Abstract
Placenta-specific 1 (PLAC1), a novel cancer-testis antigen (CTA), is expressed in a number of different human malignancies. It is frequently produced in breast cancer, serving a function in tumorigenesis. Adoptive immunotherapy using T cell receptor (TCR)-engineered T cells against CTA mediates objective tumor regression; however, to the best of our knowledge, targeting PLAC1 using engineered T cells has not yet been attempted. In the present study, the cDNAs encoding TCRα- and β-chains specific for human leukocyte antigen (HLA)-A*0201-restricted PLAC1 were cloned from a cytotoxic T-lymphocyte, generated by in vitro by the stimulation of CD8+ T cells using autologous HLA-A2+ dendritic cells loaded with a PLAC1-specific peptide (p28-36, VLCSIDWFM). The TCRα/β-chains were linked by a 2A peptide linker (TCRα-Thosea asigna virus-TCRβ), and the constructs were cloned into the lentiviral vector, followed by transduction into human cytotoxic (CD8+) T cells. The efficiency of transduction was up to 25.16%, as detected by PLAC1 multimers. TCR-transduced CD8+ T cells, co-cultured with human non-metastatic breast cancer MCF-7 cells (PLAC1+, HLA-A2+) and triple-negative breast cancer MDAMB-231 cells (PLAC1+, HLA-A2+), produced interferon γ and tumor necrosis factor α, suggesting TCR activation. Furthermore, the PLAC1 TCR-transduced CD8+ T cells efficiently and specifically identified and annihilated the HLA-A2+/PLAC1+ breast cancer cell lines in a lactate dehydrogenase activity assay. Western blot analysis demonstrated that TCR transduction stimulated the production of mitogen-activated protein kinase signaling molecules, extracellular signal-regulated kinases 1/2 and nuclear factor-κB, through phosphoinositide 3-kinase γ-mediated phosphorylation of protein kinase B in CD8+ T cells. Xenograft mouse assays revealed that PLAC1 TCR-transduced CD8+T cells significantly delayed the tumor progression in mice-bearing breast cancer compared with normal saline or negative control-transduced groups. In conclusion, a novel HLA-A2-restricted and PLAC1-specific TCR was identified. The present study demonstrated PLAC1 to be a potential target for breast cancer treatment; and the usage of PLAC1-specific TCR-engineered T cells may be a novel strategy for PLAC1-positive breast cancer treatment.
Keywords: breast cancer, placenta specific 1, T cell receptor, cytotoxic T cells, cancer immunotherapy
Introduction
Breast cancer is the most common type of cancer in females in the United States (1) and China (2) in 2015. A total of 882,900 cases were diagnosed and 324,300 mortalities occurred in developing countries in 2012, accounting for 25% of all cancer cases and 15% of all cancer-associated mortalities amongst females (3). Therapeutic treatments for breast cancer typically include surgery, chemotherapy, radiotherapy, endocrine therapy and molecular targeted therapy (4–6). Although the prognosis for patients with early-stage breast cancer has improved over previous years, the 5-year survival rate of patients with metastatic breast cancer remains <20% in the United States in 2016 (7). Notably, no targeted therapies are available for the treatment of triple-negative breast cancer (TNBC), which is defined by the lack of the expression of estrogen receptor, progesterone receptor and human epidermal growth factor receptor; therefore, chemotherapy remains the standard treatment for this cancer (8). With developments in the fields of molecular biology, immunology and pharmacogenomics, immunotherapy is developing promising breast cancer therapies, including cancer vaccines, bispecific antibodies and immune checkpoint inhibitors (5,9).
The multiple advances in immunotherapeutic strategies over previous years include the characterization, cloning and expression of tumor-specific T cell receptors (TCRs) derived from T cells ex vivo (10,11). At present, the stimulation of expression of TCRs in T cells for adoptive transfer is an established procedure (12,13). Targeting cancer-testis antigens (CTAs), including NY-ESO-1, melanoma-associated antigen 3 and glycoprotein 100, using engineered T cells has demonstrated clinical efficacy in the treatment of a number of tumor types (including synovial cell sarcoma, multiple myeloma and melanoma) (10–13), but has not yet been attempted in breast cancer.
The trophoblast-specific gene placenta-specific 1 (PLAC1) is ectopically expressed in multiple human malignancies including ovarian cancer, hepatocellular carcinoma, colorectal carcinoma and breast cancer (14–16); however, the most frequent occurrence is in breast cancer, where it is particularly associated with cancer cell proliferation, migration and invasion, resulting in the classification of PLAC1 as an oncoplacental protein (14–16). In one previous study, a total of 51/62 (82%) primary breast cancer samples scored positive for PLAC1 expression, with 15/62 (24%) with low expression, 25/62 (40%) with intermediate expression and 11/62 (17%) with high expression (16). In the same previous study, RNA interference-mediated silencing of PLAC1 in two breast cancer cell lines, MCF-7 and BT-549, profoundly impaired their motility, migration and invasion, in addition to inducting G1-S cell cycle arrest with almost complete abrogation of proliferation (16). Therefore, PLAC1 qualified as a promising candidate for targeted therapeutic methods for breast cancer. Furthermore, the identification of PLAC1-specific TCR-engineered T cells would be crucial to the immunotherapy of breast cancer.
In the present study, a human leukocyte antigen (HLA)-A*0201-restricted and PLAC1-specific TCR was isolated and used for constructing lentiviral vectors. Normal CD8+ T cells engineered using this TCR demonstrated potent antitumor effects in vitro and in vivo in response to HLA-A*0201+/PLAC1+ breast cancer cells. These results suggested that the use of this TCR for adoptive immunotherapy may be valuable for the treatment of patients with PLAC1-expressing breast cancer.
Materials and methods
Cells and lentiviral vectors
Breast cancer cell lines (MCF-7, MDAMB-231 and T47D) and the virus packaging cell line (293T) were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 U/ml streptomycin (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) in an incubator at 37°C and 5% CO2. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy donors by Ficoll gradient centrifugation with 700 × g for 20 min at 4°C, subsequent to obtaining written informed consent. The present study conformed to The Declaration of Helsinki and was approved by the Ethics Committee of Shenzhen Beike Cell Engineering Research Institute (Guangdong, China). Two male and two female patients participated in present study, with an age range from 25 to 40 years old and the mean age was 31.5 years old. The blood of healthy donors was collected at the Hengze Clinic (Shenzhen, Guangdong, China) on January 7, 2016. Subsequently, the cytotoxic (CD8+) T cells from PBMCs were selected using a negative selection procedure using a CD8+ T cell Isolation kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's protocol. These isolated cells were cultured using the T cell Activation/Expansion kit (Miltenyi Biotec GmbH) and RPMI 1640 medium supplemented with 10% human serum from donors of AB blood group (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 400 U/ml interleukin (IL)-2 (Peprotech, Inc., Rocky Hill, NJ, USA) and 400 U/ml IL-15 (Peprotech, Inc., Rocky Hill, NJ, USA) at 37°C and 5% CO2.
