Abstract
Organ damage and resulting pathologies often involve multiple deregulated pathways. MicroRNAs (miRNAs) are short, non-coding RNAs that regulate a multitude of genes at the post-transcriptional level. Since their discovery over two decades ago, miRNAs have been established as key players in the molecular mechanisms of mammalian biology including the maintenance of normal homeostasis and the regulation of disease pathogenesis. In recent years, there has been substantial progress in innovative techniques to measure miRNAs along with advances in targeted delivery of agents modulating their expression. This has expanded the scope of miRNAs from being important mediators of cell signaling to becoming viable quantitative biomarkers and therapeutic targets. Currently, miRNA therapeutics are in clinical trials for multiple disease areas and vast numbers of patents have been filed for miRNAs involved in various pathological states. In this review, we summarize miRNAs involved in organ injury and repair, specifically with regards to organs that are the most susceptible to injury: the liver, heart and kidney. In addition, we review the current state of knowledge on miRNA biology, miRNA biomarkers and nucleotide-based therapeutics designed to target miRNAs to prevent organ injury and promote repair.
INTRODUCTION
MicroRNAs (miRNAs) are short, non-coding RNAs that regulate gene expression at the post-transcriptional level. In recent years, miRNAs have been shown to be integral in regulating not just normal homeostasis, development and physiology but also disease pathogenesis in terms of initiation, progression or resolution of the disease[1, 2]. According to miRBase version 21, there are more than 2500 annotated human miRNAs that are each predicted to regulate many mRNAs, and each mRNA can be targeted by a large number of different miRNAs[1, 3, 4]. Importantly, most miRNAs are evolutionarily conserved, making the translation of findings from preclinical models more likely than some other molecular targets. These fundamental characteristics allow miRNAs to serve as potential biomarkers of disease progression and targets for therapeutic intervention.
MiRNAs are expressed from genomic DNA and processed in multiple steps before becoming ~22 nucleotide mature miRNAs (Figure 1). First, miRNAs are transcribed by RNA polymerase II and can originate from discrete miRNA-coding genes or from within introns of protein-coding genes (deemed “miRtrons”)[5]. Many diverse transcriptional regulators have been shown to contribute to miRNA expression including HIF1α, SMADs, p53, MYC, NF-κB and CREB[6]. After transcription, long stem-loop structures called primary miRNAs (pri-miRNAs) undergo cleavage in the nucleus by an RNase-III-type enzyme, Drosha, to form 60-to-70-nucleotide precursor miRNAs (pre-miRNAs). Alternatively, miRtrons can bypass Drosha and form pre-miRNAs directly through spliceosome-mediated processing[7]. The Ran GTPase Exportin-5 complex then shuttles all pre-miRNAs through nuclear pore complexes into the cytosol. Pre-miRNAs are further processed by Dicer to generate miRNA duplexes. Then, miRNA duplexes will dissociate with the help of Argonaute 2 and passenger strands may degrade while the guide strands can remain active. Mature miRNAs then form the RNA-induced silencing complex (RISC), which include additional proteins necessary for mRNA silencing activity[8]. Mature miRNAs will canonically match their “seed regions” (nucleotides 2 through 8 on the 5′ end of the miRNA) to a complementary region on the 3′ untranslated region (UTR) of a target mRNA[2]. However, evidence has emerged that miRNAs can bind to 5′ UTRs and within the mRNA coding sequence[9, 10]. These interactions promote mRNA degradation or inhibit protein synthesis and are responsible for regulating gene expression[11]. In addition, though most miRNAs act in the cytosol to regulate translation some mature miRNAs can reenter the nucleus to regulate transcription, miRNA expression and RNA processing[12, 13].
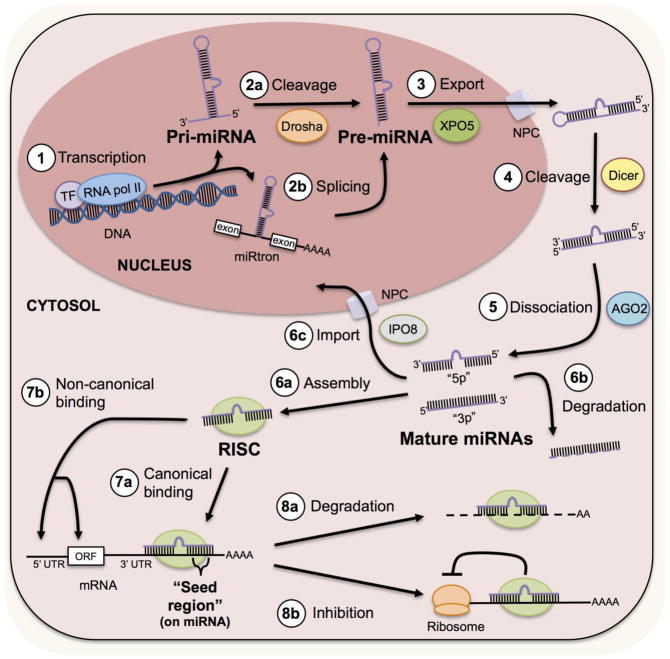
Figure 1. MiRNA expression, processing and function are highly organized.
MicroRNA biogenesis begins with transcription of genomic DNA by RNA polymerase II and can be mediated by many common transcription factors. Often, large RNA stem-loop structures called pri-miRNAs are formed directly. Drosha then cleaves pri-miRNAs to form intermediate stem-loop structures called pre-miRNAs. Alternatively, pre-miRNAs can form through an intermediate called a “miRtron” from splicing of mRNA. Exportin 5 (XPO5) then transports pre-miRNAs through nuclear pore complexes (NPC) into the cytosol. In the cytosol, Dicer cleaves pre-miRNAs to form microRNA duplexes. Argonaute 2 (AGO2) then mediates the dissociation of the miRNA duplex to form two mature miRNAs. These two miRNAs are deemed “5p” or “3p” depending on whether they originate from the 5′ or 3′ end of the pre-miRNA, respectively. Depending on the context or specific miRNA, mature miRNAs will either assemble within RNA-induced silencing complexes (RISC), degrade or may reenter the nucleus via Importin 8 (IPO8) to regulate transcription. After RISC assembly, miRNAs can bind to mRNAs with their complementary seed region to the 3′ untranslated region (UTR), 5′ UTR or the open reading frame (ORF). MiRNAs then regulate protein production either by promoting mRNA degradation or by inhibiting translation.
When organs experience injury or disease, affected cells may release specific miRNAs into circulation and miRNA biomarkers can be detected in the blood, urine and various other body fluids[14]. MiRNA biomarkers are remarkably stable due to packaging within microvesicles and/or binding to RISC protein components or high-density lipoproteins[15]. In addition, nucleotide-based therapeutics have been developed that can either mimic or inhibit miRNA expression in vivo. MiRNA therapeutics can resist degradation in systemic circulation due to modifications to the nucleotide backbone or by being packaged within microvesicles before dosing[16–18]. Clinical trials are currently underway for a miR-122 inhibitor to treat hepatitis C virus (HCV)[19], a miR-21 inhibitor to treat Alport nephropathy[20] and a miR-34a mimic to treat liver cancer[21]. These aspects highlight the transformative potential of miRNAs as mechanistic biomarkers and therapeutic targets in translational medicine. In this review, we discuss the role of miRNAs in organ damage and repair with a particular focus on the liver, heart and kidney, which are organs that are most susceptible to injury.
MiRNAs IN LIVER INJURY AND REPAIR
MiRNAs in liver injury
Drug induced liver injury (DILI) accounts for more than half of all cases of acute liver failure in many Western countries and acetaminophen (APAP) overdose is a major cause in the United States[22]. DILI is also a major reason for attrition during drug development and is one of the main causes of withdrawing drugs from the market[23]. Other causes of liver injury include chronic viral hepatitis C (HCV) and non-alcoholic fatty liver disease (NAFLD), which are two of the leading causes of chronic liver disease worldwide[24, 25]. The liver encounters toxic stress due to its anatomical location and first-pass metabolism and biotransformation that results in generation of toxic reactive metabolites. Current methods of detecting liver damage include measuring aminotransferases, such as alanine aminotransferase (ALT), and total bilirubin in the blood. However, these methods lack sensitivity and specificity, especially with regards to early detection and prognoses of liver disease, and other factors can affect the quantities of these markers in the blood[26]. Therefore, miRNAs have been explored as biomarkers and as mediators in the mechanisms of pathogenesis for a number of these liver ailments.
MiR-122
MiR-122 is the most abundant miRNA in the liver and has been implicated in basic liver physiology, disease and regeneration[27]. MiR-122 has been shown to be expressed up to 100,000-times higher in the normal liver compared to other tissues such as brain, heart, kidney and lung[28]. MiR-122 knockout mice were shown to develop steatohepatitis, fibrosis and hepatocellular carcinoma, which indicates the protective role of miR-122 in pathogenesis of liver disease [29, 30]. Furthermore, miR-122 has emerged as a promising biomarker of liver damage and can be measured in blood (Figure 2). MiR-122 has been demonstrated to be more sensitive and with a greater dynamic range than ALT in both animals and humans. In mice subjected to APAP-induced liver injury, miR-122 was decreased in liver tissue but highly increased in the plasma in a dose-dependent manner[31]. In humans with APAP-induced hepatotoxicity, miR-122 was significantly increased in the sera compared to healthy controls and has shown its utility to diagnose APAP toxicity in at least one clinical case[32, 33].
