Abstract
It is well known that autophagy serves a crucial role in renal tubular epithelial cell (RTEC) injury in the pathogenesis of chronic kidney disease (CKD). The accumulation of advanced oxidation protein products (AOPPs) also participates in the progression of CKD. However, the effects of AOPPs on autophagy remain unknown. To clarify the underlying mechanism of RTEC injury in CKD, the effect of AOPPs on HK-2 cells, an RTEC cell line, was investigated. The results of the present study revealed that AOPP exposure downregulated the expression of B-cell lymphoma-2-interacting myosin-like coiled-coil protein 1, reduced the conversion of microtubule-associated proteins 1 light chain 3B (LC3)-I to LC3-II and the formation of autophagosomes, and lead to an accumulation of p62. These results suggest that AOPPs may inhibit the autophagic activity of HK-2 cells. Furthermore, the aforementioned changes were mediated by the AOPP-phosphorylated phosphoinositide 3-kinase3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway; this was verified by treatment with LY294002, a PI3K inhibitor, which reversed the AOPP-induced changes. The present study also demonstrated that the activation of autophagy with rapamycin led to an improvement in the AOPP-induced overexpression of kidney injury molecule 1 and neutrophil gelatinase-associated lipocalin, two biomarkers of RTEC injury, whereas inhibiting autophagy with chloroquine further increased their expression during AOPP treatment. Collectively, these results indicate that AOPPs may inhibit autophagy in RTECs via activation of the PI3K/AKT/mTOR pathway and that autophagy inhibition serves a role in AOPP-induced RTEC injury.
Keywords: advanced oxidation protein products, autophagy, chronic kidney disease, phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway, renal tubular epithelial cell injury
Introduction
Chronic kidney disease (CKD) is a worldwide health concern with high morbidity and mortality (1). Tubulointerstitial fibrosis is a major pathological characteristic of CKD (2) and renal tubular epithelial cell (RTEC) damage induces interstitial fibrosis (3). Therefore, elucidating the mechanism responsible for RTEC injury is critical for developing a treatment to prevent fibrosis and interrupt the progression of CKD.
It has previously been reported that macroautophagy (autophagy) is associated with RTEC damage (4). Autophagy is a highly regulated lysosomal protein degradation process in which damaged organelles and long-lived proteins are removed in order to maintain intracellular homeostasis and cellular integrity (5). During autophagy activation, the biosynthesis of microtubule-associated protein 1 light chain 3B-II (LC3-II) (6) and B-cell lymphoma-2 (Bcl-2)-interacting myosin-like coiled-coil protein (Beclin1) (7) increases. These proteins are associated with the extent of autophagosome formation (8). p62 is known to serve a role in linking polyubiquitinated protein aggregates in the autophagic machinery and upregulated protein expression indicates inhibited autophagy (9). Yang et al (10) reported that the nephrotoxic drug cisplatin induced autophagic activation to protect RTEC from apoptosis. By contrast, Suzuki et al (11) demonstrated that, in renal ischemia/reperfusion injury, increased autophagic activity resulted in RTEC death. As such, it is not known whether autophagy has a protective or detrimental effect on cell survival.
In the pathogenesis of CKD, autophagy serves a role in RTEC injury when exposed to harmful conditions, including excessive proteinuria (12) and high glucose (13). Advanced oxidation protein products (AOPPs), which are primarily formed by the reaction of plasma albumin with chlorinated oxidants (14), were revealed to be elevated in the plasma of patients with CKD and the level of AOPPs is positively associated with the deterioration of renal function in CKD (15). Zhou et al (16) demonstrated that AOPPs induced glomerular podocyte apoptosis and albuminuria in normal rats and Li et al (17) suggested that AOPPs induced renal fibrosis in the remnant kidneys of 5/6 nephrectomy rats. A previous study by our group demonstrated that AOPPs induced RTEC injury, including hypertrophy and epithelial-to-mesenchymal transition (EMT) (18). However, the effects of AOPPs on RTEC autophagy are yet to be investigated.
Notably, autophagy activation is modulated by cross-linked signaling pathways (19). The kinase mammalian target of rapamycin (mTOR) is the key modulator of autophagy and acts as a ‘gatekeeper’ of various upstream signaling pathways (20). In recent years, researchers have taken great interest in the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mTOR pathway, which is a crucial negative modulator of autophagy (21). Furthermore, a number of studies have demonstrated that activation of this signaling pathway serves a crucial role in the pathogenesis of CKD (22,23). Therefore, it is reasonable to hypothesize that the PI3K/AKT/mTOR pathway may be associated with the effects of AOPPs on RTEC autophagy.
The aim of the present study was to examine the effect of AOPPs on autophagy and the probable modulatory pathway in cultured HK-2 cells in vitro in order to elucidate the mechanism of AOPP-induced RTEC injury.
