Abstract
Background: Many studies have been shown an important role of glutamatergic system as well microglial activa-tion in the pathophysiology of major depressive disorder (MDD). In humans most resistant to the development of psychiat-ric disorders, including MDD, are observed a greater degree of resilience resulting from stress. Less resilience is associated with neuroendocrine and neuroinflammatory markers, as well as with glutamatergic system dysregulation. Thus, this review we highlighted findings from literature identifying the function of glutamatergic system, microglial activation and inflamma-tion in resilience.
Methods: We conducted a review of computerized databases from 1970 to 2017.
Results: There is an association between microglial activation and glutamatergic system activation with stress vulnerability and resilience.
Conclusions: Glutamate neurotransmission, including neurotransmitter synthesis, signalling, and glutamate receptor func-tions and expression all seem to be involved with both stress vulnerability and resilience. Moreover, inflammation and mi-croglial activation mediate individual differences in resilience and the risk of stress-induced MDD.
Keywords: Glutamatergic system, AMPA receptor, NMDA receptor, microglia, neuroinflammation, resilience, stress vulnerability, major depressive disorder
1. INTRODUCTION
Mental illnesses are serious disorders that cause a high degree of burden around the world, even surpassing coronary diseases and cancer [1]. Major depressive disorder (MDD) is a pathology that affects many people worldwide, and is a major cause of disability [2]. Epidemiological data estimates that globally, around 121 million people are affected by MDD, with a prevalence of approximately 17% in the United States of America [3]. The symptoms of MDD include depressed mood, loss of concentration, anhedonia, as well as changes in appetite and sleep [4]. Furthermore, around one million people commit suicide each year, resulting in 3.000 deaths per day [5].
From the middle of the 21st century, scientists working within the psychiatric field have been undertaking research looking for new drugs that can reduce depressive symptoms by regulating the levels of monoamines, especially serotonin, norepinephrine and dopamine [6]. The diversified physiopathology of MDD has proven difficult to elucidate, with many hypotheses being suggested over the years. Historically, the monoaminergic deficiency theory has been used to explain the depressive symptoms seen in MDD. This occurs when the levels of monoamine neurotransmitters are decreased within the synaptic cleft [6-8]. Van Praag and Korf [9] defended the serotoninergic hypothesis soon after the dopaminergic hypothesis was created by Willner et al. [10]. Evidence suggests that genetic variations within the serotonin system can affect the stress response and increase the risk of developing MDD [11]. The critical brain circuitry underlying stressor reactivity and the regulation of emotion encompasses the amygdala, the ventromedial prefrontal corte and the dorsal raphe nucleus [12].
Historically, pharmacotherapy with antidepressants shows an efficacy of only 60-70% in patients that are diagnosed with MDD [13]. Even though these drugs have side effects, delayed actions and low remission rates, antidepressant drugs based on the monoaminergic theory are the first line of treatment used in MDD therapy. Due to this, there is a huge need for innovative new antidepressants in the treatment of this disorder [7, 14, 15].
Since the moanoaminergic system presents many limitations in explaining the pathophysiology of MDD, other pathways and systems have been investigated. Studies have been undertaken looking at the bioenergetic alterations detected in mood disorders, and this has been used to formulate a hypothesis based on mitochondrial pathogenetics [16, 17]. This hypothesis gives more evidence to the involvement of genetic factors being involved in the onset of MDD. The presence of a dysfunction involving mitochondrial bioenergetics increases the levels of oxidative stress and damage to brain tissue. Interestingly, studies have shown the involvement of oxidative stress in the pathophysiology of MDD [18, 19]. However, the pathophysiology of depression cannot be explained solely by the presence of oxidative stress. Notably, clinical data has shown that individuals with atypical depression have higher levels of inflammatory markers in their circulation when compared to controls and those with melancholic depression [20, 21]. The molecular mechanisms by which cytokines may impact behaviour are manifold, and influence the metabolism and function of noradrenalin, serotonin, dopamine, and neuroendocrine, leading to a flattening of the cortisol curve and increased evening concentrations of cortisol. Consequently, evidence indicates the existence of a link between the inflammatory processes and the hypothalamic–pituitary–adrenal (HPA) axis.
Studies have also indicated that decreased levels of brain derived-neurotrophic factor (BDNF) and neurogenesis are found in patients suffering from stress and MDD, and these changes are connected in part to the induction of innate immune cytokines [22-24]. The involvement of inflammatory processes could justify the alterations found in the microglia cells and glutamatergic system, as reported in patients with MDD [25-27]. Thus, this review also aims to highlight the roles that the glutamatergic system and microglial activation play in the brain’s vulnerability or resilience to stress, which could be key factors in the development of MDD.
2. RESILIENCE
Although many studies have managed to demonstrate some of the pathological mechanisms involved in the development and progression of MDD, the neurobiological mechanisms involved in this disorder are still far from being understood. Combinations of multiple genetic factors may be related to the development of MDD, since a defect in a single gene does not normally induce the expression of the depressive symptoms [28]. In addition, several non-genetic factors, such as stress, affective trauma and viral infections increase the complexity of the pathogenesis of MDD [29]. Some individuals, even after exposure to several stressors, do not develop MDD, presenting a condition known as resilience.
Resilience is defined as the adaptive maintenance of normal physiology, development and behaviour in the face of stress and adversity [30, 31]. In the literature, there are innumerable examples of adults and children, who despite significant psychological stress, present minimal changes in their emotional well-being or behavioural disorders [32-35]. Resilience is probably the result of the body’s successful response to adaptive stress, allowing it to maintain the status quo. The biological processes underlying resilience are often collectively termed “alostase”, and constitute variations in body systems which maintain homeostasis in response to a stressor [36]. However, for different reasons, some individuals do not maintain the capacity to develop resilience. Likewise, resilience probably results from successful allosteric mechanisms within the HPA axis, autonomic nervous system, immune system and brain [36]. In fact, in vulnerable individuals, stress can lead to monoaminergic dysfunction, decreased levels of BDNF and changes in the HPA axis [37, 38]. However, other brain regions and neurotransmitters are also being studied to elucidate the relationship between MDD and resilience. In a recent study with rats which were exposed to chronic stress, the vulnerability of gabaergic neurons to stress was evaluated [39]. The results showed that there was an involvement of gabaergic neurons in the nucleus accumbens, with a decrease in gabaergic release and expansion capacity as well as excitatory function, all of which are associated with MDD [39]. On the other hand, the invulnerability of these structures to chronic stress may be related to resilience [39].
An adequate response to stress involves the coordinated activity of the autonomic nervous system and the HPA axis, as well as the neural circuits in the hypothalamus, brainstem and prefrontal regions [40]. The association between MDD and changes in the HPA axis has been described over the years, as well as its involvement in neurogenesis [41]. The hypothalamus secretes a corticotrophin releasing hormone (CRH) which is transported to the anterior pituitary, where it stimulates adrenocorticotrophic hormone secretion (ACTH). This is then released and transported to the adrenal gland, where it stimulates the production and release of the glucocorticoid cortisol. Cortisol interacts with its receptors in the HPA axis, being responsible for negative feedback, so inhibiting CHR and ACTH and helping to maintain the body's homeostasis and its response to stress. Studies have shown that in depressed patients, the HPA axis may be affected by hyperactivity or hypoactivity, thus resulting in increased or decreased basal concentrations of hormones. Its activity may also be dysregulated, and this change may occur due to increased concentrations of one of the hormones at different times of the day, and unchanged or decreased levels of others [41, 42].
The relationship between childhood trauma and increased peripheral and central inflammation seems to be associated with epigenetic changes in FKBP5, a gene associated with MDD, as well as glucocorticoid sensitivity. Decreased activation of glucocorticoid receptor sensitivity and increased activation of regulated NF-κB genes are both a constant in the effects of chronic stress [43]. NkB is a nuclear transcription factor that transcribes DNA precursors and pro-inflammatory molecules.
Administration of glucocorticoids after exposure to a trauma has emerged as a potential treatment for individuals who are vulnerable to post-traumatic stress disorder (PTSD), since it appears to control the overactive response of fear, as well as the memory consolidation of fear [44]. This strategy has shown positive results in hospital patients and veterans exposed to combat [45, 46].
The alteration of the sex hormones also seems to be involved in MDD and stress resilience. In fact, oestrogen seems to play a part in the cognitive processes, affecting the neurotransmission and metabolism of monoamines, as well as in the expression of transcription factors and neurotrophins [47, 48]. Perimenopause associated with fluctuations of estradiol is thought to contribute towards a vulnerability for MDD. On the other hand, testosterone has emerged as a potential pro-resilience factor in men [49]. In addition, the levels of testosterone in the blood and saliva decrease after stress [47, 50] and low levels of testosterone are often found in individuals suffering with PTSD [51].
Several factors have shown to be involved in resilience. The identification of the characteristics involved in this condition can help to understand the pathophysiology of MDD and to suggest new perspectives for treatments.
3. GLUTAMATERGIC SYSTEM
Glutamate is the main excitatory neurotransmitter in the central nervous system (CNS), and under regular conditions, plays a role in synaptic plasticity, learning and memory [52]. Glutamate can be synthesized by the Krebs/ tricarboxylic acid cycle using glucose [53], or recaptured by surrounding astrocytes, where it is catalyzed into glutamine by the glutamine sinthetase enzyme, after which it can return to neurons to be resynthesized in glutamate [54, 55].
Recent studies have proposed that increased levels of extracellular glutamate are associated with MDD [7, 14, 56], which can cause neuronal damage or death by excitotoxicity [57, 58]. However, the response of the glutamatergic system to classical antidepressants and modulators can be related to the low levels of glutamine and biochemical cascades that are associated with glutamatergic signaling [26].
Vesicular glutamate transporters (VGLUTs) store glutamate in vesicles that are activated by Ca2+ binding [59], and then release glutamate via exocytosis into the synaptic cleft with the support of the soluble N-ethylmaleimide-sensitive factor-attachment protein receptor (SNARE) complex proteins [60]. Glutamate interacts with ionotropic (iGluRs) and metabotropic (mGluRs) receptors. mGluRs are receptors that are coupled to G proteins. On the other hand, iGluRs are ion channels that promote depolarization of the neuron through the influx of Ca2+ and Na+, IGluRs being subdivided into N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), and kainate [61].