The lentiviral vector pCDH-EF1-MCS-Thosea asigna virus (T2A)-Puro and three packaging vectors, pLP1, pLP2 and pLP-VSVG, were obtained from Addgene, Inc. (Cambridge, MA, USA).
Generation of PLAC1-specific T lymphocytes
In order to generate cytotoxic T-lymphocytes (CTLs), the monocyte-derived dendritic cells (DC) of an HLA-A2+ donor were loaded with 5 µg/ml PLAC128–36-peptide (VLCSIDWFM) (GenScript, Jiangsu, China) for the stimulation of autologous CD8+ T cells. Following two stimulations, the HLA-A2+/PLAC128–36-multimer+ CD8+ T cells were sorted by flow cytometry (FACSAria; BD Biosciences, San Jose, CA, USA), and the data was analyzed using BD FACSDiva software (version 6.0; BD Biosciences). Subsequently, the sorted T cells were cloned using a limiting dilution as previously described (17,18) and after 2 weeks, screened for specific recognition of MCF-7 cells (PLAC1+, HLA-A2+) based on specific interferon γ (IFN-γ) secretion (data not shown), which was typically stimulated by antigen encounters, and was ascribed to the major histocompatibility complex (MHC)-dependent TCR activation (19). Then, the PLAC1-reactive clones were expanded using the T cell Activation/Expansion kit and RPMI 1640 supplemented with 10% human serum from donors of AB blood group, 400 U/ml IL-2, and 400 U/ml IL-15 at 37°C and 5% CO2.
To identify the sequences of the TCR genes, a 5′-RACE-PCR GeneRacer kit (Invitrogen; Thermo Fisher Scientific, Inc.) amplifying the TCRα- and TCRβ-chains was performed using RNA isolated from the T cell clones using an RNA Isolation Kit (cat. no. R6731; Omega Bio-tek, Inc., Norcross, GA, USA) according to the manufacturer's protocol. The RACE-PCR products were sequenced, the TCRα- and TCRβ-chains were linked by a 2A peptide linker (TCRα-T2A-TCRβ) as previously described (17,18), and the whole constructs were codon-optimized and synthesized by GenScript. These synthesized fragments were cloned into the lentiviral vector pCDH-EF1-MCS-T2A-Puro via EcoRI and BamHI restriction sites.
A virus packaging cell line, 293T, was seeded at a density of 1×107 cells/150-mm dish and then incubated at 37°C with 5% CO2 for 24 h. After 24 h, the cells were subjected to transfection at 37°C for 4 h with Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) and 20.0 µg pCDH-TCR construct (or pCDH-EF1-MCS-T2A-Puro empty vector) with the pLP1, pLP2, and pLP-VSVG packaging vectors. After culturing the cells for 48 h, the filtered culture supernatants were used for infection. The CD8+ T cells from the PBMCs of healthy donors were transduced using the lentiviral vectors carrying TCR or control empty vector [negative control (NC)] in the presence of 5 µg/ml polybrene (Sigma-Aldrich; Merck KGaA) for 12 h, followed by incubation at 37°C for 48 h in RPMI 1640 supplemented with 10% human serum from donors of AB blood group, 400 U/ml IL-2 and 400 U/ml IL-15. The TCR-transduced CD8+ T cells were evaluated for the expression of appropriate TCR by multimer staining at 4°C for 20 min and flow cytometry.
Flow cytometry
A total of 1×106 T cells were collected by centrifugation with 650 × g for 10 min at room temperature, fixed with paraformaldehyde for 30 min at room temperature, washed twice with PBS, and then blocked with fluorescence-activated cell sorter (FACS) solution (PBS containing 2% FBS and 0.1% NaN3) for 30 min at 4°C. Then cells were stained using fluorescein isothiocyanate (FITC)-labeled monoclonal antibody (mAb) against human CD8 (cat. no. 555366; dilution, 1:10; BD Biosciences, San Jose, CA, USA), PerCP-Cy5.5-labeled mAb against human CD3 (cat no. 560835; dilution, 1:10; BD Biosciences), and phycoerythrin (PE)-labeled PLAC1-multimer (code, F2A-G; dilution, 1:10; Proimmune, Ltd., Oxford, UK) diluted with FACS solution for 20 min at 4°C. Cancer cells were digested with 0.25% trypsin for 3 min at 37°C, collected by centrifugation at 300 × g for 10 min at room temperature, fixed with paraformaldehyde for 30 min at room temperature and washed twice with PBS. Then, cells were blocked with FACS solution for 30 min at 4°C. HLA-A2 expression in the cultured cancer cells was evaluated by flow cytometry using the PE-labeled HLA-A2 antibody (cat no. 558570; dilution, 1:10; BD Biosciences) and PE-labeled isotype control (cat no. 555743; dilution, 1:10; BD Biosciences) diluted with FACS solution for 20 min at 4°C. Isotype control antibody was used in order to eliminate nonspecific combinations in a previous test (data not shown). The cell was analyzed using flow cytometry (FACS Calibur; BD Biosciences) and the data was analyzed using CellQuest Pro.app software (version 6.0; BD Biosciences).
Cytokine release assay
TCR-, NC-transduced or untransduced CD8+ T cells were serially diluted with RPMI 1640 medium supplemented with 10% FBS and co-cultured with 1×104 target cells including MCF-7, MDA-MB-231 and T47D in 100 µl RPMI 1640 medium supplemented with 10% FBS, resulting in a gradient effector cell to target cell (E:T) ratio (20:1, 10:1 and 5:1, respectively). After 4 h, the culture supernatants were collected, and the level of IFN-γ and tumor necrosis factor α (TNF-α) determined using a commercial enzyme-linked immunosorbent assay kit (cat. no. DIF50 and DTA00C; R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer's protocol.
Cytotoxicity assay
For evaluating the efficacy of TCR- or NC-transduced CD8+ T cell-mediated lysis, the cells were serially diluted with RPMI 1640 medium supplemented with 10% FBS and co-cultured with 1×104 target cells including MCF-7, MDA-MB-231 and T47D, resulting in a gradient E:T ratio as described above. After 4 h of co-culture at 37°C, the cytolysis was determined using a lactate dehydrogenase (LDH) activity assay (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's protocol, as previously described (20). The absorbance was measured on a microplate reader at 490 nm (BioTek Instruments, Inc., Winooski, VT, USA). Following background subtraction, the percentage lysis was calculated using the following equation: 100% × [(experimental release-effector spontaneous release-target spontaneous release)/(target maximum release-target spontaneous release)].