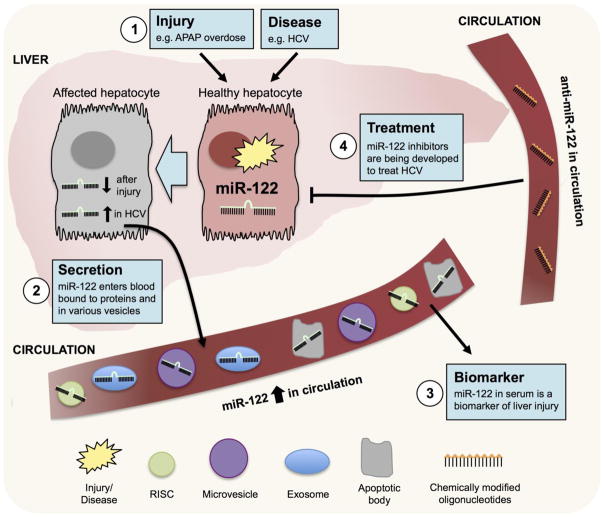
Figure 2. MiR-122 is a biomarker and therapeutic target in liver disease.
MiR-122 is the most abundant miRNA in the liver and can increase or decrease in the liver tissue depending on the injury or disease. In diverse forms of liver injury and disease, miR-122 is secreted into the blood within small vesicles, dead cells or bound to RISC proteins or high-density lipoproteins. MiR-122 has been successfully utilized as a biomarker of various forms of liver injury and liver diseases such as HCV. Because of its involvement in HCV replication, miR-122 has also been successfully targeted pharmacologically with an inhibitor, Mirvirsen, which is currently in clinical trials.
Importantly, miR-122 upregulation in the blood is responsive to diverse etiologies of DILI. Specifically, miR-122 allowed for early detection of liver injury following exposure alpha-naphthylisothiocyanate[34, 35], methapyriline[35], tri-chlorobromomethane[28], carbon tetrachloride (CCl4)[28] and dioxin[36]. In addition, miR-122 has been used as a diagnostic biomarker in other disease settings including NAFLD[37], alcoholic liver disease[38] and chronic HCV infection[39, 40]. In HCV, miR-122 was found to play a critical role in promoting the viral life cycle and when inhibited interferes with viral replication[19]. Thus, a miR-122 inhibitor, Miravirsen, is currently in clinical trials to treat HCV in human patients[41]. Though many of the targets of miR-122 are currently unknown, miR-122 is clearly a critical miRNA in the liver and is promising to diagnose and treat liver diseases.
MiR-192
MiR-192 is highly enriched in the liver and elevated after injury, but it is also expressed highly in other tissues including small intestine, colon and kidney, which is different from the liver-specific miR-122[42]. Following diverse etiologies of DILI in both animal and in human sera, miR-192 has been shown to track with miR-122 expression; however, miR-192 is generally not as highly expressed as miR-122[31, 32, 43, 44]. In addition, miR-192 and miR-122 were effective serum biomarkers of liver injury in rats that experienced acute liver transplantation rejection[45]. In a recent study, miR-192 was increased in the sera of humans with acute liver failure and in mice subjected to hepatic ischemia-reperfusion injury or liver injury induced by CCl4[46]. In addition, miR-192 in sera was correlated with increasing liver damage. However, miR-192 was downregulated in liver tissues of mice and humans with liver injury. In a mouse hepatoma cell line, miR-192 was shown to target Zeb2, an anti-apoptotic gene, and increased susceptibility to oxidative stress[46].
MiR-125b
MiR-125b-5p was increased in the blood of patients with APAP overdose and HCV[47, 48]. Recently, miR-125b overexpression was shown to prevent liver injury and improve the survival of mice following APAP-induced liver failure[49]. To uncover the role of miR-125b in hepatocyte survival an unbiased screen of 302 miRNA mimics was performed using primary mouse hepatocytes. The cells were transfected with miRNA mimic library, subjected to APAP toxicity and analyzed for improvements in cell viability, where miR-125b was found as potential hit. Remarkably, administration of a hepatocyte-specific pri-miR-125b vector into mice before APAP overdose protected them from liver failure and death by targeting Kelch-like ECH-associated protein 1 (Keap1). Lastly, miR-125b was shown to be cytoprotective in human hepatocytes and was downregulated in biopsies from patients with acute liver failure[49]. This suggests that a miR-125b mimic may be therapeutically beneficial in patients with increased risk of liver failure.
MiR-34a
MiR-34a [50] is an established tumor suppressor implicated in promoting apoptosis and p21 expression in cancer cells[51]. Though miR-34a is not liver-specific, it has been shown to be upregulated at the termination phase of liver regeneration in rats and inhibit proliferation by targeting inhibin αB and Met when overexpressed in rat hepatocytes[52]. In addition, miR-34a has been shown to be significantly upregulated in animals and humans with Non-Alcoholic Fatty Liver Disease (NAFLD)[53, 54]. In rat hepatocytes, miR-34a overexpression increased apoptosis when challenged with ursodeoxycholic acid via directly targeting Sirtuin 1 (SIRT1), an NAD-dependent deacetylase for p53[54]. In an in vivo study, miR-34a was shown to target SIRT1 and peroxisome proliferator-activated receptor-α (PPARα), an essential modulator of lipid transport and metabolism[55]. Importantly, when injected with a miR-34a inhibitor, mice with NAFLD had less lipid accumulation and improvements in levels of steatosis[55]. Taken together, miR-34a is dysregulated in liver disease and may be targeted therapeutically to promote hepatocyte survival and limit steatosis.
MiRNAs in liver development and regeneration
Despite the liver’s vulnerability to toxic insult, it is also the one mammalian organ that is capable of fully regenerating to achieve baseline size and function after acute or chronic injury[56]. Normally, hepatocytes are highly differentiated and quiescent and will only enter the cell cycle once initiated by ischemia, toxic exposure or infection. Interestingly, mice with hepatocyte-specific Dicer knockout exhibit normal development and hepatic function at birth, suggesting the absence of mature miRNAs does not greatly affect liver development[57]. However, within four months after birth these mice experience progressive liver damage, decreased function and aberrant hepatocyte proliferation and apoptosis[57]. In addition, these mice were shown to have deficiencies in correct liver zonation, metabolic pathways and tumor suppression[58, 59]. This suggests miRNAs are critical in basic liver function. Furthermore, specific miRNAs have been found that regulate processes of liver regeneration as well as the response toxic stress as will be discussed below (Table 1).
Table 1.
Additional miRNAs involved in in vivo liver regeneration
| miRNA | Expression during liver regeneration | Target(s) | Model | Effect of miRNA | Citation |
|---|---|---|---|---|---|
| miR-23b | downregulated at termination phase | SMAD3 | Rats | Inhibition of miRNA inhibited liver cell proliferation | [135] |
| miR-26a | downregulated | Cyclin D2*, Cyclin E2* | Mice | Inhibition of miRNA promotes hepatocyte proliferation | [136] |
| miR-127 | downregulated | BCL2*, SETD8* | Rats | Inhibition of miRNA promoted liver cell proliferation | [137] |
| miR-221 | upregulated | p27, p57, Arnt, Puma | Mice | Overexpression of miRNA promoted hepatocyte proliferation | [138–140] |
| miR-382 | upregulated | PTEN | Mice, humans | Overexpression of miRNA promoted liver cell proliferation | [141, 142] |
Asterisk (*) represents that only correlation between miRNA and target was shown, not direct interaction.
MiR-21
MiR-21 is among the most upregulated miRNAs during liver regeneration. Previously, miR-21 has been identified as an onco-miRNA that has multiple targets that normally inhibit proliferation. These targets include phosphatase and tensin homolog (PTEN), B-cell translocation gene 2 (BTG2), and Ras homolog gene family member B (RhoB) [60–63]. Therefore, when miR-21-specific antisense oligonucleotides were injected into mice following two-thirds hepatectomy, early liver regeneration was impaired because of a decrease in hepatocyte proliferation[63]. In this study, miR-21 was found to target RhoB and increase Akt and mamalian target of rapamycin (mTOR) signaling during early regeneration, which increased global translation in the cell via increased eukaryotic initiation factor (eIF)-4F activation. Also, upregulation of miR-21 increased cell cycle progression through G1 to S phase via accelerated translation of cyclin D1 mRNA[63]. A later study showed miR-21 was upregulated during in vitro hepatocyte proliferation and also targeted PTEN to enhance PI3K/Akt activity[60]. Interestingly, another study showed that deoxycholic acid (DCA) – a toxic bile acid that may lead to NAFLD – inhibited miR-21 expression in rats. When miR-21 was overexpressed in hepatocytes in vitro DCA-induced cell death was reduced[64]. Overall, evidence suggests that miR-21 promotes liver regeneration and may protect from pathological liver diseases such as NAFLD.