Glutamate binds to AMPA and kainate receptors inducing a conformational alteration and subsequent influx of Na+, promoting a fast synaptic transmission [62, 63]. The NMDA receptor is blocked by a magnesium ion during cell resting. The removal of the magnesium ion requires the depolarization of the cell that happens after the binding of glutamate to the AMPA or kainate receptors [64, 65]. The activation of NMDA receptor requires the binding of the co-agonist glycine or D-serine [66, 67].
Several mental illnesses and neurological disorders such as schizophrenia, MDD and stroke, are related to a dysfunction in NMDA receptor activity, which is due to extrasynaptic NMDA receptor activation [68]. The glutamatergic system has proven to be an extremely important novel target for the antidepressant drugs that are used in the treatment of monoamine treatment-resistant patients. In fact, studies have shown that a partial agonist of the NMDA receptors, D-cycloserine, exhibited antidepressant effects [69, 70]. Ketamine, a dissociative anaesthetic which has high affinity for the NMDA receptor, acts as a non-competitive antagonist that avoids excessive entry of extracellular Ca2+ and cellular damage [71]. In preclinical studies, ketamine has shown a rapid antidepressant-like behaviour in animal models of depression [72-75]. In human studies, a single intravenous injection of ketamine (0.5 mg/kg) leads to a decrease of depressive symptoms in treatment-resistant patients when compared to standard antidepressants [76-79]. The mechanisms by which ketamine achieves its rapid antidepressant effects have been discussed and reported by several studies. BDNF, a neurotrophin which is important in both antidepressant responses and MDD, was found to have increased within the hippocampus after acute treatment in rats [73]. Additionally, the administration of ketamine reversed the low levels of BDNF and high levels of corticosterone caused by CMS [80].
The antidepressant effects of ketamine are also associated with the activation of AMPA receptors. The effects of a single injection of ketamine (10 mg/kg) in combination with the antidepressant fluoxetine and the antipsychotic olanzapine enhanced AMPA and NMDA-induced currents, but it was more noticeable on AMPA receptors [81]. On the other hand, using the 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f] quinoxaline-7- sulfonamide (NBQX), an AMPA receptor inhibitor, abolished the antidepressant effects of ketamine [82, 83]. The mammalian target of rapamycin (mTOR) has a role in both the pathophysiology of MDD and the antidepressant properties of ketamine [84-88]. Indeed, mTOR signaling in the prefrontal cortex was stimulated by the administration of ketamine [88]. Ketamine also induces synaptogenesis and glutamate transmission, leading to an activation of AKT and extracellular signal regulated kinase signaling, elevated levels of cAMP response element-binding protein (CREB), and elevated levels of the BDNF receptor, tropomyosin receptor kinase B (TrkB) [89].
4. MICROGLIA
Around 10% of the brain is composed of microglial cells, and these play a pivotal role in the normal development and regulation of structural and functional processes, from individual synapses to neural circuits and behaviour [90, 91]. The microglia are created in the peripheral mesodermal tissue during embryonary development, which in contrast to neurons, astrocytes, and oligodendrocytes, are derived from the neuroectoderm [92]. In the developing CNS, microglia regulate neuronal precursor cell sets, as well as synaptogenesis and the formation of neural networks [93, 94]. The “resting” microglia processes are highly mobile even under normal inactive conditions within the adult CNS. Frequently, the brief contacts that microglial processes have with presynaptic and postsynaptic neuronal elements contribute to the regulation, formation and elimination of synapses (pruning) [90, 91].
In conditions of disrupted brain homeostasis, such as: infection, injury, neurodegeneration, or markedly altered neuronal activity, the structure and function of microglial cells are rapidly and profoundly altered, and they assume an ‘activated’ status which is characterized by (I) fast and targeted movement of microglial processes in the direction of the site of infection or injury; (II) a proliferation and increase in the density of microglial cells; (III) morphological alterations, including enlargement of the soma, increase in the diameter of primary processes, shortening of distal processes, and, in fully activated microglia, complete retraction of all processes and seizure of an amoeboid morphology; (IV) enhanced phagocytic activity; and (V) production and secretion of inflammatory cytokines and other mediators [95]. It is now evident that microglial activation is not an ‘all or none’ process; in other words, microglia can undergo multiple functional alterations or programs, which confer specific adaptation for coping with diverse pathological conditions [95].
The microglia can be active or inhibited, these processes are controlled by exogenous and endogenous ‘alarm’ molecules (‘on’ signals) or (‘off’ signals) [96]. Neuroinflammation activates the microglia in an adaptive process that allows the elimination of pathogens. However, under many circumstances, including neurodegenerative diseases such as Alzheimer's and Parkinson's, activated microglia become neurotoxic to neurons and other glial cells [97, 98].
The intervention of microglial functioning can also occur in conditions that induce microglial suppression, decline, and senescence. These interferences produce changes in behaviour, cognition, and mood. They are usually transient and may be long lasting, for example, in association with chronic infections, stroke, trauma, neurodegenerative diseases, or chronic psychiatric disorders [99].
Several bacterial and viral infections are associated with a variety of depressive symptoms [100]. Many of these infectious pathogens have a special affinity for the brain, where they induce microglial activation [101]. These pathogens also induce the secretion of proinflammatory cytokines by microglia [102], and many studies have correlated the plasma levels of cytokines with depressive symptomatology [103, 104], with the experimental administration of immune challenges that are known to activate microglia [e.g., endotoxin (lipopolysaccharide, LPS) or Salmonella typhi] inducing depressive symptoms. The severity of MDD is highly correlated with elevated blood levels of inflammatory cytokines [105-107]. In fact, patients with MDD present with increased serum levels of proinflammatory cytokines, such as interleukin-1 (IL-1), IL-6, IL-8, IL-12, interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) [108, 109]. In addition, Dahl and collaborators, [110] showed that patients with MDD presented with elevated plasma levels of IL-1β, IL-1 receptor antagonists, IL-5, IL-6, IL-7, IL-8, IL-10, granulocyte colony-stimulating factor (G-CSF), and IFN-γ. Moreover, the cytokines were shown to have reduced to normal levels after 12 weeks of treatment with antidepressants [110].
Many studies have demonstrated that exposure to stress can induce microglial activation [25, 27]. Both in humans and in experimental animals, stress is causally related to the development of MDD [111-113]. Interestingly, a post-mortem study found an increase in the levels of microglial activation and macrophage recruitment within the dorsal anterior cingulate matter from individuals suffering from MDD [114]. In addition, in individuals who committed suicide, microglial activation was also higher in the ventral prefrontal white matter [115].
5. GLUTAMATERGIC SYSTEM IN THE RESILIENCE
Studies have shown that the glutamatergic system plays an important role in both the vulnerability and resilience to stress. In fact, chronic stress reduced the levels of glutamatergic neurotransmission, decreased the surface expression of AMPA receptors and induced depressive-like behaviour in rodents [116]. Additionally, Li et al. [116] also demonstrated that the modulation of AMPA receptor trafficking and synaptic plasticity mediated by caspase-1 and IL-1β was altered, and this was associated with a vulnerability to stress. Other studies have also related that the surface expression of AMPA receptors in the hippocampus is decreased when the levels of IL-1β are elevated [117, 118].
Chronic mild stress is a widely used animal model. This animal model induces depressive-like behaviour, including anhedonia, and changes in the brain structures that are shown to be involved with MDD [119-123]. Moreover, antidepressant drugs as well as new therapeutic targets with antidepressant properties are able to reverse both the depressive-like behaviour and neurochemical changes induced by chronic mild stress [80, 124-126]. Delgado y Palacios et al. [127] showed that resilient rodents subjected to chronic mild stress had increased levels of glutamate and an inward displacement in the hippocampus, when compared to anhedonic animals. Some animals that are subjected to chronic mild stress develop anhedonic behaviour; however, other animals are resilient. For example, Toth et al. [128] found anhedonic behaviour in an adult within their study, but not in the young rats following chronic mild stress. In addition, in rats with depressive-like behaviour, it has been observed that there is a decrease in neurogenesis and a drop in the levels of BDNF within the hippocampus, and also altered levels of the AMPA receptor within the hippocampus and nucleus accumbens, none of which were reported in young rats with no depressive-like behaviour [128].
AMPA receptor functioning has been shown to be important in determining vulnerability to stress. In fact, mice subjected to chronic social stress had lower mRNA expression of the AMPA receptor subunit GluR1 in the dentate gyrus and CA1 region of the hippocampus, with these effects being reported in vulnerable stressed mice, when compared to the resilient mice [129]. Interestingly, the fast antidepressant action of ketamine seems to be mediated by AMPA receptors [130, 131]. The synaptic plasticity induced by NMDA receptor expression and activation is also critical in the positive emotional learning needed to induce resilience to MDD [132]. In response to acute or chronic stress in rodents with a history of chronic stress, it was observed that the mGlu2 receptor and the NR1 subunit of the NMDA receptor are important in regulating epigenetic plasticity within the hippocampus, and these effects were associated with depressive and anxiety-like behaviours [133].
mGluR5−/− mice displayed depressive-like behaviour following experience of stressful stimulus [134]. In addition, knockout mice for the Homer I protein, which interacts with mGlu5 receptors, had more susceptibility to stress [135]. On the other hand, the activation of mGlu5 receptor in the nucleus accumbens was associated with the development of stress resilience [134].
Learned helplessness (LH) is an animal model in which rodents display depressive-like behaviour after repeated uncontrollable stress [136]. In addition, non-LH behaviour is associated with stress resilience [137, 138]. Muneoka et al. [139] studied the effects of LH on amino acid levels, and demonstrated that non-LH models had increased levels of GABA and glutamine, and LH models had a reduction in their levels of glutamine, showing that the regulation of amino acids is required during the recovery from depressive-like behaviour.
