Abstract
HRas proto-oncogene (HRAS) is one of the most commonly mutated genes in thyroid cancer, with mutations frequently occurring in the follicular and Hurthle cell subtypes. However, the contribution of mutations in HRAS to papillary thyroid carcinoma (PTC) progression and the tall-cell variant (TCV) is poorly understood. The aim of the present study was to investigate the somatic genetic variants present in HRAS in patients with PTC, and to investigate the association of these mutations with PTC. The present study is a retrospective case-control study using tumor samples collected from 139 patients with PTC and blood samples from 195 healthy individuals. All patient samples were screened for mutations in ‘hotspot’ regions of HRAS and B-raf proto-oncogene (BRAF) by single-stranded conformational polymorphism analysis, followed by direct sequencing. A novel variant (IVS1-82del gctgggcctggg) in the HRAS 5′-untranslated region was identified. There was no difference in age or sex of patients with PTC and the healthy controls; however, the HRAS variant was more frequently detected in PTC tissue than in the healthy control samples (37 vs. 26%, P=0.04). There was no association between the HRAS variant and age, sex, tumor size, encapsulation, multifocality/intra-thyroidal spread, Tumor-Node-Metastasis stage, history of Hashimoto's disease, BRAF V600E mutation or PTC subtype (all P>0.05). There were differences of BRAF V600E distribution among different subtypes (χ2=6.390, P=0.041). HRAS variant co-occurring with the BRAF V600E mutation accounted for 31.6% of the total number (P=0.196). Therefore, this novel variant of HRAS (IVS1-82del gctgggcctggg) may be associated with PTC; however, larger scale studies are required to assess the contribution of this novel HRAS variant to PTC progression.
Keywords: papillary thyroid carcinoma, HRAS proto-oncogene, variants, intron, case-control
Introduction
Carcinoma of the thyroid gland is the most common malignant tumor of the endocrine system (1). Papillary thyroid cancer (PTC) is the most common histological type of thyroid cancer, representing 80% of all cases of thyroid cancer and 85% of cases of differentiated thyroid cancer (2,3). PTC is 2.9–3.8 times more common in women than in men (4), and is more common in regions associated with a high dietary intake of iodine (5). In the United States, the incidence of PTC is 1.56–3.58/100,000 men and 4.9–10.96/100,000 women (4). PTC is usually associated with a more positive prognosis than follicular thyroid cancer; however, certain subtypes are more aggressive than others (5). Of the PTC subtypes, the tall-cell variant (TCV) is among the most aggressive (6). The 2004 World Health Organization (WHO) classification defined TCV as PTC containing ≥50% tall cells (7). Other characteristics of TCV include an eosinophilic tall cell cytoplasm and nuclear features characteristic of PTC (7). However, the molecular mechanisms that cause TCV differentiation are unclear.
In recent years, B-raf proto-oncogene (BRAF) mutation has been demonstrated to be the most common genetic alteration in PTC (8). It is a molecular marker associated with aggressive tumor behaviors, including size, extra-thyroidal extension, multifocality, lymph node metastasis, tumor recurrence and advanced disease stage (9,10).
The rat sarcoma viral oncogene homolog (RAS) genes, which include the isoforms HRAS, KRAS and NRAS, are crucial effectors in a number of signaling cascades. The mitogen activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase (PI3K) pathways, which mediate cell differentiation, proliferation, and survival, are affected by RAS genes (11,12). RAS activity is regulated by GTP-bound hydrolysis, and any mutation that results in the dysregulation of this hydrolysis results in aberrant MAPK and PI3K/(RAC serine/threonine-protein kinase (Akt) signaling, which are critical events in thyroid carcinogenesis (13).
HRAS is one of the most commonly mutated genes in PTC, particularly in variants identified in follicular (14–17) and Hurthle cells (18), reflecting its key regulatory functions. The contribution made by HRAS to PTC progression is poorly understood. Therefore, the present study aimed to investigate the presence of somatic variants in HRAS exhibited by patients with PTC as well as by healthy individuals, and to investigate their association with PTC development. The results of the present study provide an improved understanding of PTC pathogenesis and may provide novel insight for the advancement of PTC treatment.
Materials and methods
Study design and subjects
The present study involves the retrospective investigation of tumor samples collected from patients with PTC who underwent thyroidectomy at the Beijing Friendship Hospital, Capital Medical University (Beijing, China) between January 2011 and February 2016. A total of 139 PTC patients (106 females and 33 males), age (48.7±9.3) years old. The final diagnoses were made by pathological examination of the specimens. The following inclusion criteria were applied: i) No treatment for PTC prior to the surgery; ii) the absence of any other type of malignant tumor; iii) tumor size >0.5 cm; iv) no distant metastasis identified prior to surgery; v) clear results from lymph node dissection; and vi) sufficient DNA extractable from the tissue for analysis.