Materials and methods
AOPPs-bovine serum albumin (BSA) preparation and content determination
AOPPs were prepared as previously described (24). BSA was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and hypochlorous acid (HOCl) was purchased from Fluke Switzerland GmbH (Basserdorf, Switzerland). BSA solution (100 mg/ml) was combined with HOCl (200 mmol/l) at a molar ratio of 1:140 for 30 min at room temperature in the dark and in the absence of free amino acid/carbohydrates/lipids to exclude the formation of advance glycation end product-like structures. Prepared samples were dialyzed in 4°C PBS to remove free HOCl for 24 h. BSA was dissolved in PBS alone as control. All samples were passed through a Detoxi-Gel column (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to remove contaminating endotoxins. The endotoxin levels were measured using the Amebocyte lysate assay kit (Sigma Aldrich; Merck KGaA) and were demonstrated to be <0.025 EU/ml. The AOPP content was determined by measuring absorbance at 340 nm in an acidic condition after mixing 200 µl of prepared sample or chloramines-T with 20 µl of acetic acid in a 96-well plate. The sample was calibrated using chloramines-T in the presence of potassium iodide (14).
Culture of HK-2
Immortalized HK-2 cells were purchased from ATCC (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium, nutrient mixture F12 (DMEM/F12; Hyclone; GE Healthcare, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.) and an antibiotic mixture of penicillin (100 U/ml) and streptomycin (100 µg/ml) at 37°C in an atmosphere containing 5% CO2. Cells at 80% confluence cells from passages 2 to 5 were used. In the LY294002 (CST Biological Reagents Co., Ltd.), rapamycin (Sigma-Aldrich; Merck KGaA) and chloroquine (Sigma Aldrich; Merck KGaA) blocking experiments, cells were pretreated with LY294002 (10 µM), rapamycin (1 µM) or chloroquine (1 mM) for 1 h at room temperature and subsequently treated with AOPPs for 24 h at room temperature.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. cDNA was synthesized using total RNA (1 µg) with random primers and the MMLV reverse transcriptase First Strand kit at 37°C for 15 min, followed by 85°C for 5 sec and storage at 4°C (Invitrogen; Thermo Fisher Scientific, Inc.). qPCR was performed using the SYBR®Premix Ex Taq™kit (Takara Bio, Inc., Otsu, Japan). The thermocycling conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec. The following primers were used: GAPDH forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′; kidney injury molecule 1 (KIM-1) forward, 5′-GACAACGAGCATTCCAACAA-3′ and reverse, 5′-GCTGAGGTGAAGATGGTGAAG-3′; neutrophil gelatinase-associated lipocalin (NGAL) forward, 5′-ACAAAGACCCGCAAAAGATG-3′ and reverse, 5′-GCAACCTGGAACAAAAGTCC-3′. All data was normalized using the internal control GAPDH. Fold change were quantified using the 2−∆∆Cq method (25).
Western blotting
Total protein was extracted from cells using pre-cooled radioimmunoprecipitation assay lysis buffer containing cocktail protease inhibitors (Biotool; Stratech Scientific, Ltd., Newmarket, UK). Protein concentrations were determined using the Micro Bicinchoninic Acid Assay kit (CoWin Biosciences, Beijing, China). According to the expression abundance and molecular weight of the proteins, 40 µg of LC3B and p62 were separated using 12% SDS-PAGE and 20 µg of the remaining proteins were separated using 8% SDS-PAGE. Proteins were then transferred onto polyvinylidene fluoride membranes. Membranes were blocked in 5% non-fat milk at room temperature for 1–3 h, followed by incubation with primary antibodies for 2 h at room temperature and additional incubation at 4°C overnight. The primary antibodies included the following: Anti-LC3B (cat. no. 2775 1:1,000), anti-phospho-AKT (Ser 473; cat. no. 4060; 1:1,000), anti-Beclin1 (cat. no. 3738; 1:1,000), anti-AKT (cat. no. 9272; 1:2,000), anti-p62 (cat. no. 8025; 1:2,000; all CST Biological Reagents Co., Ltd.), anti-p85 PI3K (cat. no. WL01169; 1:500; Wanleibio Co., Ltd., Shanghai, China), anti-phospho-mTOR (Ser 2448; cat. no. sc-293133; 1:500), anti-mTOR (cat. no. sc8319; 1:500; both Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and anti-GAPDH (cat. no. 20301707-1; 1:5,000; Bioworld, Dublin, OH USA). The membranes were washed three times with TBS containing Tween-20 (TBST) for 10 min and subsequently incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no. bs-0295M-HRP; 1:10,000; BIOSS, Beijing, China) at room temperature for 1 h. Membranes were washed three times with TBST for 10 min and proteins were detected using an enhanced chemiluminescence system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Semi-quantitative analysis was performed using ImageJ software (version 1.48u; National Institutes of Health, Bethesda, MD, USA). GAPDH was used as an internal control.