6. MICROGLIA AND INFLAMMATION IN THE RESILIENCE TO STRESS
Microglia cells have been shown to play an important role in the pathophysiology of MDD [25, 140]. Additionally, the status of microglial activation and inflammation also appear to be associated with stress vulnerability and resilience. The activation of microglia in response to repeated stress seems to be regulated by inflammatory mediators, mainly, IL-1β and prostaglandin (PG) E2 (PGE2) [141]. Inflammation and microglial activation have been pointed out as being involved with individual differences in risk and resilience to MDD induced by stress [142]. Knockout mice for soluble epoxide hydrolase (sEH), which plays a role in inflammation, did not develop depressive-like behaviour after repeated social defeat stress, showing a resilience to stress [143]. In addition, it was found that the levels of sEH were high in chronically stressed rats, as well as in the brain samples of MDD patients, when compared to control rats or healthy individuals [143]. Another pathway that is involved with inflammation is the activation of the transcription factor Keap1-Nrf2 system. Yao et al. [144] demonstrated that nuclear factor erythroid 2-related factor 2 (Nrf2) knockout mice had an increase in their serum levels of pro-inflammatory cytokines. On the other hand, pre-treatment with an Nrf2 activator (sulforaphane) and a diet containing a sulforaphane precursor, reversed the depressive-like behaviour that was induced by repeated social defeat stress, showing the role of Nrf2 in stress resilience [144]. Lower levels of BDNF in those vulnerable to stress were associated with decreases in Nrf2 translocation and oxidative stress [145]. Contrary to this, the activation of Nrf2 translocation restored redox homeostasis and induced resilience [145]. In an elegant study by Brachman et al. [146] isolated lymphocytes from mice were subjected to chronic social defeat stress and transferred to naive Rag2 knockout mice, which have lack of mature lymphocytes. The results showed that the mice which received lymphocytes had less anxiety and an increase in social behaviour, when compared to the mice that received no cells or cells from non-stressed mice [146]. Moreover, the mice that received lymphocytes had an anti-inflammatory status mediated by microglial cells [146]. Mice susceptible to anhedonia were found to have an increase in their levels of pro-inflammatory cytokines, 5-HT transporter (SERT), and also in the numbers of Iba-1, a marker of microglial cell activation, within the prefrontal area, when compared to resilient mice [147]. In rats exposed to chronic unpredictable stress, ovarian hormones provided resilience against the development of depressive-like behaviour as well as changes in the HPA axis [148]. In the same experiment, fluoxetine treatment did not have efficacy in reversing the behavioural impairments and neuroendocrine changes induced by stress; however, fluoxetine reduced microglial activation, and increased neurogenesis and cell proliferation in the dentate gyrus of the hippocampus of stressed rats [148].
Recently, studies have demonstrated that intestinal microbiota have an important role in MDD and stress resilience [149-151]. In fact, there is a communication between gut microbiota and the brain. Gut microbes could affect brain functions by altering neurotransmitters and metabolites [152-154]. Conversely, the brain acts on the gut microbiota and immune functions by altering the composition of intestinal microbiota [155, 156]. Yang et al. [157] showed that in resilient mice, more of the probiotic bacterium, Bifidobacterium, were detected when compared to the control or susceptible mice, which had low levels of Bifidobacterium. Also, the oral intake of Bifidobacterium increased the number of resilient mice after chronic social defeat stress [157], suggesting that an elevation in gut Bifidobacterium may contribute to stress resilience.
CONCLUSION
Glutamate neurotransmission, including neurotransmitter synthesis, signalling, and glutamate receptor functions and expression all seem to be involved with both stress vulnerability and resilience. In addition, inflammation and microglial activation mediate individual differences in resilience and the risk of stress-induced MDD. However, the mechanisms involved in the resilience response mediated by microglia cells and the glutamatergic system are not fully clarified. It remains unclear whether microglial activation can lead to the onset of MDD by increasing the levels of inflammatory cytokines or by inducing a dysregulation in the glutamatergic system, or whether a genetic predisposition to stress or resilience could be a key factor for inflammation and the changes to glutamatergic system seen in response to a stressor (Fig. 1). Future studies investigating glutamate and its receptor, as well microglial activation in resilience could be important in understanding the pathophysiology of MDD, as well as in finding new novel therapeutic targets for depressive individuals. Additionally, preclinical studies investigating ketamine, which is an antagonist of the NMDA receptor, and other anti-inflammatory drugs, such as minocycline, in stress vulnerability could be noteworthy in helping to prevent MDD in vulnerable individuals.
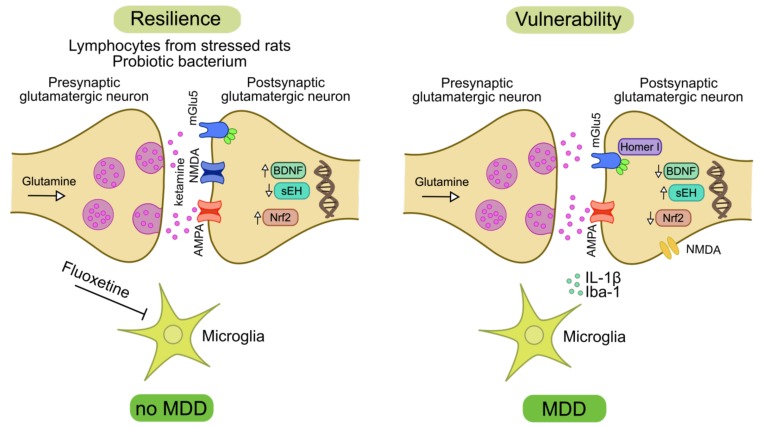
Fig. (1).
The association of glutamatergic system and microglial activation to promote resilience or stress vulnerability. Vulnerability to stress is linked to impairment in the glutamatergic system neurotransmission. A decrease in the AMPA expression mediated by increased levels of IL-1β is associated with stress vulnerability. BDNF levels and neurogenesis that are regulated in part by glutamate receptor activation are decreased in stress vulnerability. On the other hand, ketamine that acts in NMDA and AMPA receptors increases BDNF levels, thus promoting resilience. A deficiency of mGlu5 receptors in proteins that regulate mGlu5 receptor, such as Homer I protein, leads to a more stress vulnerability. Contrarily, mGlu5 receptor activation leads to resilience. The microglial activation mediated by Iba-1 marker and increased levels of IL-1β are associated with stress vulnerability. Classical antidepressants, as fluoxetine, inhibit the microglial activation. The levels of the transcription of Nrf2 are increased in the stress resilience and decreased in stress vulnerability. Augmented levels of Nrf2 are associated to a reduction in the BDNF levels. Increased levels of sEH stimulate stress vulnerability, while the inhibition of sEH promotes resilience. Probiotic bacterium and lymphocytes from stressed rats display anti-inflammatory effects and resilience. At last a dysregulation in the glutamatergic system, microglial activation, and an increase in the inflammation mediators are associated with stress vulnerability and the development of MDD. On the other hand, drugs that modulate the glutamatergic system and reduce inflammation promote resilience and prevent MDD development. AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; BDNF = brain-derived neurotrophic factor; IL-1β = interleukin-1β; MDD = major depressive disorder; NMDA = N-methyl-D-aspartate; Nrf2 = nuclear factor erythroid 2-related factor 2; sEH = soluble epoxide hydrolase.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
The Translational Psychiatry Program (USA) is funded by the Department of Psychiatry and Behavioral Sciences, McGovern Medical School, The University of Texas Health Science Center at Houston (UTHealth). Laboratory of Neurosciences (Brazil) is one of the centers of the National Institute for Molecular Medicine (INCT-MM) and one of the members of the Center of Excellence in Applied Neurosciences of Santa Catarina (NENASC). Its research is supported by grants from CNPq (JQ), FAPESC (JQ); Instituto Cérebro e Mente (JQ) and UNESC (JQ and GZR). JQ is a 1A CNPq Research Fellow. We thank Leandro D.V. Soares for making the figure.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Reeves W.C., Strine T.W., Pratt L.A., Thompson W., Ahluwalia I., Dhingra S.S., McKnight-Eily L.R., Harrison L., D’Angelo D.V., Williams L., Morrow B., Gould D., Safran M.A. Centers for Disease Control and Prevention (CDC). Mental illness surveillance among adults in the United States. MMWR Surveill. Summ. 2011;60:1–29. [PubMed] [Google Scholar]
- 2.Ferrari A.J., Charlson F.J., Norman R.E., Patten S.B., Freedman G., Murray C.J., Vos T., Whiteford H.A. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. [http://dx.doi.org/10.1371/journal.pmed.1001547]. [PMID: 24223526]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sattler R., Rothstein J.D. Targeting an old mechanism in a new disease-protection of glutamatergic dysfunction in depression. Biol. Psychiatry. 2007;61(2):137–138. doi: 10.1016/j.biopsych.2006.11.011. [http://dx.doi.org/10.1016/ j.biopsych.2006.11.011]. [PMID: 17223439]. [DOI] [PubMed] [Google Scholar]
- 4.Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Gold S.J., Monteggia L.M. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [http://dx.doi.org/10.1016/S0896-6273(02)00653-0]. [PMID: 11931738]. [DOI] [PubMed] [Google Scholar]
- 5.2012.