A total of 195 blood samples from asymptotic people undergoing routine health examinations were acquired as healthy controls. The following exclusion criteria were applied: i) Any symptom of thyroid cancer, and ii) the identification of any biochemical abnormality.
The present study was approved by the Clinical Research Ethics Committee of Beijing Friendship Hospital, Capital Medical University.
Pathological evaluation
Following surgery, formalin-fixed paraffin-embedded (FFPE) tumor-rich tissue areas were dissected from unstained 4-µm sections under the guidance of stained slides which was stained by undiluted hematoxylin and eosin (Merck KGaA, Darmstadt, Germany) for 330 sec at room temperature, with the tumor area marked under the guidance of light microscope (original magnification ×200). All histological slides were reviewed independently by experienced pathologists specialized in thyroid pathology (from the Peking University Third Hospital, Beijing, China). Diagnoses were performed according to the WHO classification (7). The tumors were classified into histological subtypes: Classic variant of papillary thyroid carcinoma (CVPTC), follicular variant of papillary thyroid carcinoma (FVPTC), and TCV.
DNA extraction
Tumor-rich areas were scraped from the paraffin sections, added to 500 µl xylene (concentration ≥99.0%; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and centrifuged 27,400 × g at room temperature for 15 min. The supernatant was discarded and 500 µl of anhydrous ethanol was used to disperse the pellet prior to centrifugation twice more 27,400 × g at room temperature for 10 min. The supernatant was discarded and 50 µl acetone was used to disperse the pellet prior to further centrifugation 27,400 × g at room temperature for 5 min. Subsequent to air drying, the pellet was suspended in 309 µl DNA extraction buffer (300 µl digestion buffer and 9 µl proteinase K; E.Z.N.A® FFPE DNA Kit; Omega Bio-Tek, Inc., Rockville, MD, USA), and incubated at 55°C for 3–5 h. The DNA was extracted from the 195 control samples using Blood DNA kits (Tiangen Biotech Co., Ltd., Beijing, China), according to the manufacturer's instructions. The DNA concentration was determined by spectrophotometric absorption (A) at 230, 260, and 280 nm, and the DNA quality was evaluated by calculating the ratio of optical density (OD) value at 260 and 280 nm or 260 and 230 nm measured by a BioSpec-nano spectrophotometer (Shimadzu Corporation, Kyoto, Japan).
Single-stranded conformational polymorphism analysis (SSCP) and direct DNA sequencing for HRAS mutations
SSCP analysis was performed to prescreen for mutations in the HRAS and BRAF exons, in which hotpoint mutations can be identified (19). Primers for SSCP-polymerase chain reaction (PCR) were designed using the Primer 3.0 software (Premier Biosoft International, Palo Alto, CA, USA; Table I. PCR was performed in a total volume of 10 µl, consisting of 1 µl DNA solution (100 ng/µl), 0.5 units of Platinum Taq DNA polymerase (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 0.1 µCi [a-33P] deoxycytidine triphosphate (ICN Biomedicals, Irvine, CA, USA; specific activity of 3,000 Ci/mmol), 1–4 mmol/l MgCl2, 0.1–0.2 mmol/l deoxynucleotide triphosphate, 0.2–0.4 mmol/l each primer, 10 mmol/l Tris-HCl (pH 8.3) and 50 mmol/l KCl in a thermal cycler (Biometra GmbH, Göttingen, Germany). The thermocycling conditions were as follows: 95°C for 5 min, 37–40 cycles of 95°C for 50 sec, 45–54°C for 60 sec and extension 72°C for 60 sec, and 72°C for 5 min. Subsequent to PCR amplification, 10 µl PCR product was mixed with 20 µl loading buffer (0.02 M NaOH, 95% formamide, 20 mmol/l EDTA, 0.05% xylene cyanol, and 0.05% bromophenol blue) and denatured at 95°C for 10 min, prior to quenching on ice. A total of 5.5 µl sample mixture was loaded onto a 12.5% polyacrylamide non-denaturing gel containing 10% glycerol. Electrophoresis was performed at 45 W for 3.5–4.5 h at room temperature with fan cooling. Gels were performed silver staining according to our previous study (20). Samples exhibiting mobility shifts in SSCP analysis were re-amplified using the same primers and PCR conditions as for SSCP analysis and sequenced to determine the HRAS and BRAF genotypes (Beijing Tianyi Huiyuan Co., Ltd., Beijing, China; Table I) (21).