ELISA assay
HK-2 cells were cultured and treated with 200 µg/ml AOPPs at room temperature for 24 h in 96-well plates. The collected cell culture medium was centrifugated at a speed of 95 × g for 5 min at room temperature to obtain the supernatant and discard the cell debris. All reagents and samples were brought to room temperature prior to use. Monoclonal antibodies [cat. no. 70-EK11181; 1:100; Hangzhou MultiSciences (Lianke) Biotech Co., Ltd., Hangzhou, China] specific to human KIM-1 or NGAL were pre-coated onto microplates with carbonate buffer of (pH 9.6) overnight at 4°C. A total of 100 µl of standards (concentrations ranging from 1–2,000 pg/ml) or samples were added to each well in triplicate, followed by 50 µl of diluted KIM-1 and NGAL detection antibodies [cat. no. 70-EK11181; 1:100; Hangzhou MultiSciences (Lianke) Biotech Co., Ltd.]. Plates were incubated at room temperature for 2 h with gentle agitation. Wells were washed six times and 100 µl of diluted streptavidin-horseradish peroxidase-conjugated secondary antibody (cat. no. 70-EK11181; 1:100; Multi Science, Hangzhou, China) was added and incubated at room temperature for 45 min with gentle agitation. Plates were washed six times and 100 µl of substrate solution [cat. no. 70-EK11181; Hangzhou MultiSciences (Lianke) Biotech Co., Ltd.] was added at room temperature for 30 min in the dark. Finally, the absorbance was read at 450 and 570 nm using a microplate reader.
Immunofluorescence
HK-2 cells were seeded at density of 1,000/cm2 in a laser confocal dish. Following treatment, the supernatant was discarded and cells were washed three times with PBS for 5 min. Cells were subsequently fixed with 4% paraformaldehyde at room temperature for 15 min. Following washing with PBS, cells were permeabilized with 0.3% Triton X-100 for 15 min and incubated in a blocking buffer containing 5% BSA for 30 min at room temperature. Cells were incubated with anti-LC3B antibody (ca. no. 2775; 1:100; CST Biological Reagents Co., Ltd.) for 1 h at room temperature and again overnight at 4°C. Secondary antibodies labeled with fluorescein (cat. no. SA00006-6; Alexa Fluor 488; 1:1,000; Proteintech Group, Inc., Chicago, IL, USA) were added and incubated for 1 h at 37°C in the dark. Cells were subsequently incubated with 0.1% DAPI for 10 min at room temperature and washed with PBS. The cells were observed using a laser confocal fluorescent microscope at a magnification of ×100. The detection of punctuated LC3B staining in the diffuse staining indicated the quantity of autophagosomes. LC3B-stained dots were counted in individual HK-2 cells and the mean of ≥30 cells was calculated.
Transmission electron microscopy
HK-2 cells were harvested gently using trypsin-EDTA (Gibco; Thermo Fisher Scientific, Inc.) following 12 h of culture. Cells were centrifuged at a speed of 95 × g for 5 min at room temperature and the sediment cells were collected, washed twice with cold PBS and fixed in 2.5% glutaraldehyde for 2 h at 4°C. Cells were conventionally dehydrated, embedded, sliced into 60 nm sections and stained with uranyl acetate for 15 min at room temperature, followed by lead citrate for 15 min at room temperature. Autophagosome (AP) and autolysosome (AL) formation was observed using transmission electron microscopy (TEM) at a magnification of ×10,000 or ×20,000. During the TEM study, images of 10 random fields were captured in a grid (top left, middle left, bottom left, center, top right, middle right and bottom right) and the number of APs, ALs and cells per field was counted.