- 6.Berton O., Nestler E.J. New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 2006;7(2):137–151. doi: 10.1038/nrn1846. [http://dx.doi.org/10.1038/nrn1846]. [PMID: 16429123]. [DOI] [PubMed] [Google Scholar]
- 7.Delgado P.L. Depression: the case for a monoamine deficiency. J. Clin. Psychiatry. 2000;61(Suppl. 6):7–11. [PMID: 10775018]. [PubMed] [Google Scholar]
- 8.Schildkraut J.J. The catecholamine hypothesis of affective disorders: a review of supporting evidence. 1965. J. Neuropsychiatry Clin. Neurosci. 1995;7(4):524–533. doi: 10.1176/jnp.7.4.524. [http://dx.doi.org/10.1176/ jnp.7.4.524]. [PMID: 8555758]. [DOI] [PubMed] [Google Scholar]
- 9.van Praag H.M., Korf J. A pilot study of some kinetic aspects of the metabolism of 5-hydroxytryptamine in depressive patients. Biol. Psychiatry. 1971;3(2):105–112. [PMID: 5089909]. [PubMed] [Google Scholar]
- 10.Willner P., Sampson D., Phillips G., Muscat R. A matching law analysis of the effects of dopamine receptor antagonists. Psychopharmacology (Berl.) 1990;101(4):560–567. doi: 10.1007/BF02244238. [http://dx.doi.org/ 10.1007/BF02244238]. [PMID: 2201990]. [DOI] [PubMed] [Google Scholar]
- 11.Pezawas L., Meyer-Lindenberg A., Drabant E.M., Verchinski B.A., Munoz K.E., Kolachana B.S., Egan M.F., Mattay V.S., Hariri A.R., Weinberger D.R. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [http://dx.doi.org/10.1038/nn1463]. [PMID: 15880108]. [DOI] [PubMed] [Google Scholar]
- 12.Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci. Biobehav. Rev. 2008;32(7):1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [http://dx.doi.org/10.1016/j.neubiorev. 2008.03.006]. [PMID: 18439676]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souery D., Amsterdam J., de Montigny C., Lecrubier Y., Montgomery S., Lipp O., Racagni G., Zohar J., Mendlewicz J. Treatment resistant depression: methodological overview and operational criteria. Eur. Neuropsychopharmacol. 1999;9(1-2):83–91. doi: 10.1016/s0924-977x(98)00004-2. [http://dx.doi.org/10.1016/S0924-977X(98)00004-2]. [PMID: 10082232]. [DOI] [PubMed] [Google Scholar]
- 14.Rogóz Z., Skuza G., Daniel W.A., Wójcikowski J., Dudek D., Wróbel A. Amantadine as an additive treatment in patients suffering from drug-resistant unipolar depression. Pharmacol. Rep. 2007;59(6):778–784. [PMID: 18195470]. [PubMed] [Google Scholar]
- 15.Trivedi M.H., Hollander E., Nutt D., Blier P. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J. Clin. Psychiatry. 2008;69(2):246–258. doi: 10.4088/jcp.v69n0211. [http://dx.doi.org/10.4088/JCP.v69n0211]. [PMID: 18363453]. [DOI] [PubMed] [Google Scholar]
- 16.DiMauro S., Schon E.A. Mitochondrial disorders in the nervous system. Annu. Rev. Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [http://dx.doi.org/ 10.1146/annurev.neuro.30.051606.094302]. [PMID: 18333761]. [DOI] [PubMed] [Google Scholar]
- 17.Gardner A., Boles R.G. Mitochondrial energy depletion in depression with somatization. Psychother. Psychosom. 2008;77(2):127–129. doi: 10.1159/000112891. [http://dx.doi.org/10.1159/000112891]. [PMID: 18230947]. [DOI] [PubMed] [Google Scholar]
- 18.Szuster-Ciesielska A., Słotwińska M., Stachura A., Marmurowska-Michałowska H., Dubas-Slemp H., Bojarska-Junak A., Kandefer-Szerszeń M. Accelerated apoptosis of blood leukocytes and oxidative stress in blood of patients with major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(3):686–694. doi: 10.1016/j.pnpbp.2007.11.012. [http://dx.doi.org/10.1016/j.pnpbp.2007.11.012]. [PMID: 18083280]. [DOI] [PubMed] [Google Scholar]
- 19.Réus G.Z., Simões L.R., Colpo G.D., Scaini G., Oses J.P., Generoso J.S., Prossin A.R., Kaddurah-Daouk R., Quevedo J., Barichello T. Ketamine potentiates oxidative stress and influences behavior and inflammation in response to lipolysaccharide (LPS) exposure in early life. Neuroscience. 2017;353:17–25. doi: 10.1016/j.neuroscience.2017.04.016. Epub ahead of print [http://dx.doi.org/10.1016/j.neuroscience.2017.04.016]. [PMID: 28433652]. [DOI] [PubMed] [Google Scholar]
- 20.Lamers F., Vogelzangs N., Merikangas K.R., de Jonge P., Beekman A.T., Penninx B.W. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry. 2013;18(6):692–699. doi: 10.1038/mp.2012.144. [http://dx.doi.org/10.1038/mp.2012.144]. [PMID: 23089630]. [DOI] [PubMed] [Google Scholar]
- 21.Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry. 2016;21(12):1696–1709. doi: 10.1038/mp.2016.3. [http://dx.doi.org/10.1038/ mp.2016.3]. [PMID: 26903267]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo J.W., Duman R.S. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. USA. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [http://dx.doi.org/10.1073/pnas. 0708092105]. [PMID: 18178625]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller A.H. Depression and immunity: a role for T cells? Brain Behav. Immun. 2010;24(1):1–8. doi: 10.1016/j.bbi.2009.09.009. [http://dx.doi.org/10.1016/ j.bbi.2009.09.009]. [PMID: 19818725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwata M., Ota K.T., Duman R.S. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [http://dx.doi.org/10. 1016/j.bbi.2012.12.008]. [PMID: 23261775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Réus G.Z., Fries G.R., Stertz L., Badawy M., Passos I.C., Barichello T., Kapczinski F., Quevedo J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–154. doi: 10.1016/j.neuroscience.2015.05.018. [http://dx.doi.org/ 10.1016/j.neuroscience.2015.05.018]. [PMID: 25981208]. [DOI] [PubMed] [Google Scholar]
- 26.Réus G.Z., Abelaira H.M., Tuon T., Titus S.E., Ignácio Z.M., Rodrigues A.L., Quevedo J. Glutamatergic NMDA receptor as therapeutic target for depression. Adv. Protein Chem. Struct. Biol. 2016;103:169–202. doi: 10.1016/bs.apcsb.2015.10.003. [http://dx.doi.org/10.1016/bs.apcsb.2015. 10.003]. [DOI] [PubMed] [Google Scholar]
- 27.Yirmiya R., Rimmerman N., Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38(10):637–658. doi: 10.1016/j.tins.2015.08.001. [http://dx. doi.org/10.1016/j.tins.2015.08.001]. [PMID: 26442697]. [DOI] [PubMed] [Google Scholar]
- 28.Burmeister M. Basic concepts in the study of diseases with complex genetics. Biol. Psychiatry. 1999;45(5):522–532. doi: 10.1016/s0006-3223(98)00316-3. [http://dx. doi.org/10.1016/S0006-3223(98)00316-3]. [PMID: 10088042]. [DOI] [PubMed] [Google Scholar]
- 29.Nemeroff C.B., Owens M.J. Treatment of mood disorders. Nat. Neurosci. 2002;5(Suppl.):1068–1070. doi: 10.1038/nn943. [http://dx.doi.org/10.1038/ nn943]. [PMID: 12403988]. [DOI] [PubMed] [Google Scholar]
- 30.Reich J.W., Zautra A., Hall J.S. Handbook of Adult Resilience. New York: The Guilford Press; 2010. [Google Scholar]
- 31.Holling C.S., Schindler D.W., Walker B.W., Roughgarden J. Biodiversity in the functioning of ecosystems: An ecological synthesis. Biodivers. Loss; 1995. pp. 44–83. [http://dx.doi.org/10.1017/ CBO9781139174329.005] [Google Scholar]
- 32.Feder A., Nestler E.J., Charney D.S. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 2009;10(6):446–457. doi: 10.1038/nrn2649. [http://dx.doi.org/10.1038/nrn2649]. [PMID: 19455174]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yehuda R., Brand S., Yang R.K. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol. Psychiatry. 2006;59(7):660–663. doi: 10.1016/j.biopsych.2005.08.027. [http://dx.doi.org/10.1016/j.biopsych.2005.08.027]. [PMID: 16325152]. [DOI] [PubMed] [Google Scholar]
- 34.Fredrickson B.L., Tugade M.M., Waugh C.E., Larkin G.R. What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. J. Pers. Soc. Psychol. 2003;84(2):365–376. doi: 10.1037//0022-3514.84.2.365. [http://dx.doi.org/10.1037/0022-3514.84.2.365]. [PMID: 12585810]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonanno G.A. Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? Am. Psychol. 2004;59(1):20–28. doi: 10.1037/0003-066X.59.1.20. [http://dx.doi.org/10. 1037/0003-066X.59.1.20]. [PMID: 14736317]. [DOI] [PubMed] [Google Scholar]
- 36.McEwen B.S. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol. Aging. 2002;23(5):921–939. doi: 10.1016/s0197-4580(02)00027-1. [http://dx.doi.org/10.1016/S0197-4580(02)00027-1]. [PMID: 12392796]. [DOI] [PubMed] [Google Scholar]
- 37.Berton O., Hahn C.G., Thase M.E. Are we getting closer to valid translational models for major depression? Science. 2012;338(6103):75–79. doi: 10.1126/science.1222940. [http://dx.doi.org/10.1126/science.1222940]. [PMID: 23042886]. [DOI] [PubMed] [Google Scholar]
- 38.Strekalova T., Couch Y., Kholod N., Boyks M., Malin D., Leprince P., Steinbusch H.M. Update in the methodology of the chronic stress paradigm: internal control matters. Behav. Brain Funct. 2011;7:9. doi: 10.1186/1744-9081-7-9. [http://dx.doi.org/10.1186/1744-9081-7-9]. [PMID: 21524310]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Z., Wang G., Ma K., Cui S., Wang J.H. GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression. Oncotarget. 2017;8(22):35933–35945. doi: 10.18632/oncotarget.16411. [PMID: 28415589]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [http://dx.doi.org/10.1038/nrn2647]. [PMID: 19469025]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vilela L.H. Relação da depressão com os eixos hipotálamo-hipófiseadrenal, hipotálamo-hipófise-tireóide e o estresse precoce. 2014.
