Abstract
Background:
Considering all the latest achievements in neonatal respiratory care, bronchopulmonary dysplasia (BPD) is still among the most prevalent morbidity causes in premature infants. Involvement in this process results in longer period of hospitalization for the newborn and in the long run makes the living conditions more difficult. Taking the multifactorial pathogenesis into account, approaches to tackle chronic lung disease (CLD) are mainly focused on interventions and prevention procedures. This study tries to investigate the potential capability of inhaled budesonide in the prevention of BPD in newborns with gestational age of <28 weeks with the respiratory distress syndrome (RDS).
Methods:
This study was a randomized clinical trial done on seventy newborns with gestational ages of 23–28 weeks with RDS in Isfahan Shahid Beheshti Educational Hospital from June 2014 to April 2016. Patients were randomly assigned to two groups of intervention with budesonide and control. There were 35 newborns in each group. Upon recording demographic characteristics, the newborns in two groups were compared based on the length of noninvasive ventilation, the need for invasive mechanical ventilation, the number of surfactant administrations, pneumothorax, intraventricular hemorrhage, patent ductus arteriosus (PDA), CLD, and death.
Results:
The length of the need for nasal continuous positive airway pressure showed no statistically significant difference between the groups (P = 0.54). The number of newborns who needed invasive mechanical ventilation also revealed no meaningful difference (P = 0.14). Similarly, the number of newborns who were characterized as affected by CLD also showed no significant difference between the groups (P = 0.053). Moreover, the number of newborns who experienced pneumothorax was not significantly different for the groups (P = 0.057). The number of newborns who received three administrations of surfactant had also no statistically meaningful difference between the groups (P = 0.69). However, the number of newborns who received two doses of surfactant was statistically lower in budesonide intervention group than the control (P = 0.041). The prevalence of intraventricular hemorrhage with degrees of I, II, and III also showed no statistically meaningful difference between the groups with P = 0.74, 0.32, and 0.49, respectively. The occurrence of PDA had no meaningful difference between the groups (P = 0.66). Relative death cases also revealed no significant difference between the groups (P = 0.53).
Conclusions:
The current study revealed a decrease in CLD prevalence for newborns in interventional group; however, this decrease was not statistically meaningful. The newborns, in the intervention group, who had received two doses of surfactant (survanta) showed a significant decrease, which can be the basis for further research in this field.
Keywords: Budesonide, chronic lung disease, respiratory distress syndrome
Introduction
Currently, the most prevalent morbidity cause among premature newborns is bronchopulmonary dysplasia (BPD), and it has affected the resources for the newborns, the family, and ultimately the society. Involvement in this disorder is accompanied by long hospitalization for the newborns and eventually results in difficulties to an extent that not only affects the quality of the newborn's life compared with that of a healthy one but also leads to later disorders and complications in the normal function of the respiratory system beyond childhood period, including decrease in lung function and limitations regarding sport activities, revealing an arrest in alveoli development in lungs.[1,2]
It was first in 1967 that Northway applied the term BPD for the gradual development of a pulmonary disease in premature newborns that had experienced respiratory distress syndrome (RDS) upon birth. Northway et al. defined and categorized radiologic criteria for this pulmonary disease and attributed its development process to the exposure of a premature lung to oxygen and mechanical ventilation.[3]
BPD is in fact a diagnosis based on a series of pathologic evidence which can undoubtedly be attributed to mechanical ventilation and exposure to oxygen in a premature newborn's lung who is involved in RDS. Regarding gestational age and weight, this disease which is also referred to as chronic lung disease (CLD) entangles a significantly different population of the newborns than the one described by Northway four decades ago; at the moment, this disease is rarely observed in newborns weighing 1500–2000 g. CLD occurrence among very premature newborns (severe BPD prevalence can be traced in 44% of newborns with 24 weeks of gestation and 20% of newborns with 27 weeks of gestation) is so highlighted these days that it is referred to as “new BPD”; currently, CLD is defined as the need for supplemental oxygen for at least 28 days from birth.[4]
Due to multifactorial nature of the pathogenesis of the diseases, approaches to tackle CLD mainly focus on prevention and interventional procedures. As mechanical ventilation, oxygen toxicity, infectious processes, and inflammation are considered as major factors in the etiology of CLD, a wide range of medicinal and nonmedicinal interventions has been proposed to treat BPD.[5]