Immunofluorescence
Immunofluorescence was performed as described previously (21,22). Briefly, MCF-7, MDA-MB-231 and T47D cells were fixed with 4% paraformaldehyde for 30 min at room temperature and permeabilized using 0.2% Triton X-100. Following blocking with 2% bovine serum albumin for 1 h at 4°C, the cells were incubated with a rabbit anti-PLAC1 antibody (cat. no. ab105395; dilution, 1:100; Abcam, Cambridge, UK) or a rabbit IgG (cat. no. A7016; dilution, 1:100; Beyotime Institute of Biotechnology, Haimen, China) overnight at 4°C. Subsequently, the cells were washed, followed by the addition of PE-conjugated goat anti-rabbit IgG (cat. no. HS121-01; dilution, 1:100; TransGen, Beijing, China) for 1 h at 37°C in the dark, and visualized using an inverted fluorescence microscope (magnification, ×100) (Olympus IX71; Olympus Corporation, Tokyo, Japan).
PLAC1 gene silencing with small interfering RNA (siRNA)
One pair of siRNA oligonucleotides corresponding to the target sequence for human PLAC1 (CACCTACCGTGTTACTGAA) were designed and synthesized by RiboBio (Guangzhou RiboBio Co., Ltd., Guangzhou, China). A negative-control siRNA (NC siRNA) (Guangzhou RiboBio Co., Ltd.) was used as the control. Cells were plated to achieve 40–60% confluency in RPMI 1640 containing 10% FBS without antibiotics. The siRNA was transfected into MCF-7 cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, the cells were analyzed 48 h after transfection, and PLAC1 protein expression was determined using western blot analysis.
Western blot analysis
Western blot analysis was performed as described previously (23). Briefly, the TCR-transduced or NC-transduced CD8+ T cells, MCF-7 cells and PLAC1 gene silencing- and NC-silencing-MCF-7 cells were lysed using radioimmunoprecipitation assay buffer (cat. no. P0013B; Beyotime Institute of Biotechnology) and the protein concentrations measured using a BCA Assay kit (Beyotime Institute of Biotechnology, Jiangsu, China). Equivalent amounts of protein (20 µg) were separated using a 10% gel and SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were probed with primary antibodies against phosphoinositide 3-kinase γ (PI3Kγ; cat. no. 5405S; 1:1,000), protein kinase B (AKT; cat. no. 9272S; 1:1,000), phosphorylated (p)-AKT (cat. no. 4060S; 1:1,000), extracellular signal-regulated kinase (ERK; cat. no. 4695S; 1:1,000), p-ERK (cat. no. 4377S; 1:1,000), p38 (cat. no. 8690S; 1:1,000), p-p38 (cat. no. 4631S; 1:1,000), c-Jun N-terminal kinases (JNK; cat. no, 9252S; 1:1,000), p-JNK (cat. no. 4668S; 1:1,000), nuclear factor-κB (NF-κB; cat. no. 4764S; 1:1,000), β-actin (cat. no. 4970L; 1:1,000) and GAPDH (cat. no. 5174S; 1:1,000) (all from Cell Signaling Technology, Inc., Danvers, MA, USA), and PLAC1 (cat. no. ab105395; 1:1,000; Abcam, Cambridge, UK) in TBS-Tween-20 (TBST) containing 5% non-fat milk (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 4°C overnight, followed by three washes in TBST for 10 min per wash. Subsequently, the membranes were incubated with the IRDye 800CW goat anti-rabbit secondary antibody (1:5,000 dilution in TBST containing 5% non-fat milk; cat. no. 926-32211; LI-COR Bioscience; Lincoln, Nebraska, USA) for 2 h at room temperature. The protein bands were visualized using an Infrared Imaging System Odyssey (LI-COR Bioscience; Lincoln, Nebraska, USA). Quantity One software (version 4.62; Bio-Rad Laboratories, Inc., Hercules, California, USA) was used to quantify of the western blots.
In vivo tumor growth assays
A total of 15 subcutaneous transplant breast cancer model female nude mice (BALB/c-nu) (4–5-week-old) weighing between 18 and 20 g, were purchased directly from the Medical Laboratory Animal Center of Guangdong Province (Guangdong, China). The mice were housed under controlled 12 h light-dark cycles, with constant temperature (22–24°C) and humidity (55–60%), and were given sterilized food and tap water ad libitum. Briefly, the mice were pre-treated with 200 µg/kg estradiol valerate (Sigma-Aldrich; Merck KGaA) by gastric perfusion, starting 1 week prior to cell implantation as previously described (24). Subsequently, each of the mice were administered ~5×106 MCF-7 cells (in 100 µl serum-free media) mixed with 100 µl Matrigel (BD Biosciences) into the left flank subcutaneously. Protocols for the treatment of nude mice were approved by the Laboratory Animal Ethics Committee of the Medical Laboratory Animal Center of Guangdong Province. When large tumors reached a mean volume of 150 mm3, the mice were randomly divided into 3 groups (5 animals/group) and subjected to intravenous tail vein transplantation with normal saline, 1×107 TCR-, or NC-transduced CD8+ T cells twice with a 1 week interval. The tumors were measured once a week using electronic calipers. The longest length and width were recorded and the tumor volume was calculated according to the formula π/6 [(length × (width)2]. The mice were anesthetized prior to cervical dislocation using 45 mg/kg pentobarbital sodium at the culmination of the experiment.
Statistical analysis
Data were expressed as the mean ± standard deviation. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used for analysis. All experiments (excluding the nude mice subcutaneous transplant tumor model experiment) were repeated at least 3 times. A one-way analysis of variance with Bonferroni correction was used for analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
CD8+ T cells may be efficiently engineered to express TCRs recognizing the HLA-A2-restricted PLAC1 peptides
cDNAs encoding TCRα- and β-chains specific for HLA-A2-restricted PLAC1 were cloned from a CTL generated by the in vitro stimulation of CD8+ T cells using a HLA-A2+ DC loaded with PLAC128–36-peptide (VLCSIDWFM). The TCRα- and TCRβ-chains were linked by a 2A peptide linker (TCRα-T2A-TCRβ), and the whole constructs were codon-optimized and synthesized. These synthesized fragments were then cloned into the lentiviral vector pCDH-EF1-MCS-T2A-Puro via EcoRI and BamHI restriction sites (Fig. 1A). To further characterize the TCR, the lentiviral vectors carrying TCR or NC were used for the transduction of the CD8+ T cells, isolated from PBMCs (99.1% purity), with a negative selection procedure using magnetic beads (Fig. 1B). The results revealed that the accurately expressing and matching efficiency of TCRα- and TCRβ-chains in CD8+ T cells was up to 25.16%, as detected by FITC-labeled CD8 mAb and PE-labeled PLAC1-multimers (Fig. 1C). These HLA-A*0201-restricted and PLAC1-specific TCR-engineered CD8+ T cells were then used for subsequent experiments.
