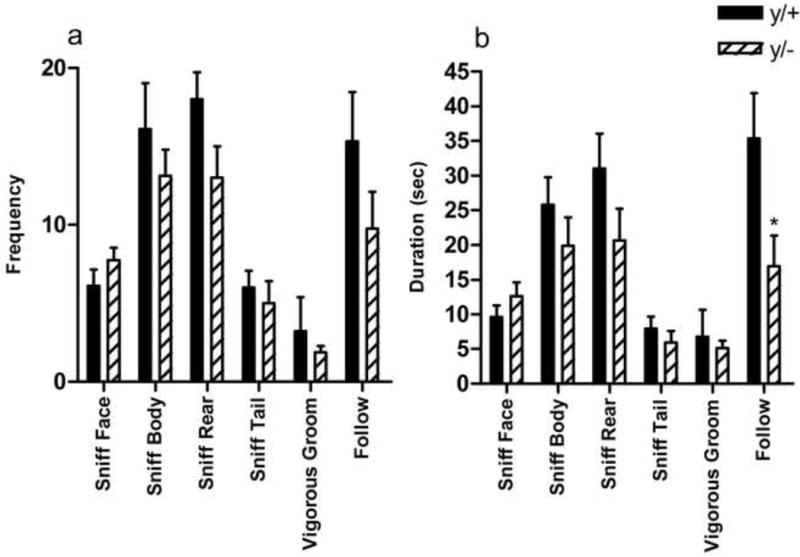
Mecp2 mutant mice (y/+) show similar frequencies of behavior to an unfamiliar intruder compared to their wild-type littermates (y/+) (a). Mutant mice show decrease duration of following behavior (b). *p<0.05. Wild-type (y/+) N=9, mutant (y/−) N=8.
4.0 Discussion
Mutations of the MECP2 gene in humans and laboratory animals are associated with a variety of neurological and behavioral alterations. However, the specific role of this gene and the Mecp2 protein in normal variation in behavior and in disease states remains to be elucidated. A major goal of this set of studies was to unravel the specific types of abnormalities in social behaviors and stereotypies in male mice with targeted mutations in Mecp2. Since existing reports are inconsistent with regards to the effect of mutation of this gene on social behavior, we performed a battery of tasks designed to reveal subtle differences in social motivation under diverse social contexts. In the VBS, a lab-based semi-natural environment in which subjects are free to establish their own time budgets, Mecp2308/Y mutant males preferentially approach one another from the front rather than the back, a preference which appears to be associated with pro-social motivation (Arakawa et al. 2007). Mutant Mecp2308/Y mice also spent a greater amount of time huddling during the dark phase. In the three-chamber apparatus, both genotypes spent more time in the side containing an unfamiliar stimulus mouse. This result is similar to those noted in Mecp21lox and Mecp2flox mutants (Fyffe et al. 2008; Samaco et al. 2008, 2009; Schaevitz et al. 2010) albeit with some possible differences in interpretation, due to other results from these studies (see below). Kerr et al. (2008) noted enhanced social novelty preference for Mecp2lox/y mutants. However, a limitation of the Kerr et al. (2008) study is that they did not counterbalance the social novelty phase, possibly confounding social and object novelty preferences (Pearson et al. 2010).
Mutant Mecp2308/Y mice have been reported to be hypoactive in the dark but hyperactive in the light phase (De Filippis et al. 2010; Moretti et al. 2005). However, in the present study, huddling behavior in mutants in the light phase, when mutants tend to be hyperactive, was comparable to that of wild-type mice, while allogrooming was elevated in the dark phase, the period when mutants tend to be hypoactive. We found no genotype differences in locomotor activity in the habituation phase of the repetitive novel object contact task or in the entries or contacts with the stimulus cups in the three-chamber task; both tasks were assessed in the light phase. Thus, although elevated huddling in the VBS during the dark phase might result from general hypoactivity, light cycle differences in activity patterns seem an insufficient explanation for the present results. The affiliative inclination of Mecp2 mutants does not appear to solely reflect hypoactivity in the dark phase and hyperactivity in the light.