MiRNAs IN HEART INJURY AND REPAIR
MiRNAs in heart injury
Many drugs and environmental chemicals are known to induce cardiotoxicity specifically by targeting cardiomyocytes and can contribute to the development of coronary heart disease[65]. Cardiotoxicity is a major obstacle to drug development largely because of the failure to predict it in preclinical studies[66]. Currently, pre-clinical and clinical cardiotoxicity biomarkers include measuring the electrical efficiency of the heart using electrocardiography, visualizing the heart with echocardiograms and hERG assays to assess QT prolongation. In addition, methods for assessing cardiac dysfunction include measuring cardiac troponins or performing endomyocardial biopsies, which are invasive[67]. Therefore, miRNAs have been studied as being more sensitive, specific and stable biomarkers of heart disease (Table 2). In addition, many groups have looked at miRNAs that respond to direct cardiac injury and are involved in mechanisms of cardiomyocyte cell death.
Table 2.
MiRNA biomarkers that respond to cardiac injury.
| miRNA | Expression during heart injury | Biofluid | Model | Citation |
|---|---|---|---|---|
| miR-486-3p | upregulated in MI | sera | human | [143] |
| miR-150-3p | upregulated in MI | sera | human | [143] |
| miR-126-3p | downregulated in MI | sera | human | [143] |
| miR-26a-5p | downregulated in MI | sera | human | [143] |
| miR-191-5p | downregulated in MI | sera | human | [143] |
| miR-1 | upregulated in MI | plasma | human, mice | [144, 145] |
| miR-499 | upregulated in MI | plasma | human, mice | [145–147] |
| miR-133a | upregulated in MI | plasma | human, mice | [145] |
| miR-133b | upregulated in MI | plasma | human, mice | [145] |
| miR-122 | downregulated in MI | plasma | human | [145] |
| miR-375 | downregulated in MI | plasma | human | [145] |
| miR-208b | upregulated in MI | plasma | human | [147] |
MiR-34a
In a mouse model of chronic cardiotoxicity using doxorubicin, miRNA sequencing identified that miR-34a was the only miRNA upregulated across five time points and showed higher expression with increasing doses of doxorubicin[68]. In a different study, miR-34a was increased in the serum exosomes of patients who suffered myocardial infarction (MI) and developed heart failure within one year, showing its potential as a prognostic biomarker[69]. In addition, miR-34a has been implicated in aging-associated cardiomyocyte cell death and when inhibited in mice after MI reduced cell death and improved recovery of cardiac function[70]. Taken together, miR-34a acts similarly in the heart as was previously discussed in the liver by promoting cell death and tissue dysfunction.
MiR-208a
MiR-208a is a cardiac-specific miRNA that is generated from the intronic region of the gene that codes for a major protein that regulates myocardial contractility in the adult heart, α-myosin heavy chain (α-MHC)[71]. MiR-208a has been shown to regulate critical cardiac transcription factors and is required for proper cardiac function[72]. Specifically, overexpression of miR-208a resulted in increased α-MHC expression and was associated with arrhythmias, fibrosis and hypertrophy in mice[72]. Following isoproteronol-induced cardiac injury in rats, miR-208a was shown to have stable upregulation in plasma and was superior to cardiac troponins in indicating injury[73, 74]. MiR-208a was also increased in the human heart tissue of patients who died from MI[75]. Importantly, miR-208a was upregulated in plasma and exhibited a greater sensitivity than cardiac troponins in patients following MI[76]. As such, miR-208a has also been evaluated as a possible therapeutic target for heart injury. In one study, inhibiting miR-208a before doxorubicin treatment prevented cardiomyocyte apoptosis and improved cardiac function as determined by echocardiography[77]. In addition, in a rat model of congestive heart failure, miR-208a inhibition prevented pathological cardiac remodeling and improved cardiac function and survival[78].
MiRNAs in heart development and regeneration
During development, the heart increases in size due to proliferation of cardiomyocytes. Despite some limited renewal throughout life, mammalian cardiomyocytes generally exit the cell cycle after birth and any further increase in heart size is due to enlargement of existing cells[79]. The failure of cardiomyocytes to proliferate after injury can lead to persistent thinning of the myocardial wall, an expansion of cardiac fibroblasts that can lead to fibrosis and an overall decrease in cardiac function. MiRNAs have been shown to be necessary for proper heart development and cardiac function. Previously, mice with cardiac progenitor cell-specific Dicer deletion were shown to be embryonically lethal due to malfunctions in ventricular myocardium development, outflow tract morphogenesis and chamber septation[80, 81]. Likewise, three week-old juveniles with myocardium-specific, tamoxifen-inducible Dicer knockout resulted in ventricular remodeling and premature death within one week. Also using these mice, it was shown that inducing Dicer knockout in adults led to severe hypertrophy, myofiber disarray and ventricular fibrosis[82]. Recently, many miRNAs have been identified as being critical for the normal development of the heart and in enhancing processes of repair after injury.
MiR-15 family
In mice, there is short period (<7 days) after birth when the heart can sustain ischemic injury and cardiomyocytes will proliferate to facilitate full regeneration; however, these regeneration mechanisms are silenced after this period[83]. Previously, the miR-15 family was shown to help regulate this process (Figure 3). The miR-15 family is composed of six members (including miR-15a, miR-15b, miR-16-1, miR-16-2, miR-195 and miR-497) that all have the same seed sequence. Interestingly, the miR-15 family generally, and miR-195 specifically, are among the most highly expressed miRNAs immediately after the postnatal period when mice lose the ability to regenerate the heart. Overexpression of miR-195 has been shown to promote even earlier premature cardiomyocyte cell cycle withdrawal by targeting checkpoint kinase 1 (Chek1), which regulates the progression through G2/M and spindle assembly checkpoints in the cell cycle[84]. Conversely, inhibition of miR-15b and miR-16 prolonged the postnatal regeneration period by increasing the expression of Chek1[85]. In addition, the miR-15 family is highly upregulated after MI and was shown to promote apoptosis by targeting B-cell lymphoma 2 (Bcl-2) and led to mitochondrial dysfunction by targeting ADP-ribosylation factor-like protein 2 (Arl2). Impressively, using anti-miRs complementary to the miR-15 family was able to protect adult mice and pigs from MI[86].
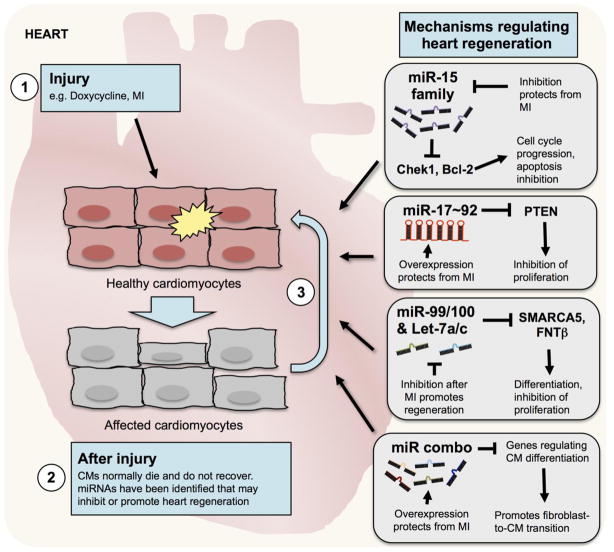
Figure 3. Diverse approaches have been developed using miRNAs that can promote heart regeneration.
Cardiomyocytes (CMs) may be the target of injury from drugs, environmental chemicals or from myocardial infarction (MI). Following injury, cardiomyocytes die and do not normally recover. Many different approaches have been developed with specific miRNAs or combinations of miRNAs that promote cardiac regeneration and recovery.
miR-17~92 cluster
Identified as the first onco-miRNA (“oncomiR-1”), the miR-17~92 cluster has been implicated in promoting cardiac differentiation and proliferation (Figure 3)[87]. This polycistronic cluster encodes six mature miRNAs (including miR-17, miR-18a, miR-19a, miR-19b-1, miR-20a and miR-92-1), all of which are expressed differentially due to selective post-transcriptional processing mechanisms[88]. Using miR-17~92 knockout mice and in vitro knockdown strategies, the cluster was shown to target cardiac progenitor genes and promoted proper myocardial differentiation[89]. Interestingly, cardiac-specific miR-17~92 knockout mice were shown to have smaller hearts due to a decrease in total cardiomyocytes, but most mice survived until adulthood despite decreased cardiac function[90]. Conversely, cardiac-specific miR-17~92 transgenic mice had increased postnatal cardiomyocyte proliferation through direct PTEN suppression that resulted in larger hearts as well as cardiomyocyte proliferation into adulthood. Importantly, transgenic mice were protected from MI as shown by echocardiogram and increased cardiomyocyte proliferation[90].
miR-99/100 & Let-7a/c
Unlike mammals, many lower vertebrates including amphibians and fish retain the ability of cardiac regeneration throughout their entire lives[91, 92]. Specifically, adult zebrafish can sustain an amputation of up to 20% of the ventricle and the remaining cardiomyocytes will still dedifferentiate, proliferate and redifferentiate to completely regenerate the heart[92, 93]. Some groups have postulated that this ability may still be present in mammals and active during development but is somehow silenced in adulthood. A recent study explored this concept further by analyzing miRNAs in regenerating zebrafish hearts post-amputation[94]. They found two specific miRNA families, miR-99/100 and Let-7a/c, that were conserved in higher vertebrates and downregulated during zebrafish heart regeneration (Figure 3). These miRNAs were shown to target SMARCA5 and FNTβ which are key regulators of differentiation. In mice, however, miR-99/100 and Let-7a/c levels increase and SMARCA5 and FNTβ decrease during development, but do not change following induction of MI in adults. Incredibly, inhibiting miR-99/100 after MI was sufficient to induce dedifferentiation and proliferation of cardiomyocytes, reduce infarct size and improve cardiac function[94].