Caffeine, this methylxanthine is widely used to treat apnea of prematurity (AOP), as AOP occurs in at least 85% of newborns with gestational ages of <34 weeks. In a study done by Schmidt et al., the BPD prevalence decreased from 47% to 36% in newborns weighing <1250 g.[6]
Diuretics, pulmonary edema, caused by the liquid leakage from lung capillaries upon damage from mechanical ventilation, infectious processes, left-to-right shunt (as a result of patent of ductus arteriosus), and excessive administration of liquids during the RDS sets grounds for the administration of diuretics. In a meta-analysis done by Cochrane in 2002, it was revealed that long-term administration of Thiazides has improved the pulmonary function of newborns with RDS.[7,8]
Bronchodilators
During BPD, due to hyperactivity and hypertrophy of smooth muscles, airway resistance increases. Bronchodilators have proven to decrease the pulmonary resistance and eventually increase the lungs’ dynamic compliance. These drugs lay under two categories of adrenergic and anticholinergic.[9]
Mast cell stabilizers
Sodium cromolyn has been used to decrease the migration of neutrophils and, as a result, decrease the inflammation in the lungs involved with BPD; some of these studies have also used prophilaxy. Studies incorporating such interventions have not revealed any significant effect on the decrease in mortality or the need for mechanical ventilation.[10]
Vitamin A
A meta-analysis done by Cochrane on eight extensive studies has revealed that high-dose Vitamin A intramuscular administration in premature newborns weighing <1000 g decreases BPD prevalence up to 8%.[11]
Inositol
This phospholipid improves the synthesis and secretion of surfactants; a meta-analysis done by Cochrane has shown that intravascular (IV) administration of inositol in extremely low-weight newborns has decreased the prevalence of BPD and death cases.[12]
Antioxidants
Intrathecal administration of CuZn-SOD, as a superoxide dismutase, in newborns with RDS with gestational age of <27 weeks has decreased BPD prevalence. Moreover, IV administration of N-acetylcysteine, as a forerunner for glutathione, in newborns with RDS weighing <1000 g has decreased the prevalence and severity of BPD. As Vitamins E and C act like scavengers against free oxygen radicals, they have also been proposed as a preventive means against BPD; anyway, the philosophy of administering antioxidants in premature newborns roots in the sensitivity of premature newborns’ tissues to preoxidation which results from exposure to high densities of inhaled oxygen and their limitations in the case of endogen scavengers.[13,14,15]
Stem cells
As you know, stem cells divide into two categories of embryonic and somatic; in lung tissue, there are islands of somatic stem cells (adult stem cells [ASCs]) which have shown the capability to restore the damaged epithelium in the lungs. In the last 5 years, extensive research has been done in this field, and progenitors have been found in four categories of ASCs including proximal airway stem, distal lung epithelial stem, mesenchymal stem, and endothelial stem.[2]
Inhaled nitric oxide
Administration of inhaled nitric oxide (iNO) not only decreases the pulmonary vascular resistance but also acts as an inflammatory process suppressant. This combination has been studied for interventional and prevention approaches to handle BPD. In one of the biggest studies of this kind done by Ballrad to prevent CLD, iNO administration has shown a decrease in the prevalence of BPD in the study population.[16]
Steroids
The most important pathogenesis in the process of BPD is inflammation. Steroids, as an anti-inflammation, not only decrease the edema in the airways and thus prevent pulmonary fibrosis but also improve pulmonary function (gas exchange) through improvement in surfactant production, stabilization of capillary walls and prevention of capillary leakage, and finally improvement in β-adrenergic system.[17] Corticosteroid administration has been studied as an intervention to treat CLD as well as preventing BPD. Regarding prevention, the effect of corticosteroid administration has been investigated in several studies based on whether it is administered early (in the 1st week) or late (after 1 week).[18]
Currently, there is an extensive research being done in European Union on the effect of inhaled budesonide on the prevention of BPD in newborns with RDS with gestational ages of <28 weeks. The mentioned study started under the title of Neonatal European Study of Inhaled Steroid by seven workgroups in the member countries of the European Union in 2009. Moreover, Cochrane foundation also published a review on the potential of inhaled corticosteroids to prevent BPD in very low-weight newborns in 2011, which in turn called for more extensive research in the field.[19,20]
Due to the fact that the administration of inhaled corticosteroids as a means to prevent BPD is still regarded as an open research area by Cochrane, we decided to conduct a clinical trial to challenge the effect of inhaled budesonide on the prevention of CLD.
