Abstract
Background:
One of the main causes of mortality is hepatocellular carcinoma (HCC) which accounts for the third leading cause of deaths and one in forty deaths worldwide. The flavonoids, natural antioxidant compounds, account for a major group of polyphenolic compounds. One of the major isoflavones in soybean is genistein (GE) which can inhibit proliferation and induce apoptosis. Isoflavones, major type of phenolic materials, derived from dietary plants and medicinal herbs play a significant role in cancer prevention and treatment. Correlation between dietary habits and cancer risk including breast, prostate, and colon cancer has been reported. Various bioactivities of these compounds such as anticarcinogenic and antioxidant are responsible for their chemopreventive activities by which induce migration, proliferation, cell cycle arrest, and apoptosis. GE, one of the major isoflavones, is considered as a potent chemopreventive agent against cancer. The aim of this study was to investigate the inhibitory and apoptotic effects of GE on HCC Hepa1-6 cell line.
Methods:
Cell viability assay and cell cycle analysis with flow cytometry were used to evaluate proliferative and apoptotic effect GE.
Results:
GE inhibited the growth of Hepa1-6 cells and induced apoptosis with a concentration and time-dependent fashion. During GE treatment for 24, the half maximal inhibitory concentration (IC50) was 20 μM, and the maximum inhibition of cell growth was 52% (P < 0.01). The percentage of apoptotic cells with a concentration of 20 μM of GE after 24, 48, and 72 h was 35, 42, and 65%, respectively (P < 0.01).
Conclusions:
Our finding clearly indicated that GE can significantly inhibit proliferation of hepatocellular carcinoma Hepa 1-6 cell line and induce apoptosis in this cell line.
Keywords: Apoptosis, genistein, hepatocellular carcinoma, proliferation
Introduction
One of the main causes of mortality is hepatocellular carcinoma (HCC) which accounts for the third leading cause of deaths and one in forty deaths worldwide.[1] This cancer occurs more often among men than women. The main risk factors of HCC are hepatitis C virus and hepatitis B virus.[2] The flavonoids, natural antioxidant compounds, account for a major group of polyphenolic compounds. These compounds constitute distinct groups including flavans, anthocyanidins, flavonols, flavanones, proanthocyanidins, neoflavonoids, isoflavones, and flavones which present in fruits, vegetables, legumes, herbs, cereals, stems and flowers, seeds, nuts, spices, and also in cocoa, wine, and tea.[3,4,5,6,7,8] The incidence rate of colon, prostate, and breast cancers in the Asian people who consume diets rich in flavonoids is lower than populations in the Western hemisphere.[9] The major isoflavones in soybean are genistein (GE) and daidzein. It has been reported that GE can inhibit the cell cycle of certain cells at G2-M.[10] GE significantly inhibited the growth of MHCC97-H hepatocellular carcinoma (HCC) cells in vitro.[11] Previously, we reported that GE can inhibit proliferation and induce apoptosis in HCC Hepg2 and PLC/PRF5 cells.[12,13] Few studies have evaluated the antiproliferation and apoptotic effect of GE on HCC Hepa 1-6 cell. Therefore, this study was designed to define the antiproliferation and apoptotic effects of GE on HCC Hepa 1-6 cell.
Methods
Human HCC cells (Hepa 1-6 cell line) were purchased from the National Cell Bank of Iran, Pasteur Institute. GE was obtained from Sigma-Aldrich (St. Louis, MO, USA) diluted to 1, 5, 10, 15, 20, 25, 50, 75, and 100 μM in DMSO (Fisher Chemicals, Fair Lawn, NJ, USA) and stored in small aliquots at − 20°C. Dulbecco minimal essential medium (DMEM) and (3-4 5-dimethyl-2-thiazolyl]-2, 5-diphenyl-2Htetrazolium [MTT] bromide) and fetal bovine serum (FBS) purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The human HCC Hepa 1-6 cell was used in this experiment. These cells were cultured in DMEM (pH 7.2–7.4) containing 1% sodium pyruvate (Gibco, California, USA), 3.7 mg/ml sodium bicarbonate (Sigma), 10% FBS, and 1% antibiotics which include 10,000 units/ml penicillin G sodium, 10,000 ug/ml streptomycin sulfate, and 25 ug/ml amphotericin B with culture condition at 37°C in a humidified atmosphere containing 50 mL/L CO2 in air.