Figure 1.
Generation of PLAC1-specific TCR-engineered CD8+ T cells. (A) Schematic illustration of the lentiviral vector encoding an anti-PLAC1 TCR expression cassette. TCRα- and TCRβ-chains were linked with a 2A peptide sequence. (B) CD8+ T cells, selected from peripheral blood mononuclear cells with a negative selection procedure using magnetic beads, were analyzed using flow cytometry for CD3 and CD8 expression via FITC-labeled mAb against human CD8 and PerCP-Cy5.5-labeled mAb against human CD3. (C) CD8+ T cells transduced with PLAC1-specific TCR or negative control or untransduced were stained with PE-labeled PLAC1-multimer (PE-multimer) and FITC-labeled CD8 mAb (CD8-FITC). The results are representative of three independent experiments. PLAC1, placenta-specific 1; TCR, T cell receptor; CD8+ T cell, cytotoxic T cell; CD, cluster of differentiation; FITC, fluorescein isothiocyanate; PE, phycoerythrin; mAb, monoclonal antibody.
Identification of PLAC1 and HLA-A2 serotype-positive breast cancer cell lines
Human non-metastatic breast cancer cells (MCF-7) and TNBC cells (MDAMB-231) were examined for the baseline expression of PLAC1 by immunofluorescence. As presented in Fig. 2A, the two cell lines expressed PLAC1. Next, these two different cancer cell lines were serotyped with an HLA-A2 antibody by flow cytometry and were revealed to be HLA-A2 positive. The HLA-A2 expression efficiency in MCF-7 and MDAMB-231 cell lines reached 92.07 and 98.63%, respectively (Fig. 2B), thereby resulting in the selection of these 2 cell lines for further studies.
Figure 2.
Identification of PLAC1 and HLA-A2 serotype-positive breast cancer cell lines. (A) Immunofluorescence staining with rabbit anti-PLAC1 primary antibody or a rabbit IgG and PE-conjugated secondary antibody in the human non-metastatic breast cancer cell line MCF-7 and triple-negative breast cancer cell line MDAMB-231. The scale bar indicates 50 µm. (B) MCF-7 and MDAMB-231 cells were analyzed using flow cytometry for HLA-A2 expression using the PE-labeled HLA-A2 antibody and PE-labeled isotype control. The results are representative of three independent experiments. PLAC1, placenta-specific 1; HLA, human leukocyte antigen; PE, phycoerythrin; IgG, immunoglobulin G.
Evaluation of the function of PLAC1 TCR-engineered CD8+ T cells
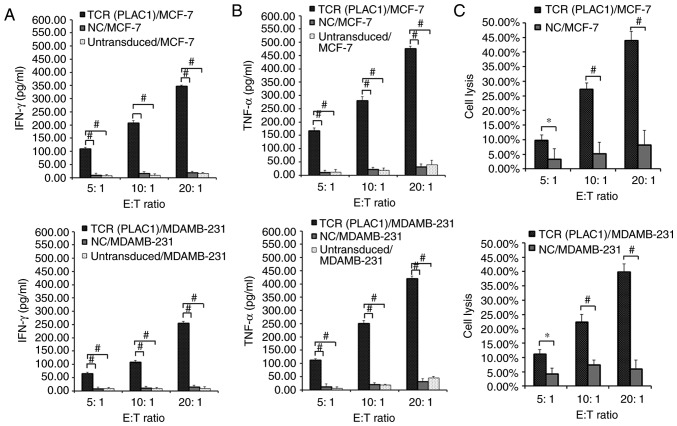
In order to evaluate the recognition of PLAC1 TCRs, transduced CD8+ T cells were subjected to a co-culture assay with MCF-7 (PLAC1+, HLA-A2+) and MDAMB-231 (PLAC1+, HLA-A2+) cells. A significantly higher release of IFN-γ and TNF-α was observed in TCR-engineered CD8+ T cells co-cultured with HLA-A2+/PLAC1+ MCF-7 and MDAMB-231 cell lines compared with NC-transduced and untransduced CD8+ T cells co-cultured with these two cell lines (P<0.01; Fig. 3A and B). As NC-transduced and untransduced CD8+ T cells could not mediate the release of effector cytokines when co-cultured with HLA-A2+/PLAC1+ MCF-7 and MDAMB-231 cell lines, only the NC-transduced CD8+T cells were selected as the control in subsequent studies.
Figure 3.
Evaluation of the function of PLAC1 TCR-engineered CD8+ T cells. Human leukocyte antigen-A2-restricted and PLAC1-specific TCR-, NC-transduced or untransduced CD8+ T cells were co-cultured for 4 h with 1×104 MCF-7 and MDAMB-231 cells respectively at a range of E:T ratios (5:1, 10:1 and 20:1). Concentration of (A) IFN-γ and (B) TNF-α secreted into the culture medium were measured using an enzyme-linked immunosorbent assay. (C) TCR- or NC-transduced CD8+ T cells were co-cultured for 4 h with 1×104 MCF-7 and MDAMB-231 cells. Cytolysis was determined using a lactate dehydrogenase activity assay. Subsequent to background subtraction, the percentage lysis was calculated by 100% × [(experimental release-effector spontaneous release-target spontaneous release)/(target maximum release-target spontaneous release)]. *P<0.05 and #P<0.01 with comparisons shown by lines. PLAC1, placenta-specific 1; TCR, T cell receptor; CD8+ T cell, cytotoxic T cell; NC, negative control; E:T, effector cell to target cell; IFN-γ, interferon γ; TNF-α, tumor necrosis factor α.
Subsequently, the specific lysis of breast cancer cell lines by the engineered CD8+ T cells was measured using an LDH assay. PLAC1 TCR-transduced CD8+ T cells demonstrated an significantly increased lytic function against HLA-A2+/PLAC1+ MCF-7 and MDAMB-231 cells compared with NC-transduced CD8+ T cells, which demonstrated little reactivity against any of the target cells (P<0.05; Fig. 3C). PLAC1 TCR-transduced CD8+ T cells were not capable of lysing T47D cells, which were PLAC1-positive, however, this cell line was identified to be HLA-A2 negative in the immunofluorescence and flow cytometry assays (Fig. 4A-C). As PLAC1 is generally expressed in the breast cancer cells, it is difficult to identify a PLAC1-negative cell line. Therefore, the PLAC1 gene was silenced using siRNA in MCF-7 cells, to further detect the function of PLAC1 TCR-engineered CD8+ T cells. The results revealed that the significant PLAC1 gene silencing in MCF-7 cells compared with NC MCF-7 cells (P<0.01; Fig. 4D) resulted in the significant decrease in the specific lysis of breast cancer cells by the engineered CD8+ T cells compared with NC cells (P<0.05; Fig. 4E). These results indicated that the PLAC1 TCR-engineered CD8+ T cells may efficiently and specifically recognize and kill breast cancer cells.
