Abstract
Objective:
The present study aimed to evaluate the influences of combined traffic noise (CTN) on the ability of learning and memory in mice.
Materials and Methods:
The Institute of Cancer Research (ICR) mice were exposed to CTN from highways and high-speed railways for 42 days, whose day–night equivalent continuous A-weighted sound pressure level (Ldn) was 70 dB(A). On the basis of behavioral reactions in Morris water maze (MWM) and the concentrations of amino acid neurotransmitters in the hippocampus, the impacts of CTN on learning and memory in mice were examined.
Results:
The MWM test showed that the ability of learning and memory in mice was improved after short-term exposure (6–10 days, the first batch) to 70 dB(A) CTN, which showed the excitatory effect of stimuli. Long-term exposure (26–30 days, the third batch; 36–40 days, the fourth batch) led to the decline of learning and memory ability, which indicated the inhibitory effect of stimuli. Assays testing amino acid neurotransmitters showed that the glutamate level of the experimental group was higher than that of the control group in the first batch. However, the former was lower than the latter in the third and fourth batches. Both, behavioral reactions and the concentrations of amino acid neurotransmitters, testified that short-term exposure and long-term exposure resulted in excitatory effect and inhibitory effect on the ability of learning and memory, respectively.
Conclusion:
The effects of 70 dB(A) CTN on the ability of learning and memory were closely related to the exposure duration. Furthermore, those effects were regulated and controlled by the level of glutamate in the hippocampus.
Keywords: Combined traffic noise, high-speed railway noise, highway noise, learning and memory, neurotransmitters
Introduction
With the increase of highway and high-speed railway mileage in China, the density of traffic network has increased continuously in recent years. To save land resources, the highway and high-speed railway are always constructed in parallel, which results in an increasing number of population being influenced by combined traffic noise (CTN). There are many differences in both time- and frequency-domain properties between single traffic noise and CTN from highway and high-speed railway. Highway traffic noise is relatively continuous. However, high-speed railway produces intermittent noise, which is characterized by shorter duration, higher-peak sound level, and more low-frequency components.[1,2] The effects of noise with different acoustic characteristics on organisms are different. Di et al.[3] investigated the annoyance of residents induced by railway dominant noise, road traffic dominant noise, and road–rail CTN in Dalian. In addition, they found that when the day–night equivalent continuous A-weighted sound pressure level (L dn) was greater than 63.5 dB, the percentage of highly annoyed individuals due to the road–rail CTN (the difference in L dn between the two sources was less than or equal to 5 dB) was significantly higher than that due to the one dominant noise (the difference in L dn between the two sources was larger than 5 dB, and the larger one was the dominant noise) with the same L dn. Öhrström et al.[4] also supported the aforementioned findings. The researches on biological effects induced by traffic noise mainly focused on single traffic noise;[5,6] nonetheless, researches concentrating on CTN are scarce.
The ability of learning and memory is an advanced cognitive function of individuals and easily influenced by noise.[7] Haines et al.[8] investigated the cognitive ability of children who were chronically exposed to the aircraft noise at the Heathrow Airport in Britain and found that the cognitive ability of children was influenced by chronic aircraft noise exposure and did not habituate over a 1-year period. Studies by Kempen et al.[9] indicated that children in school where noise level induced by road traffic was high had a high error rate in cognitive behavior test. However, Stansfeld et al.[10] found that the road traffic noise whose highest equivalent continuous A-weighted sound pressure level (L Aeq) was 71 dB(A) could improve the ability of episodic memory in children. All the aforementioned studies used the method of epidemiological survey to explore the influences of traffic noise on cognitive ability. In addition to acoustic factors, the results of epidemiological survey were also influenced by the personal factors of participants (e.g., age, educational level, and physical condition) and social factors (e.g., the level of economic development), which led to an inconsistency in the findings regarding the effects of traffic noise on cognitive ability. Hygge et al.[11] and Bronzaft and Mccarthy[12] explored the influences of traffic noise on the cognitive ability of individuals through the method employing human exposure to noise in a controllable laboratory. The exposure dose in the aforementioned method is quantitative and controllable compared with that in the method of field investigation. Because the exposure objects of this method are human beings, restricted by ethical factors, they cannot be exposed to chronic and high sound level noise.[13] The method employing animal exposure to noise in a controllable laboratory is a good way to make up this shortage. However, previous studies adopting the aforementioned method commonly used nonenvironmental noise as the stimulus (such as white noise, narrow band noise, or pure tone), which is different from the actual-environmental noise.[14,15,16]
In this study, the Institute of Cancer Research (ICR) mice were selected as the exposure object because of the similarity in the central nervous system (CNS) between mice and human beings. CTN of actual sampling was used as the stimulus. On the basis of the behavioral reactions of the mice in Morris water maze (MWM) and the concentrations of the amino acid neurotransmitters in the hippocampus, the influences of CTN from highway and high-speed railway on the ability of learning and memory were examined.