Statistical analysis
All experiments were performed in triplicate. Data are presented as the mean ± standard deviation. Differences between groups were assessed using one-way analysis of variance followed by a post hoc Tukey's test. When comparing 2 groups, Fisher's Least Significant Difference method was used. When the assumption of equal variance did not hold, the Dunnett's T3 method was used. Statistical analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
AOPPs inhibit autophagic activity in HK-2 cells
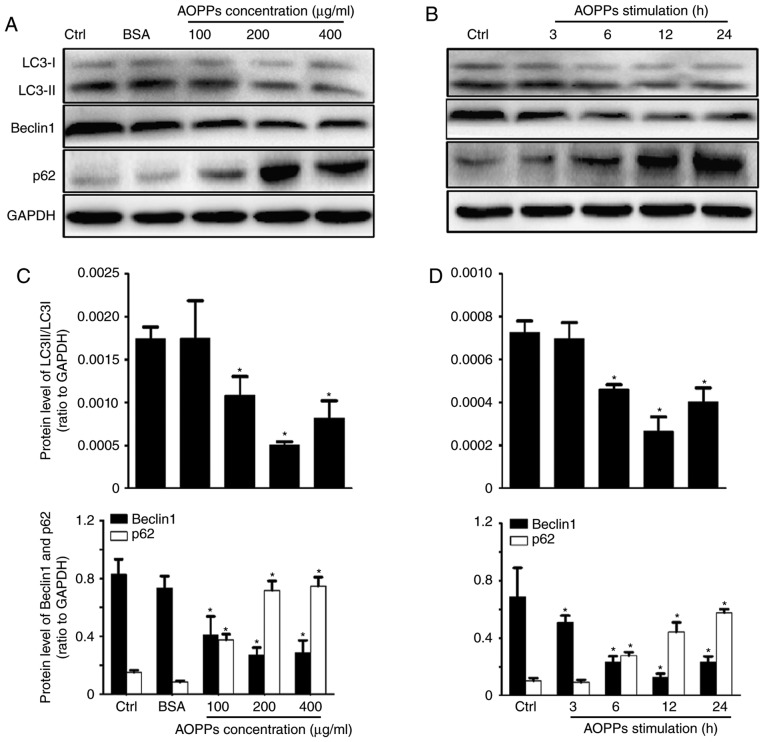
To investigate the effects of AOPPs on the autophagy of RTECs, western blotting was performed to examine the expression of LC3B, Beclin1 and p62 proteins (Fig. 1A and B). AOPPs treatment significantly upregulated the expression of p62, whereas the expression of Beclin1 and conversion of LC3-I to LC3-II were significantly downregulated compared with the control group. However, no significant differences were observed between the control and unmodified BSA groups. The optimal concentration and duration of treatment were determined to be 200 µg/ml and 12 h, respectively (Fig. 1C and D). HK-2 cells were therefore treated with control medium or AOPPs (200 µg/ml) for 12 h in subsequent experiments.
Figure 1.
AOPPs inhibit autophagic activity in HK-2 cells. HK-2 cells were treated with (A) vehicle control, unmodified BSA (200 µg/ml) and BSA + AOPPs for 12 h, or (B) 200 µg/ml of AOPPs for 3, 6, 12 or 24 h and levels of LC3-I, LC3-II, Beclin1 and p62 were assessed using western blotting. (C and D) Quantified results of western blotting. Data are expressed as the mean ± standard deviation of three independent experiments; *P<0.05 vs. Ctrl. AOPPs, advanced oxidation protein products; BSA, bovine serum albumin; LC3, microtubule-associated proteins 1 light chain 3B; Beclin1, B-cell lymphoma-2-interacting myosin-like coiled-coil protein; Ctrl, control.
Autophagic activity of the HK-2 cells was assessed using immunofluorescence technology and TEM (Fig. 2). Immunofluorescent staining revealed that LC3B-positive staining was significantly decreased in the AOPP-treated cells compared with the control group (Fig. 2A and C). Similarly, the TEM results demonstrated a decrease in the number of typical APs, which are recognized as double-membrane vacuoles engulfing cytoplasmic structures and ALs, characterized as single-membrane vacuole structures containing high-density materials, in AOPP-treated cells compared with the control group (Fig. 2B and D). Collectively, these results suggest that AOPPs inhibit the activation of autophagy in HK-2 cells.
Figure 2.
Immunofluorescent staining and TEM observation of autophagy. HK-2 cells were treated with the vehicle control or 200 µg/ml AOPPs for 12 h in the absence or presence of LY294002 (10 µM). (A) Indirect immunofluorescent staining revealed that AOPP treatment decreased positive LC3B staining, whereas LY294002 addition reversed this effect. Magnification, ×100. Scale bar, 10 µm. (B) TEM visualization revealed that AOPP treatment inhibited autophagic vacuole formation, whereas LY294002 treatment improved it. Top row, magnification, ×10,000 and scale bar, 2 µm; bottom row, magnification, ×20,000 and scale bar, 1 µm (C) LC3B-positive cells were counted in individual HK-2 cells and the mean of ≥30 cells was calculated. (D) Images of 10 random TEM fields were captured in a grid (top left, middle left, bottom left, center, top right, middle right and bottom right) and the number of autophagosomes, autolysosomes and cells were counted. Data are expressed as the mean ± standard deviation of three independent experiments; *P<0.05 vs. control, #P<0.05 vs. AOPP-treatment group. TEM, transmission electron microscopy; AOPPs, advanced oxidation protein products; LC3B, microtubule-associated proteins 1 light chain 3B; Ctrl, control.