The social proximity task was designed to differentiate close-quarter differences in social interactions (Defensor et al. 2011). In the current study, Mecp2308/Y mice showed elevated frequencies and/or durations of crawl-under, crawl-over behaviors, and nose-to-face investigation. Notably, low-social BTBR T+tf/J (BTBR) mice (Defensor et al, 2011) also displayed increases in crawl-over and -under behaviors, which were interpreted as attempts to avoid the other pair-member in a situation in which physical distance from that animal could not be achieved; a view that was supported by findings that diazepam significantly reduced this behavior in BTBRs. In the present, highly social, Mecp2308/Y mutants, these crawl-under/over results are consonant with their enhanced social approach, investigation, and huddling in the VBS. An intriguing possibility, however, is that enhanced crawl under/over may nonetheless reflect a subtle social deficiency, in that control (C57BL/6J) mice in the Defensor et al (2011) study tended to orient to the pair-partner mouse and move around it, rather than crawling under or over it, as might be more appropriate for an inanimate object.
These data, including VBS indices, the three-chamber task, and social proximity findings, suggest that this specific partial loss of function mutation of the Mecp2 gene in male mice produces an up-regulation of affiliative social behavior. An additional goal of this set of studies was to better understand the motivational components of these enhanced behaviors. Many commonly utilized social behavior tasks (ie. three chamber) do not inherently discriminate aggressive motivations in approach and proximity variables. This is a particular issue for Mecp2 mutants, as brain region and cell-specific Mecp2 mutants (Fyffe et al. 2008; Samaco et al. 2009) were more aggressive in a resident-intruder situation, and also spent more time in social proximity in the partition test, suggesting an aggressive motivation in such approaches to the stimulus mouse. Present findings that the 308 mutant mice show higher levels of allogrooming are consonant with this interpretation, in that intense bouts of allogrooming are often interpreted as agonistic behaviors reflective of dominance motivations (vigorous or “rough grooming”; Litvin et al. 2007; Long et al. 1972). Similarly, increased scent marking for Mecp2308/Y in this study suggests an interpretation in terms of enhanced aggressive or dominance motivations. This interpretation is not supported by findings that Mecp2308/Y mice failed to show attack in the resident-intruder task. However, overt attack may not be the most sensitive measure of dominance or competitive motives, and an overall evaluation of the present data suggests that the contribution of such motivation to social approach for the 308 mutant, cannot currently be dismissed.
An additional aspect of social interaction, relevant to interpretation in terms of autism-like behaviors, is reciprocity. Much of the early work with Mecp2308/Y mutant mice indicated that wild-type mice seem to avoid contact with the mutant mice in the tube test and resident intruder task (Shahbazian et al. 2002). One possibility is that it is the lack of reciprocation on the part of the recipient (stimulus) mouse that drives this enhanced sociability in the mutants: Another is that the Mecp2308/Y mutants are deficient in some behavioral, or perhaps physiological, characteristics that elicit sociality in WT mice. In this context, the above-mentioned enhanced crawl under/over behaviors may suggest such a social deficiency. These possibilities have yet to be tested, but suggest some additional and potentially important aspects to the complex set of events that constitute sociality in mammals. However, the present study, in contrast to Shahbazian et al. (2002), found no differences in the frequency of directed investigation towards mutant vs wild-type residents; and a slight augmentation of duration of olfactory investigation including a significant increase in duration of anogenital sniffing of mutants by intruder wild-types (data not shown) in the resident intruder paradigm. The original 308/Y mutants in the Shahbazian et al. (2002) study were on a mixed background, while those in the current study were on a C57BL/6J. Since there are substantial strain differences in three-chamber sociability it is important to take this into account in social behavior tests on mutants of varying backgrounds (Bolivar et al, 2007 Moy et al, 2004; 2007).
Mouse models of ASD commonly display patterns of restricted repetitive behaviors (Moy et al, 2008; Pearson et al, 2011). Published studies have characterized hindlimb clasping when lifted by the tail, and forelimb stereotypies in Mecp2 mutants, and it is thought that these might represent face validity in models of Rett syndrome (Chen et al, 2001; Gemelli et al, 2006; Guy et al, 2001; Moretti et al, 2005; Shahbazian et al, 2002). We found no disturbances in patterns of object investigation, and only minor alterations in forepaw- and anogenital-directed grooming behaviors. The latter results may extend previous discoveries of altered stereotypy in Mecp2 mutants by noting that qualitative and region-specific differences in grooming may exist, but the cephalo-caudal patterning appears to be normal.