Combination of miRNAs
Instead of increasing proliferation of surviving cardiomyocytes after injury, some groups have focused on inducing cardiac fibroblasts or embryonic stem cells (ESCs) to become cardiomyocytes and injecting them into the injured areas of the heart. In this case, however, combinations of miRNAs have been examined that regulate cardiomyocyte differentiation. Specifically, a set of miRNAs deemed “miR combo” (including miR-1, miR-133, miR-208a and miR-299) have been utilized due to their established roles in cardiac-specific development and function (Figure 3)[95]. Previously, both MiR-1 and miR-133 were shown to be co-transcribed, specific to cardiac muscle and skeletal muscle and integral for promoting proper heart development[96–98]. Also, ESCs have been transfected with miR-1 and improved cardiac function when transplanted following MI[99]. However, there is evidence of miR-1 and miR-133 having opposing functions in cardiomyocyte differentiation, suggesting they likely have different but perhaps complementary roles in cardiac functions[100, 101]. The other members of miR combo, MiR-208 and miR-499, are intronic miRNAs both within the α- and β-MHC primary transcript and have been shown to control MHC expression[72, 102]. Through empirical experimentation, miR combo was discovered to induce significant fibroblast reprogramming to cardiomyocyte-like cells in vitro[95]. Importantly, following MI and the subsequent injection of lentivirus-encoding miR combo in mice, cardiac fibroblasts became positive for cardiomyocyte markers. Injection of miR-1 alone was sufficient to promote this cardiac fibroblasts reprogramming in vivo, but the addition of miR-133, 208 and 499 – especially when JAK inhibitor was included – further improved these effects and resulted in improved cardiac function[103].
MiRNAS IN KIDNEY INJURY AND REPAIR
MiRNAs in kidney injury
The kidney is a critical organ for the excretion of waste products from the body and therefore, like the liver, is a main target of toxicity or ischemic insult. As a result, acute kidney injury (AKI) is growing as a major problem for clinicians around the world[104]. In fact, a recent meta-analysis found that AKI occurred in one-in-five adults and one-in-three children worldwide who were hospitalized with acute illnesses[105]. AKI is believed to be increasing in part due to growth in the aging population and health events such as diabetes mellitus, cardiovascular disease, invasive surgeries, sepsis, chronic kidney disease (which predisposes people to AKI) and exposure to environmental toxicants and nephrotoxic drugs[106]. Therefore, because no specific treatments have yet been developed to ameliorate AKI, miRNAs have been investigated that may have a role. Proximal tubule-specific Dicer knockout mice were shown to be protected from renal ischemia reperfusion injury (IRI), suggesting that mature miRNAs are critical in regulating pathways that mediate the response to kidney injury[107]. Many specific miRNAs have also been identified that regulate kidney injury and offer therapeutic benefits when modulated (Table 3).
Table 3.
Additional miRNAs described in kidney injury.
| miRNA | Expression during kidney injury | Target(s) | Model | Effect of miRNA | Citation |
|---|---|---|---|---|---|
| miR-24 | upregulated by IRI | H2AX, HO-1 | Mice | Inhibition of miRNA reduced tubular injury, decreased fibrosis & increased survival | [148] |
| miR-34a | upregulated by cisplatin & IRI | ATG4B | Mice, mouse PTECs | Inhibition of miRNA increased apoptosis of cisplatin-treated mouse PTECs & decreased autophagy | [149, 150] |
| miR-155 | upregulated following IRI, UUO & gentamycin-induced injury | c-Fos* | Rats, mice | Knockout mice had increased kidney injury & decreased function | [113, 151] |
| miR-489 | upregulated following IRI | PARP1 | Mice | Inhibition of miRNA increased apoptosis in cells, exacerbated kidney injury | [152] |
| miR-494 | upregulated following IRI | ATF3 | Mice | Overexpression of miRNA increased apoptosis and inflammation, decreasing renal function | [153] |
Asterisk (*) represents that only correlation between miRNA and target was shown, not direct interaction. AA: aristolochic acid; IRI: ischemia-reperfusion injury; PTECs: proximal tubular epithelial cells; UUO: unilateral ureteral obstruction
Current methods of detecting AKI rely on non-specific and insensitive biomarkers such as serum creatinine (SCr) and blood urea nitrogen (BUN)[108]. In 2008, FDA and European Medicines Agency qualified seven biomarkers for kidney toxicity in preclinical studies and the evaluation of these in humans is currently ongoing[109]. These studies provided compelling evidence that showed proteins excreted in the urine, such as Kidney Injury Molecule-1 (KIM-1), outperform BUN and SCr and correlate with histopathology in the rats. This has prompted investigators to conduct additional discovery studies to identify miRNAs as sensitive biomarkers or potentially as companion biomarkers that can also provide mechanistic information regarding pathway dysregulation[110].
MiR-21
MiR-21 is the most studied miRNA in kidney disease and is promising as both a biomarker and therapeutic target (Figure 4). Mice with miR-21 deletion or treated with miR-21 inhibitors after the induction of unilateral ureteral obstruction (UUO)-induced kidney injury were protected from fibrosis development[111]. In this study, miR-21 was shown to directly target PPARα and Mpv17I, an inhibitor or reactive oxygen species (ROS) generation in the mitochondria, and therefore miR-21 knockout mice had increased lipid oxidation, higher activation of metabolic pathways and decreased ROS following injury. In a mouse model of Alport syndrome, a genetic disorder involving progressive fibrosis that results in end-stage renal failure in young adults, miR-21 inhibition significantly reduced the development of fibrosis[112]. Because of these promising preclinical results, a miR-21 inhibitor, RG-012, is currently in Phase 2 clinical trials for this indication[20]. In addition, our lab was among the first to measure miR-21 in the urine supernatant in mice and patients following AKI[113]. Later, our lab was able to effectively differentiate AKI patients from healthy patients by measuring miR-21 combined with miR-200c, miR-423 and miR-4640 in urine[114]. Since then, miR-21 has been found to be a reliable biomarker of drug-induced kidney injury[110] and fibrosis[115], and was associated with kidney injury from multiple chronic diseases including type 2 diabetes and IgA nephropathy[116, 117].
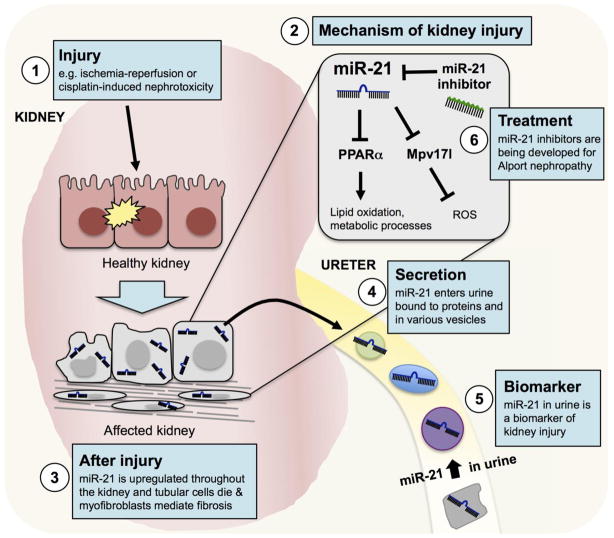
Figure 4. MiR-21 mediates kidney injury and causes the progression of fibrosis.
Following diverse forms of kidney injury, miR-21 is upregulated in kidney cells including the tubular epithelium, glomerular cells, myofibroblasts and infiltrating macrophages. MiR-21 mediates kidney injury by decreasing lipid oxidation, deregulating metabolic processes and increasing reactive oxygen species (ROS). In addition, miR-21 is secreted in the urine within small vesicles, dead cells or bound to RISC proteins or high-density lipoproteins. MiR-21 can be measured in the sediment or supernatant of urine samples as a biomarker of kidney injury. Because of the role of miR-21 in promoting fibrosis, a miR-21 inhibitor, RG-012, is in clinical trials to treat Alport nephropathy.