Table I.
Primers used for HRAS variant screening in PTC.
| Gene (exon) | Forward sequence, 5′-3′ | Reverse sequence, 5′-3′ | Product size, bp |
|---|---|---|---|
| HRAS (1) | cagtccttgctgcctggc | atggttctggatcagctgga | 264 |
| HRAS (2) | cctgtctcctgcttcctctag | tggcaaacacacacaggaag | 298 |
| BRAF (15) | aactcttcataatgcttgctctga | agtaactcagcagcatctcagg | 251 |
HRAS, HRas proto-oncogene; PTC, papillary thyroid carcinoma; BRAF, B-raf proto-oncogene.
Statistical analysis
χ2 test was used to identify the association between HRAS and BRAF variants, the different subtypes of PTC, and lymph node metastasis. All statistical analyses were performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Subject characteristics
Table II presents the clinical characteristics of the subjects. There were no differences in age and sex between the 139 patients with PTC and the 195 healthy individuals. However, HRAS variants were more frequent in patients with PTC compared with healthy individuals (37 vs. 26%, P=0.04).
Table II.
Characteristics of the subjects.
| Variable | PTC samples, n (%) | Control samples, n (%) | P-value |
|---|---|---|---|
| Total | 139 | 195 | |
| Age, years | 0.14 | ||
| ≤45 | 78 (56.1) | 126 (64.6) | |
| >45 | 61 (44.9) | 69 (35.4) | |
| Sex | 0.25 | ||
| Female | 106 (76.3) | 136 (69.7) | |
| Male | 33 (23.7) | 59 (30.3) | |
| HRAS variant | 51 (36.7) | 51 (26.2) | 0.04 |
PTC, papillary thyroid carcinoma; HRAS, HRas proto-oncogene.
Molecular analysis of HRAS and BRAF
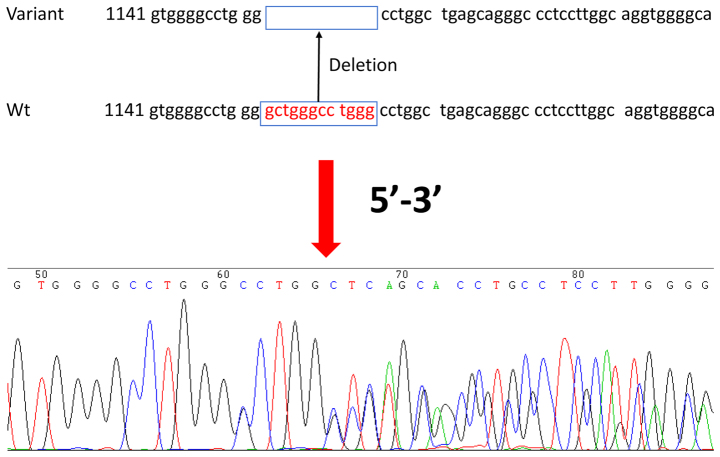
A novel variant of HRAS (IVS1-82del gctgggcctggg; Fig. 1) was identified to the best of our knowledge for the first time in PTC and adjacent non-tumor tissue: 51/139 (37%) patients with PTC were heterozygous for the IVS1-82del gctgggcctggg variant, compared with 51/195 (26%) healthy controls (P=0.04; Table II). The HRAS variant was not specific to PTC but occurred more frequently in patients with PTC compared with healthy individuals. The frequency of the HRAS variant did not differ among PTC subtypes (P=0.95). There were no associations between the HRAS variant and age, sex, tumor size, encapsulation, multifocality/intrathyroidal spread, Tumor-Node-Metastasis stage (22), thyroid nodule status, Hashimoto history, BRAF mutation or PTC subtype (all P>0.05; Table III). There were significant differences in the number of BRAF mutations among the different subtypes (P=0.041; Table IV). The presence of the BRAF V600E mutant was not associated with that of the HRAS variant (P=0.196; Table V).
Figure 1.
Identification and analysis of a novel HRAS variant in papillary thyroid carcinoma. (A) heterozygous variant with 12 bp deletion in the 5′-untranslated region (IVS1-82del gctgggcctggg) in the HRAS gene. Wt, wild-type.
Table III.