PI3K/AKT/mTOR signaling pathway activation mediates AOPP-inhibited autophagy
Next, it was assessed whether the PI3K/AKT/mTOR signaling pathway is associated with the AOPP-induced inhibition of HK-2 autophagy. The expression of p85-PI3K, p-AKT (Ser473), AKT, p-mTOR (Ser2448) and mTOR proteins in cells was measured following exposure to AOPPs (Fig. 3). Treatment with AOPPs induced the phosphorylation of PI3K, AKT and mTOR. Specifically, at 6 h post-treatment, the significant phosphorylation of PI3K, AKT and mTOR was observed and this increase peaked at 12 h post-treatment (Fig. 3D and F). Furthermore, the optimal concentration of AOPPs was determined to be 200 µg/ml (Fig. 3C and E). No significant differences were observed between control and unmodified BSA-treated cells. Collectively, these results suggest that AOPPs trigger the PI3K/AKT/mTOR signaling pathway.
Figure 3.
AOPPs activate the PI3K/AKT/mTOR signaling pathway. HK-2 cells were treated with (A) vehicle, unmodified BSA (200 µg/ml) and 100, 200 or 400 µg/ml AOPPs for 12 h, or (B) 200 µg/ml of AOPPs for 3, 6, 12 or 24 h and the phosphorylation of PI3K, AKT and mTOR was assessed using western blotting (C-F). Western blotting results were quantified using densitometry. Data are presented as the mean ± standard deviation of three independent experiments; *P<0.05 vs. Ctrl. AOPPs, advanced oxidation protein products; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; BSA, bovine serum albumin; Ctrl, control; p, phosphorylated; t, total.
Cells were subsequently treated with AOPPs for 12 h in the presence or absence of LY294002 (10 µM), a PI3K inhibitor that blocks the PI3K/AKT/mTOR pathway (Fig. 4). The AOPP-induced downregulation of Beclin1 and LC3-II expression, as well as the AOPP-induced upregulation of p62 was significantly reversed by treatment with LY294002 (Fig. 4D). Furthermore, LC3B-positive staining was significantly increased in cells treated with LY294002 compared with those treated with AOPPs alone (Fig. 2A and C). Electron microscopy analysis demonstrated that LY294002 also partially ameliorated the AOPP-induced decrease in APs and ALs (Fig. 2B and D). These results suggest that the PI3K/AKT/mTOR signaling pathway mediates the AOPP-induced inhibition of autophagic activity in HK-2 cells.
Figure 4.
AOPPs inhibit autophagy via the PI3K/AKT/mTOR signaling pathway. (A) HK-2 cells were treated with the vehicle control or 200 µg/ml AOPPs for 12 h in the absence or presence of 10 µM LY294002 and the expression of (B) PI3K, (C) AKT and mTOR was quantified. The PI3K/AKT/mTOR signaling pathway was significantly blocked by LY294002. (D) Western blotting revealed that the AOPP-induced decrease in LC3-I to LC3-II conversion, suppression of Beclin1 and overexpression of p62 were partly reversed by treatment with LY294002. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05 vs. Ctrl, #P<0.05 vs. AOPP-treatment group. AOPPs, advanced oxidation protein products; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; LC3, microtubule-associated proteins 1 light chain 3B; Beclin1, B-cell lymphoma-2-interacting myosin-like coiled-coil protein; Ctrl, control; p, phosphorylated; t, total.