Additional miRNA biomarkers for AKI
Investigators looking at miRNA biomarkers of AKI have focused largely on measuring changes in the urine, since the kidney’s role is to produce urine and its collection is noninvasive. In addition, miRNAs are remarkably stable in this medium[118]. Multiple groups have described urinary miRNA expression changes in preclinical rodent models and patients with drug-induced kidney injury[110]. In one study, male Wistar rats treated with a single dose of the nephrotoxicant cisplatin were shown to have a greater than 20-fold increase in 11 miRNAs in the urine – including miR-15, miR-16, miR-20a, miR-192, miR-193 and miR-210 – over multiple time points[119]. Another study using cisplatin in Sprague-Dawley rats found let-7g-5p, miR-93, miR-191a and miR-192 significantly increased in the urine after injury[120]. Though kidney biopsies are rarely acquired in the clinic, sequencing studies conducted on biopsies of human kidney allografts have suggested specific miRNAs that may be used as biomarkers of acute rejection. In a study comparing 12 acute rejection biopsies to 21 normal, six miRNAs were differentially regulated including miR-142-5p, miR-155 and miR-223 (all upregulated) and miR-10b, miR-30a-3p and let-7c (all downregulated)[121]. In a later study, several miRNAs including miR-21, miR-142-3p, miR-142-5p and the miR-506 cluster were significantly increased in allograft biopsies with tubulointerstitial fibrosis compared to normal while miR-30a, miR-30d and miR-30e were all significantly downregulated[122]. Though more cross-validation and translational studies are needed, these studies show the potential of miRNA biomarkers for AKI.
MiRNAs in kidney development and regeneration
Following an episode of AKI the kidney has a remarkable ability to regenerate, though repeated injury or incomplete repair could lead to chronic kidney disease[123]. During mammalian development, Six2-positive progenitor cells give rise to all of the diverse cell types that constitute the nephron[124]. Previous studies in mice using Six2-specific Dicer ablation have shown that miRNAs are critical in regulating proper nephrogenesis[125]. In addition, Six2-specific miR-17~92 knockout mice had decreased nephrogenesis and developed albuminuria and glomerulosclerosis by three months[126]. Likewise, Dicer knockouts in other kidney cell subtypes have had differential effects on kidney function. For example, proximal tubule-specific Dicer knockout mice had no apparent effects on kidney function under normal conditions[107]. Conversely, when Dicer was knocked out in podocytes (cells that are essential in forming the glomerular filtration barrier), mice appeared to develop normally but rapidly acquired significant glomerular and tubular injury, proteinuria and died within four weeks after birth[127]. Ablating Dicer in juxtaglomerular cells (cells in the glomerulus that are responsible for renin synthesis and release) resulted in decreased cell number, renin signaling and lowered blood pressure[128]. Taken together, miRNAs are clearly critical in development but knowledge about specific miRNAs that regulate processes of regeneration is limited.
Compared to other organs discussed in this review, more is known about miRNAs that mediate kidney injury than about specific miRNAs involved in kidney repair. Nonetheless, some efforts have been initiated through temporal miRNA sequencing experiments following AKI to identify miRNAs that are elevated during periods of repair. Our lab recently conducted small RNA sequencing in mice subjected to folic acid-induced nephropathy and identified 108 miRNAs that were differentially regulated across seven time points[129]. Using principal component analysis, we found that there were unique expression patterns of miRNAs in the injury phase versus the repair and fibrotic phases. In addition, at early time points following injury when repair processes are initiated (days 1 through 3), miR-18a and miR-132 were highly expressed throughout the renal cortex and in the injured tubules. Similar temporal expression patterns were seen in renal IRI and UUO models. Independent of our lab, a different miRNA sequencing experiment conducted on multiple early time points following induction of renal IRI identified miR-18a as possibly being involved in repair[130]. Interestingly, in a different study miR-132 inhibition following UUO was shown to increase the number of proliferating proximal tubular cells and reduce the development of fibrosis, but inhibition of miR-132 in vitro resulted in less proliferation[131]. In addition to these miRNAs, a recent study showed that miR-687 induced cell cycle progression by targeting PTEN following IRI in mice[132]. Interestingly, however, inhibiting miR-687 resulted in less tubular apoptosis and improved kidney function. Much more work must be conducted on kidney regeneration in general and how miRNAs specifically mediate these processes.
OTHER miRNAs INVOLVED ORGAN INJURY AND REPAIR
In addition to the liver, heart and kidney, there is emerging evidence of miRNAs offering therapeutic potential in the injury and repair of other organs. For example, miRNAs have been identified that are associated with the injury and repair of lungs, skin and the central nervous system (Table 4).
Table 4.
MiRNAs that regulate injury and repair processes in other organs.
| miRNA LUNG | Expression | Target(s) | Model | Effect of miRNA | Citation |
|---|---|---|---|---|---|
| miR-146a | Upregulated by LPS | IRAK-1, TRAF-6 | Rats | Overexpression of miRNA suppressed pro-inflammatory signals in alveolar macrophages | [154] |
| miR-155 | Upregulated in ventilator- induced injury | C/EBPα | Mice | Inhibition of miRNA decreased BAL, cells in BAL and pro-inflammatory cytokines | [155, 156] |
| miR-21 | Upregulated by ventilator- induced injury | BMPR2*, PTEN* | Mice | Inhibition of miRNA decreased BAL and improved oxygen exchange | [155] |
| miR-127 | Downregulated by bleomycin | CD64 | Mice | Overexpression reduced pro-inflammatory signals in vivo | [157] |
| SKIN | |||||
| miR-31 | Upregulated by wounding & TGF-α | EMP-1 | Human | Overexpression of miRNA induced keratinocyte proliferation & migration | [158] |
| miR-132 | Upregulated by wounding & TGF-α | HB-EGF | Human, mouse | Inhibition of miRNA prevented epithelialization in human and mouse wound model | [159] |
| miR-200b | Downregulated by wounding | GATA2, VEFGR2 | Mice | Inhibition of miRNA mediated angiogenesis in vivo | [160] |
| miR-21 | Upregulated in chronic wounds | LepR, EGR3 | Rats, human | Overexpression of miRNA inhibited epithelialization in human & rat wound model | [161] |
| CENTRAL NERVOUS SYSTEM | |||||
| miR-21 | Upregulated by TBI | PTEN* | Rat | Inhibition of miRNA increased apoptosis and decreased angiogenesis after injury | [162] |
| miR-23b | Downregulated in plasma of TBI patients & brain of rats | ATG12 | Rats, human | Overexpression of miRNA reduced apoptosis, lesion volume & edema in brain | [163] |
| miR-223 | Upregulated in injured spinal cord | GluR2* | Rats | Inhibition of miRNA reduced apoptosis and improved motor function | [164] |
| miR-497 | Upregulated by cerebral ischemia | Bcl-2, Bcl-w | Mice | Inhibition of miRNA reduced infarct volume, improve neurological score | [165] |
Asterisk (*) represents that only correlation between miRNA and target was shown, not direct interaction. BAL: bronchoalveolar lavage; LPS: lipopolysaccharide; TBL: traumatic brain injury
FUTURE PERSPECTIVES
The rapid growth in miRNA research has made clear that miRNAs are critical players in the molecular mechanisms of mammalian biology. There is ample evidence that miRNAs can serve as stable and specific biomarkers of various disease states and efficacious targets to manipulate therapeutically. As a result, there has been a surge of patent applications that is approaching 500 annually and there are many miRNA therapeutics in various stages of clinical development[133]. Still, challenges exist in oligonucleotide therapeutic development and delivery. Because of the ability of miRNAs to regulate multiple pathways and their different functions depending on the tissue and context, identifying miRNAs that can be modulated efficaciously and safely in patients may be difficult. In addition, although different miRNA targeting strategies are being developed there are currently no examples of miRNA-specific oral therapeutics, and half-life and specificity remains an important issue. Nonetheless, chemically-modified oligonucleotides and liposomal delivery methods have made enormous progress in recent years. Diverse strategies of therapeutic delivery include restoring miRNA function with mimics or overexpression vectors or inhibiting miRNAs with antisense oligonucleotides, vectors that express miRNA “sponges” or small molecule inhibitors that inhibit miRNA pathways[134]. Moreover, proprietary chemical modifications to nucleotide backbones have been developed that confer resistance to serum nucleases and have improved target-binding affinities. Taken together, manipulating miRNAs in human disease settings offers a promising path forward, particularly with regards to organ injury and repair.
Acknowledgments
We thank Dr. Mira Pavkovic and Dr. Mariana Cardenas-Gonzalez for invaluable suggestions during the write up of this review article. Work in the Vaidya laboratory was supported by Outstanding New Environmental Sciences (ONES) award from NIH/NIEHS (ES017543) and Innovation in Regulatory Science Award from Burroughs Wellcome Fund (BWF-1012518).