Association of HRAS variant, IVS1-82del gctgggcctggg, with clinical features in patients with PTC.
| HRAS status, n (%) | ||||
|---|---|---|---|---|
| Variable | Patients with PTC, n (%) | Wild-type | Variant | P-value |
| Total | 139 | 88 | 51 | |
| Sex | 0.68 | |||
| Female | 106 (76.3) | 66 (75.0) | 40 (78.4) | |
| Male | 33 (23.7) | 22 (25.0) | 11 (21.6) | |
| Age, years | 0.11 | |||
| ≤45 | 78 (56.1) | 54 (31.4) | 24 (47.1) | |
| >45 | 61 (43.9) | 34 (68.6) | 27 (52.9) | |
| Tumor size, mm | 0.11 | |||
| ≤10 | 79 (56.8) | 55 (62.5) | 24 (47.1) | |
| >10 | 60 (43.2) | 33 (37.5) | 27 (52.9) | |
| Encapsulation | 54 (38.9) | 32 (63.6) | 22 (53.1) | 0.47 |
| Multifocality/intrathyroidal spread | 32 (23.0) | 19 (21.6) | 13 (25.5) | 0.68 |
| Lymph node metastasis | 66 (47.5) | 39 (44.3) | 27 (72.7) | 0.40 |
| TNM stage | 0.56 | |||
| I/II | 99 (71.2) | 61 (69.3) | 38 (74.5) | |
| III/IV | 40 (28.8) | 27 (30.7) | 13 (25.5) | |
| Hashimoto's disease | 77 (55.3) | 51 (57.9) | 26 (50.9) | 0.49 |
| PTC subtype | 0.95 | |||
| CVPTC | 34 (24.5) | 22 (25.0) | 12 (23.5) | |
| FVPTC | 36 (25.9) | 22 (25.0) | 14 (27.5) | |
| TCV | 69 (49.6) | 44 (50.0) | 25 (49.0) | |
HRAS, HRas proto-oncogene; TNM, Tumor-Node-Metastasis; PTC, papillary thyroid carcinoma; CVPTC, classic variant of papillary thyroid carcinoma; FVPTC, follicular variant of papillary thyroid carcinoma; TCV, tall-cell variant.
Table IV.
BRAF V600E mutation occurrence in different subtypes of papillary thyroid carcinoma.
| Subtype | Mutation, n (%) | Wild-type, n (%) | χ2 | P-value |
|---|---|---|---|---|
| CVPTC | 25 (73.5) | 9 (26.4) | 0.041 | |
| FVPTC | 24 (66.7) | 12 (33.3) | 6.390 | |
| TCV | 60 (86.9) | 9 (13.0) |
BRAF, B-raf proto-oncogene; CVPTC, classic variant of papillary thyroid carcinoma; FVPTC, follicular variant of papillary thyroid carcinoma; TCV, tall-cell variant.
χ2 test.
Table V.
Association between BRAF mutation and the novel HRAS variant.
| HRAS−, n | HRAS+, n | Total, n | χ2 | P-value | |
|---|---|---|---|---|---|
| BRAF− | 20 | 7 | 27 | 1.672 | 0.196 |
| BRAF+ | 68 | 44 | 112 | ||
| Total | 88 | 51 | 139 |
BRAF, B-raf proto-oncogene; HRAS, HRas proto-oncogene; HRAS−, HRAS wild-type; BRAF−, BRAF wild-type; HRAS+, HRAS variant (IVS1-82del gctgggcctggg); BRAF+, BRAF mutation exhibited.
χ2 test.
Discussion
HRAS is one of the most commonly mutated genes in thyroid cancer, particularly the follicular and Hurthle cell subtypes. However, its contribution to PTC and the TCV is poorly understood. Therefore, the present study aimed to investigate the presence of somatic variants in HRAS in patients with PTC and healthy controls, and to investigate their association with PTC development. The results demonstrated that a novel HRAS variant (IVS1-82del gctgggcctggg) could be associated with PTC. Larger studies are required to assess the distribution of this novel HRAS variant and to validate the results of the present study.
PTC is the most common form of thyroid cancer (2,3). In the present study, the percentage of TCV samples harboring the HRAS variant was 49.6%. The most common etiological factor associated with onset of PTC is radiation; however, other factors, including genetic susceptibility, have been demonstrated to be associated with PTC development (23), as have predispositions such as Hashimoto's thyroiditis (HT) (24). HT has been recognized as a common autoimmune thyroid disorder associated with various antibodies, including thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) (25). If patients present with diffuse goiter (Graves disease), and their TPOAb and TgAb levels are simultaneously increased, an HT diagnosis can be made. However, in the present study, no significant association between HT and the novel HRAS variant was identified.