Materials and Methods
Experimental animals
A total of 80 healthy male ICR mice (28 days old, weighing 14.4 ± 1.1 g) were obtained from the Experimental Animal Centre of Zhejiang Province (Hangzhou, China), and were randomly divided into four batches. Each batch (n = 20) consisted of two groups, that is, the experimental group (n = 10) and the control group (n = 10). The living conditions of the mice were controlled as follows. Five mice were housed in one cage and were kept in a room with constant temperature (22 ± 2°C), humidity (50–60%), and a 12 h/12 h light/dark cycle (lights on at 08:00, lights off at 20:00). Food and water were available to the mice ad libitum. All procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals established by the National Institutes of Health (1996). Every effort was made to minimize the suffering of the animals and to reduce the number of animals used.
Combined traffic noise sampling and exposure
A four-channel dynamic signal analyzer (Photon II, Royston, England) was used to sample highway and high-speed railway noise in different periods. The two single traffic noises collected were rationally arranged and superimposed to get CTN according to the 24-h traffic flux of highway (2092 pcu/h) and high-speed railway (24 vehicles per hour in the daytime and 12 vehicles per hour at night).
The CTN was played through a dodecahedron nondirectional sound source (Nor270, Norsonic, Lierskogen, Norway) in a sound insulation laboratory. The L dn, noise exposure intensity for the experimental group was 70 ± 1.5 dB(A) (the exposure intensity of noise varied slightly in different cages, and 70 dB was the average exposure intensity in all cages, while ±1.5 dB was the range of exposure intensity difference). The L Aeq of the background noise was not more than 35 dB(A), which was the intensity presented to the control group. After an adaptation for 7 days in the laboratory, the mice of the experimental group were continuously exposed to the CTN for 42 days, while for the control group, this was not done.
Morris water maze test and hippocampal amino acid neurotransmitters assays
Among all behavioral tests of animals investigating the ability of spatial learning and memory, the MWM test was the most classic.[17] It mainly included a circular pool, hidden platform, video camera, and analysis system. The circular pool was 42 cm high, and its diameter was 120 cm. To prevent animals from seeing the platform when they were tested, black ink was used to make the water opaque. The hidden platform was 25 cm high, and its diameter was 6 cm. The surface of water was 1 cm higher than the platform, and the video camera was hung in the pool to record the behaviors of animals.
In the present study, the MMW test was performed in mice from four different batches at four different time points during the period of noise exposure, that is, 6–10 days (the first batch), 16–20 days (the second batch), 26–30 days (the third batch), and 36–40 days (the fourth batch). The MWM test included a hidden platform test and a probe trail test. The hidden platform test was used to assess the learning ability of animals, in which latency was the common indicator. The probe trail test was used to assess the memory ability of animals, in which the common indicator was the percentage of time spent in a target quadrant.[18] The hidden platform test lasted for four training days, and four trials were performed per day for each mouse. During the hidden platform test, the mice were placed into the water at one of four starting positions (northwest, north, east, or southeast) alternated in a clockwise manner and in a manner such that their faces were towards the wall of the circular pool. The duration for the trial was 90 s. If the mice succeeded in locating the platform within 90 s, the video camera would automatically stop recording. If the mice failed to locate the platform, the mice were manually placed on the platform for 15 s and put at the next starting position. When a mouse finished its four trials at four starting positions, the next mouse would start its test. The probe trail test was performed on the fifth day. During the probe trail test, the platform was moved away from the circular pool, and each mouse was placed in the pool once for 90 s. After all the tests, the latency and the percentage of time spent in the target quadrant were automatically analyzed by a video analysis system.
The latencies of mice in the hidden platform test showed a downtrend with an increase in the number of training days. A faster declining speed of latency indicated a faster learning of the mice.[19] Therefore, the learning rate could be represented by the absolute value of linear fitting slope between latencies and training days (that was the declining quantity of latency in unit time). The greater the absolute value of the linear fitting slope, the faster was the learning rate.[20] On the basis of this, a linear fitting between latencies and training days for each mouse was done, and the absolute value of the linear fitting slope was used to represent its learning rate.