Cell viability assay
The proliferative effect of GE was evaluated by MTT assay. First, 5 × 105 cells per well were cultured in a 96-well plate and treated with various doses of GE (1, 5, 10, 15, 20, 25, 50, 75, and 100 μM) after 24 h of culture. The control groups were received DMSO only which the concentration was 0.2%. After 24 h of treatment, cell viability was accessed by MTT assay according to protocol. For this purpose, the treated cells were washed with PBS, and then, a fresh medium in combination with MTT (0.5 mg/mL) was added and after 4 h, the formazan crystals formed in this process were dissolved in acidic isopropanol, and finally, the absorbance was measured at 540 nm. All tests were done three times independently.
Cell cycle analysis with flow cytometry
Hepa1-6 cells were seeded at the density of 5 × 105/well in in 24-well plates. After 24 h, the medium was changed and the cells were treated with or without GE with indicated concentrations. After treatment times (24, 48 and 72 h), all treated cells were trypsinized with 0.05% trypsin by which the cells were collected, washed with cold phosphate-buffered saline (PBS), and resuspended in binding buffer (1×). Annexin-V-(FITC) and propidium iodide (PI, Becton-Dickinson, San Diego, CA) were utilized to staining according to protocol. Finally, the apoptotic cells were counted by FACScan flow cytometry (Becton Dickinson, Heidelberg, Germany).
Statistical analysis
The database was setup with the SPSS 16.0 software package (SPSS Inc., Chicago, Illinois, USA) for analysis. The data were acquired from three tests and are shown as means ± standard deviations. Statistical comparisons between groups were performed with ANOVA (one-way ANOVA) and Turkey test. A significant difference was considered as P < 0.05.
Results
Effect of genistein on growth of Hepa1-6 cells
To investigate the growth inhibitory effect of GrE, Hepa1-6 cells were treated with different concentrations (1, 5, 10, 15, 20, 25, 50, 75, and 100 μM) of GE for 24 h. After treatment time, cell viability was tested by the MTT assay, which showed that GE (with all indicated concentrations) decreased the cell numbers of Hepa1-6 cells in a dose- and time-dependent manner significantly [Figure 1]. The effective dose of the GE that inhibited 50% of the Hepa1-6 cancer cell lines was 20 μM and the maximum inhibition of cell growth was 52% (P < 0.001) [Figure 2].
Figure 1.
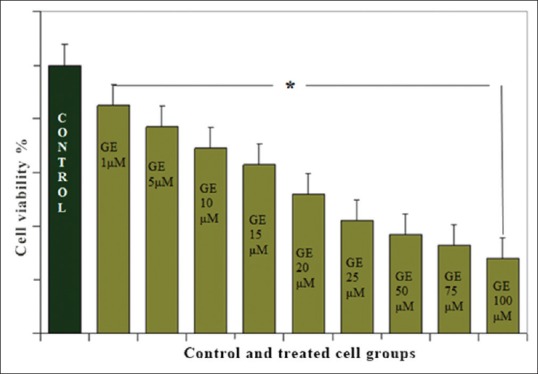
Inhibitory effect of genistein on proliferation of Hepa1-6. Hepa1-6 cells were treated with various concentration of genistein (as indicated in graph). The data are presented as the percentage change compared with the control group. *P < 0.001, significantly different from the control group
Figure 2.