Mutations associated with phenotypic susceptibility are popular in oncology research; however, such research often requires a large sample size to obtain reliable results. Furthermore, the identification of novel variants often requires DNA sequencing, which is an expensive technology with limited availability in certain countries. The most commonly used method is SSCP (26,27), which is an efficient and sensitive technique used for the identification of single-base mutations.
Mutations in the genes of the RAS family members are known to be associated with thyroid carcinogenesis; RAS mutations have been identified in PTC, follicular carcinoma, follicular adenoma, and medullary thyroid carcinoma (17,28–32). Previous studies have demonstrated that various types of thyroid carcinoma, particularly FVPTC, harbor somatic mutations in HRAS (15,16,33). The HRAS gene is also often activated in urinary tract tumors (34). The 81T>C polymorphism in the HRAS gene is associated with increased risk of skin (35), oral (36), bladder (37), and gastric (38) cancer. It has been demonstrated that the 81T>C polymorphism, which increases protein expression without changing its function, was associated with aneuploidy in thyroid cancer (39). Previous studies have reported that the frequency of RAS variants was 10–43% in PCT (40–43).
The BRAF V600E mutation has been demonstrated to be the most common genetic alteration in PTC (8). BRAF is a member of the RAF family and is involved in the MAPK pathway (28). Briefly, the MAPK cascade is initiated upon RAS activation, which recruits BRAF to the plasma membrane. The present study demonstrated that BRAF mutations were more frequent in TCV than in other subtypes, and that the HRAS variant occurred concomitantly with the BRAF mutation in 31.6% of PTC samples (P=0.196). The concomitant mutations are typically present in the CVPTC and TCV subtypes (29.4 vs. 30.4%). This indicates that the concomitant mutations may be associated with aggressive disease behavior and poor prognosis; however, further studies are required to confirm this.
Two different mechanisms may be responsible for the carcinogenic effect of HRAS mutations: Modified protein function or increased protein expression (43,44–46). As RAS proteins are involved in cell differentiation, proliferation, and survival, increased expression or activity of HRAS may enhance these activities, which are associated with carcinogenesis. Indeed, increased RAS activation leads to constitutive activation of the downstream targets of RAS proteins, i.e., the MAPK and PI3K/Akt signaling pathways (13). The novel HRAS variant identified in the present study occurs at the 5′ end of the sequence, which may affect the selective splicing of HRAS and could be associated with tumor pathogenesis. However, the exact effect of this variant on protein expression remains to be determined.
Concomitant BRAF and RAS mutations may allow simultaneous activation of the MAPK and PI3K/Akt signaling pathways in cancer cells, providing a growth advantage (47,48). Long-term follow-up revealed that patients with concomitant mutations had a poorer response to treatment and reduced disease-free survival times (49), indicating that activation of the two genes may have a synergistic effect on disease progression (50).
One previous study revealed no association between HRAS variants and tumor biology (51), whereas other studies have reported associations between HRAS variants and poorly differentiated tumors (51,52). In the present study, HRAS mutations were demonstrated to be associated with follicular thyroid lesions (32). HRAS has been demonstrated to be frequently mutated in Hurthle cells, which are believed to represent a common metaplastic change in damaged thyroid follicular epithelium (53). Hurthle cells can often develop into Hurthle cell cancer, which is categorized as an oncocytic variant of follicular carcinoma (54). The present study did not include follicular carcinoma or Hurthle cell cancer clinical cases; however, it is possible that the HRAS variant arises from follicular or Hurthle cells in PTCs. In addition, the results of the present study indicated that the novel HRAS variant tends to occur in the TCV. Additional studies are required to fully elucidate the role of the novel HRAS variant in tumor biology.
The present study is limited by the number of patients, retrospective nature, and constrained follow-up information. Furthermore, the potential cellular mechanisms of mutation functions in PTC were not determined. In conclusion, a novel variant of HRAS (IVS1-82del gctgggcctggg) was associated with PTC. Further studies are required to assess the distribution of this novel HRAS variant and to validate the results of the present study.
Acknowledgements
The authors would like to thank the team of Professor Jian Huang for providing technical support. This study was supported by a grant from the National Natural Science Foundation of China (grant no. 81541132).