Figure 4.
PLAC1 TCR-engineered CD8+ T cells specifically recognize and kill breast cancer cells. (A) Immunofluorescence staining with rabbit anti-PLAC1 primary antibody or a rabbit IgG and PE-conjugated secondary antibody in the human breast cancer cell lines T47D. Scale bar indicates 50 µm. The results are representative of three independent experiments. (B) T47D cells were analyzed using flow cytometry for HLA-A2 expression using the PE-labeled HLA-A2 antibody and PE-labeled isotype control. The results are representative of three independent experiments. (C) Lysis of T47D breast cancer cells by the engineered CD8+ T cells was determined using a LDH activity assay. Data are expressed as the means ± SD of three independent experiments. (D) MCF-7 cells were transfected with PLAC1 siRNA or NC siRNA and then analyzed using western blot analysis for PLAC1 expression following normalization to β-actin. The results are representative of three independent experiments. Bars represent the relative protein level as compared with the MCF-7 cells alone group. #P<0.01 vs. MCF-7 + NC siRNA group. (E) Lysis of MCF-7-NC siRNA and MCF-7-PLAC1 siRNA cells by the engineered CD8+ T cells was determined using a LDH activity assay. Data are expressed as the means ± SD of three independent experiments. *P<0.05 and #P<0.01 with comparisons shown by lines. PLAC1, placenta-specific 1; PE, phycoerythrin; IgG, immunoglobulin G; E:T, effector cell to target cell; TCR, T cell receptor; NC, negative control; siRNA, small interfering RNA; CD8+ T cell, cytotoxic T cell; HLA, human leukocyte antigen; SD, standard deviation; LDH, lactate dehydrogenase.
Mechanism of enhanced activation of TCR-transduced CD8+ T cells
A previous study demonstrated that PI3Kγ kinase activity is essential for optimal T cell activation and differentiation, in addition to an efficient T cell-mediated immune response (25). Upon TCR engagement, multiple signaling mechanisms, including mitogen-activated protein kinase (MAPK) and NF-κB, modulated by the PI3K/AKT signaling pathway, are activated during the process of T cell activation, which results in gene induction and cell cycle progression (26–29). In order to investigate the mechanism of the enhanced activation of TCR-transduced CD8+ T cells, western blot analysis was performed and demonstrated that the expression of PI3Kγ, and the phosphorylation of AKT (p-AKT) and ERK1/2 (p-ERK) in addition to the expression of NF-κB, but not the expression of JNK, p-JNK, P-38 and p-p38 in TCR-transduced CD8+ T cells were enhanced as compared with the NC-transduced CD8+ T cells (Fig. 5). These results suggested that the PI3K pathway possessed the capacity to develop the T cells in order to respond to the engineered TCR stimulation.
Figure 5.
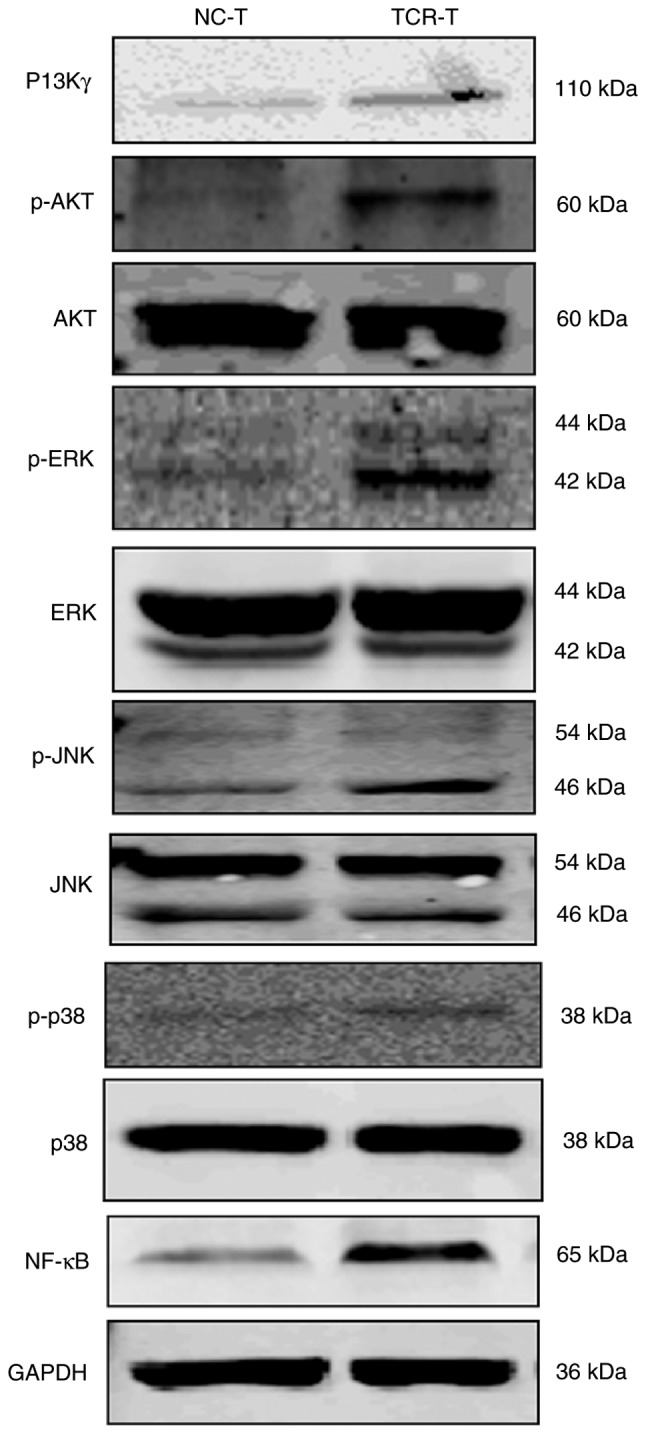
Mechanism of enhanced activation of TCR-transduced CD8+ T cells. Cell lysates of TCR- or NC-transduced CD8+ T cells were analyzed by western blot analysis for the expression of PI3Kγ, AKT, p-AKT, ERK1/2, p-ERK1/2, JNK, p-JNK, p38, p-p38, NF-κB and GAPDH. The results are representative of three independent experiments. NC, negative control; TCR, T cell receptor; CD8+ T cells, cytotoxic T cells; PI3Kγ, phosphoinositide 3-kinase γ; AKT, protein kinase B; ERK1/2, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinases; NF-κB, nuclear factor κB; p-, phosphorylated.