- 42.Cunha C.F., Silva I.N. Resposta hipofisária-adrenal ao teste de estímulo com o hormônio liberador da corticotrofina em crianças hospitalizadas. Arq. Bras. Endocrinol. Metabol. 2002;46:161–166. [http://dx.doi.org/10.1590/S0004-27302002000200008]. [Google Scholar]
- 43.Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [http://dx.doi.org/10.1038/nri.2015. 5]. [PMID: 26711676]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kearns M.C., Ressler K.J., Zatzick D., Rothbaum B.O. Early interventions for PTSD: a review. Depress. Anxiety. 2012;29(10):833–842. doi: 10.1002/da.21997. [http://dx.doi.org/10.1002/da.21997]. [PMID: 22941845]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schelling G., Roozendaal B., Krauseneck T., Schmoelz M.D.E., Quervain D., Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Ann. N. Y. Acad. Sci. 2006;1071:46–53. doi: 10.1196/annals.1364.005. [http://dx.doi. org/10.1196/annals.1364.005]. [PMID: 16891561]. [DOI] [PubMed] [Google Scholar]
- 46.Surís A., North C., Adinoff B., Powell C.M., Greene R. Effects of exogenous glucocorticoid on combat-related PTSD symptoms. Ann. Clin. Psychiatry. 2010;22(4):274–279. [PMID: 21180658]. [PMC free article] [PubMed] [Google Scholar]
- 47.Pfau M.L., Russo S.J. Peripheral and central mechanisms of stress resilience. Neurobiol. Stress. 2015;1:66–79. doi: 10.1016/j.ynstr.2014.09.004. [http://dx.doi.org/ 10.1016/j.ynstr.2014.09.004]. [PMID: 25506605]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shanmugan S., Epperson C.N. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum. Brain Mapp. 2014;35(3):847–865. doi: 10.1002/hbm.22218. [http://dx.doi.org/10.1002/hbm.22218]. [PMID: 23238908]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russo S.J., Murrough J.W., Han M.H., Charney D.S., Nestler E.J. Neurobiology of resilience. Nat. Neurosci. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. [http://dx.doi.org/10.1038/nn.3234]. [PMID: 23064380]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan C.A., III, Wang S., Mason J., Southwick S.M., Fox P., Hazlett G., Charney D.S., Greenfield G. Hormone profiles in humans experiencing military survival training. Biol. Psychiatry. 2000;47(10):891–901. doi: 10.1016/s0006-3223(99)00307-8. [http://dx.doi.org/10.1016/S0006-3223(99) 00307-8]. [PMID: 10807962]. [DOI] [PubMed] [Google Scholar]
- 51.Mulchahey J.J., Ekhator N.N., Zhang H., Kasckow J.W., Baker D.G., Geracioti T.D., Jr Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology. 2001;26(3):273–285. doi: 10.1016/s0306-4530(00)00052-4. [http:// dx.doi.org/10.1016/S0306-4530(00)00052-4]. [PMID: 11166490]. [DOI] [PubMed] [Google Scholar]
- 52.Sanacora G., Zarate C.A., Krystal J.H., Manji H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008;7(5):426–437. doi: 10.1038/nrd2462. [http://dx.doi.org/10.1038/nrd2462]. [PMID: 18425072]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musazzi L., Treccani G., Mallei A., Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol. Psychiatry. 2013;73(12):1180–1188. doi: 10.1016/j.biopsych.2012.11.009. [http://dx.doi.org/10.1016/j.biopsych.2012.11.009]. [PMID: 23273725]. [DOI] [PubMed] [Google Scholar]
- 54.Cahoy J.D., Emery B., Kaushal A., Foo L.C., Zamanian J.L., Christopherson K.S., Xing Y., Lubischer J.L., Krieg P.A., Krupenko S.A., Thompson W.J., Barres B.A. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28(1):264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [http://dx.doi.org/10.1523/JNEUROSCI. 4178-07.2008]. [PMID: 18171944]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serafini G., Pompili M., Innamorati M., Dwivedi Y., Brahmachari G., Girardi P. Pharmacological properties of glutamatergic drugs targeting NMDA receptors and their application in major depression. Curr. Pharm. Des. 2013;19(10):1898–1922. doi: 10.2174/13816128113199990293. [http:// dx.doi.org/10.2174/13816128113199990293]. [PMID: 23173582]. [DOI] [PubMed] [Google Scholar]
- 56.Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [http://dx.doi.org/10.1038/ nature07455]. [PMID: 18923511]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olney J.W., de Gubareff T. Glutamate neurotoxicity and Huntington’s chorea. Nature. 1978;271(5645):557–559. doi: 10.1038/271557a0. [http://dx. doi.org/10.1038/271557a0]. [PMID: 146165]. [DOI] [PubMed] [Google Scholar]
- 58.Olney J.W., Labruyere J., de Gubareff T. Brain damage in mice from voluntary ingestion of glutamate and aspartate. Neurobehav. Toxicol. 1980;2(2):125–129. [PMID: 7290308]. [PubMed] [Google Scholar]
- 59.Meloni D., Gambarana C., De Montis M.G., Dal Prá P., Taddei I., Tagliamonte A. Dizocilpine antagonizes the effect of chronic imipramine on learned helplessness in rats. Pharmacol. Biochem. Behav. 1993;46(2):423–426. doi: 10.1016/0091-3057(93)90374-3. [http://dx.doi.org/10.1016/0091-3057(93)90374-3]. [PMID: 7903459]. [DOI] [PubMed] [Google Scholar]
- 60.Lesch K.P., Schmitt A. Antidepressants and gene expression profiling: how to SNARE novel drug targets. Pharmacogenomics J. 2002;2(6):346–348. doi: 10.1038/sj.tpj.6500150. [http://dx.doi.org/10.1038/sj.tpj.6500150]. [PMID: 12629500]. [DOI] [PubMed] [Google Scholar]
- 61.Dingledine R., Borges K., Bowie D., Traynelis S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51(1):7–61. [PMID: 10049997]. [PubMed] [Google Scholar]
- 62.Chittajallu R., Vignes M., Dev K.K., Barnes J.M., Collingridge G.L., Henley J.M. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379(6560):78–81. doi: 10.1038/379078a0. [http://dx.doi.org/10.1038/379078a0]. [PMID: 8538745]. [DOI] [PubMed] [Google Scholar]
- 63.Tang C.M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989;243(4897):1474–1477. doi: 10.1126/science.2467378. [http://dx.doi.org/ 10.1126/science.2467378]. [PMID: 2467378]. [DOI] [PubMed] [Google Scholar]
- 64.Laube B., Hirai H., Sturgess M., Betz H., Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18(3):493–503. doi: 10.1016/s0896-6273(00)81249-0. [http://dx.doi.org/ 10.1016/S0896-6273(00)81249-0]. [PMID: 9115742]. [DOI] [PubMed] [Google Scholar]
- 65.Lester R.A., Clements J.D., Westbrook G.L., Jahr C.E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346(6284):565–567. doi: 10.1038/346565a0. [http://dx.doi. org/10.1038/346565a0]. [PMID: 1974037]. [DOI] [PubMed] [Google Scholar]
- 66.Henneberger C., Papouin T., Oliet S.H., Rusakov D.A. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463(7278):232–236. doi: 10.1038/nature08673. [http://dx.doi.org/10.1038/ nature08673]. [PMID: 20075918]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panatier A., Theodosis D.T., Mothet J.P., Touquet B., Pollegioni L., Poulain D.A., Oliet S.H. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125(4):775–784. doi: 10.1016/j.cell.2006.02.051. [http://dx.doi.org/10.1016/j.cell.2006.02.051]. [PMID: 16713567]. [DOI] [PubMed] [Google Scholar]
- 68.Parsons M.P., Raymond L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014;82(2):279–293. doi: 10.1016/j.neuron.2014.03.030. [http://dx.doi.org/10.1016/j.neuron.2014.03.030]. [PMID: 24742457]. [DOI] [PubMed] [Google Scholar]
- 69.Crane G.E. Cyloserine as an antidepressant agent. Am. J. Psychiatry. 1959;115(11):1025–1026. doi: 10.1176/ajp.115.11.1025. [http://dx.doi.org/10.1176/ajp.115. 11.1025]. [PMID: 13637281]. [DOI] [PubMed] [Google Scholar]
- 70.Papp M., Moryl E. Antidepressant-like effects of 1-amino-cyclopropanecarboxylic acid and D-cycloserine in an animal model of depression. Eur. J. Pharmacol. 1996;316(2-3):145–151. doi: 10.1016/s0014-2999(96)00675-9. [http:// dx.doi.org/10.1016/S0014-2999(96)00675-9]. [PMID: 8982680]. [DOI] [PubMed] [Google Scholar]
- 71.Harrison N.L., Simmonds M.A. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br. J. Pharmacol. 1985;84(2):381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [http://dx.doi.org/ 10.1111/j.1476-5381.1985.tb12922.x]. [PMID: 2858237]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaturvedi H.K., Chandra D., Bapna J.S. Interaction between N-methyl-D-aspartate receptor antagonists and imipramine in shock-induced depression. Indian J. Exp. Biol. 1999;37(10):952–958. [PMID: 10783752]. [PubMed] [Google Scholar]
- 73.Garcia L.S., Comim C.M., Valvassori S.S., Réus G.Z., Barbosa L.M., Andreazza A.C., Stertz L., Fries G.R., Gavioli E.C., Kapczinski F., Quevedo J. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(1):140–144. doi: 10.1016/j.pnpbp.2007.07.027. [http:// dx.doi.org/10.1016/j.pnpbp.2007.07.027]. [PMID: 17884272]. [DOI] [PubMed] [Google Scholar]
- 74.Garcia L.S., Comim C.M., Valvassori S.S., Réus G.Z., Andreazza A.C., Stertz L., Fries G.R., Gavioli E.C., Kapczinski F., Quevedo J. Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels. Basic Clin. Pharmacol. Toxicol. 2008;103(6):502–506. doi: 10.1111/j.1742-7843.2008.00210.x. [http://dx.doi.org/10.1111/j.1742-7843.2008.00210.x]. [PMID: 19067677]. [DOI] [PubMed] [Google Scholar]
- 75.Réus G.Z., Nacif M.P., Abelaira H.M., Tomaz D.B., dos Santos M.A., Carlessi A.S., da Luz J.R., Gonçalves R.C., Vuolo F., Dal-Pizzol F., Carvalho A.F., Quevedo J. Ketamine ameliorates depressive-like behaviors and immune alterations in adult rats following maternal deprivation. Neurosci. Lett. 2015;584:83–87. doi: 10.1016/j.neulet.2014.10.022. [http://dx.doi.org/10.1016/j.neulet.2014.10.022]. [PMID: 25459283]. [DOI] [PubMed] [Google Scholar]
- 76.Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [http://dx.doi.org/10.1016/S0006-3223(99)00230-9]. [PMID: 10686270]. [DOI] [PubMed] [Google Scholar]
- 77.Zarate C.A., Jr, Singh J.B., Carlson P.J., Brutsche N.E., Ameli R., Luckenbaugh D.A., Charney D.S., Manji H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [http://dx.doi.org/10.1001/archpsyc.63.8.856]. [PMID: 16894061]. [DOI] [PubMed] [Google Scholar]