References
- 1.Hou P, Bojdani E, Xing M. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. J Clin Endocrinol Metab. 2010;95:820–828. doi: 10.1210/jc.2009-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ron E, Schneider AB. Thyroid Cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. Oxford University Press; New York: 2006. [DOI] [Google Scholar]
- 3.Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol. 2010;6:1771–1779. doi: 10.2217/fon.10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011;21:125–134. doi: 10.1089/thy.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider DF, Chen H. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin. 2013;63:374–394. doi: 10.3322/caac.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghossein R, Livolsi VA. Papillary thyroid carcinoma tall cell variant. Thyroid. 2008;18:1179–1181. doi: 10.1089/thy.2008.0164. [DOI] [PubMed] [Google Scholar]
- 7.Livolsi VA, Albores-Saavedra J, Asa SL, Baloch ZW, Sobrinho-Simoes M, Wenig B, DeLellis RA, Cady B, Mazzaferri EL, Hay I, et al. Papillary Carcinoma. In: DeLellis RA, Lloyd RV, Heitz R, Eng C, editors. Pathology and Genetics: Tumours of Endocrine Organs. World Health Organization Classification of Tumours. IARC Press; Lyon: 2004. [Google Scholar]
- 8.Kim KB, Cabanillas ME, Lazar AJ, Williams MD, Sanders DL, Ilagan JL, Nolop K, Lee RJ, Sherman SI. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF (V600E) mutation. Thyroid. 2013;23:1277–1283. doi: 10.1089/thy.2013.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joo JY, Park JY, Yoon YH, Choi B, Kim JM, Jo YS, Shong M, Koo BS. Prediction of occult central lymph node metastasis in papillary thyroid carcinoma by preoperative BRAF analysis using fine-needle aspiration biopsy: A prospective study. J Clin Endocrinol Metab. 2012;97:3996–4003. doi: 10.1210/jc.2012-2444. [DOI] [PubMed] [Google Scholar]
- 10.Kurtulmus N, Duren M, Ince U, Cengiz Yakicier M, Peker O, Aydin O, Altiok E, Giray S, Azizlerli H. BRAF (V600E) mutation in Turkish patients with papillary thyroid cancer: Strong correlation with indicators of tumor aggressiveness. Endocrine. 2012;42:404–410. doi: 10.1007/s12020-012-9651-x. [DOI] [PubMed] [Google Scholar]
- 11.Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: A fine balance. Biochem Soc Trans. 2012;40:139–146. doi: 10.1042/BST20110609. [DOI] [PubMed] [Google Scholar]
- 12.Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid. 2010;20:697–706. doi: 10.1089/thy.2010.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular mutation of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120:71–77. doi: 10.1309/ND8D9LAJTRCTG6QD. [DOI] [PubMed] [Google Scholar]
- 15.Park JY, Kim WY, Hwang TS, Lee SS, Kim H, Han HS, Lim SD, Kim WS, Yoo YB, Park KS. BRAF and RAS mutations in follicular mutation of papillary thyroid carcinoma. Endocr Pathol. 2013;24:69–76. doi: 10.1007/s12022-013-9244-0. [DOI] [PubMed] [Google Scholar]
- 16.Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, Ghossein RA. Molecular genotyping of papillary thyroid carcinoma follicular mutation according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23:1191–1200. doi: 10.1038/modpathol.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, Biddinger PW, Nikiforov YE. Correlation between genetic alterations and microscopic features, clinical manifestations and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–222. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 18.Liu RT, Hou CY, You HL, Huang CC, Hock-Liew, Chou FF, Wang PW, Cheng JT. Selective occurrence of ras mutations in benign and malignant thyroid follicular neoplasms in Taiwan. Thyroid. 2004;14:616–621. doi: 10.1089/1050725041692882. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Pang J, Watanabe T, Ng HK, Ohgaki H. Whole genome amplification for array comparative genomic hybridization using DNA extracted from formalin-fixed, paraffin-embedded histological sections. J Mol Diagn. 2009;11:109–116. doi: 10.2353/jmoldx.2009.080143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Zhao YP, Li Q, Zhang JX, Wang Y, Zhang B. Association of single nucleotide polymorphisms of NBS1 gene with genetic susceptibility to primary liver cancer in a Chinese Han population. Prog Biochem Biophys. 2012;39:678–686. doi: 10.3724/SP.J.1206.2011.00536. [DOI] [Google Scholar]