Taken together, the behavioral studies outlined here illustrate one of the many conceptual issues arising from models of pervasive developmental disorders-that autism-relevant features may occur without actual reductions in affiliative social behavior. Mouse models of symptom- or domain-specific alterations in neurodevelopmental disorders may be as informative as those that are generated in an attempt to create an entire syndrome. To that end, a future goal might be to clarify the distinct effects of the variety of region and allele targeted mutants of Mecp2 on specific forms of social behavior, and the downstream targets of Mecp2 protein which influence behavior, particularly throughout the developmental span. These results contribute to a growing body of research that suggests Mecp2 is critical in the bi-directional fine-tuning of behavioral responses, possibly up to, and including complex social interactions.
Acknowledgments
Project funded by NIH MH081845 to RJB. Amy Vasconcellos and Larry Oasay assisted in data collection and behavioral scoring.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. 2000. Diagnostic criteria for 299.00 Autistic Disorder. text revision (DSM-IV-TR) [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Blanchard DC, Blanchard RJ. Colony formation of C57BL/6J mice in visible burrow system: identification of eusocial behaviors in a background strain for genetic animal models of autism. Behav Brain Res. 2007;176(1):27–39. doi: 10.1016/j.bbr.2006.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Social features of scent-donor mice modulate scent marking of C57BL/6J recipient males. Behav Brain Res. 2009;205(1):138–145. doi: 10.1016/j.bbr.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu T, Carrie A, de Roux N, Vinet MC, Jonveaux P, Couvert P, Villard L, Arzimanoglou A, Beldjord C, Fontes M, Tardieu M, Chelly J. MECP2 mutations account for most cases of typical forms of Rett syndrome. Hum Mol Genet. 2000;9(9):1377–1384. doi: 10.1093/hmg/9.9.1377. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176(1):21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfa G, Percy AK, Pozzo-Miller L. Experimental models of Rett syndrome based on Mecp2 dysfunction. Exp Biol Med. 2011;236(1):3–19. doi: 10.1258/ebm.2010.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of Autism Spectrum Disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006. 2009;58(SS10):1–20. [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320(5880):1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468(7321):263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27(3):327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J, Watson P, Ramsden S, Black GC. Somatic mutation in MECP2 as a non-fatal neurodevelopmental disorder in males. Lancet. 2000;356(9232):830–832. doi: 10.1016/s0140-6736(00)02661-1. [DOI] [PubMed] [Google Scholar]
- De Filippis B, Ricceri L, Laviola G. Early postnatal behavioral changes in the Mecp2-308 truncation mouse model of Rett syndrome. Genes Brain Behav. 2010;9(2):213–223. doi: 10.1111/j.1601-183X.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- Defensor EB, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav Brain Res. 2011;217(2):302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti MT, Orrico A, De Stefano N, Battisti C, Sicurelli F, Severi S, Lam CW, Galli L, Sorrentino V, Federico A. A Rett syndrome MECP2 mutation that causes mental retardation in men. Neurology. 2002;58(2):226–230. doi: 10.1212/wnl.58.2.226. [DOI] [PubMed] [Google Scholar]
- Fyffe SL, Neul JL, Samaco RC, Chao HT, Ben-Shachar S, Moretti P, McGill BE, Goulding EH, Sullivan E, Tecott LH, Zoghbi HY. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron. 2008;59(6):947–958. doi: 10.1016/j.neuron.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol Psychiatry. 2006;59(5):468–476. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27(3):322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol. 1983;14(4):471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Hagberg B. Rett’s syndrome: prevalence and impact on progressive severe mental retardation in girls. Acta Paediatr Scand. 1985;74(3):405–408. doi: 10.1111/j.1651-2227.1985.tb10993.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen HF, Bird A. MeCP2 and other methyl-CpG binding proteins. Ment Retard Dev Disabil Res Rev. 2002;8(2):87–93. doi: 10.1002/mrdd.10021. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc. 2007;2(10):2538–2544. doi: 10.1038/nprot.2007.367. [DOI] [PubMed] [Google Scholar]