Autophagy inhibition mediates AOPP-induced injury to RTECs
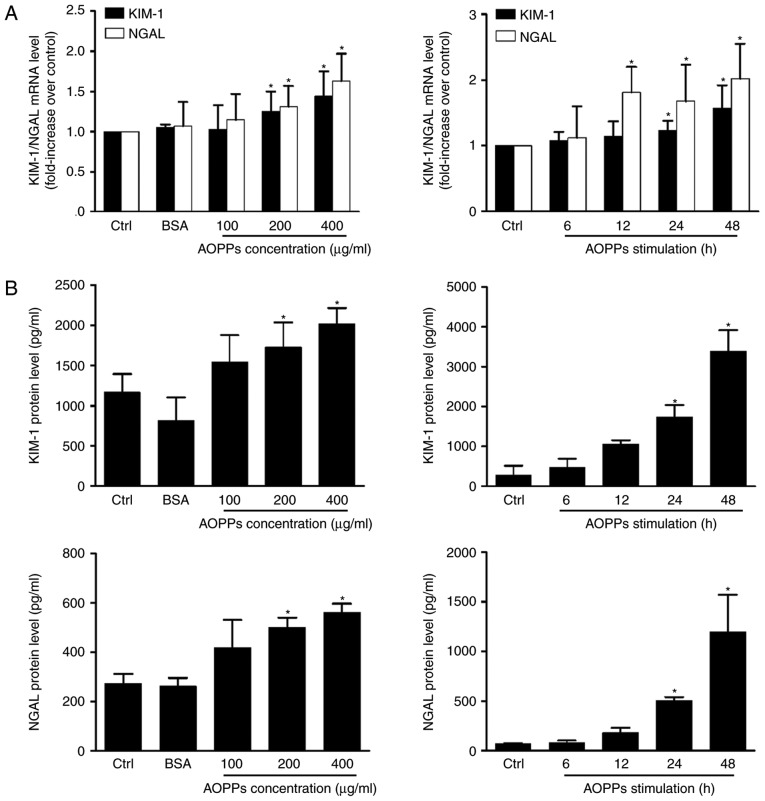
To explore the effect of autophagy inhibition on AOPP-induced RTEC injury, RTEC injury was assessed by measuring the expression of two novel renal tubular injury markers, KIM-1 and NGAL, using RT-qPCR and ELISA. KIM-1 is overexpressed on the surface of proximal tubular cells and NGAL is produced predominantly by neutrophils and in part by proximal tubular cells; both are produced in response to harmful stimuli and act as indicators of RTEC injury (26). Compared with the control group, the expression of KIM-1 and NGAL mRNA and protein was significantly upregulated in cells treated with AOPPs (Fig. 5). No significant difference was observed in unmodified BSA-treated cells, indicating that the overexpression of KIM-1 and NGAL was associated with advanced oxidation of BSA. Additionally, a significant increase in KIM-1 and NGAL levels was observed in the cell supernatant at 24 h post-treatment, which is consistent with the excretion characteristics of the two proteins (26).
Figure 5.
AOPPs induce HK-2 cell injury. HK-2 cells were cultured in control medium, native BSA (200 µg/ml) and 100, 200 and 400 µg/ml AOPPs for 24 h, or 200 µg/ml of AOPPs for 6, 12, 24 and 48 h. (A) Reverse transcription-quantitative polymerase chain reaction demonstrated that AOPP treatment increases the expression of KIM-1 and NGAL mRNA. (B) ELISA assays revealed that AOPP treatment ≥200 µg/ml induced a significant increase in KIM-1 and NGAL expression following 24 h. Data is expressed as the mean ± standard deviation of three independent experiments. *P<0.05 vs. Ctrl. AOPPs, advanced oxidation protein products; BSA, bovine serum albumin; KIM-1, kidney injury molecular; NGAL, neutrophil gelatinase-associated lipocalin; PI3K, phosphoinositide 3-kinase; ctrl, control.
Cells were pretreated with rapamycin, an autophagy inducer, or chloroquine, an autophagy inhibitor, for 1 h prior to treatment with AOPPs for 24 h. The results of western blotting revealed that rapamycin (1 µM) significantly increased autophagy, whereas chloroquine (1 mM) inhibited it (Fig. 6A). RT-qPCR (Fig. 6B) and ELISA (Fig. 6C and D) detection of KIM-1 and NGAL revealed a partial but significant decrease in the rapamycin-treatment group as well as an increase in the chloroquine-treatment group compared with AOPP treatment alone. These data suggest that autophagy inhibition serves a role in AOPP-induced HK-2 cell injury.
Figure 6.
Autophagy inhibition mediates AOPP-induced HK-2 injury. HK-2 cells were treated with vehicle control or 200 µg/ml AOPPs for 24 h, in the absence or presence of rap (1 µM) or CQ (1 mM). (A) Western blotting demonstrated that rap increased autophagy in AOPP-treated HK-2 cells, whereas CQ decreased it. (B) Reverse transcription-polymerase chain reaction revealed that the AOPP-induced over-expression of KIM-1 and NGAL mRNA was reversed by rap and further aggravated by CQ. ELISA assays demonstrated that the AOPP-induced over-expression of (C) KIM-1 and (D) NGAL protein was reversed by rap or further aggravated by CQ. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05 vs. Ctrl, #P<0.05 vs. AOPP-treatment group. AOPP, advanced oxidation protein product; rap, rapamycin; CQ, chloroquine; KIM-1, kidney injury molecular; NGAL, neutrophil gelatinase-associated lipocalin; PI3K, phosphoinositide 3-kinase; Beclin1, B-cell lymphoma-2-interacting myosin-like coiled-coil protein; LC3, microtubule-associated proteins 1 light chain 3B;ctrl, control.