Being performed by the cooperation of the different regions in the brain, the ability of spatial learning and memory in mice is highly depended on the hippocampus. The balance of the hippocampal excitatory and inhibitory amino acid neurotransmitters was closely related to the ability of spatial learning and memory. Glutamate (Glu) was the main excitatory amino acid neurotransmitter of CNS, and gamma-aminobutyric acid (GABA) was the main inhibitory amino acid neurotransmitter,[21] which led to the measurement of Glu and GABA levels in the hippocampus. To diminish the influences of the MWM test on the levels of neurotransmitters in mice, the levels of amino acid neurotransmitters in the hippocampus were measured after the MWM test for 2 days in each batch of mice. Specific procedures were as follows. The bilateral hippocampi rapidly stripped away from the brain tissue of the mice, after the mice were beheaded, were put in a glass homogenizer. Thereafter, the hippocampal tissue was manually homogenized in precooling physiological saline, whose volume value was nine times the mass value of the hippocampal tissue in quantity. The mixture was then allowed to stand for 15 min and transferred to a 1.5-mL centrifuge tube for refrigerated centrifuging at 14,000 rpm for 15 min at 4°C. Next, 100 μL of the supernatants was extracted, to which isovolumetric perchloric acid of 0.4 M was added. The mixture was thoroughly shaken and allowed to stand for 20 min to fully precipitate the proteins of the hippocampal homogenate, followed by centrifugation at 14,000 rpm for 15 min. A volume of 150 μL was extracted from the supernatants for measuring the levels of Glu and GABA by OPA (O-phthalaldehyd) precolumn derivatization high-performance liquid chromatography.
Statistical analysis
All data from the MWM test and assays testing the hippocampal amino acid neurotransmitters were expressed as mean ± SEM (Standard Error of Mean) and analyzed by the Statistical Package for the Social Sciences version 20.0 software (SPSS Inc., Chicago, IL, United States). Differences in latency between the experimental and control groups were examined by analysis of variance with repeated measures. Differences in the percentage of time spent in a target quadrant, learning rate, and the levels of the hippocampal amino acid neurotransmitters between the experimental and control groups were examined by an independent samples t-test. Significance was accepted at the level of P < 0.05.
Results
The Morris water maze test
The relationships between the latencies and the training days of the mice from the experimental and control groups in different batches are shown in Figure 1. The latencies of the mice from the experimental group on 1–4 training days were all lower than those of the mice from the control group and showed significant difference on fourth training day (P < 0.05) in the first batch (6–10 days) [Figure 1a]. Figure 1b showed that the latencies of the mice from the experimental group on 1–4 training days were approximate to those of the mice from the control group in the second batch (16–20 days). However, in the third (26–30 days) and fourth (36–40 days) batches, the latencies of the mice from the experimental group on 1–4 training days were higher than those of the mice from the control group [Figure 1c and d].
Figure 1.
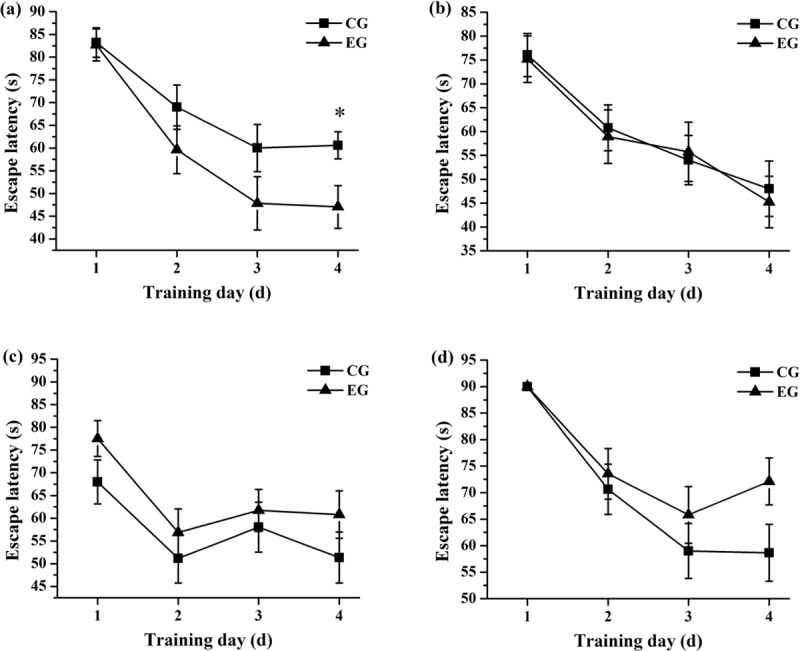
The relationships between the latencies and the training days of the mice from the experimental group (EG) and control group (CG) in the first batch (a), second batch (b), third batch (c), and fourth batch (d) (*P < 0.05)
The learning rates of the mice from the experimental and control groups in different batches are shown in Figure 2. The learning rate of the mice from the experimental group was significantly higher than that for the mice from the control group in the first batch (6–10 days) (P < 0.05). The learning rate of the mice from the experimental group was approximate to that for the mice from the control group in the second batch (16–20 days). However, in the third (26–30 days) and fourth (36–40 days) batches, the learning rates of the mice from the experimental group were lower than those of the mice from the control group and showed significant difference in the fourth batch (P < 0.05). Obviously, the ability of learning in mice was improved after short-term exposure (6–10 days) to 70 dB(A) CTN, but the long-term exposure (26–30 days, 36–40 days) led to a decline in the learning ability of mice.