Methods
This study is a prospective randomized clinical trial done on newborns with gestational age of 23–28 weeks involved in RDS at the Neonatal Intensive Care Unit at Shahid Beheshti Educational Hospital (affiliate of Isfahan University of Medical Sciences) from June 2014 to March 2016. Thirty-five newborns were placed in the interventional group and were treated with inhaled budesonide while 35 other newborns were placed in the control group. To randomize the sampling, the newborns with even document numbers entered the interventional group (with inhaled budesonide) and the newborns with odd document numbers entered the control group and sampling continued until the required number of infants (n = 35) was achieved in both groups. The inclusion criteria included newborns who showed clinical symptoms of RDS within 2 h from birth (tachypnea, intercostal retraction, nasal flaring, grunting, and the need for supplementary oxygen) and thus received surfactant while under nasal continuous positive airway pressure (nCPAP).[21,22,23,24] Consent form was filled for the newborns, and the newborns with congenital malformations or prenatal asphyxia (5 min Apgar score of 0–3 and umbilical pH of <7 with umbilical bicarbonate of <12 mEq/L) were excluded from the study.[25]
At birth, the newborns were placed under nCPAP respiratory support with a T-piece (Infant T-Piece resuscitator-Fisher and Paykel Healthcare, Auckland, New Zealand) and Argyle nasal prong (Covidien, Dublin, Ireland); with continuous distending pressure (CDP) set on 5–6 cm H2O, the newborns were transferred to nursery while covered in plastic.[21,22]
In neonatal nursery, the newborns were placed under nCPAP with flow-driven continuous positive airway pressure system, including Medin prong interface and Medijet (Medin Innovations GmbH, Germany) injector; to maintain the current in the circuit and to enable the jet nebulizer capability, Christina (Stephan Medizintechnik, Dusseldorf, Germany) ventilator was used. This ventilator has the essential accessories for aerosolized spacer in the circuit. The flow was gradually increased to reach CDP = 6 cm H2O and FiO2 = 30%.[23] In case Fio2 of more than 40% was needed to maintain the oxygen saturation in the right hand in the range of 90%–95%, Survanta would be administered with the dose of 100 mg/kg, and the newborn would eventually enter the study. To administer the Survanta, the newborns received surfactant without detachment from nCPAP, through a vascular catheter, an approach which is also referred to as less invasive surfactant administration. In case the newborn's need for the FiO2 of more than 40% to maintain oxygen saturation in the acceptable range, Survanta would be administered again after 6 h from the previous administration, and if necessary, the course of treatment (4 days) would be completed; newborns were monitored for capillary blood gas (CBG) before and after the administration of the surfactant; and then on, at each 12 h, and necessary steps would be taken to manage the mechanical ventilation.[24]
In the interventional group, 500 μg doses of inhaled budesonide (Pulmicort Respules, AstraZeneca group, Australia) were administered each 12 h starting 12 h from birth, and this procedure continued as long as the newborn was under nCPAP (maximum of 7 days). To administer budesonide, while the Aerochamber (Intersurgical Incorporated, Liverpool, UK) was placed at the end of the inspiratory limb, the jet nebulizer knob in Christina ventilator was activated, and the medicine was aerosolized.[19,22,26]
During the respiratory management, in case the newborn's need for FiO2 of <50% to maintain oxygen saturation in the acceptable range continued for more than 4 h, CDP was decreased 1 or 2 cm H2O at a time, and the newborn was eventually detached from nCPAP respiratory support at CDP = 4 cmH2O and FiO2 <30%.[27]
The realization of each of the following indicators resulted in the discontinuation of the noninvasive respiratory support and the start of intubation and invasive respiratory support:
Despite CDP = 8 cm H2O and FiO2 ≤75%, the newborn was unable to maintain oxygen saturation in the range of 90%–95% in the right hand[28]
CBG indicators reflecting respiratory failure (pH < 7.2 and PCO2 >65 mmHg)[27]
More than three times apnea per hour necessitating stimulation or ventilation with bag and mask.[27]
Brain sonography was done for these newborns at 3, 7, and 14 days after birth to monitor intraventricular hemorrhage (IVH) and periventricular leukomalacia. On the 3rd day from birth, echocardiography was done on newborns. The demographic characteristics questionnaire registered the length of noninvasive respiratory support, surfactant administrations and dosage, the need for mechanical ventilation, the length of oxygen support (if the newborn needed oxygen for more than 28 days from birth, CLD was diagnosed for the newborn), and pneumothorax incidence.