TCR-transduced CD8+ T cells inhibit tumor growth in vivo
To examine the in vivo efficacy of PLAC1 TCR-transgenic CD8+ T cells, 5×106 MCF-7 cells were inoculated into BALB/c-nu mice to establish a subcutaneous transplant tumor model. Upon achieving a mean tumor volume of 150 mm3, the mice were randomly divided into 3 groups (5 animals/group) and subjected to intravenous tail vein transplantation of either normal saline, 1×107 PLAC1 TCR-transduced CD8+ T cells or NC-transduced CD8+ T cells, twice with a 1 week interval. Slowing of tumor growth was observed 14 days post-injection. At day 28, the tumors in the PLAC1 TCR-transduced cells group were significantly smaller, ~50% smaller compared with the normal saline or NC-transduced cells group (P<0.05; Fig. 6). These results indicated that TCR-transduced CD8+ T cells inhibited the tumor growth in vivo.
Figure 6.

TCR-transduced CD8+ T cells inhibit tumor growth in vivo. MCF-7 cells (5×106) were inoculated subcutaneously into 15 BALB/c-nu mice to establish a subcutaneous transplant tumor model. When tumors reached a mean volume of 150 mm3, the mice underwent intravenous tail vein transplantation with either normal saline, 1×107 PLAC1 TCR-transduced CD8+ T cells or NC-transduced CD8+ T cells twice with a 1-week interval. The tumors were measured once/week using electronic calipers. The longest length and width were recorded, and the tumor volume was calculated according to the formula (π/6) (length) × (width)2. *P<0.05 compared with the TCR (PLAC1) group, with comparisons shown by lines. TCR, T cell receptor; CD8+ T cells, cytotoxic T cells; PLAC1, placenta-specific 1; NC, negative control.
Discussion
Toxicity to normal tissues is a potential negative side-effect in TCR-based adoptive immunotherapy targeting tumor antigens that are additionally expressed in certain normal tissues (12,30). Adoptive immunotherapy should strive to produce T cells that target tumor-specific antigens that are not expressed in normal tissues. Therefore, CTA is the ideal candidate due to its overexpression in multiple tumor types and limited expression in normal tissue (31). As a novel member of the CTA group, PLAC1 has been revealed to be expressed in a wide range of human malignancies including ovarian cancer, hepatocellular carcinoma, colorectal carcinoma and breast cancer (14–16). PLAC1 was identified to be frequently expressed in breast cancer, but not in normal tissues except the testes and placenta, and was involved in the proliferation, migration and invasion of breast cancer (32), thereby indicating its key function in cancer tumorigenesis. Germ cells do not present antigens due to their lack of expression of MHC molecules. Therefore, germ cells cannot be targeted by TCR-engineered T cells (33). Thus, the immune responses directed against CTAs by PLAC1 are unlikely to result in the recognition of these tissues. In addition, HLA-A*0201 is the most widely expressed HLA-I molecule in the Chinese population (34). Therefore, the identification of HLA-A*0201-restricted and PLAC1-specific TCR-engineered T cells would be critical to the immunotherapy of breast cancer; however, it has not yet been well-studied. In the present study, an HLA-A*0201-restricted and PLAC1-specific TCR was identified, which was transduced into CD8+T cells in order to investigate the antitumor effects of PLAC1-specific TCR-engineered CD8+T cells in breast cancer cells in vitro and in vivo.
In order to generate PLAC1-specific T cell clones, protocols similar to the method described by Sommermeyer et al (18) were employed: Autologous CD8+ T cells were stimulated with HLA-A2+ DC and loaded with PLAC128–36-peptide (VLCSIDWFM). Liu et al (34) demonstrated that the HLA-A*0201-restricted T cell epitope, PLAC128–36-peptide (VLCSIDWFM), may induce the most potent peptide-specific CTLs and serve as a novel candidate epitope for the development of peptide vaccines against PLAC1-positive breast cancer. In the present study, CTL clones against PLAC1 (VLCSIDWFM) were additionally isolated successfully. Furthermore, the codon-optimized PLAC1-TCR gene was isolated and synthesized. The TCRα- and TCRβ-chains were linked by a 2A peptide linker (TCRα-T2A-TCRβ). T2A and porcine teschovirus-1 (P2A) are small ‘self-cleaving’ peptides. The first small ‘self-cleaving’ peptide F2A was identified in the foot-and-mouth disease virus, a member of the picornavirus group (35,36). Subsequently, ‘2A like’ peptides from equine rhinitis A virus, P2A and T2A were identified, and their activities in proteolytic cleavage were certified in various in vitro and in vivo eukaryotic systems (35,36). Subsequently, the PLAC1-TCR gene was expressed in primary human CD8+T cells using a negative selection process using magnetic beads. The 25.16% expressing and matching efficiency of TCRα- and TCRβ-chains detected by PLAC1-multimers indicated the accurate expression of TCR by CD8+ T cells. It was hypothesized that a longer time would be required to enrich T cells when the puromycin resistance gene carried by the lentiviral vector pCDH-EF1-MCS-T2A-Puro were used compared to those without the puromycin resistance gene, while the proliferation ability of T cells decreased with the increase in days (data not shown). Therefore, the puromycin resistance gene was not used in the present study. The puromycin resistance gene and the concentrated lentiviral particles will be used to improve the transduction efficiency in future studies. Furthermore, a limitation of the present study is that the mispairing between the TCRα- and β chain was not detected, as the accuracy of the expression and matching of TCRα- and TCRβ-chains important for the functions of TCR (37). Studies by Mizote et al (37) and Rapoport et al (10) additionally detected the structure of TCR using Streptamer or Dextramer reagents. The percentage of variable region of β-chains positive cells and mispairing will be examined in future studies.