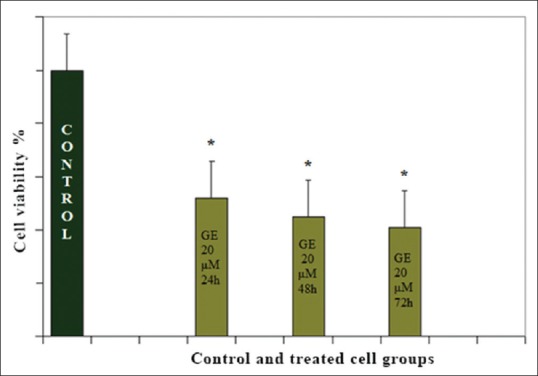
Antiproliferative effects of genistein (20 μM) on Hep1-6 cells in different time periods (24, 48, and 72 h). The data are presented as the percentage change compared with the control group. *P < 0.001, significantly different from the control group
Genistein induction of apoptosis
GE induces cell cycle arrest in Hep1-6 cells. Hepa1-6 cells were treated with 20 μM of GE for different time periods (24, 48, and 72 h). The percentage of apoptotic cells was also examined by flow cytometry. GE induced cell cycle arrest in the treated groups versus control in a time-dependent manner. The percentage of apoptotic cells was 35, 42, and 65% after 24, 48, and 72 h, respectively (P < 0.01). The best result (maximal apoptotic effect) was obtained in the group which treated for 72 h as shown in Figure 3.
Figure 3.
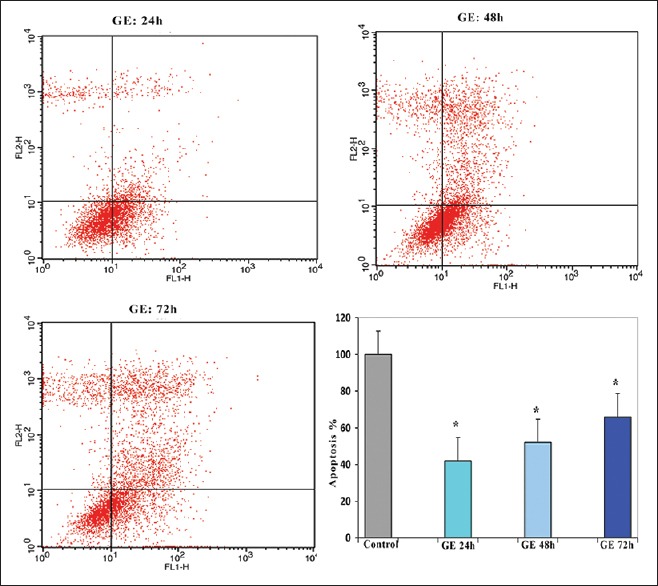
Effects of genistein on Hepa1-6 cell apoptosis. The cells were treated with genistein (20 μM) for 24, 48, and 72 h and the apoptosis inducing effect of genistein was investigated by flow cytometric analysis of Hepa1-6 cells stained with Annexin V and propidium iodide. Results were obtained from three independent experiments and were expressed as mean ± standard error of the mean. P < 0.001
Discussion
A lower incidence of cancer has been reported by epidemiological studies in Asian people which consume soy products.[14,15] In fact, many studies have reported that soy product, GE, has anticancer effect in many organs.[16,17,18]
Previously, we reported that GE can inhibit proliferation and induce apoptosis in HCC Hepg2 and PLC/PRF5 cells.[12,13] The results of the present study showed that GE inhibits proliferation and induces apoptosis in HCC Hepa1-6 cell with a dose- and time-dependent manner.
Similar to our result, antiproliferative and apoptotic effect of GE has been reported in other tissues and cell lines. This effect has been shown in colon cancer HT-29 cells,[19] human breast carcinoma cell line, MDA-MB-435,[20] SKBR3, and ZR751 cells,[21,22,23,24,25] LNCaP and DU-145 human prostate cancer cell.[26,27,28,29,30,31] Further studies have reported that GE exerts strong anti-invasive effects in vitro.[32] Other studies have demonstrated separate anti-invasive, antiadhesion, and antiproliferative effects of GE on other carcinoma cell lines.[33,34] These reports are consistent with our result that revealed GE exerts an apoptotic and antiproliferative effect. On the other hand, in other reports, the biphasic effect of GE (proliferative and antiproliferative) was observed. In human uterine leiomyoma cells, low concentration (<1 mg/ml) of GE stimulates proliferation.[35] GE exerts a biphasic effect on growth of MCF-7 cells, proliferative effect at low and antiproliferative effect at high concentrations.[36] Similarly, it has been demonstrated that GE has estrogenic activity and inhibitory effects at a physiological concentration (10 nM–20 μM).[37] in vitro experiment has been indicated that GE promotes metastasis in advanced human prostate cancer transplant line.[38] We did not evaluate the mechanism of apoptotic and antiproliferative effect of GE in this work.