- 78.Liebrenz M., Stohler R., Borgeat A. Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. World J. Biol. Psychiatry. 2009;10(4 Pt 2):640–643. doi: 10.1080/15622970701420481. [http://dx. doi.org/10.1080/15622970701420481]. [PMID: 17853274]. [DOI] [PubMed] [Google Scholar]
- 79.aan het Rot, M.; Collins, K.A.; Murrough, J.W.; Perez, A.M.; Reich, D.L.; Charney, D.S.; Mathew, S.J. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry. 2010;67(2):139–145. doi: 10.1016/j.biopsych.2009.08.038. [http://dx.doi.org/ 10.1016/j.biopsych.2009.08.038]. [PMID: 19897179]. [DOI] [PubMed] [Google Scholar]
- 80.Garcia L.S., Comim C.M., Valvassori S.S., Réus G.Z., Stertz L., Kapczinski F., Gavioli E.C., Quevedo J. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(3):450–455. doi: 10.1016/j.pnpbp.2009.01.004. [http://dx.doi.org/10.1016/ j.pnpbp.2009.01.004]. [PMID: 19439250]. [DOI] [PubMed] [Google Scholar]
- 81.Björkholm C., Jardemark K., Schilström B., Svensson T.H. Ketamine-like effects of a combination of olanzapine and fluoxetine on AMPA and NMDA receptor-mediated transmission in the medial prefrontal cortex of the rat. Eur. Neuropsychopharmacol. 2015;25(10):1842–1847. doi: 10.1016/j.euroneuro.2015.07.002. [http://dx.doi.org/10.1016/j.euroneuro. 2015.07.002]. [PMID: 26233606]. [DOI] [PubMed] [Google Scholar]
- 82.Koike H., Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav. Brain Res. 2014;271:111–115. doi: 10.1016/j.bbr.2014.05.065. [http://dx.doi.org/10.1016/j.bbr.2014.05.065]. [PMID: 24909673]. [DOI] [PubMed] [Google Scholar]
- 83.Nishitani N., Nagayasu K., Asaoka N., Yamashiro M., Shirakawa H., Nakagawa T., Kaneko S. Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int. J. Neuropsychopharmacol. 2014;17(8):1321–1326. doi: 10.1017/S1461145714000649. [http://dx.doi.org/10.1017/ S1461145714000649]. [PMID: 24852262]. [DOI] [PubMed] [Google Scholar]
- 84.Abelaira H.M., Réus G.Z., Neotti M.V., Quevedo J. The role of mTOR in depression and antidepressant responses. Life Sci. 2014;101(1-2):10–14. doi: 10.1016/j.lfs.2014.02.014. [http://dx.doi.org/10.1016/j.lfs.2014.02.014]. [PMID: 24582593]. [DOI] [PubMed] [Google Scholar]
- 85.Abelaira H.M., Réus G.Z., Ignácio Z.M., Dos Santos M.A., de Moura A.B., Matos D., Demo J.P., da Silva J.B., Danielski L.G., Petronilho F., Carvalho A.F., Quevedo J. Ketamine exhibits different neuroanatomical profile after mammalian target of rapamycin inhibition in the prefrontal cortex: the role of inflammation and oxidative stress. Mol. Neurobiol. 2017;54(7):5335–5346. doi: 10.1007/s12035-016-0071-4. [http://dx.doi.org/10.1007/s12035-016-0071-4]. [PMID: 27590136]. [DOI] [PubMed] [Google Scholar]
- 86.Abelaira H.M., Réus G.Z., Ignácio Z.M., Dos Santos M.A., de Moura A.B., Matos D., Demo J.P., da Silva J.B., Michels M., Abatti M., Sonai B., Dal Pizzol F., Carvalho A.F., Quevedo J. Effects of ketamine administration on mTOR and reticulum stress signaling pathways in the brain after the infusion of rapamycin into prefrontal cortex. J. Psychiatr. Res. 2017;87:81–87. doi: 10.1016/j.jpsychires.2016.12.002. [http://dx. doi.org/10.1016/j.jpsychires.2016.12.002]. [PMID: 28017918]. [DOI] [PubMed] [Google Scholar]
- 87.Hoeffer C.A., Tang W., Wong H., Santillan A., Patterson R.J., Martinez L.A., Tejada-Simon M.V., Paylor R., Hamilton S.L., Klann E. Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60(5):832–845. doi: 10.1016/j.neuron.2008.09.037. [http://dx.doi.org/10.1016/j.neuron.2008. 09.037]. [PMID: 19081378]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li N., Lee B., Liu R.J., Banasr M., Dwyer J.M., Iwata M., Li X.Y., Aghajanian G., Duman R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [http://dx.doi.org/ 10.1126/science.1190287]. [PMID: 20724638]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Réus G.Z., Vieira F.G., Abelaira H.M., Michels M., Tomaz D.B., dos Santos M.A., Carlessi A.S., Neotti M.V., Matias B.I., Luz J.R., Dal-Pizzol F., Quevedo J. MAPK signaling correlates with the antidepressant effects of ketamine. J. Psychiatr. Res. 2014;55:15–21. doi: 10.1016/j.jpsychires.2014.04.010. [http://dx.doi.org/10.1016/j.jpsychires.2014.04. 010]. [PMID: 24819632]. [DOI] [PubMed] [Google Scholar]
- 90.Tremblay M.È., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. The role of microglia in the healthy brain. J. Neurosci. 2011;31(45):16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [http://dx.doi.org/10.1523/ JNEUROSCI.4158-11.2011]. [PMID: 22072657]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wake H., Moorhouse A.J., Miyamoto A., Nabekura J. Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 2013;36(4):209–217. doi: 10.1016/j.tins.2012.11.007. [http://dx. doi.org/10.1016/j.tins.2012.11.007]. [PMID: 23260014]. [DOI] [PubMed] [Google Scholar]
- 92.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., Samokhvalov I.M., Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [http://dx.doi.org/10.1126/science.1194637]. [PMID: 20966214]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., Ragozzino D., Gross C.T. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [http://dx.doi.org/10.1126/science.1202529]. [PMID: 21778362]. [DOI] [PubMed] [Google Scholar]
- 94.Schafer D.P., Lehrman E.K., Kautzman A.G., Koyama R., Mardinly A.R., Yamasaki R., Ransohoff R.M., Greenberg M.E., Barres B.A., Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [http://dx.doi.org/10.1016/j.neuron.2012.03.026]. [PMID: 22632727]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kettenmann H., Hanisch U.K., Noda M., Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [http:// dx.doi.org/10.1152/physrev.00011.2010]. [PMID: 21527731]. [DOI] [PubMed] [Google Scholar]
- 96.Hanisch U.K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [http://dx.doi.org/10.1038/ nn1997]. [PMID: 17965659]. [DOI] [PubMed] [Google Scholar]
- 97.Heneka M.T., Kummer M.P., Latz E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014;14(7):463–477. doi: 10.1038/nri3705. [http://dx.doi.org/10.1038/nri3705]. [PMID: 24962261]. [DOI] [PubMed] [Google Scholar]
- 98.Heppner F.L., Ransohoff R.M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16(6):358–372. doi: 10.1038/nrn3880. [http://dx.doi.org/10.1038/nrn3880]. [PMID: 25991443]. [DOI] [PubMed] [Google Scholar]
- 99.Prinz M., Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014;15(5):300–312. doi: 10.1038/nrn3722. [http://dx.doi.org/10.1038/nrn3722]. [PMID: 24713688]. [DOI] [PubMed] [Google Scholar]
- 100.Yirmiya R., Weidenfeld J., Pollak Y., Morag M., Morag A., Avitsur R., Barak O., Reichenberg A., Cohen E., Shavit Y., Ovadia H. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv. Exp. Med. Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [http://dx.doi.org/10.1007/978-0-585-37970-8_16]. [PMID: 10442179]. [DOI] [PubMed] [Google Scholar]
- 101.Rock R.B., Gekker G., Hu S., Sheng W.S., Cheeran M., Lokensgard J.R., Peterson P.K. Role of microglia in central nervous system infections. Clin. Microbiol. Rev. 2004;17(4):942–964. doi: 10.1128/CMR.17.4.942-964.2004. [http://dx.doi.org/10.1128/CMR.17.4.942-964.2004]. [PMID: 15489356]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vollmer-Conna U., Fazou C., Cameron B., Li H., Brennan C., Luck L., Davenport T., Wakefield D., Hickie I., Lloyd A. Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behaviour in humans. Psychol. Med. 2004;34(7):1289–1297. doi: 10.1017/s0033291704001953. [http://dx.doi.org/10.1017/S0033291704001953]. [PMID: 15697055]. [DOI] [PubMed] [Google Scholar]
- 103.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [http://dx.doi. org/10.1016/j.biopsych.2009.09.033]. [PMID: 20015486]. [DOI] [PubMed] [Google Scholar]
- 104.Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [http://dx. doi.org/10.1016/j.bbi.2015.06.001]. [PMID: 26065825]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reichenberg A., Yirmiya R., Schuld A., Kraus T., Haack M., Morag A., Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [http://dx.doi.org/10.1001/archpsyc.58.5.445]. [PMID: 11343523]. [DOI] [PubMed] [Google Scholar]
- 106.Harrison N.A., Brydon L., Walker C., Gray M.A., Steptoe A., Critchley H.D. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [http://dx.doi.org/10.1016/j. biopsych.2009.03.015]. [PMID: 19423079]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grigoleit J.S., Kullmann J.S., Wolf O.T., Hammes F., Wegner A., Jablonowski S., Engler H., Gizewski E., Oberbeck R., Schedlowski M. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One. 2011;6(12):e28330. doi: 10.1371/journal.pone.0028330. [http://dx.doi.org/10.1371/journal.pone.0028330]. [PMID: 22164271]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schiepers O.J., Wichers M.C., Maes M. Cytokines and major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29(2):201–217. doi: 10.1016/j.pnpbp.2004.11.003. [http://dx.doi.org/10.1016/j.pnpbp.2004.11.003]. [PMID: 15694227]. [DOI] [PubMed] [Google Scholar]
- 109.O’Brien S.M., Scully P., Fitzgerald P., Scott L.V., Dinan T.G. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J. Psychiatr. Res. 2007;41(3-4):326–331. doi: 10.1016/j.jpsychires.2006.05.013. [http://dx.doi.org/10.1016/j.jpsychires. 2006.05.013]. [PMID: 16870211]. [DOI] [PubMed] [Google Scholar]
- 110.Dahl J., Ormstad H., Aass H.C., Malt U.F., Bendz L.T., Sandvik L., Brundin L., Andreassen O.A. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. 2014;45:77–86. doi: 10.1016/j.psyneuen.2014.03.019. [http://dx.doi.org/10.1016/j.psyneuen.2014. 03.019]. [PMID: 24845179]. [DOI] [PubMed] [Google Scholar]
- 112.Goshen I., Yirmiya R. 2009.