The activation of T cell antigens triggers the intracellular pathways that result in cytotoxic activity and/or the production of immunomodulatory cytokines (including IFN-γ and TNF-α) (38–40). In the present study, as the CD8+ T cells were transduced with lentiviral vectors expressing the HLA-A2-restricted and PLAC1-specific TCR, the effector T cell functions were measured via cytokine release and cell lysis in vitro subsequent to co-culturing with PLAC1+/HLA-A2+, PLAC1+/HLA-A2- and PLAC1 gene silenced/HLA-A2+ target cells. The TCR-transduced CD8+ T cells, when co-cultured with a human non-metastatic breast cancer cell line (MCF-7; PLAC1+ and HLA-A2+) and a TNBC cell line (MDAMB-231; PLAC1+ and HLA-A2+) produced IFN-γ and TNF-α, suggesting TCR stimulation. Although the E:T ratio (5:1) was low, the enhanced ability to lyse tumor cells of TCR T cells compared to NC-transduced T-cells were detected, demonstrating the notable properties of these engineered T cells. Furthermore, a limitation of the present study was that TCR affinity was not examined. The present study only evaluated the functions of PLAC1 TCR-transduced CD8+ T cells and original T cells by detecting the specific release of IFN-γ and TNF-α when the TCR-engineered CD8+ T cells were co-cultured with HLA-A2+/PLAC1+ MCF-7 and MDAMB-231 cell lines (Fig. 3A and B). Experiments associated with TCR affinity will be performed in future studies. Upon TCR engagement, multiple signaling mechanisms, including MAPK and NF-κB modulated by the PI3K/AKT signaling pathway, are activated during T cell stimulation that result in gene induction and cell cycle progression (26–29). In the present study, whether the PI3K pathway may result in a responsiveness in T cells towards the engineered TCR stimulation was preliminarily detected. It was observed that the expression of PI3Kγ, the phosphorylation of AKT and ERK1/2, and the expression of NF-κB in TCR-transduced CD8+ T cells were enhanced compared with the NC-transduced CD8+ T cells. Therefore, these results confirmed that the PI3K pathway may effectuate a response in T cells towards the engineered TCR stimulation; the underlying mechanism of TCR activating the PI3K pathway post-transfection into T cell will be illustrated in future studies.
To examine the in vivo efficacy of TCR-transgenic T cells, xenograft mouse assays were performed. Following transplantation, the result demonstrated that PLAC1-TCR-transduced CD8+ T cells significantly delayed the tumor progression in mice bearing breast cancer compared with normal saline or N-transduced mice (P<0.05). These experiments will be repeated with a larger number of animals in future studies. As T cells may infiltrate the melanoma xenograft tumor masses in mice (41) and the IFN-γ and TNF-α production by TCR-T cells were enhanced (Fig. 3A and B), it was hypothesized that PLAC1-TCR T cells may hone in on the tumor site, kill the tumor cells and modulate the microenvironment by producing IFN-γ and TNF-α. Furthermore, the results of the present study revealed that TCR T cells may substantially inhibit the growth of small and medium tumor types, while shortening the anti-tumor effect on larger tumor types, thereby indicating that these PLAC1-TCR T cells may exert a superior anti-tumor effect on early breast cancer compared with that on malignant breast cancer in clinical therapy.
To conclude, a novel HLA-A*0201-restricted and PLAC1-specific TCR was identified. The present study demonstrated that PLAC1 was a putative target in breast cancer treatment, and the PLAC1-specific TCR-engineered T cells is a robust and novel strategy for the treatment of PLAC1-positive breast cancer.
Acknowledgements
The present study was supported by the Special Funds of Shenzhen For The Development of Strategic New Industries (grant no. CXZZ20150430152511042).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Jiang X, Tang H, Chen T. Epidemiology of gynecologic cancers in China. J Gynecol Oncol. 2018;29:e7. doi: 10.3802/jgo.2018.29.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistic, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Mondal D, Sharma DN. External beam radiation techniques for breast cancer in the new millennium: New challenging perspectives. J Egypt Natl Canc Inst. 2016;28:211–218. doi: 10.1016/j.jnci.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Yu LY, Tang J, Zhang CM, Zeng WJ, Yan H, Li MP, Chen XP. New immunotherapy strategies in breast cancer. Int J Environ Res Public Health. 2017;14:E68. doi: 10.3390/ijerph14010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernal-Estévez D, Sanchez R, Tejada RE, Parra-López C. Chemotherapy and radiation therapy elicits tumor specific T cell responses in a breast cancer patient. BMC Cancer. 2016;16:591. doi: 10.1186/s12885-016-2625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakai K, Hung MC, Yamaguchi H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am J Cancer Res. 2016;6:1609–1623. [PMC free article] [PubMed] [Google Scholar]
- 8.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 9.Migali C, Milano M, Trapani D, Criscitiello C, Esposito A, Locatelli M, Minchella I, Curigliano G. Strategies to modulate the immune system in breast cancer: Checkpoint inhibitors and beyond. Ther Adv Med Oncol. 2016;8:360–374. doi: 10.1177/1758834016658423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B, Finklestein J, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunert A, Straetemans T, Govers C, Lamers C, Mathijssen R, Sleijfer S, Debets R. TCR-Engineered T cells meet new challenges to treat solid tumors: Choice of antigen, t cell fitness and sensitization of tumor milieu. Front Immunol. 2013;4:363. doi: 10.3389/fimmu.2013.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinnasamy N, Wargo JA, Yu Z, Rao M, Frankel TL, Riley JP, Hong JJ, Parkhurst MR, Feldman SA, Schrump DS, et al. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J Immunol. 2011;186:685–696. doi: 10.4049/jimmunol.1001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chodon T, Comin-Anduix B, Chmielowski B, Koya RC, Wu Z, Auerbach M, Ng C, Avramis E, Seja E, Villanueva A, et al. Adoptive transfer of MART-1 T cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res. 2014;20:2457–2465. doi: 10.1158/1078-0432.CCR-13-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koslowski M, Tureci O, Biesterfeld S, Seitz G, Huber C, Sahin U. Selective activation of trophoblast-specific PLAC1 in breast cancer by CCAAT/enhancer-binding protein beta (C/EBPbeta) isoform 2. J Biol Chem. 2009;284:28607–28615. doi: 10.1074/jbc.M109.031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Old LJ. Cancer is a somatic cell pregnancy. Cancer Immun. 2007;7:19. [PMC free article] [PubMed] [Google Scholar]