Figure 2.
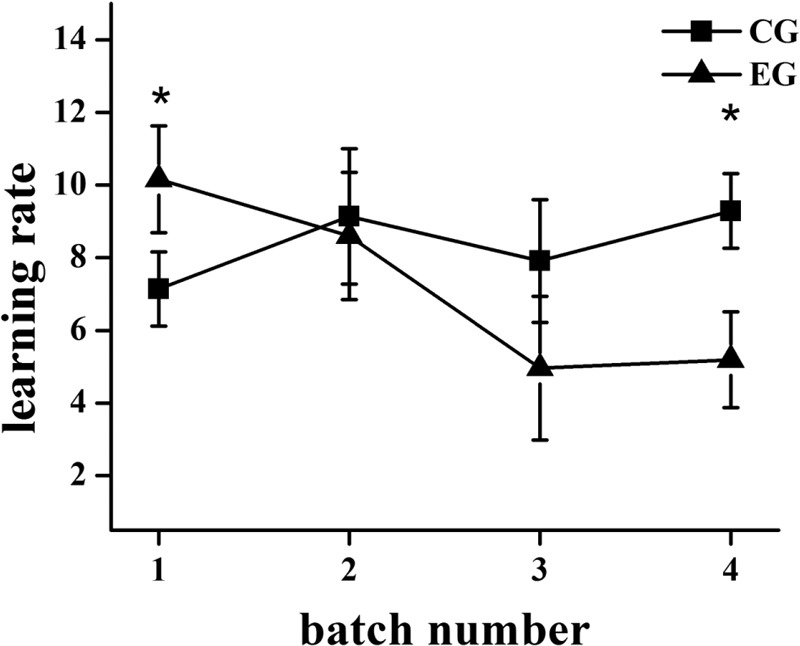
The learning rates of the mice from the experimental group (EG) and control group (CG) in different batches (*P < 0.05)
The percentages of time spent in a target quadrant for the mice from the experimental and control groups in different batches are shown in Figure 3. The percentage of time spent in a target quadrant for the mice from the experimental group was significantly higher than that of the mice from the control group in the first batch (6–10 days) (P < 0.05). The percentage of time spent in a target quadrant for the mice from the experimental group was approximate to that of the mice from the control group in the second batch (16–20 days). However, in the third (26–30 days) and fourth (36–40 days) batches, the percentages of time spent in a target quadrant for the mice from the experimental group were lower than those for the mice from the control group. Thus, the ability of memory in mice was improved after short-term exposure (6–10 days) to 70 dB(A) CTN, but long-term exposure (26–30 days, 36–40 days) led to the decline of the memory ability in mice.
Figure 3.