- 113.Kreisel T., Frank M.G., Licht T., Reshef R., Ben-Menachem-Zidon O., Baratta M.V., Maier S.F., Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry. 2014;19(6):699–709. doi: 10.1038/mp.2013.155. [http://dx.doi.org/10.1038/mp.2013.155]. [PMID: 24342992]. [DOI] [PubMed] [Google Scholar]
- 114.Torres-Platas S.G., Cruceanu C., Chen G.G., Turecki G., Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [http://dx.doi.org/10.1016/j.bbi.2014.05.007]. [PMID: 24858659]. [DOI] [PubMed] [Google Scholar]
- 115.Schnieder T.P., Trencevska I., Rosoklija G., Stankov A., Mann J.J., Smiley J., Dwork A.J. Microglia of prefrontal white matter in suicide. J. Neuropathol. Exp. Neurol. 2014;73(9):880–890. doi: 10.1097/NEN.0000000000000107. [http:// dx.doi.org/10.1097/NEN.0000000000000107]. [PMID: 25101704]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li M.X., Zheng H.L., Luo Y., He J.G., Wang W., Han J., Zhang L., Wang X. Ni; L.; Zhou, H.Y.; Hu, Z.L1.; Wu, P.F.; Jin, Y.; Long, L.H.; Zhang, H.; Hu, G.; Chen, J.G.; Wang, F. Gene deficiency and pharmacological inhibition of caspase-1 confers resilience to chronic social defeat stress via regulating the stability of surface AMPARs. Mol. Psychiatry. doi: 10.1038/mp.2017.76. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bellinger F.P., Madamba S., Siggins G.R. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628(1-2):227–234. doi: 10.1016/0006-8993(93)90959-q. [http://dx.doi. org/10.1016/0006-8993(93)90959-Q]. [PMID: 8313151]. [DOI] [PubMed] [Google Scholar]
- 118.Lai A.Y., Swayze R.D., El-Husseini A., Song C. Interleukin-1 beta modulates AMPA receptor expression and phosphorylation in hippocampal neurons. J. Neuroimmunol. 2006;175(1-2):97–106. doi: 10.1016/j.jneuroim.2006.03.001. [http://dx.doi.org/10.1016/j.jneuroim.2006.03.001]. [PMID: 16626814]. [DOI] [PubMed] [Google Scholar]
- 119.Lucca G., Comim C.M., Valvassori S.S., Réus G.Z., Vuolo F., Petronilho F., Dal-Pizzol F., Gavioli E.C., Quevedo J. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem. Int. 2009;54(5-6):358–362. doi: 10.1016/j.neuint.2009.01.001. [http://dx.doi.org/10. 1016/j.neuint.2009.01.001]. [PMID: 19171172]. [DOI] [PubMed] [Google Scholar]
- 120.Lucca G., Comim C.M., Valvassori S.S., Réus G.Z., Vuolo F., Petronilho F., Gavioli E.C., Dal-Pizzol F., Quevedo J. Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J. Psychiatr. Res. 2009;43(9):864–869. doi: 10.1016/j.jpsychires.2008.11.002. [http://dx.doi.org/10.1016/j.jpsychires. 2008.11.002]. [PMID: 19100996]. [DOI] [PubMed] [Google Scholar]
- 121.Réus G.Z., Abaleira H.M., Michels M., Tomaz D.B., dos Santos M.A., Carlessi A.S., Matias B.I., Leffa D.D., Damiani A.P. Gomes, Vde.C.; Andrade, V.M.; Dal-Pizzol, F.; Landeira-Fernadez, J.; Quevedo, J. Anxious phenotypes plus environmental stressors are related to brain DNA damage and changes in NMDA receptor subunits and glutamate uptake. Mutat. Res. 2015;772:30–37. doi: 10.1016/j.mrfmmm.2014.12.005. [http://dx.doi.org/10.1016/j.mrfmmm.2014.12.005]. [PMID: 25772108]. [DOI] [PubMed] [Google Scholar]
- 122.Valvassori S.S., Resende W.R., Budni J., Dal-Pont G.C., Bavaresco D.V., Réus G.Z., Carvalho A.F., Gonçalves C.L., Furlanetto C.B., Streck E.L., Quevedo J. Sodium butyrate, a histone deacetylase inhibitor, reverses behavioral and mitochondrial alterations in animal models of depression induced by early- or late-life stress. Curr. Neurovasc. Res. 2015;12(4):312–320. doi: 10.2174/1567202612666150728121121. [http://dx.doi. org/10.2174/1567202612666150728121121]. [PMID: 26216027]. [DOI] [PubMed] [Google Scholar]
- 123.Ortmann C.F., Réus G.Z., Ignácio Z.M., Abelaira H.M., Titus S.E., de Carvalho P., Arent C.O., Dos Santos M.A., Matias B.I., Martins M.M., de Campos A.M., Petronilho F., Teixeira L.J., Morais M.O., Streck E.L., Quevedo J., Reginatto F.H. Enriched flavonoid fraction from Cecropia pachystachya Trécul Leaves exerts antidepressant-like behavior and protects brain against oxidative stress in rats subjected to chronic mild stress. Neurotox. Res. 2016;29(4):469–483. doi: 10.1007/s12640-016-9596-6. [http://dx.doi.org/10.1007/s12640-016-9596-6]. [PMID: 26762362]. [DOI] [PubMed] [Google Scholar]
- 124.Fortunato J.J., Réus G.Z., Kirsch T.R., Stringari R.B., Fries G.R., Kapczinski F., Hallak J.E., Zuardi A.W., Crippa J.A., Quevedo J. Effects of beta-carboline harmine on behavioral and physiological parameters observed in the chronic mild stress model: further evidence of antidepressant properties. Brain Res. Bull. 2010;81(4-5):491–496. doi: 10.1016/j.brainresbull.2009.09.008. [http://dx.doi.org/10.1016/j. brainresbull.2009.09.008]. [PMID: 19772900]. [DOI] [PubMed] [Google Scholar]
- 125.Arent C.O., Réus G.Z., Abelaira H.M., Ribeiro K.F., Steckert A.V., Mina F., Dal-Pizzol F., Quevedo J. Synergist effects of n-acetylcysteine and deferoxamine treatment on behavioral and oxidative parameters induced by chronic mild stress in rats. Neurochem. Int. 2012;61(7):1072–1080. doi: 10.1016/j.neuint.2012.07.024. [http://dx.doi.org/10.1016/ j.neuint.2012.07.024]. [PMID: 22898295]. [DOI] [PubMed] [Google Scholar]
- 126.Réus G.Z., Abelaira H.M., Stringari R.B., Fries G.R., Kapczinski F., Quevedo J. Memantine treatment reverses anhedonia, normalizes corticosterone levels and increases BDNF levels in the prefrontal cortex induced by chronic mild stress in rats. Metab. Brain Dis. 2012;27(2):175–182. doi: 10.1007/s11011-012-9281-2. [http://dx.doi.org/10.1007/s11011-012-9281-2]. [PMID: 22327556]. [DOI] [PubMed] [Google Scholar]
- 127.Delgado y Palacios R., Campo A., Henningsen K., Verhoye M., Poot D., Dijkstra J., Van Audekerke J., Benveniste H., Sijbers J., Wiborg O., Van der Linden A. Magnetic resonance imaging and spectroscopy reveal differential hippocampal changes in anhedonic and resilient subtypes of the chronic mild stress rat model. Biol. Psychiatry. 2011;70(5):449–457. doi: 10.1016/j.biopsych.2011.05.014. [http://dx.doi.org/10.1016/ j.biopsych.2011.05.014]. [PMID: 21762877]. [DOI] [PubMed] [Google Scholar]
- 128.Toth E., Gersner R., Wilf-Yarkoni A., Raizel H., Dar D.E., Richter-Levin G., Levit O., Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J. Neurochem. 2008;107(2):522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [http://dx.doi.org/10.1111/j.1471-4159.2008.05642.x]. [PMID: 18752645]. [DOI] [PubMed] [Google Scholar]
- 129.Schmidt M.V., Trümbach D., Weber P., Wagner K., Scharf S.H., Liebl C., Datson N., Namendorf C., Gerlach T., Kühne C., Uhr M., Deussing J.M., Wurst W., Binder E.B., Holsboer F., Müller M.B. Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. J. Neurosci. 2010;30(50):16949–16958. doi: 10.1523/JNEUROSCI.4668-10.2010. [http://dx.doi.org/10. 1523/JNEUROSCI.4668-10.2010]. [PMID: 21159965]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang K., Xu T., Yuan Z., Wei Z., Yamaki V.N., Huang M., Huganir R.L., Cai X. Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci. Signal. 2016;9(458):ra123. doi: 10.1126/scisignal.aai7884. [http://dx.doi.org/10.1126/scisignal.aai7884]. [PMID: 27965425]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aleksandrova L.R., Phillips A.G., Wang Y.T. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J. Psychiatry Neurosci. 2017;42(4):222–229. doi: 10.1503/jpn.160175. [http://dx.doi.org/10.1503/jpn. 160175]. [PMID: 28234212]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burgdorf J., Colechio E.M., Stanton P., Panksepp J. Positive emotional learning induces resilience to depression: A role for NMDA receptor-mediated synaptic plasticity. Curr. Neuropharmacol. 2017;15(1):3–10. doi: 10.2174/1570159X14666160422110344. [http://dx.doi.org/10.2174/1570159X14666 160422110344]. [PMID: 27102428]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nasca C., Zelli D., Bigio B., Piccinin S., Scaccianoce S., Nisticò R., McEwen B.S. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc. Natl. Acad. Sci. USA. 2015;112(48):14960–14965. doi: 10.1073/pnas.1516016112. [http://dx.doi.org/10.1073/pnas.1516016112]. [PMID: 26627246]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shin S., Kwon O., Kang J.I., Kwon S., Oh S., Choi J., Kim C.H., Kim D.G. mGluR5 in the nucleus accumbens is critical for promoting resilience to chronic stress. Nat. Neurosci. 2015;18(7):1017–1024. doi: 10.1038/nn.4028. [http://dx.doi.org/10.1038/nn.4028]. [PMID: 26005851]. [DOI] [PubMed] [Google Scholar]