References
- 1.Breving K, Esquela-Kerscher A. The complexities of microRNA regulation: mirandering around the rules. The international journal of biochemistry & cell biology. 2010;42(8):1316–1329. doi: 10.1016/j.biocel.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Ding XC, Weiler J, Grosshans H. Regulating the regulators: mechanisms controlling the maturation of microRNAs. Trends in biotechnology. 2009;27(1):27–36. doi: 10.1016/j.tibtech.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Fromm B, et al. A Uniform System for the Annotation of Vertebrate microRNA Genes and the Evolution of the Human microRNAome. Annual review of genetics. 2015;49:213–242. doi: 10.1146/annurev-genet-120213-092023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffiths-Jones S, Saini HK, van Dongen S. miRBase: tools for microRNA genomics. Nucleic acids…. 2008 doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marco A, Ninova M, Griffiths-Jones S. Multiple products from microRNA transcripts. Biochemical Society transactions. 2013;41(4):850–854. doi: 10.1042/BST20130035. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, et al. TransmiR: a transcription factor-microRNA regulation database. Nucleic acids research. 2010;38(Database issue):22. doi: 10.1093/nar/gkp803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruby JG, Jan CH, Bartel DP. Intronic microRNA precursors that bypass Drosha processing. Nature. 2007;448(7149):83–86. doi: 10.1038/nature05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su H, et al. Essential and overlapping functions for mammalian Argonautes in microRNA silencing. Genes & development. 2009;23(3):304–317. doi: 10.1101/gad.1749809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ørom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Molecular cell. 2008;30(4):460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Brümmer A, Hausser J. MicroRNA binding sites in the coding region of mRNAs: extending the repertoire of post-transcriptional gene regulation. BioEssays : news and reviews in molecular, cellular and developmental biology. 2014;36(6):617–626. doi: 10.1002/bies.201300104. [DOI] [PubMed] [Google Scholar]
- 11.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nature Reviews Genetics. 2011;12(2):99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 12.Wei Y, et al. Importin 8 Regulates the Transport of Mature MicroRNAs into the Cell Nucleus. Journal of Biological Chemistry. 2014;289(15):10270–10275. doi: 10.1074/jbc.C113.541417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang R, et al. Mouse miRNA-709 directly regulates miRNA-15a/16-1 biogenesis at the posttranscriptional level in the nucleus: evidence for a microRNA hierarchy system. Cell research. 2012;22(3):504–515. doi: 10.1038/cr.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saikumar J, Ramachandran K, Vaidya VS. Noninvasive Micromarkers. Clinical Chemistry. 2014;60(9):1158–1173. doi: 10.1373/clinchem.2013.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrill AH, et al. MicroRNA Biomarkers of Toxicity in Biological Matrices. Toxicological sciences : an official journal of the Society of Toxicology. 2016;152(2):264–272. doi: 10.1093/toxsci/kfw090. [DOI] [PubMed] [Google Scholar]
- 16.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circulation research. 2012;110(3):496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, et al. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Advanced drug delivery reviews. 2015;81:142–160. doi: 10.1016/j.addr.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Beavers KR, Nelson CE, Duvall CL. MiRNA inhibition in tissue engineering and regenerative medicine. Advanced drug delivery reviews. 2015;88:123–137. doi: 10.1016/j.addr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebert LF, et al. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic acids research. 2014;42(1):609–621. doi: 10.1093/nar/gkt852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.clinicaltrials.gov. Study of Weekly RG-012 Injections in Patients With Alport Syndrome (HERA) 2016 Available from: https://clinicaltrials.gov/ct2/show/NCT02855268?term=rg-012&rank=1.
- 21.Bouchie A. First microRNA mimic enters clinic. Nature biotechnology. 2013 doi: 10.1038/nbt0713-577. [DOI] [PubMed] [Google Scholar]
- 22.Lee WM. Etiologies of acute liver failure. Seminars in liver disease. 2008;28(2):142–152. doi: 10.1055/s-2008-1073114. [DOI] [PubMed] [Google Scholar]
- 23.Lee WM. Drug-induced hepatotoxicity. The New England journal of medicine. 2003;349(5):474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- 24.de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. Journal of hepatology. 2008;48(Suppl 1):12. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Mohd Hanafiah K, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology (Baltimore, Md) 2013;57(4):1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 26.Ozer J, et al. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245(3):194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Bandiera S, et al. miR-122--a key factor and therapeutic target in liver disease. Journal of hepatology. 2015;62(2):448–457. doi: 10.1016/j.jhep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Laterza OF, et al. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clinical chemistry. 2009;55(11):1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- 29.Tsai W-CC, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. The Journal of clinical investigation. 2012;122(8):2884–2897. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu S-HH, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. The Journal of clinical investigation. 2012;122(8):2871–2883. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang K, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starkey Lewis PJ, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology (Baltimore, Md) 2011;54(5):1767–1776. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- 33.Dear JW, et al. Early detection of paracetamol toxicity using circulating liver microRNA and markers of cell necrosis. British journal of clinical pharmacology. 2014;77(5):904–905. doi: 10.1111/bcp.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Church RJ, et al. Beyond miR-122: identification of microRNA alterations in blood during a time course of hepatobiliary injury and biliary hyperplasia in rats. Toxicological …. 2015 doi: 10.1093/toxsci/kfv260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaura Y, et al. Plasma microRNA profiles in rat models of hepatocellular injury, cholestasis, and steatosis. PloS one. 2012;7(2) doi: 10.1371/journal.pone.0030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshioka W, Higashiyama W, Tohyama C. Involvement of microRNAs in dioxin-induced liver damage in the mouse. Toxicological sciences : an official journal of the Society of Toxicology. 2011;122(2):457–465. doi: 10.1093/toxsci/kfr130. [DOI] [PubMed] [Google Scholar]
- 37.Miyaaki H, et al. Significance of serum and hepatic microRNA-122 levels in patients with non-alcoholic fatty liver disease. Liver …. 2014 doi: 10.1111/liv.12429. [DOI] [PubMed] [Google Scholar]
- 38.Bala S, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology (Baltimore, Md) 2012;56(5):1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Köberle V, Waidmann O, Kronenberger B. Serum microRNA-122 kinetics in patients with chronic hepatitis C virus infection during antiviral therapy. Journal of viral …. 2013 doi: 10.1111/jvh.12075. [DOI] [PubMed] [Google Scholar]
- 40.Meer AJ, Farid WRR, Sonneveld MJ. Sensitive detection of hepatocellular injury in chronic hepatitis C patients with circulating hepatocyte-derived microRNA-122. Journal of viral …. 2013 doi: 10.1111/jvh.12001. [DOI] [PubMed] [Google Scholar]
- 41.Janssen HL, et al. Treatment of HCV infection by targeting microRNA. The New England journal of medicine. 2013;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins RH, et al. Transforming growth factor β1 represses proximal tubular cell microRNA-192 expression through decreased hepatocyte nuclear factor DNA binding. The Biochemical journal. 2012;443(2):407–416. doi: 10.1042/BJ20111861. [DOI] [PubMed] [Google Scholar]
- 43.Nagano T, et al. Liver-specific microRNAs as biomarkers of nanomaterial-induced liver damage. Nanotechnology. 2013;24(40):405102. doi: 10.1088/0957-4484/24/40/405102. [DOI] [PubMed] [Google Scholar]
- 44.Krauskopf J, et al. Application of high-throughput sequencing to circulating microRNAs reveals novel biomarkers for drug-induced liver injury. Toxicological sciences : an official journal of the Society of Toxicology. 2015;143(2):268–276. doi: 10.1093/toxsci/kfu232. [DOI] [PubMed] [Google Scholar]
- 45.Hu J, et al. Plasma microRNA, a potential biomarker for acute rejection after liver transplantation. Transplantation. 2013;95(8):991–999. doi: 10.1097/TP.0b013e31828618d8. [DOI] [PubMed] [Google Scholar]
- 46.Roy S, et al. Down-regulation of miR-192-5p protects from oxidative stress-induced acute liver injury. Clinical science (London, England : 1979) 2016;130(14):1197–1207. doi: 10.1042/CS20160216. [DOI] [PubMed] [Google Scholar]
- 47.Ward J, et al. Circulating microRNA profiles in human patients with acetaminophen hepatotoxicity or ischemic hepatitis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(33):12169–12174. doi: 10.1073/pnas.1412608111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ninomiya M, et al. The expression of miR-125b-5p is increased in the serum of patients with chronic hepatitis B infection and inhibits the detection of hepatitis B virus surface antigen. Journal of viral hepatitis. 2016;23(5):330–339. doi: 10.1111/jvh.12522. [DOI] [PubMed] [Google Scholar]
- 49.Yang D, et al. MicroRNA-125b-5p mimic inhibits acute liver failure. Nature communications. 2016;7:11916. doi: 10.1038/ncomms11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferreira DM, et al. c-Jun N-terminal kinase 1/c-Jun activation of the p53/microRNA 34a/sirtuin 1 pathway contributes to apoptosis induced by deoxycholic acid in rat liver. Molecular and cellular biology. 2014;34(6):1100–1120. doi: 10.1128/MCB.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamakuchi M, Ferlito M. miR-34a repression of SIRT1 regulates apoptosis. Proceedings of the …. 2008 doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H, et al. Mir-34a is upregulated during liver regeneration in rats and is associated with the suppression of hepatocyte proliferation. PloS one. 2011;6(5) doi: 10.1371/journal.pone.0020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheung O, et al. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. 2008 doi: 10.1002/hep.22569. …. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castro RE, et al. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. Journal of hepatology. 2013;58(1):119–125. doi: 10.1016/j.jhep.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Ding J, et al. Effect of miR-34a in regulating steatosis by targeting PPARα expression in nonalcoholic fatty liver disease. Scientific reports. 2015;5:13729. doi: 10.1038/srep13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michalopoulos G, DeFrances M. Liver regeneration. Science. 1997;276(5309):60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 57.Hand NJ, et al. Hepatic function is preserved in the absence of mature microRNAs. Hepatology. 2009;49(2):618–626. doi: 10.1002/hep.22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sekine S, et al. Disruption of Dicer1 induces dysregulated fetal gene expression and promotes hepatocarcinogenesis. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sekine S, et al. Dicer is required for proper liver zonation. The Journal of Pathology. 2009;219(3):365–372. doi: 10.1002/path.2606. [DOI] [PubMed] [Google Scholar]