Conclusions
Our work clearly demonstrated that GE can inhibit cell proliferation and induce cell apoptosis in human Hepa 1-6 cell line which can provide a new strategy for HCC treatment.
Financial support and sponsorship
Adjutancy of research of Jahrom Medical University, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This article was supported by adjutancy of research of Jahrom Medical University, Iran.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer incidence in five continents. IARC Sci Publ. 2007;9:898–905. [Google Scholar]
- 3.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann K. Flavonols and flavones in food plants: A review. J Food Technol. 1976;11:433–48. [Google Scholar]
- 5.Kühnau J. The flavonoids. A class of semi-essential food components: Their role in human nutrition. World Rev Nutr Diet. 1976;24:117–91. [PubMed] [Google Scholar]
- 6.Crozier A, Burns J, Aziz AA, Stewart AJ, Rabiasz HS, Jenkins GI, et al. Antioxidant flavonols from fruits, vegetables and beverages: Measurements and bioavailability. Biol Res. 2000;33:79–88. doi: 10.4067/s0716-97602000000200007. [DOI] [PubMed] [Google Scholar]
- 7.Kefford JF, Chandler BV, editors. New York: Academic Press; 1970. The Chemical Constituents of Citrus Fruits. [Google Scholar]
- 8.Wesley F Taylor, Ehsan Jabbarzadeh. The use of natural products to target cancer stem cells. Am J Cancer Res. 2017;7:1588–1605. [PMC free article] [PubMed] [Google Scholar]
- 9.Adlercreutz H, Honjo H, Higashi A, Fotsis T, Hämäläinen E, Hasegawa T, et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr. 1991;54:1093–100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 11.Gu Y, Zhu CF, Dai YL, Zhong Q, Sun B. Inhibitory effects of genistein on metastasis of human hepatocellular carcinoma. World J Gastroenterol. 2009;15:4952–7. doi: 10.3748/wjg.15.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanaei M, Kavoosi F, Arezoo M. Apoptotic effect of genistein on hepatocellular carcinoma HepG 2 cell line. Glob J Med Res Stud. 2016;3:1–8. [Google Scholar]
- 13.Dastjerdi MN, Kavoosi F, Valiani A, Esfandiari E, Sanaei M, Sobhanian S, et al. Inhibitory effect of genistein on PLC/PRF5 hepatocellular carcinoma cell line. Int J Prev Med. 2015;6:54. doi: 10.4103/2008-7802.158914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adlercreutz CH, Goldin BR, Gorbach SL, Höckerstedt KA, Watanabe S, Hämäläinen EK, et al. Soybean phytoestrogen intake and cancer risk. J Nutr. 1995;125(3 Suppl):757S–70S. doi: 10.1093/jn/125.3_Suppl.757S. [DOI] [PubMed] [Google Scholar]
- 15.Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: Evidence from epidemiological and laboratory studies. Toxicol Lett. 2004;150:43–56. doi: 10.1016/j.toxlet.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Wang HK. The therapeutic potential of flavonoids. Expert Opin Investig Drugs. 2000;9:2103–19. doi: 10.1517/13543784.9.9.2103. [DOI] [PubMed] [Google Scholar]
- 17.Barnes S, Peterson TG. Biochemical targets of the isoflavone genistein in tumor cell lines. Proc Soc Exp Biol Med. 1995;208:103–8. doi: 10.3181/00379727-208-43840. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21:744–57. doi: 10.1081/cnv-120023773. [DOI] [PubMed] [Google Scholar]
- 19.Qi W, Weber CR, Wasland K, Roy H, Wali R, Joshi S, et al. Tumor suppressor FOXO3 mediates signals from the EGF receptor to regulate proliferation of colonic cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G264–72. doi: 10.1152/ajpgi.00416.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr Cancer. 1996;26:167–81. doi: 10.1080/01635589609514473. [DOI] [PubMed] [Google Scholar]