Discussion
In the present study it was demonstrated that autophagy inhibition mediates AOPP-induced HK-2 cell injury in CKD, which is modulated by the PI3K/AKT/mTOR signaling pathway. Autophagy is a dynamic process that comprises autophagosome formation, lysosome fusion and turnover in the lysosome (5). Although the autophagosome is formed during the initiation of autophagy, autophagic flux may be blocked if the fusion and/or turnover processes fail (9,27). Liu et al (12) demonstrated that proteinuria exposure increased the expression of LC3-II and decreased p62 levels, suggesting that proteinuria may induce autophagy initiation as well as autophagosome degradation. Huang et al (13) reported that high glucose induced the overexpression of p62 in synchrony with LC3-II upregulation, which indicates that high glucose inhibits the turnover of autophagosomes. The results of the present study indicate that the expression of p62 is upregulated when LC3-II and Beclin1 expression, as well as autophagosome formation, are downregulated in cells treated with AOPP. This suggests that AOPPs inhibit the initiation of autophagy.
Autophagy activation is modulated by complex upstream signaling pathways (28). As AOPPs may inhibit the initiation of autophagy in RTECs, the probable modulatory pathway was investigated. In agreement with previous studies, which have reported that the PI3K/AKT/mTOR signaling pathway serves a crucial role in the pathogenesis of CKD (20,29), it was demonstrated that this well-known negative modulatory pathway mediated AOPP-inhibited autophagy in RTECs.
A number of studies have reported that autophagy serves a role in CKD (30,31). Yamahara et al (32) reported that obesity-mediated autophagy insufficiency exacerbated proteinuria-induced tubulointerstitial lesions, while Kitada et al (33) demonstrated that RTEC autophagy was impaired in diabetic nephropathy and dietary restriction improved autophagy to alleviate RTEC injury. AOPPs accumulation is a sign of renal failure as well as an executor of renal deterioration (14), which also induced an increase in RTEC injury biomarkers KIM-1 and NGAL via inhibiting autophagy in the present study. KIM-1 and NGAL have previously been reported to be useful biomarkers for the assessment of renal tubular injury (26). Liu et al (12) demonstrated that these biomarkers were significantly increased when HK-2 cells were treated with urinary protein. However, Huang et al (34) reported that limited overexpression of KIM-1 and NGAL was observed when cells were exposed to nephrotoxic substances, including cisplatin. Therefore, only the KIM-1 and NGAL alone are insufficient to determine how autophagy mediates HK-2 cells injury. Future studies should investigate the correlation between autophagy and hypertrophy and EMT, as well as the injury phenotype of RTEC, in order to further elucidate the effect of autophagy on AOPP-induced RTEC injury. The findings presented here were obtained from an in vitro study only; appropriate animals models and primary cell models should be constructed to determine the role of AOPPs in vivo. Finally, autophagy induction is modulated by cross-linked upstream signaling pathways and the PI3K/AKT/mTOR signaling pathway is highly regulated by multiple mechanisms, so more experiments should be performed to clarify the relevant pathways and the function of the PI3K/AKT/mTOR signaling pathway in AOPP-treated cells.
In conclusion, the results of the present study suggest that AOPPs inhibit autophagy via activation of the PI3K/AKT/mTOR pathway in HK-2 cells. On the basis of these findings, AOPP-induced autophagy inhibition appears to be associated with RTEC injury. These findings suggest that targeting autophagy may be an effective therapeutic strategy for inhibiting CKD.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AOPPs
advanced oxidation protein products
- AKT
protein kinase B
- Beclin1
B-cell lymphoma-2-interacting myosin-like coiled-coil protein
- KIM-1
kidney injury molecular
- LC3B
microtubule-associated proteins 1 light chain 3B
- mTOR
mammalian target of rapamycin
- NGAL
neutrophil gelatinase-associated lipocalin
- PI3K
phosphoinositide 3-kinase
Funding
The present study was supported by the National Science Foundation of China (grant no. 81202842).
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JZ designed the study. XX conducted most of the experiments and helped design the study. SS assisted with the culture of cells and qPCR experiments. CZ and YL jointly helped with the immunofluorescence experiment. TJ and WZ jointly helped with the transmission electron microscopy. TG was responsible for the data analysis. XL and XT were responsible for revising and submitting the manuscript and figures, gave their final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the study. All authors collaborated to interpret the results and develop the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors have no conflict of interest to declare.