- 21.Huang J, Grotzer MA, Watanabe T, Hewer E, Pietsch T, Rutkowski S, Ohgaki H. Mutations in the Nijmegen breakage syndrome gene in medulloblastomas. Clin Cancer Res. 2008;14:4053–4058. doi: 10.1158/1078-0432.CCR-08-0098. [DOI] [PubMed] [Google Scholar]
- 22.Qing W, Fang WY, Ye L, Shen LY, Zhang XF, Fei XC, Chen X, Wang WQ, Li XY, Xiao JC, Ning G. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid. 2012;22:905–910. doi: 10.1089/thy.2011.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma mutation. Head Neck Pathol. 2011;5:51–56. doi: 10.1007/s12105-010-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okayasu I, Fujiwara M, Hara Y, Tanaka Y, Rose NR. Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese and white and African Americans. Cancer. 1995;76:2312–2318. doi: 10.1002/1097-0142(19951201)76:11<2312::AID-CNCR2820761120>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 25.Hiromatsu Y, Satoh H, Amino N. Hashimoto's thyroiditis: History and future outlook. Hormones (Athens) 2013;12:12–18. doi: 10.1007/BF03401282. [DOI] [PubMed] [Google Scholar]
- 26.Long J, Wang Y, Li M, Tong WM, Jia JD, Huang J. Correlation of TP53 mutations with HCV positivity in hepatocarcinogenesis: Identification of a novel TP53 microindel in hepatocellular carcinoma with HCV infection. Oncol Rep. 2013;30:119–124. doi: 10.3892/or.2013.2430. [DOI] [PubMed] [Google Scholar]
- 27.Huang MD, Chen XF, Xu G, Wu QQ, Zhang JH, Chen GF, Cai Y, Qi FZ. Genetic variation in the NBS1 gene is associated with hepatic cancer risk in a Chinese population. DNA Cell Biol. 2012;31:678–682. doi: 10.1089/dna.2011.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 29.Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A, Máximo V, Botelho T, Seruca R, Sobrinho-Simões M. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 30.Frattini M, Ferrario C, Bressan P, Balestra D, De Cecco L, Mondellini P, Bongarzone I, Collini P, Gariboldi M, Pilotti S, et al. Alternative mutations of BRAF, RET and NTRK1 are associated with similar but distinct gene expression patterns in papillary thyroid cancer. Oncogene. 2004;23:7436–7440. doi: 10.1038/sj.onc.1207980. [DOI] [PubMed] [Google Scholar]
- 31.Dockhorn-Dworniczak B, Caspari S, Schroder S, Bocker W, Dworniczak B. Demonstration of activated oncogenes of the ras family in human thyroid tumors using the polymerase chain reaction. Verh Dtsch Ges Pathol. 1990;74:415–418. [PubMed] [Google Scholar]
- 32.Schulten HJ, Al-Maghrabi J, Al-Ghamdi K, Salama S, Al-Muhayawi S, Chaudhary A, Hamour O, Abuzenadah A, Gari M, Al-Qahtani M. Mutational screening of RET, HRAS, KRAS, NRAS, BRAF, AKT1 and CTNNB1 in medullary thyroid carcinoma. Anticancer Res. 2011;31:4179–4183. [PubMed] [Google Scholar]
- 33.Rivera M, Ricarte-Filho J, Tuttle RM, Ganly I, Shaha A, Knauf J, Fagin J, Ghossein R. Molecular, morphologic and outcome analysis of thyroid carcinomas according to degree of extrathyroid extension. Thyroid. 2010;20:1085–1093. doi: 10.1089/thy.2010.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita J, Yoshida O, Yuasa Y, Rhim JS, Hatanaka M, Aaronson SA. Ha-ras oncogenes are activated by somatic alterations in human urinary tract tumours. Nature. 1984;309:464–466. doi: 10.1038/309464a0. [DOI] [PubMed] [Google Scholar]
- 35.Kreimer-Erlacher H, Seidl H, Back B, Kerl H, Wolf P. High mutation frequency at Ha-ras exons 1–4 in squamous cell carcinomas from PUVA-treated psoriasis patients. Photochem Photobiol. 2001;74:323–330. doi: 10.1562/0031-8655(2001)074<0323:HMFAHR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Sathyan KM, Nalinakumari KR, Abraham T, Kannan S. Influence of single nucleotide polymorphisms in H-Ras and cyclin D1 genes on oral cancer susceptibility. Oral Oncol. 2006;42:607–613. doi: 10.1016/j.oraloncology.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Johne A, Roots I, Brockmoller J. A single nucleotide polymorphism in the human H-ras proto-oncogene determines the risk of urinary bladder cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:68–70. [PubMed] [Google Scholar]