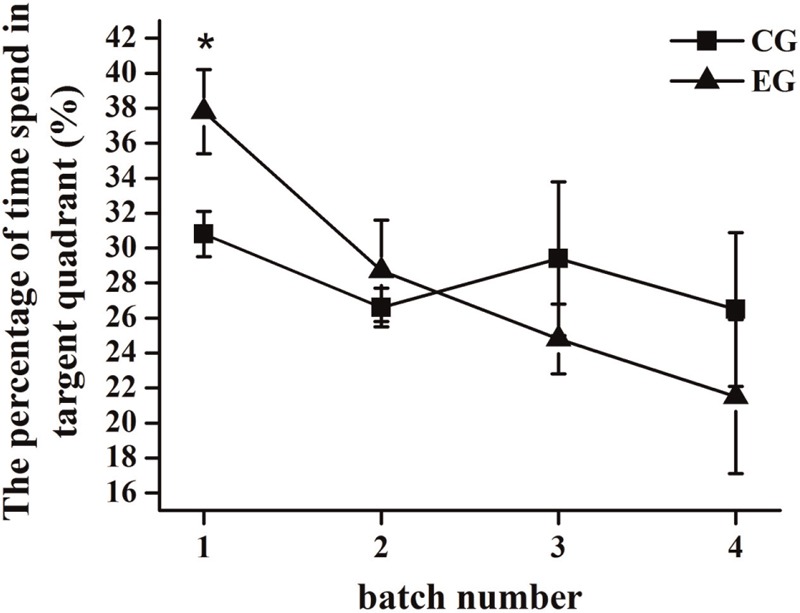
The percentages of time spent in a target quadrant for the mice from the experimental group (EG) and control group (CG) in different batches (*P < 0.05)
Levels of the amino acid neurotransmitters in the hippocampus
The hippocampal amino acid neurotransmitters of the mice from the experimental and control groups in different batches are shown in Figure 4. The GABA levels of the mice from the experimental group were approximate to those of the mice from the control group in all batches. However, the differences between the mice from the experimental and control groups with regard to the Glu levels were larger than that with regard to the GABA levels. Specifically, the Glu level of the mice from the experimental group was higher than that of the mice from the control group in the first batch (6–10 days). The Glu level of the mice from the experimental group was approximate to that of the mice from the control group in the second batch (16–20 days). However, in the third (26–30 days) and fourth (36–40 days) batches, the Glu levels of the mice from the experimental group were lower than those of the mice from the control group. Obviously, the level of Glu in mice rose after short-term exposure (6–10 days) to 70 dB(A) CTN, but the long-term exposure (26–30 days, 36–40 days) led to a decrease in the Glu level in mice.
Figure 4.
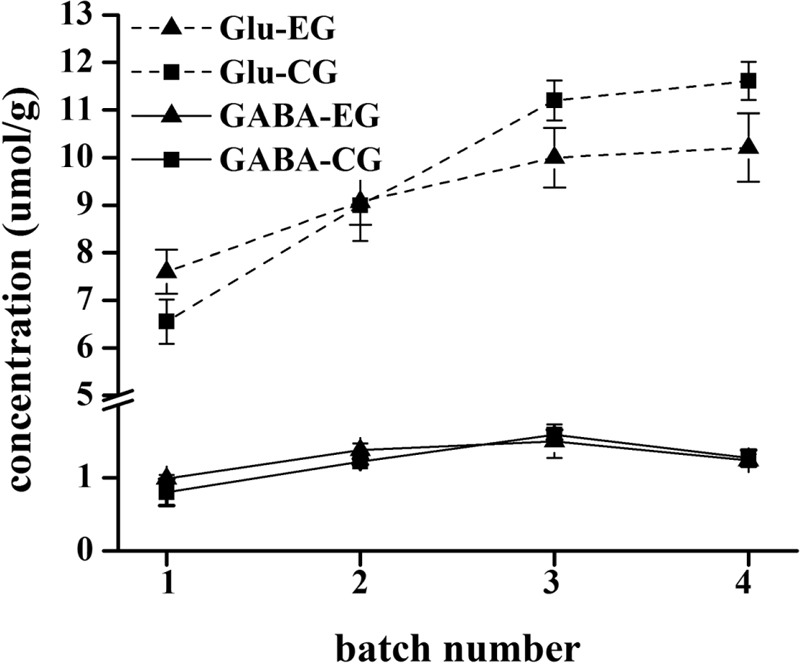
The hippocampal amino acid neurotransmitters of the mice from the experimental group (EG) and control group (CG) in different batches
Discussion
Hormesis was the common biphasic dose-effect model in toxicology and was characterized by low-dose excitement and high-dose inhibition.[22] It was manifested by various metabolic processes (including cell division and protein synthesis) of many species (including animals, plants, and microorganisms) in response to a wide range of stressors (chemicals, biological molecules, and physical stimuli).[23] The dose-effect curve of hormesis could be drawn as an inverted U-shaped or U-shaped curve.[24] Corresponding to the dose of toxicity that was ingested by organisms, the exposure dose of noise for organisms was the value of exposure sound level times exposure duration.[25] The results of the MWM test showed that the ability of learning and memory in mice was improved after short-term exposure (6–10 days) to 70 dB(A) CTN, which showed the excitatory effect of stimuli. Long-term exposure (26–30 days, 36–40 days) led to a decline in the learning and memory ability of mice, which showed the inhibitory effect of stimuli. This biphasic dose-effect conformed to the characteristics of hormesis. Calabreseci[26] found that hormesis was caused by an overcompensation mechanism and was a kind of adaptive response by organisms. After the external stimuli acted on organisms, the homeostasis related to one kind of biological function was broken, and organisms would produce a compensatory response to maintain this balance. Under the condition of low-dose stimulation, the compensatory response produced by organisms could be greater than the inhibitory response of stimuli, which resulted in the excitatory effect of stimuli. Under the condition of high-dose stimulation, the inhibitory response of stimuli was greater than the compensatory response, which led to an inhibitory effect of stimuli. In the present research, one kind of balance related to the ability of learning and memory in mice was broken by the exposure to 70 dB(A) CTN. It was speculated that the compensatory response was greater than the inhibitory response of CTN in mice. This speculation was made on the basis of the results of the MWM test, that is, the ability of learning and memory was improved after the short-term exposure (6–10 days, low dose) to CTN. However, long-term exposure (26–29 days, 36–39 days, high dose) to CTN led to a decline in the learning and memory ability of mice. It could be caused by a gradual exhaustion of compensatory response.[27] Accordingly, the inhibitory response of CTN would be greater than the compensatory response in mice.