- 135.Wagner K.V., Hartmann J., Labermaier C., Häusl A.S., Zhao G., Harbich D., Schmid B., Wang X.D., Santarelli S., Kohl C., Gassen N.C., Matosin N., Schieven M., Webhofer C., Turck C.W., Lindemann L., Jaschke G., Wettstein J.G., Rein T., Müller M.B., Schmidt M.V. Homer1/mGluR5 activity moderates vulnerability to chronic social stress. Neuropsychopharmacology. 2015;40(5):1222–1233. doi: 10.1038/npp.2014.308. [http://dx.doi.org/10.1038/npp.2014.308]. [PMID: 25409593]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Willner P. Validation criteria for animal models of human mental disorders: learned helplessness as a paradigm case. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1986;10(6):677–690. doi: 10.1016/0278-5846(86)90051-5. [http:// dx.doi.org/10.1016/0278-5846(86)90051-5]. [PMID: 3809518]. [DOI] [PubMed] [Google Scholar]
- 137.Kohen R., Kirov S., Navaja G.P., Happe H.K., Hamblin M.W., Snoddy J.R., Neumaier J.F., Petty F. Gene expression profiling in the hippocampus of learned helpless and nonhelpless rats. Pharmacogenomics J. 2005;5(5):278–291. doi: 10.1038/sj.tpj.6500322. [http://dx.doi.org/10.1038/ sj.tpj.6500322]. [PMID: 16010284]. [DOI] [PubMed] [Google Scholar]
- 138.Taneja M., Salim S., Saha K., Happe H.K., Qutna N., Petty F., Bylund D.B., Eikenburg D.C. Differential effects of inescapable stress on locus coeruleus GRK3, alpha2-adrenoceptor and CRF1 receptor levels in learned helpless and non-helpless rats: a potential link to stress resilience. Behav. Brain Res. 2011;221(1):25–33. doi: 10.1016/j.bbr.2011.02.018. [http://dx.doi.org/10.1016/j.bbr.2011.02.018]. [PMID: 21333691]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muneoka K., Shirayama Y., Horio M., Iyo M., Hashimoto K. Differential levels of brain amino acids in rat models presenting learned helplessness or non-learned helplessness. Psychopharmacology (Berl.) 2013;229(1):63–71. doi: 10.1007/s00213-013-3080-2. [http://dx.doi.org/10.1007/ s00213-013-3080-2]. [PMID: 23568578]. [DOI] [PubMed] [Google Scholar]
- 140.Brisch R., Steiner J., Mawrin C., Krzyżanowska M., Jankowski Z., Gos T. Microglia in the dorsal raphe nucleus plays a potential role in both suicide facilitation and prevention in affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2017;267(5):403–415. doi: 10.1007/s00406-017-0774-1. [http://dx.doi.org/10.1007/s00406-017-0774-1]. [PMID: 28229240]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Furuyashiki T. Roles of dopamine and inflammation-related molecules in behavioral alterations caused by repeated stress. J. Pharmacol. Sci. 2012;120(2):63–69. doi: 10.1254/jphs.12r09cp. [http://dx.doi.org/10.1254/jphs. 12R09CP]. [PMID: 22986366]. [DOI] [PubMed] [Google Scholar]
- 142.Felger J.C., Haroon E., Miller A.H. Risk and resilience: animal models shed light on the pivotal role of inflammation in individual differences in stress-induced depression. Biol. Psychiatry. 2015;78(1):7–9. doi: 10.1016/j.biopsych.2015.04.017. [http://dx.doi.org/10.1016/j.biopsych.2015.04.017]. [PMID: 26051639]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ren Q., Ma M., Ishima T., Morisseau C., Yang J., Wagner K.M., Zhang J.C., Yang C., Yao W., Dong C., Han M., Hammock B.D., Hashimoto K. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc. Natl. Acad. Sci. USA. 2016;113(13):E1944–E1952. doi: 10.1073/pnas.1601532113. [http://dx.doi.org/10.1073/pnas.1601532113]. [PMID: 26976569]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yao W., Zhang J.C., Ishima T., Dong C., Yang C., Ren Q., Ma M., Han M., Wu J., Suganuma H., Ushida Y., Yamamoto M., Hashimoto K. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci. Rep. 2016;6:30659. doi: 10.1038/srep30659. [http://dx.doi.org/10.1038/srep30659]. [PMID: 27470577]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bouvier E., Brouillard F. in press.
- 146.Brachman R.A., Lehmann M.L., Maric D., Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J. Neurosci. 2015;35(4):1530–1538. doi: 10.1523/JNEUROSCI.2278-14.2015. [http://dx.doi.org/10.1523/JNEUROSCI.2278-14.2015]. [PMID: 25632130]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Couch Y., Anthony D.C., Dolgov O., Revischin A., Festoff B., Santos A.I., Steinbusch H.W., Strekalova T. Microglial activation, increased TNF and SERT expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain Behav. Immun. 2013;29:136–146. doi: 10.1016/j.bbi.2012.12.017. [http://dx.doi.org/ 10.1016/j.bbi.2012.12.017]. [PMID: 23305936]. [DOI] [PubMed] [Google Scholar]
- 148.Mahmoud R., Wainwright S.R., Chaiton J.A., Lieblich S.E., Galea L.A. Ovarian hormones, but not fluoxetine, impart resilience within a chronic unpredictable stress model in middle-aged female rats. Neuropharmacology. 2016;107:278–293. doi: 10.1016/j.neuropharm.2016.01.033. [http://dx.doi.org/ 10.1016/j.neuropharm.2016.01.033]. [PMID: 27018449]. [DOI] [PubMed] [Google Scholar]
- 149.Abautret-Daly Á., Dempsey E., Parra-Blanco A., Medina C., Harkin A. Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr. 2017;8:1–22. doi: 10.1017/neu.2017.3. [http://dx.doi.org/10.1017/neu.2017.3]. [PMID: 28270247]. [DOI] [PubMed] [Google Scholar]
- 150.Yu M., Jia H., Zhou C., Yang Y., Zhao Y., Yang M., Zou Z. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharm. Biomed. Anal. 2017;138:231–239. doi: 10.1016/j.jpba.2017.02.008. [http://dx.doi.org/10.1016/j.jpba.2017.02.008]. [PMID: 28219800]. [DOI] [PubMed] [Google Scholar]
- 151.Rook G.A., Lowry C.A., Raison C.L. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol. Med. Public Health. 2013;2013(1):46–64. doi: 10.1093/emph/eot004. [http://dx.doi.org/10.1093/emph/eot004]. [PMID: 24481186]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kiraly D.D., Walker D.M., Calipari E.S., Labonte B., Issler O., Pena C.J., Ribeiro E.A., Russo S.J., Nestler E.J. Alterations of the host microbiome affect behavioral responses to cocaine. Sci. Rep. 2016;6:35455. doi: 10.1038/srep35455. [http://dx.doi.org/10.1038/srep35455]. [PMID: 27752130]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Borrelli L., Aceto S., Agnisola C., De Paolo S., Dipineto L., Stilling R.M., Dinan T.G., Cryan J.F., Menna L.F., Fioretti A. Probiotic modulation of the microbiota-gut-brain axis and behaviour in Zebrafish. Sci. Rep. 2016;6:30046. doi: 10.1038/srep30046. [http://dx.doi.org/10. 1038/srep30046]. [PMID: 27416816]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith P.A. The tantalizing links between gut microbes and the brain. Nature. 2015;526(7573):312–314. doi: 10.1038/526312a. [http://dx.doi.org/10. 1038/526312a]. [PMID: 26469024]. [DOI] [PubMed] [Google Scholar]
- 155.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28(2):203–209. [PMID: 25830558]. [PMC free article] [PubMed] [Google Scholar]
- 156.Ha C.W., Lam Y.Y., Holmes A.J. Mechanistic links between gut microbial community dynamics, microbial functions and metabolic health. World J. Gastroenterol. 2014;20(44):16498–16517. doi: 10.3748/wjg.v20.i44.16498. [http://dx.doi.org/10.3748/wjg.v20.i44.16498]. [PMID: 25469018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang C., Fujita Y., Ren Q., Ma M., Dong C., Hashimoto K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci. Rep. 2017;7:45942. doi: 10.1038/srep45942. [http:// dx.doi.org/10.1038/srep45942]. [PMID: 28368029]. [DOI] [PMC free article] [PubMed] [Google Scholar]