Results
Table 1 depicts the demographic information of the participants in both groups. Each group involves a total of 35 newborns, with average gestational ages of 27.3 ± 0.8 and 26.9 ± 1.3 weeks in control and interventional groups, respectively. The difference in average gestational age was not significant (P = 0.09). The average Apgar score at 1 and 5 min was, respectively, 5.18 ± 2.59 and 7.56 ± 1.21 for the control and 4.74 ± 2.2 and 7.71 ± 1.18 for the interventional group, which showed no statistically meaningful difference. One newborn in the control group and two newborns in the interventional group had experienced long-term (equal or more than 18 h) rupture of membrane, which was not significantly different (P = 0.56).
Table 1.
Demographic characteristics of newborns in both groups

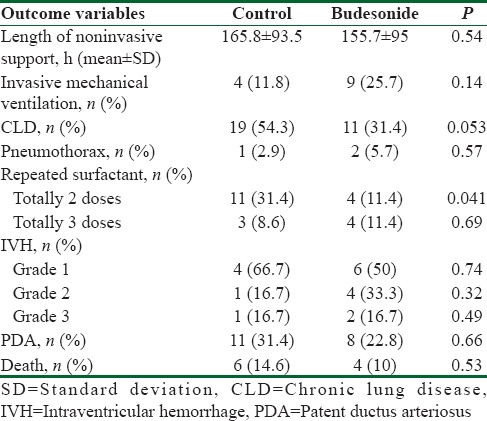
Table 2 compares the prevalence of the outcome in both groups. The length of the need for nCPAP was not statistically different in both groups (P = 0.54). The number of newborns who needed invasive mechanical ventilation also showed no significant difference (P = 0.14). The number of newborns who were diagnosed with CLD also had no statistically meaningful difference (P = 0.053). Pneumothorax prevalence had no significant difference in the groups (P = 0.57). The number of newborns who received three administrations of surfactant was not significantly different in both groups (P = 0.69); however, the number of newborns receiving two administrations of surfactant showed a statistically significant decrease in the interventional group (P = 0.041). The prevalence of IVH of grades I (P = 0.74), II (P = 0.32), and III (P = 0.49) revealed no significant difference between the groups. Patent ductus arteriosus relative prevalence had no statistical difference between the groups (P = 0.66). Relative prevalence of death also showed no significant difference between groups (P = 0.53).
Table 2.
Respiratory and clinical outcome in both groups
Discussion
Most of the articles were focused on the IV administration of corticosteroids to prevent BPD, such as the study done by Ventro in 2004 on twenty newborns with gestational ages of <33 weeks who were under mechanical ventilation as a result of RDS. From the 4th day of birth, the newborns were treated with IV administered dexamethasone for a week. The newborns in this group showed a statistically significant decrease in the need for supplementary oxygen at 36 weeks from postmenstrual age (PMA); however, a decrease was observed in the prevalence of both hypertension and hyperglycemia.[29]
In a study done by Soll on 542 newborns weighing 500–1000 g who were under mechanical ventilation and received surfactant as a result of RDS, the newborns were treated with IV dexamethasone from 12 h from birth for 12 days. In the course of the study, each 3 days, the dosage of administered dexamethasone (starting at 0.5 mg/kg/day) was cut back by half. This study revealed that BPD prevalence, death at 28 days from birth, and retinopathy of prematurity showed a statistically meaningful decrease in the interventional group than the control. However, hyperglycemia, hypertension, and digestive system perforation also showed a significant increase in the experimental group.[30]
In the article mentioned above, although surfactant administration decreased the prevalence of BPD, the side effects of administering systemic corticosteroids were inevitable. In this regard, and to decrease the side effects of the administration of systemic corticosteroids, aerosol therapy of these corticosteroids has been in focus.
In an extensive multicenter study done by Cole et al. from 1999 to 2000, the newborns with gestational ages of <33 weeks, who were under mechanical ventilation, received daily doses of beclomethasone inhaler from 3 days to 14 days from birth, even if they were detached from the mechanical ventilation. Although the study results indicated a decrease in the need for supplementary oxygen at 28 days from birth in the interventional group (123 newborns) compared to the control group (130 newborns), however, this decrease was not statistically significant.[31]
In another study done by Jónsson et al., thirty newborns with gestational ages of 23–29 weeks, who ventilated invasively by nCPAP at 7 days from birth, received budesonide inhaler by jet nebulizer for 14 consecutive days. At 36 weeks of PMA, 61% of the newborns in the interventional group still relied on oxygen supplement while the oxygen supplement dependency was observed in 79% of the newborns in control group, yet this difference was not statistically significant.[32]
With the goal of preventing BPD spread, Jangaard et al. studied the newborns weighing <1250 g who were under mechanical ventilation due to RDS; in the mentioned study, thirty newborns received beclomethasone inhaler as metered dose inhaler from 3 days from birth, and the target accumulative dose of 0.2 mg/kg was set. At 28 days from birth, the results revealed a 33% dependence on supplementary oxygen among the newborns in the interventional group compared to 36% in the control group.[33]
Conclusions
The current study showed a slight decrease in the prevalence of BPD in the study participants. Nevertheless, this decrease, just like the results of the studies done by Cole, Jonsson, and Jangaard, was not statistically significant. However, as Ventro and Soll reported no side effects for systematic corticosteroids, the conduction of such studies on a larger scale seems plausible. In the current study, the newborns who received two administrations of surfactant revealed a significant decrease in the interventional group; the point which can be considered as a focus for further studies in this regard.