Glu is one of the excitatory amino acid neurotransmitters in CNS. It was released from the presynaptic membrane, through the synaptic cleft, and would combine with the N-methyl-d-aspartate (NMDA) receptor in the postsynaptic membrane. Subsequently, Mg2+, which blocked the channel of Ca2+, was removed, and the Ca2+ flowed into the cell, which induced the change of synaptic plasticity.[21] GABA is an important inhibitory amino acid neurotransmitter in the CNS. It is converted from Glu via decarboxylation by glutamic acid decarboxylase.[28] GABA could strengthen the blocking effect of Mg2+ on the NMDA receptor and make difficulties in changing synaptic plasticity mediated by the NMDA receptor.[29] The change of synaptic plasticity was the basis for individual ability in learning and memory. During the process of learning and memory, synaptic plasticity concomitantly changed.[30] In recent years, the results related to the effects of Glu and GABA levels in the hippocampus on the ability of learning and memory had been basically consistent.[31] The individual ability of learning and memory was upregulated by Glu level in the hippocampus, that is, increasing the release of Glu in the hippocampus enhanced the individual ability of learning and memory. Nonetheless, too much release of Glu could cause an excitatory toxic effect on the neurons, which resulted in a decline in the learning and memory ability. The individual ability of learning and memory was downregulated by the GABA level in the hippocampus, that is, increasing the release of GABA in the hippocampus led to a decline in the individual ability of learning and memory. However, when the neurons were subjected to excitatory toxic effects, increasing the release of GABA would avoid a decline in the learning and memory ability. In this study, the level of GABA in the hippocampus was not influenced by exposure to CTN. However, the level of Glu in the hippocampus rose after the mice were short-term exposed (6–10 days) to CTN. The level of Glu in the hippocampus decreased after the mice were long-term exposed (26–30 days, 36–40 days) to CTN. The level of Glu was between 5 and 15 μmol/g in the hippocampus of normal mice.[32] In the present study, the levels of Glu in the hippocampus of mice from the experimental group were all in this range. On the basis of this, it could be ruled out that too much release of Glu led to an excitatory toxic effect on the neurons in the hippocampus. Therefore, a rise in Glu level after short-term exposure (6–10 days) to 70 dB(A) CTN conduced to improve the learning and memory ability in mice. A decrease in Glu level after long-term exposure (26–30 days, 36–40 days) to 70 dB(A) CTN led to a decline in the learning and memory ability of mice. These findings were in accordance with the results of behavioral experiment from WMW test for determining the ability of learning and memory in mice. Thus, it could be speculated that the effects of CTN on the ability of learning and memory in mice were regulated and controlled by the level of Glu in the hippocampus.Similar effects were also found in the behavioral experiments of humans under short-term noise exposure and epidemiological surveys on long-term noise exposure. For instance, Rausch et al.[33] tested the memory ability of humans who were exposed to 70 dB(A) white noise for a short term (<1 h) through a cognitive behavior test and found that the memory ability of the participants were enhanced. By a verbal episodic recall test, Söderlund et al.[34] found that white noise (the exposure duration was shorter than 1 h) could improve the recall accuracy of inattentive children. This excitatory effect of stimuli under short-term noise exposure in humans as aforementioned was similar to that in mice in the present study. Epidemiological surveys conducted by both Haines and Kempen indicated that the cognitive ability of children declined after long-term (1–2 years) exposure to aircraft noise or road traffic noise.[8,9] This inhibitory effect of stimuli under long-term noise exposure in humans was similar with that in mice in the present study. On the basis of the aforementioned correlational studies in humans, it could be speculated that the cognitive ability of humans would probably be improved after short-term exposure to 70 dB(A) CTN, and long-term exposure would lead to a decline in the cognitive ability of humans. Of course, this speculation needs to be verified by additional cognitive behavior tests in humans exposed to 70 dB(A) CTN.