- 16.Koslowski M, Sahin U, Mitnacht-Kraus R, Seitz G, Huber C, Tureci O. A placenta-specific gene ectopically activated in many human cancers is essentially involved in malignant cell processes. Cancer Res. 2007;67:9528–9534. doi: 10.1158/0008-5472.CAN-07-1350. [DOI] [PubMed] [Google Scholar]
- 17.Leisegang M, Engels B, Meyerhuber P, Kieback E, Sommermeyer D, Xue SA, Reuss S, Stauss H, Uckert W. Enhanced functionality of T cell receptor-redirected T cells is defined by the transgene cassette. J Mol Med (Berl) 2008;86:573–583. doi: 10.1007/s00109-008-0361-z. [DOI] [PubMed] [Google Scholar]
- 18.Sommermeyer D, Conrad H, Krönig H, Gelfort H, Bernhard H, Uckert W. NY-ESO-1 antigen-reactive T cell receptors exhibit diverse therapeutic capability. Int J Cancer. 2013;132:1360–1367. doi: 10.1002/ijc.27792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosati SF, Parkhurst MR, Hong Y, Zheng Z, Feldman SA, Rao M, Abate-Daga D, Beard RE, Xu H, Black MA, et al. A Novel murine T cell receptor targeting NY-ESO-1. J Immunother. 2014;37:135–146. doi: 10.1097/CJI.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SY, Sin JI. MC32 tumor cells acquire Ag-specific CTL resistance through the loss of CEA in a colon cancer model. Hum Vaccin Immunother. 2015;11:2012–2020. doi: 10.1080/21645515.2015.1016669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Liu G, Shao D, Wang J, Yuan H, Chen T, Zhai R, Ni W, Tai G. Mucin1 mediates autocrine transforming growth factor beta signaling through activating the c-Jun N-terminal kinase/activator protein 1 pathway in human hepatocellular carcinoma cells. Int J Biochem Cell Biol. 2015;59:116–125. doi: 10.1016/j.biocel.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Wang F, Li Q, Ni W, Fang F, Sun X, Xie F, Wang J, Wang F, Gao S, Tai G. Expression of human full-length MUC1 inhibits the proliferation and migration of a B16 mouse melanoma cell line. Oncol Rep. 2013;30:260–268. doi: 10.3892/or.2013.2440. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Wang F, Liu G, Yuan H, Chen T, Wang J, Xie F, Zhai R, Wang F, Guo Y, et al. Impact of Mucin1 knockdown on the phenotypic characteristics of the human hepatocellular carcinoma cell line SMMC-7721. Oncol Rep. 2014;31:2811–2819. doi: 10.3892/or.2014.3136. [DOI] [PubMed] [Google Scholar]
- 24.Yun BL, Cho N, Li M, Jang MH, Park SY, Kang HC, Kim B, Song IC, Moon WK. Intratumoral heterogeneity of breast cancer xenograft models: Texture analysis of diffusion-weighted MR imaging. Korean J Radiol. 2014;15:591–604. doi: 10.3348/kjr.2014.15.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladygina N, Gottipati S, Ngo K, Castro G, Ma JY, Banie H, Rao TS, Fung-Leung WP. PI3Kγ kinase activity is required for optimal T cell activation and differentiation. Eur J Immunol. 2013;43:3183–3196. doi: 10.1002/eji.201343812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huse M. The T cell-receptor signaling network. J Cell Sci. 2009;122:1269–1273. doi: 10.1242/jcs.042762. [DOI] [PubMed] [Google Scholar]
- 27.Prinz PU, Mendler AN, Masouris I, Durner L, Oberneder R, Noessner E. High DGK-α and disabled MAPK pathways cause dysfunction of human tumor-infiltrating CD8+ T cells that is reversible by pharmacologic intervention. J Immunol. 2012;188:5990–6000. doi: 10.4049/jimmunol.1103028. [DOI] [PubMed] [Google Scholar]
- 28.Robertson LK, Mireau LR, Ostergaard HL. A role for phosphatidylinositol 3-kinase in TCR-stimulated ERK activation leading to paxillin phosphorylation and CTL degranulation. J Immunol. 2005;175:8138–8145. doi: 10.4049/jimmunol.175.12.8138. [DOI] [PubMed] [Google Scholar]
- 29.Radoja S, Frey AB, Vukmanovic S. T cell receptor signaling events triggering granule exocytosis. Crit Rev Immunol. 2006;26:265–290. doi: 10.1615/CritRevImmunol.v26.i3.40. [DOI] [PubMed] [Google Scholar]
- 30.Adamina M. When gene therapy meets adoptive cell therapy: Better days ahead for cancer immunotherapy? Expert Rev Vaccines. 2010;9:359–363. doi: 10.1586/erv.10.24. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson BJ, Iseli C, Panji S, Zahn-Zabal M, Hide W, Old LJ, Simpson AJ, Jongeneel V. Rapid evolution of cancer/testis genes on the X chromosome. BMC Genomics. 2007;8:129. doi: 10.1186/1471-2164-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cocchia M, Huber R, Pantano S, Chen EY, Ma P, Forabosco A, Ko MS, Schlessinger D. PLAC1, an Xq26 gene with placenta-specific expression. Genomics. 2000;68:305–312. doi: 10.1006/geno.2000.6302. [DOI] [PubMed] [Google Scholar]
- 33.Gjerstorff MF, Andersen MH, Ditzel HJ. Oncogenic cancer/testis antigens: Prime candidates for immunotherapy. Oncotarget. 2015;6:15772–15787. doi: 10.18632/oncotarget.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Zhai M, Wu Z, Qi Y, Wu Y, Dai C, Sun M, Li L, Gao Y. Identification of a novel HLA-A2-restricted cytotoxic T lymphocyte epitope from cancer-testis antigen PLAC1 in breast cancer. Amino Acids. 2012;42:2257–2265. doi: 10.1007/s00726-011-0966-3. [DOI] [PubMed] [Google Scholar]
- 35.De FP, Luke GA, Hughes LE, Gani D, Halpin C, Ryan MD. E unum pluribus: Multiple proteins from a self-processing polyprotein. Trends Biotechnol. 2006;24:68–75. doi: 10.1016/j.tibtech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Tang X, Liu X, Tao G, Qin M, Yin G, Suo J, Suo X. ‘Self cleaving’ 2A peptide from porcine teschovirus 1 mediates cleavage of dual fluorescent proteins in transgenic Eimeria tenella. Vet Res. 2016;47:68. doi: 10.1186/s13567-016-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizote Y, Uenaka A, Isobe M, Wada H, Kakimi K, Saika T, Kita S, Koide Y, Oka M, Nakayama E. Production of NY-ESO-1 peptide/DRB1*08:03 tetramers and ex vivo detection of CD4 T cell responses in vaccinated cancer patients. Vaccine. 2014;32:957–964. doi: 10.1016/j.vaccine.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 38.Malyguine AM, Strobl S, Dunham K, Shurin MR, Sayers TJ. ELISPOT assay for monitoring cytotoxic T lymphocytes (CTL) activity in cancer vaccine clinical trials. Cells. 2012;1:111–126. doi: 10.3390/cells1020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colagiovanni A, Di Renzo L, Sarlo F, Schiavino D, De Lorenzo A. Role of TNF-alpha polymorphism in patients with nickel allergy: A marker of susceptibility to contact polysensitization. Eur Rev Med Pharmacol Sci. 2016;20:2663–2666. [PubMed] [Google Scholar]
- 40.Wang S, Campos J, Gallotta M, Gong M, Crain C, Naik E, Coffman RL, Guiducci C. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells; Proc Natl Acad Sci USA; 2016; pp. E7240–e7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gammaitoni L, Giraudo L, Leuci V, Todorovic M, Mesiano G, Picciotto F, Pisacane A, Zaccagna A, Volpe MG, Gallo S, et al. Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features. Clin Cancer Res. 2013;19:4347–4358. doi: 10.1158/1078-0432.CCR-13-0061. [DOI] [PubMed] [Google Scholar]