- 60.Yan-nan B, et al. MicroRNA-21 accelerates hepatocyte proliferation in vitro via PI3K/Akt signaling by targeting PTEN. Biochemical and biophysical research communications. 2014;443(3):802–807. doi: 10.1016/j.bbrc.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 61.Marquez RT, Wendlandt E, Galle CS. MicroRNA-21 is upregulated during the proliferative phase of liver regeneration, targets Pellino-1, and inhibits NF-κB signaling. American Journal of …. 2010 doi: 10.1152/ajpgi.00338.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song G, et al. MicroRNAs control hepatocyte proliferation during liver regeneration. 2010 doi: 10.1002/hep.23547. …. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng R, et al. A microRNA-21 surge facilitates rapid cyclin D1 translation and cell cycle progression in mouse liver regeneration. The Journal of …. 2012 doi: 10.1172/JCI46039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodrigues PM, et al. Inhibition of NF-κB by deoxycholic acid induces miR-21/PDCD4-dependent hepatocellular apoptosis. Scientific Reports. 2015;5:17528. doi: 10.1038/srep17528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang YJ. Molecular and cellular mechanisms of cardiotoxicity. Environmental health perspectives. 2001;109(Suppl 1):27–34. doi: 10.1289/ehp.01109s127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qureshi ZP, et al. Market withdrawal of new molecular entities approved in the United States from 1980 to 2009. Pharmacoepidemiology and Drug Safety. 2011;20(7):772–777. doi: 10.1002/pds.2155. [DOI] [PubMed] [Google Scholar]
- 67.Koturbash I, et al. microRNAs as pharmacogenomic biomarkers for drug efficacy and drug safety assessment. Biomarkers in medicine. 2015;9(11):1153–1176. doi: 10.2217/bmm.15.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Desai VG, et al. Early biomarkers of doxorubicin-induced heart injury in a mouse model. Toxicology and applied pharmacology. 2014;281(2):221–229. doi: 10.1016/j.taap.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Matsumoto S, et al. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circulation research. 2013;113(3):322–326. doi: 10.1161/CIRCRESAHA.113.301209. [DOI] [PubMed] [Google Scholar]
- 70.Boon RA, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495(7439):107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 71.Oliveira-Carvalho V, Carvalho VO, Bocchi EA. The emerging role of miR-208a in the heart. DNA and cell biology. 2013;32(1):8–12. doi: 10.1089/dna.2012.1787. [DOI] [PubMed] [Google Scholar]
- 72.Callis TE, et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. The Journal of clinical investigation. 2009;119(9):2772–2786. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu L, et al. miR-208a as a biomarker of isoproterenol-induced cardiac injury in Sod2+/-and C57BL/6J wild-type mice. Toxicologic pathology. 2014;42(7):1117–1129. doi: 10.1177/0192623314525684. [DOI] [PubMed] [Google Scholar]
- 74.Nishimura Y, et al. Plasma miR-208 as a useful biomarker for drug-induced cardiotoxicity in rats. Journal of applied toxicology : JAT. 2015;35(2):173–180. doi: 10.1002/jat.3044. [DOI] [PubMed] [Google Scholar]
- 75.Bostjancic E, et al. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology. 2009;115(3):163–169. doi: 10.1159/000268088. [DOI] [PubMed] [Google Scholar]
- 76.Wang G-KK, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. European heart journal. 2010;31(6):659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 77.Tony H, Yu K, Qiutang Z. MicroRNA-208a Silencing Attenuates Doxorubicin Induced Myocyte Apoptosis and Cardiac Dysfunction. Oxidative medicine and cellular longevity. 2015;2015:597032. doi: 10.1155/2015/597032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Montgomery RL, et al. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124(14):1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saxena A, Tabin CJ. miRNA-processing enzyme Dicer is necessary for cardiac outflow tract alignment and chamber septation. Proceedings of the National Academy of Sciences. 2010;107(1):87–91. doi: 10.1073/pnas.0912870107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao Y, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129(2):303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 82.da Martins CPA, Bourajjaj M, Gladka M. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.769984. [DOI] [PubMed] [Google Scholar]
- 83.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science (New York, NY) 2011;331(6020):1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Porrello ER, Johnson BA, Aurora AB. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circulation …. 2011 doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Porrello ER, et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(1):187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hullinger TG, et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circulation research. 2012;110(1):71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death & Differentiation. 2013;20(12) doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du P, et al. A Biogenesis Step Upstream of Microprocessor Controls miR-17~92 Expression. Cell. 2015;162(4):885–899. doi: 10.1016/j.cell.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang J, et al. Bmp signaling regulates myocardial differentiation from cardiac progenitors through a MicroRNA-mediated mechanism. Developmental cell. 2010;19(6):903–912. doi: 10.1016/j.devcel.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J, et al. mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circulation research. 2013;112(12):1557–1566. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. The Journal of experimental zoology. 1974;187(2):249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 92.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science (New York, NY) 2002;298(5601):2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 93.Jopling C, et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010 doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aguirre A, et al. In vivo activation of a conserved microRNA program induces mammalian heart regeneration. Cell stem cell. 2014;15(5):589–604. doi: 10.1016/j.stem.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jayawardena TM, et al. MicroRNA-Mediated In Vitro and In Vivo Direct Reprogramming of Cardiac Fibroblasts to Cardiomyocytes. Circulation Research. 2012;110(11):1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436(7048):214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 97.Heidersbach A, et al. microRNA-1 regulates sarcomere formation and suppresses smooth muscle gene expression in the mammalian heart. Elife. 2013 doi: 10.7554/eLife.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu N, Bezprozvannaya S, Williams AH. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes & …. 2008 doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glass C, Singla DK. MicroRNA-1 transfected embryonic stem cells enhance cardiac myocyte differentiation and inhibit apoptosis by modulating the PTEN/Akt pathway in the infarcted heart. American Journal of Physiology-Heart …. 2011 doi: 10.1152/ajpheart.00271.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ivey KN, et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell stem cell. 2008;2(3):219–229. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen JF, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nature …. 2006 doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Rooij E, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Developmental cell. 2009;17(5):662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hodgkinson CP, et al. MicroRNAs and Cardiac Regeneration. Circulation research. 2015;116(10):1700–1711. doi: 10.1161/CIRCRESAHA.116.304377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lameire NH, et al. Acute kidney injury: an increasing global concern. Lancet (London, England) 2013;382(9887):170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 105.Susantitaphong P, et al. World incidence of AKI: a meta-analysis. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(9):1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nature reviews. Nephrology. 2014;10(4):193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 107.Wei Q, et al. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. Journal of the American Society of Nephrology : JASN. 2010;21(5):756–761. doi: 10.1681/ASN.2009070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Waikar SS, et al. Imperfect gold standards for kidney injury biomarker evaluation. Journal of the American Society of Nephrology : JASN. 2012;23(1):13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vaidya VS, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nature Biotechnology. 2010;28(5):478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pavkovic M, Vaidya VS. MicroRNAs and drug-induced kidney injury. Pharmacology & therapeutics. 2016;163:48–57. doi: 10.1016/j.pharmthera.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chau BN, et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Science translational medicine. 2012;4(121) doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gomez IG, et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. The Journal of clinical investigation. 2015;125(1):141–156. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saikumar J, et al. Expression, circulation, and excretion profile of microRNA-21,-155, and-18a following acute kidney injury. Toxicological Sciences. 2012;129(2):256–267. doi: 10.1093/toxsci/kfs210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramachandran K, et al. Human miRNome profiling identifies microRNAs differentially present in the urine after kidney injury. Clinical chemistry. 2013;59(12):1742–1752. doi: 10.1373/clinchem.2013.210245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Glowacki F, et al. Increased circulating miR-21 levels are associated with kidney fibrosis. PloS one. 2013;8(2) doi: 10.1371/journal.pone.0058014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhong X, et al. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56(3):663–674. doi: 10.1007/s00125-012-2804-x. [DOI] [PubMed] [Google Scholar]