Conclusions
The ability of learning and memory in mice was improved after short-term exposure (6–10 days) to 70 dB(A) CTN, which showed the excitatory effect of stimuli. On the contrary, the ability of learning and memory in mice declined after long-term exposure (26–30 days, 36–40 days), which indicated the inhibitory effect of stimuli. This biphasic dose-effect conformed to the characteristics of hormesis and was regulated and controlled by the level of Glu (an excitatory amino acid neurotransmitter) in the hippocampus.
Correlational studies including behavioral experiments under short-term noise exposure and epidemiological surveys on long-term noise exposure also showed this biphasic dose-effect in humans. It could be speculated that the cognitive ability of a human would probably be improved after short-term exposure to 70 dB(A) CTN, and long-term exposure would lead to a decline in the cognitive ability of humans.
On the basis of this study, it could be speculated that introducing environmental noise having appropriate sound pressure level and duration, matching daily work or living environment, could improve individual cognitive ability.
Financial support and sponsorship
This work is supported by the National Natural Science Foundation of China (Grant No. 11174251).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors sincerely thank the National Nature Science Foundation of China (Grant No. 11174251) for funding this research and express special gratitude to the teachers and students who have participated in animal feeding, data collection, and analysis.
REFERENCES
- 1.Di GQ, Zheng Y. Effects of high-speed railway noise on the synaptic ultrastructure and phosphorylated-CaMKII expression in the central nervous system of SD rats. Environ Toxicol Pharmacol. 2013;35:93–9. doi: 10.1016/j.etap.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Di GQ, He LJ. Behavioral and plasma monoamine responses to high-speed railway noise stress in mice. Noise Health. 2013;15:13–9. doi: 10.4103/1463-1741.113506. [DOI] [PubMed] [Google Scholar]
- 3.Di GQ, Liu XY, Lin QL, Zheng Y, He LJ. The relationship between urban combined traffic noise and annoyance: An investigation in Dalian, north of China. Sci Total Environ. 2012;432:189–94. doi: 10.1016/j.scitotenv.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Öhrström E, Barregård L, Andersson E, Skånberg A, Svensson H, Ängerheim P. Annoyance due to single and combined sound exposure from railway and road traffic. J Acoust Soc Am. 2007;122:2642–52. doi: 10.1121/1.2785809. [DOI] [PubMed] [Google Scholar]
- 5.Di GQ, Liu XY, Shi X, Li ZG, Lin QL. Investigation of the relationship between aircraft noise and community annoyance in China. Noise Health. 2012;14:52–7. doi: 10.4103/1463-1741.95132. [DOI] [PubMed] [Google Scholar]
- 6.Aasvang GM, Øverland B, Ursin R, Moum T. A field study of effects of road traffic and railway noise on polysomnographic sleep parameters. J Acoust Soc Am. 2011;129:3716–26. doi: 10.1121/1.3583547. [DOI] [PubMed] [Google Scholar]
- 7.Ruvalcaba-Delgadillo Y, Luquín S, Ramos-Zúñiga R, Feria-Velasco A, González-Castañeda RE, Pérez-Vega MI, et al. Early-life exposure to noise reduces mPFC astrocyte and T-maze alternation/discrimination task performance in adult male rats. Noise Health. 2015;17:216–26. doi: 10.4103/1463-1741.160703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haines MM, Stansfeld SA, Job RF, Berglund B, Head J. A follow-up study of effects of chronic aircraft noise exposure on child stress responses and cognition. Int J Epidemiol. 2001;30:839–45. doi: 10.1093/ije/30.4.839. [DOI] [PubMed] [Google Scholar]
- 9.Kempen EV, Kamp IV, Lebret E, Lammers J, Emmen H, Stansfeld S. Neurobehavioral effects of transportation noise in primary schoolchildren: A cross-sectional study. Environ Health. 2010;9:25. doi: 10.1186/1476-069X-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stansfeld SA, Berglund B, Clark C, Lopez-Barrio I, Fischer P, Öhrström E, et al. Aircraft and road traffic noise and children’s cognition and health: A cross-national study. Lancet. 2005;365:1942–9. doi: 10.1016/S0140-6736(05)66660-3. [DOI] [PubMed] [Google Scholar]