- 21.Choi EJ, Kim GH. Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Mol Med Rep. 2013;7:781–4. doi: 10.3892/mmr.2013.1283. [DOI] [PubMed] [Google Scholar]
- 22.Fritz WA, Coward L, Wang J, Lamartiniere CA. Carcinogenesis. 1998. Dietary genistein: Perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat; pp. 2151–8. [DOI] [PubMed] [Google Scholar]
- 23.Lamartiniere CA, Zhao YX, Fritz WA. Genistein: Mammary cancer chemoprevention, in vivo mechanisms of action, potential for toxicity, and bioavailability in rats. J Womens Cancer. 2000;2:11–9. [Google Scholar]
- 24.Lamartiniere CA, Moore JB, Brown NM, Thompson R, Hardin MJ, Barnes S. Genistein suppresses mammary cancer in rats. Carcinogenesis. 1995;16:2833–40. doi: 10.1093/carcin/16.11.2833. [DOI] [PubMed] [Google Scholar]
- 25.Murrill WB, Brown NM, Zhang JX, Manzolillo PA, Barnes S, Lamartiniere CA. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis. 1996;17:1451–7. doi: 10.1093/carcin/17.7.1451. [DOI] [PubMed] [Google Scholar]
- 26.Peterson G, Barnes S. Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate. 1993;22:335–45. doi: 10.1002/pros.2990220408. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Eltoum IE, Lamartiniere CA. Dietary genistein suppresses chemically induced prostate cancer in Lobund-Wistar rats. Cancer Lett. 2002;186:11–8. doi: 10.1016/s0304-3835(01)00811-4. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gingrich JR, Greenberg NM. A transgenic mouse prostate cancer model. Toxicol Pathol. 1996;24:502–4. doi: 10.1177/019262339602400414. [DOI] [PubMed] [Google Scholar]
- 30.Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–91. [PubMed] [Google Scholar]
- 31.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61:6777–82. [PubMed] [Google Scholar]
- 32.Shao ZM, Wu J, Shen ZZ, Barsky SH. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998;58:4851–7. [PubMed] [Google Scholar]
- 33.Santibáñez JF, Navarro A, Martínez J. Genistein inhibits proliferation and in vitro invasive potential of human prostatic cancer cell lines. Anticancer Res. 1997;17:1199–204. [PubMed] [Google Scholar]
- 34.Traganos F, Ardelt B, Halko N, Bruno S, Darzynkiewicz Z. Effects of genistein on the growth and cell cycle progression of normal human lymphocytes and human leukemic MOLT-4 and HL-60 cells. Cancer Res. 1992;52:6200–8. [PubMed] [Google Scholar]
- 35.Moore AB, Castro L, Yu L, Zheng X, Di X, Sifre MI, et al. Stimulatory and inhibitory effects of genistein on human uterine leiomyoma cell proliferation are influenced by the concentration. Hum Reprod. 2007;22:2623–31. doi: 10.1093/humrep/dem185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miodini P, Fioravanti L, Di Fronzo G, Cappelletti V. The two phyto-oestrogens genistein and quercetin exert different effects on oestrogen receptor function. Br J Cancer. 1999;80:1150–5. doi: 10.1038/sj.bjc.6690479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zava DT, Duwe G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr Cancer. 1997;27:31–40. doi: 10.1080/01635589709514498. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura H, Wang Y, Kurita T, Adomat H, Cunha GR, Wang Y. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PLoS One. 2011;6:e20034. doi: 10.1371/journal.pone.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]