References
- 1.Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 2.Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:353–365. doi: 10.1053/j.ackd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber TB, Edelstein CL, Hartleben B, Inoki K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, et al. Emerging role of autophagy in kidney function, diseases and aging. Autophagy. 2012;8:1009–1031. doi: 10.4161/auto.19821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine B, Klionsky DJ. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/S1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 6.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 8.Mizushima N. Autophagy: Process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 9.Bjorkoy G, Lamark T, Pankiv S, Øvervatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009;452:181–197. doi: 10.1016/S0076-6879(08)03612-4. [DOI] [PubMed] [Google Scholar]
- 10.Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;294:F777–F787. doi: 10.1152/ajprenal.00590.2007. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki C, Isaka Y, Takabatake Y, Tanaka H, Koike M, Shibata M, Uchiyama Y, Takahara S, Imai E. Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun. 2008;368:100–106. doi: 10.1016/j.bbrc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 12.Liu WJ, Luo MN, Tan J, Chen W, Huang LZ, Yang C, Pan Q, Li B, Liu HF. Autophagy activation reduces renal tubular injury induced by urinary proteins. Autophagy. 2014;10:243–256. doi: 10.4161/auto.27004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Lin MZ, Cheng D, Braet F, Pollock CA, Chen XM. Thioredoxin-interacting protein mediates dysfunction of tubular autophagy in diabetic kidneys through inhibiting autophagic flux. Lab Invest. 2014;94:309–320. doi: 10.1038/labinvest.2014.2. [DOI] [PubMed] [Google Scholar]
- 14.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 15.Kalousová M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 2002;51:597–604. [PubMed] [Google Scholar]
- 16.Zhou LL, Hou FF, Wang GB, Yang F, Xie D, Wang YP, Tian JW. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int. 2009;76:1148–1160. doi: 10.1038/ki.2009.322. [DOI] [PubMed] [Google Scholar]
- 17.Li HY, Hou FF, Zhang X, Chen PY, Liu SX, Feng JX, Liu ZQ, Shan YX, Wang GB, Zhou ZM, et al. Advanced oxidation protein products accelerate renal fibrosis in a remnant kidney model. J Am Soc Nephrol. 2007;18:528–538. doi: 10.1681/ASN.2006070781. [DOI] [PubMed] [Google Scholar]
- 18.Tang X, Rong G, Bu Y, Zhang S, Zhang M, Zhang J, Liang X. Advanced oxidation protein products induce hypertrophy and epithelial-to-mesenchymal transition in human proximal tubular cells through induction of endoplasmic reticulum stress. Cell Physiol Biochem. 2015;35:816–828. doi: 10.1159/000369740. [DOI] [PubMed] [Google Scholar]
- 19.Yang YP, Liang ZQ, Gu ZL, Qin ZH. Molecular mechanism and regulation of autophagy. Acta Pharmacol Sin. 2005;26:1421–1434. doi: 10.1111/j.1745-7254.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 20.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009;20:2493–2502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 21.Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26:2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Satriano J, Sharma K. Autophagy and metabolic changes in obesity-related chronic kidney disease. Nephrol Dial Transplant. 2013;28(Suppl 4):S29–S36. doi: 10.1093/ndt/gft229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieberthal W, Levine JS. Mammalian target of rapamycin and the kidney. II. Pathophysiology and therapeutic implications. Am J Physiol Renal Physiol. 2012;303:F180–F191. doi: 10.1152/ajprenal.00015.2012. [DOI] [PubMed] [Google Scholar]
- 24.Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers P, Drüeke T, Descamps-Latscha B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524–2532. [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C-T method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Caplin B, Nitsch D. Urinary biomarkers of tubular injury in chronic kidney disease. Kidney Int. 2017;91:21–23. doi: 10.1016/j.kint.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mavroeidi V, Petrakis I, Stylianou K, Katsarou T, Giannakakis K, Perakis K, Vardaki E, Stratigis S, Ganotakis E, Papavasiliou S, Daphnis E. Losartan affects glomerular AKT and mTOR phosphorylation in an experimental model of type 1 diabetic nephropathy. J Histochem Cytochem. 2013;61:433–443. doi: 10.1369/0022155413482925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Choi ME. Autophagy in kidney health and disease. Antioxid Redox Signal. 2014;20:519–537. doi: 10.1089/ars.2013.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takabatake Y, Kimura T, Takahashi A, Isaka Y. Autophagy and the kidney: Health and disease. Nephrol Dial Transplant. 2014;29:1639–1647. doi: 10.1093/ndt/gft535. [DOI] [PubMed] [Google Scholar]
- 32.Yamahara K, Kume S, Koya D, Tanaka Y, Morita Y, Chin-Kanasaki M, Araki H, Isshiki K, Araki S, Haneda M, et al. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol. 2013;24:1769–1781. doi: 10.1681/ASN.2012111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitada M, Takeda A, Nagai T, Ito H, Kanasaki K, Koya D. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: A model of type 2 diabetes. Exp Diabetes Res. 2011;2011:908185. doi: 10.1155/2011/908185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang JX, Kaeslin G, Ranall MV, Blaskovich MA, Becker B, Butler MS, Little MH, Lash LH, Cooper MA. Evaluation of biomarkers for in vitro prediction of drug-induced nephrotoxicity: Comparison of HK-2, immortalized human proximal tubule epithelial and primary cultures of human proximal tubular cells. Pharmacol Res Perspect. 2015;3:e00148. doi: 10.1002/prp2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.