Financial support and sponsorship
The study was funded by Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Trembath A, Laughon MM. Predictors of bronchopulmonary dysplasia. Clin Perinatol. 2012;39:585–601. doi: 10.1016/j.clp.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Reilly M, Thébaud B. Cell-based strategies to reconstitute lung function in infants with severe bronchopulmonary dysplasia. Clin Perinatol. 2012;39:703–25. doi: 10.1016/j.clp.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tin W, Wiswell TE. Drug therapies in bronchopulmonary dysplasia: Debunking the myths. Semin Fetal Neonatal Med. 2009;14:383–90. doi: 10.1016/j.siny.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Shou-Yien W, Tessy J, Medha K, Suma P, Tsu-Fuh Y. Postnatal corticosteroid to prevent or treat chronic lung disease in preterm infants. Neonatol Today. 2009;4:1. [Google Scholar]
- 5.Tropea K, Christou H. Current pharmacologic approaches for preventation and treatment of bronchopulmonary. Int J Pediatr. 2012;1:1. doi: 10.1155/2012/598606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–21. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 7.Kao LC, Durand DJ, McCrea RC, Birch M, Powers RJ, Nickerson BG. Randomized trial of long-term diuretic therapy for infants with oxygen-dependent bronchopulmonary dysplasia. J Pediatr. 1994;124:772–81. doi: 10.1016/s0022-3476(05)81373-3. [DOI] [PubMed] [Google Scholar]
- 8.Brion LP, Primhak RA, Ambrosio-Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev. 2002;1:CD001817. doi: 10.1002/14651858.CD001817. [DOI] [PubMed] [Google Scholar]
- 9.Denjean A, Paris-Llado J, Zupan V, Debillon T, Kieffer F, Magny JF, et al. Inhaled salbutamol and beclomethasone for preventing broncho-pulmonary dysplasia: A randomised double-blind study. Eur J Pediatr. 1998;157:926–31. doi: 10.1007/s004310050969. [DOI] [PubMed] [Google Scholar]
- 10.Viscardi RM, Hasday JD, Gumpper KF, Taciak V, Campbell AB, Palmer TW. Cromolyn sodium prophylaxis inhibits pulmonary proinflammatory cytokines in infants at high risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1997;156:1523–9. doi: 10.1164/ajrccm.156.5.9611088. [DOI] [PubMed] [Google Scholar]
- 11.Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short and long-term morbidity in very low birthweight infants. Cochrane Database Syst Rev. 2007;4:CD000501. doi: 10.1002/14651858.CD000501.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Howlett A, Ohlsson A. Inositol for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2000;4:CD000366. doi: 10.1002/14651858.CD000366. [DOI] [PubMed] [Google Scholar]
- 13.Mamo LB, Suliman HB, Giles BL, Auten RL, Piantadosi CA, Nozik-Grayck E. Discordant extracellular superoxide dismutase expression and activity in neonatal hyperoxic lung. Am J Respir Crit Care Med. 2004;170:313–8. doi: 10.1164/rccm.200309-1282OC. [DOI] [PubMed] [Google Scholar]
- 14.Sandberg K, Fellman V, Stigson L, Thiringer K, Hjalmarson O. N-acetylcysteine administration during the first week of life does not improve lung function in extremely low birth weight infants. Biol Neonate. 2004;86:275–9. doi: 10.1159/000080089. [DOI] [PubMed] [Google Scholar]
- 15.Berger TM, Frei B, Rifai N, Avery ME, Suh J, Yoder BA, et al. Early high dose antioxidant vitamins do not prevent bronchopulmonary dysplasia in premature baboons exposed to prolonged hyperoxia: A pilot study. Pediatr Res. 1998;43:719–26. doi: 10.1203/00006450-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–53. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 17.Bancalari E. Corticosteroids and neonatal chronic lung disease. Eur J Pediatr. 1998;157(Suppl 1):S31–7. doi: 10.1007/pl00014290. [DOI] [PubMed] [Google Scholar]