- 11.Hygge S, Boman E, Enmarker I. The effects of road traffic noise and meaningful irrelevant speech on different memory systems. Scand J Psychol. 2003;44:13–21. doi: 10.1111/1467-9450.00316. [DOI] [PubMed] [Google Scholar]
- 12.Bronzaft AL, Mccarthy DP. The effect of elevated train noise on reading ability. Environ Behav. 1975;7:517–27. [Google Scholar]
- 13.Miller FG, Rosenstein DL. Reporting of ethical issues in publications of medical research. Lancet. 2002;360:1326–8. doi: 10.1016/S0140-6736(02)11346-8. [DOI] [PubMed] [Google Scholar]
- 14.Samson J, Sheela DR, Ravindran R, Senthilvelan M. Effect of noise stress on free radical scavenging enzymes in brain. Environ Toxicol Pharmacol. 2005;20:142–8. doi: 10.1016/j.etap.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 15.Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, et al. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–26. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goble TJ, Møller AR, Thompson LT. Acute high-intensity sound exposure alters responses of place cells in hippocampus. Hearing Res. 2009;253:52–9. doi: 10.1016/j.heares.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Lindner MD. Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze. Neurobiol Learn Mem. 1997;68:203–20. doi: 10.1006/nlme.1997.3782. [DOI] [PubMed] [Google Scholar]
- 18.Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 20.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 21.Cui B, Wu M, She X, Liu H. Impulse noise exposure in rats causes cognitive deficits and changes in hippocampal neurotransmitter signaling and tau phosphorylation. Brain Res. 2012;1427:35–43. doi: 10.1016/j.brainres.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese EJ, Baldwin LA. The hormetic dose-response model is more common than the threshold model in toxicology. Toxicol Sci. 2003;71:246–50. doi: 10.1093/toxsci/71.2.246. [DOI] [PubMed] [Google Scholar]
- 23.Kendig EL, Le HH, Belcher SM. Defining hormesis: Evaluation of a complex concentration response phenomenon. Int J Toxicol. 2010;29:235–46. doi: 10.1177/1091581810363012. [DOI] [PubMed] [Google Scholar]
- 24.Calabrese EJ, Baldwin LA. Hormesis U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001;22:285–91. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- 25.Lasley SM, Gilbert ME. Rat hippocampal glutamate and GABA release exhibit biphasic effects as a function of chronic lead exposure level. Toxicol Sci. 2002;66:139–47. doi: 10.1093/toxsci/66.1.139. [DOI] [PubMed] [Google Scholar]
- 26.Calabreseci EJ. Evidence that hormesis represents an “overcompensation” response to a disruption in homeostasis. Ecotoxicol Environ Saf. 1999;42:135–7. doi: 10.1006/eesa.1998.1729. [DOI] [PubMed] [Google Scholar]
- 27.Cui B, Wu M, She X. Effects of chronic noise exposure on spatial learning and memory of rats in relation to neurotransmitters and NMDAR2B alteration in the hippocampus. J Occup Health. 2009;51:152–8. doi: 10.1539/joh.l8084. [DOI] [PubMed] [Google Scholar]
- 28.Kazi AI, Oommen A. Chronic noise stress-induced alterations of glutamate and gamma-aminobutyric acid and their metabolism in the rat brain. Noise Health. 2014;16:343–9. doi: 10.4103/1463-1741.144394. [DOI] [PubMed] [Google Scholar]
- 29.Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–11. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- 30.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 31.Vizi ES, Kiss JP. Neurochemistry and pharmacology of the major hippocampal transmitter systems: Synaptic and nonsynaptic interactions. Hippocampus. 1998;8:566–607. doi: 10.1002/(SICI)1098-1063(1998)8:6<566::AID-HIPO2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 32.Erecińska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245–96. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 33.Rausch VH, Bauch EM, Bunzeck N. White noise improves learning by modulating activity in dopaminergic midbrain regions and right superior temporal sulcus. J Cogn Neurosci. 2014;26:1469–80. doi: 10.1162/jocn_a_00537. [DOI] [PubMed] [Google Scholar]
- 34.Söderlund GB, Sikström S, Loftesnes JM, Sonuga-Barke EJ. The effects of background white noise on memory performance in inattentive school children. Behav Brain Funct. 2010;6:55. doi: 10.1186/1744-9081-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]