- Kerr B, Alvarez-Saavedra M, Sáez MA, Saona A, Young JI. Defective body-weight regulation, motor control and abnormal social interactions in Mecp2 hypomorphic mice. Hum Molec Genet. 2008;17(12):1707–1717. doi: 10.1093/hmg/ddn061. [DOI] [PubMed] [Google Scholar]
- Kleefstra T, Yntema HG, Oudakker AR, Romein T, Sistermans E, Nillessen W, van Bokhoven H, de Vries BB, Hamel BC. De novo MECP2 frameshift mutation in a boy with moderate mental retardation, obesity, and gynaecomastia. Clin Genet. 2002;61(5):359–362. doi: 10.1034/j.1399-0004.2002.610507.x. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci. 2008;28(28):7137–7142. doi: 10.1523/JNEUROSCI.1345-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, Bird A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69(6):905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- Litvin Y, Blanchard DC, Pentkowski NS, Blanchard RJ. A pinch or a lesion: a reconceptualization of biting consequences in mice. Aggress Behav. 2007;33(6):545–551. doi: 10.1002/ab.20222. [DOI] [PubMed] [Google Scholar]
- Long SY. Hair-nibbling and whisker-trimming as indicators of social hierarchy in mice. Anim Behav. 1972;20(1):10–12. doi: 10.1016/s0003-3472(72)80167-2. [DOI] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14(2):205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3(5):287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Halloway LP, Barbaro RP, Barbaro JR, West LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of ten inbred strains. Behav Brain Res. 2007;176(1):4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188(1):178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrico A, Lam C, Galli L, Dotti MT, Hayek G, Tong SF, Poon PM, Zappella M, Federico A, Sorrentino V. MECP2 mutation in male patients with non-specific X-linked mental retardation. FEBS Lett. 2000;481(3):285–288. doi: 10.1016/s0014-5793(00)01994-3. [DOI] [PubMed] [Google Scholar]
- Pearson BL, Defensor EB, Blanchard DC, Blanchard RJ. C57BL/6J mice fail to display preference for social novelty in the three-chamber apparatus. Behav Brain Res. 2010;213(2):189–194. doi: 10.1016/j.bbr.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Pobbe RL, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, Blanchard RJ. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10(2):228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, Radziewic T, Hayward M, Lahooti H, Christodoulou J, Tam PPL. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129(4):887–898. doi: 10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214(2):443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri L, De Filippis B, Laviola G. Mouse models of Rett syndrome: from behavioural phenotyping to preclinical evaluation of new therapeutic approaches. Behav Pharmacol. 2008;19(5-6):501–517. doi: 10.1097/FBP.0b013e32830c3645. [DOI] [PubMed] [Google Scholar]
- Samaco RC, Fryer JD, Ren J, Fyffe S, Chao HT, Sun Y, Greer JJ, Zoghbi HY, Neul JL. A partial loss of function allele of Methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet. 2008;17(12):1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, Hyland K, Thaller C, Maricich SM, Humphreys P, Greer JJ, Percy A, Glaze DG, Zoghbi HY, Neul JL. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci USA. 2009;106(51):21966–21971. doi: 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaevitz LR, Moriuchi JM, Nag N, Mellot TJ, Berger-Sweeney J. Cognitive and social functions and growth factors in a mouse model of Rett syndrome. Physiol Behav. 2010;100(3):255–263. doi: 10.1016/j.physbeh.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35(2):243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- Wu H, Tao J, Chen PJ, Shahab A, Ge W, Hart RP, Ruan X, Ruan Y, Sun YE. Genomewide analysis reveals methyl-CpG-binding protein 2-dependent regulation of microRNAs in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2010;107(42):18161–18166. doi: 10.1073/pnas.1005595107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JI, Hong EP, Castle JC, Crespo-Barreto J, Bowman AB, Rose MF, Kang D, Richman R, Johnson JM, Berget S, Zoghbi HY. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc Natl Acad Sci USA. 2005;102(49):17551–17558. doi: 10.1073/pnas.0507856102. [DOI] [PMC free article] [PubMed] [Google Scholar]