- 18.D'Angio CT, Maniscalco WM. Bronchopulmonary dysplasia in preterm infants: Pathophysiology and management strategies. Paediatr Drugs. 2004;6:303–30. doi: 10.2165/00148581-200406050-00004. [DOI] [PubMed] [Google Scholar]
- 19.Bassler D, Halliday HL, Plavka R, Hallman M, Shinwell ES, Jarreau PH, et al. The neonatal European study of inhaled steroids (NEUROSIS): An eu-funded international randomised controlled trial in preterm infants. Neonatology. 2010;97:52–5. doi: 10.1159/000227294. [DOI] [PubMed] [Google Scholar]
- 20.Shah VS, Ohlsson A, Halliday HL, Dunn M. Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database Syst Rev. 2012;5:CD001969. doi: 10.1002/14651858.CD001969.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Kattwinkel J. Textbook of Neonatal Resuscitation. AAP. (6th ed) 2011:274–6. [Google Scholar]
- 22.Fuchs H, Schilleman K, Hummler HD, Tepas AB. Techniques and devices to improve noninvasive ventilation in the delivery room. Neoreviews. 2012;13:355–7. [Google Scholar]
- 23.Kahn DJ, Habib RH, Courtney SE. Effects of flow amplitudes on intraprong pressures during bubble versus ventilator-generated nasal continuous positive airway pressure in premature infants. Pediatrics. 2008;122:1009–13. doi: 10.1542/peds.2007-3416. [DOI] [PubMed] [Google Scholar]
- 24.Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev. 2007;4:CD003063. doi: 10.1002/14651858.CD003063.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kribs A, Pillekamp F, Hünseler C, Vierzig A, Roth B. Early administration of surfactant in spontaneous breathing with nCPAP: Feasibility and outcome in extremely premature infants (postmenstrual age </=27 weeks) Paediatr Anaesth. 2007;17:364–9. doi: 10.1111/j.1460-9592.2006.02126.x. [DOI] [PubMed] [Google Scholar]
- 26.Mazela J, Polin RA. Aerosol delivery to ventilated newborn infants: Historical challenges and new directions. Eur J Pediatr. 2011;170:433–44. doi: 10.1007/s00431-010-1292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzella M, Bellini C, Calevo MG, Campone F, Massocco D, Mezzano P. et al. A randomised control study comparing the Infant Flow Driver with nasal continuous positive airway pressure in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;85:F86–90. doi: 10.1136/fn.85.2.F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: A randomized, controlled trial. Pediatrics. 2009;123:137–42. doi: 10.1542/peds.2007-3501. [DOI] [PubMed] [Google Scholar]
- 29.Vento G, Matassa PG, Zecca E, Tortorolo L, Martelli M, De Carolis MP, et al. Effect of dexamethasone on tracheobronchial aspirate fluid cytology and pulmonary mechanics in preterm infants. Pharmacology. 2004;71:113–9. doi: 10.1159/000077444. [DOI] [PubMed] [Google Scholar]
- 30.Soll RF. Current trials in the treatment of respiratory failure in preterm infants. Neonatology. 2009;95:368–72. doi: 10.1159/000209303. [DOI] [PubMed] [Google Scholar]
- 31.Cole CH, Colton T, Shah BL, Abbasi S, MacKinnon BL, Demissie S, et al. Early inhaled glucocorticoid therapy to prevent bronchopulmonary dysplasia. N Engl J Med. 1999;340:1005–1. doi: 10.1056/NEJM199904013401304. [DOI] [PubMed] [Google Scholar]
- 32.Jónsson B, Eriksson M, Söder O, Broberger U, Lagercrantz H. Budesonide delivered by dosimetric jet nebulization to preterm very low birthweight infants at high risk for development of chronic lung disease. Acta Paediatr. 2000;89:1449–55. doi: 10.1080/080352500456633. [DOI] [PubMed] [Google Scholar]
- 33.Jangaard KA, Stinson DA, Allen AC, Vincer MJ. Early prophylactic inhaled beclomethasone in infants less than 1250 g for the prevention of chronic lung disease. Paediatr Child Health. 2002;7:13–9. doi: 10.1093/pch/7.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]