- 38.Zhang Y, Jin M, Liu B, Ma X, Yao K, Li Q, Chen K. Association between H-RAS T81C genetic polymorphism and gastrointestinal cancer risk: A population based case-control study in China. BMC Cancer. 2008;8:256. doi: 10.1186/1471-2407-8-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castro P, Soares P, Gusmão L, Seruca R, Sobrinho-Simoes M. H-RAS 81 polymorphism is significantly associated with aneuploidy in follicular tumors of the thyroid. Oncogene. 2006;25:4620–4627. doi: 10.1038/sj.onc.1209491. [DOI] [PubMed] [Google Scholar]
- 40.Esapa CT, Johnson SJ, Kendall-Taylor P, Lennard TW, Harris PE. Prevalence of Ras mutations in thyroid neoplasia. Clin Endocrinol (Oxf) 1999;50:529–535. doi: 10.1046/j.1365-2265.1999.00704.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120:71–77. doi: 10.1309/ND8D9LAJTRCTG6QD. [DOI] [PubMed] [Google Scholar]
- 42.Santarpia L, Myers JN, Sherman SI, Trimarchi F, Clayman GL, El-Naggar AK. Genetic alterations in the RAS/RAF/mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways in the follicular variant of papillary thyroid carcinoma. Cancer. 2010;116:2974–2983. doi: 10.1002/cncr.25061. [DOI] [PubMed] [Google Scholar]
- 43.Khan MS, Pandith AA, Ul Hussain M, Iqbal M, Khan NP, Wani KA, Masoodi SR, Mudassar S. Lack of mutational events of RAS genes in sporadic thyroid cancer but high risk associated with HRAS T81C single nucleotide polymorphism (case-control study) Tumour Biol. 2013;34:521–529. doi: 10.1007/s13277-012-0577-y. [DOI] [PubMed] [Google Scholar]
- 44.Bos JL. ras oncogenes in human cancer: A review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 45.Bos JL. The ras gene family and human carcinogenesis. Mutat Res. 1988;195:255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto-Gotoh T, Kikuno R, Takahashi M, Honkawa H. Possible role of the first intron of c-H-ras in gene expression: anti-cancer elements in oncogenes. Anticancer Res. 1988;8:851–859. [PubMed] [Google Scholar]
- 47.Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, Savchenko V, Miyakawa M, Isozaki O, Murakami H, Tsushima T, et al. Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet. 2004;41:161–170. doi: 10.1136/jmg.2003.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janku F, Lee JJ, Tsimberidou AM, Hong DS, Naing A, Falchook GS, Fu S, Luthra R, Garrido-Laguna I, Kurzrock R. PIK3CA mutations frequently coexist with RAS and BRAF mutations in patients with advanced cancers. PLoS One. 2011;6:e22769. doi: 10.1371/journal.pone.0022769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou M, Baitei EY, Alzahrani AS, BinHumaid FS, Alkhafaji D, Al-Rijjal RA, Meyer BF, Shi Y. Concomitant RAS RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid. 2014;24:1256–1266. doi: 10.1089/thy.2013.0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira C, Velho S, Moutinho C, Ferreira A, Preto A, Domingo E, Capelinha AF, Duval A, Hamelin R, Machado JC, et al. KRAS and BRAF oncogenic mutations in MSS colorectal carcinoma progression. Oncogene. 2007;26:158–163. doi: 10.1038/sj.onc.1209758. [DOI] [PubMed] [Google Scholar]
- 51.Suarez HG, du Villard JA, Severino M, Caillou B, Schlumberger M, Tubiana M, Parmentier C, Monier R. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene. 1990;5:565–570. [PubMed] [Google Scholar]
- 52.Pilotti S, Collini P, Mariani L, Placucci M, Bongarzone I, Vigneri P, Cipriani S, Falcetta F, Miceli R, Pierotti MA, Rilke F. Insular carcinoma: A distinct de novo entity among follicular carcinomas of the thyroid gland. Am J Surg Pathol. 1997;21:1466–1473. doi: 10.1097/00000478-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Ganly I, Ricarte Filho J, Eng S, Ghossein R, Morris LG, Liang Y, Socci N, Kannan K, Mo Q, Fagin JA, Chan TA. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. J Clin Endocrinol Metab. 2013;98:E962–E972. doi: 10.1210/jc.2012-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeLellis RA, Lloyd RV, Heitz PU. World Health Organization Classification of Tumors. Pathology and Genetics: Tumors of Endocrine Organs. IARC Press; Lyon, France: 2004. pp. 69–72. [Google Scholar]