- 117.Hennino M-F, et al. miR-21-5p renal expression is associated with fibrosis and renal survival in patients with IgA nephropathy. Scientific Reports. 2016;6:27209. doi: 10.1038/srep27209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mall C, et al. Stability of miRNA in human urine supports its biomarker potential. Biomarkers in Medicine. 2013;7(4):623–631. doi: 10.2217/bmm.13.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pavkovic M, Riefke B, Ellinger-Ziegelbauer H. Urinary microRNA profiling for identification of biomarkers after cisplatin-induced kidney injury. Toxicology. 2014;324:147–157. doi: 10.1016/j.tox.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 120.Kanki M, et al. Identification of urinary miRNA biomarkers for detecting cisplatin-induced proximal tubular injury in rats. Toxicology. 2014 doi: 10.1016/j.tox.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 121.Anglicheau D, et al. MicroRNA expression profiles predictive of human renal allograft status. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(13):5330–5335. doi: 10.1073/pnas.0813121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ben-Dov IZ, et al. MicroRNA sequence profiles of human kidney allografts with or without tubulointerstitial fibrosis. Transplantation. 2012;94(11):1086–1094. doi: 10.1097/TP.0b013e3182751efd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bonventre JV. Primary proximal tubule injury leads to epithelial cell cycle arrest, fibrosis, vascular rarefaction, and glomerulosclerosis. Kidney international supplements. 2014;4(1):39–44. doi: 10.1038/kisup.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kobayashi A, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell stem cell. 2008;3(2):169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagalakshmi VK, et al. Dicer regulates the development of nephrogenic and ureteric compartments in the mammalian kidney. Kidney international. 2011;79(3):317–330. doi: 10.1038/ki.2010.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marrone AK, et al. MicroRNA-17~92 is required for nephrogenesis and renal function. Journal of the American Society of Nephrology : JASN. 2014;25(7):1440–1452. doi: 10.1681/ASN.2013040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ho J, et al. Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. Journal of the American Society of Nephrology : JASN. 2008;19(11):2069–2075. doi: 10.1681/ASN.2008020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sequeira-Lopez MS, et al. The MicroRNA-Processing Enzyme Dicer Maintains Juxtaglomerular Cells. Journal of the American Society of Nephrology. 2010;21(3):460–467. doi: 10.1681/ASN.2009090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pellegrini KL, et al. Application of small RNA sequencing to identify microRNAs in acute kidney injury and fibrosis. Toxicology and applied pharmacology. 2015 doi: 10.1016/j.taap.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cui R, et al. Global miRNA expression is temporally correlated with acute kidney injury in mice. PeerJ. 2016:4. doi: 10.7717/peerj.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bijkerk R, et al. Silencing of microRNA-132 reduces renal fibrosis by selectively inhibiting myofibroblast proliferation. Kidney international. 2016;89(6):1268–1280. doi: 10.1016/j.kint.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 132.Bhatt K, et al. MicroRNA-687 Induced by Hypoxia-Inducible Factor-1 Targets Phosphatase and Tensin Homolog in Renal Ischemia-Reperfusion Injury. Journal of the American Society of Nephrology : JASN. 2015;26(7):1588–1596. doi: 10.1681/ASN.2014050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Christopher AF, et al. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspectives in clinical research. 2016;7(2):68–74. doi: 10.4103/2229-3485.179431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nature reviews. Drug discovery. 2014;13(8):622–638. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 135.Yuan B, et al. Down-regulation of miR-23b may contribute to activation of the TGF-β1/Smad3 signalling pathway during the termination stage of liver regeneration. FEBS …. 2011 doi: 10.1016/j.febslet.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 136.Zhou J, et al. Down-regulation of microRNA-26a promotes mouse hepatocyte proliferation during liver regeneration. PloS one. 2012;7(4) doi: 10.1371/journal.pone.0033577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pan C, et al. Down-regulation of MiR-127 facilitates hepatocyte proliferation during rat liver regeneration. PloS one. 2012;7(6) doi: 10.1371/journal.pone.0039151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fornari F, et al. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene. 2008;27(43):5651–5661. doi: 10.1038/onc.2008.178. [DOI] [PubMed] [Google Scholar]
- 139.Yuan Q, et al. MicroRNA-221 overexpression accelerates hepatocyte proliferation during liver regeneration. Hepatology (Baltimore, Md) 2013;57(1):299–310. doi: 10.1002/hep.25984. [DOI] [PubMed] [Google Scholar]
- 140.Sharma AD, et al. MicroRNA-221 regulates FAS-induced fulminant liver failure. Hepatology (Baltimore, Md) 2011;53(5):1651–1661. doi: 10.1002/hep.24243. [DOI] [PubMed] [Google Scholar]
- 141.Bei Y, et al. miR-382 targeting PTEN-Akt axis promotes liver regeneration. Oncotarget. 2016;7(2):1584–1597. doi: 10.18632/oncotarget.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Seok J-KK, et al. MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the tumor suppressor phosphatase and tensin homolog. Nucleic acids research. 2014;42(12):8062–8072. doi: 10.1093/nar/gku515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hsu A, et al. Systemic Approach to Identify Serum microRNAs as Potential Biomarkers for Acute Myocardial Infarction. BioMed Research International. 2014;2014:1–13. doi: 10.1155/2014/418628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ai J, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochemical and biophysical research communications. 2010;391(1):73–77. doi: 10.1016/j.bbrc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 145.D’Alessandra Y, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. European heart journal. 2010;31(22):2765–2773. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Adachi T, et al. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clinical chemistry. 2010;56(7):1183–1185. doi: 10.1373/clinchem.2010.144121. [DOI] [PubMed] [Google Scholar]
- 147.Corsten MF, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circulation. Cardiovascular genetics. 2010;3(6):499–506. doi: 10.1161/CIRCGENETICS.110.957415. [DOI] [PubMed] [Google Scholar]
- 148.Lorenzen JM, et al. MicroRNA-24 antagonism prevents renal ischemia reperfusion injury. Journal of the American Society of Nephrology : JASN. 2014;25(12):2717–2729. doi: 10.1681/ASN.2013121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bhatt K, et al. MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Molecular medicine (Cambridge, Mass) 2010;16(9–10):409–416. doi: 10.2119/molmed.2010.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu X-JJ, et al. MicroRNA-34a Suppresses Autophagy in Tubular Epithelial Cells in Acute Kidney Injury. American journal of nephrology. 2015;42(2):168–175. doi: 10.1159/000439185. [DOI] [PubMed] [Google Scholar]
- 151.Pellegrini KL, et al. MicroRNA-155 deficient mice experience heightened kidney toxicity when dosed with cisplatin. Toxicological sciences : an official journal of the Society of Toxicology. 2014;141(2):484–492. doi: 10.1093/toxsci/kfu143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wei Q, et al. MicroRNA-489 Induction by Hypoxia-Inducible Factor-1 Protects against Ischemic Kidney Injury. Journal of the American Society of Nephrology : JASN. 2016 doi: 10.1681/ASN.2015080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lan Y-FF, et al. MicroRNA-494 reduces ATF3 expression and promotes AKI. Journal of the American Society of Nephrology : JASN. 2012;23(12):2012–2023. doi: 10.1681/ASN.2012050438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zeng Z, et al. Upregulation of miR-146a contributes to the suppression of inflammatory responses in LPS-induced acute lung injury. Experimental lung research. 2013;39(7):275–282. doi: 10.3109/01902148.2013.808285. [DOI] [PubMed] [Google Scholar]
- 155.Vaporidi K, Vergadi E, Kaniaris E. Pulmonary microRNA profiling in a mouse model of ventilator-induced lung injury. American Journal of …. 2012 doi: 10.1152/ajplung.00370.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guo Z, et al. Antisense oligonucleotide treatment enhances the recovery of acute lung injury through IL-10-secreting M2-like macrophage-induced expansion of CD4+ regulatory T cells. Journal of immunology (Baltimore, Md : 1950) 2013;190(8):4337–4348. doi: 10.4049/jimmunol.1203233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xie T, et al. MicroRNA-127 inhibits lung inflammation by targeting IgG Fcγ receptor I. Journal of immunology (Baltimore, Md : 1950) 2012;188(5):2437–2444. doi: 10.4049/jimmunol.1101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li D, et al. MicroRNA-31 Promotes Skin Wound Healing by Enhancing Keratinocyte Proliferation and Migration. The Journal of investigative dermatology. 2015;135(6):1676–1685. doi: 10.1038/jid.2015.48. [DOI] [PubMed] [Google Scholar]
- 159.Li D, et al. MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. The Journal of clinical investigation. 2015;125(8):3008–3026. doi: 10.1172/JCI79052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chan YC, et al. Downregulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(6):1372–1382. doi: 10.1161/ATVBAHA.112.248583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pastar I, et al. Induction of specific microRNAs inhibits cutaneous wound healing. The Journal of biological chemistry. 2012;287(35):29324–29335. doi: 10.1074/jbc.M112.382135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ge X-TT, et al. miR-21 improves the neurological outcome after traumatic brain injury in rats. Scientific reports. 2014;4:6718. doi: 10.1038/srep06718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun L, et al. miR-23b improves cognitive impairments in traumatic brain injury by targeting ATG12-mediated neuronal autophagy. Behavioural Brain Research. 2016 doi: 10.1016/j.bbr.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 164.Liu D, et al. Administration of Antagomir-223 Inhibits Apoptosis, Promotes Angiogenesis and Functional Recovery in Rats with Spinal Cord Injury. Cellular and Molecular Neurobiology. 2015;35(4):483–491. doi: 10.1007/s10571-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yin KJJ, et al. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiology of disease. 2010;38(1):17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]