Abstract
Tripartite motif containing 29 (TRIM29) dysregulation serves an important function in the progression of numerous types of cancer, but its function in the prognosis of patients with gastric cancer remains unknown. The present study assessed the prognostic value of TRIM29 in patients with gastric cancer following surgical resection. A total of 243 fresh gastric adenocarcinoma and adjacent normal tissues were continuously retrieved from patients who underwent curative surgery for gastric cancer at the Cancer Hospital of Henan Province (Zhengzhou, China) between January 2005 and December 2011. The reverse transcription-quantitative polymerase chain reaction was performed to assess TRIM29 expression. The association between TRIM29 expression and clinicopathological features and prognosis was subsequently evaluated. The results of the present study revealed that the expression of TRIM29 was increased in the gastric cancer tissues compared with the normal adjacent tissues, and that upregulated expression of TRIM29 was associated with tumor cell differentiation, tumor stage, lymph node metastasis, and tumor-node-metastasis (TNM) stage. In the training and validation data, high TRIM29 expression was associated with poor overall survival in patients with gastric cancer. Furthermore, multivariate analysis identified that TRIM29 expression was an independent prognostic factor for overall survival, in addition to TNM stage and Lauren classification. Combining TRIM29 expression with the TNM staging system generated a novel predictive model that exhibited improved prognostic accuracy for overall survival in patients with gastric cancer. The present study revealed that TRIM29 was an independent adverse prognostic factor in patients with gastric cancer. Incorporating TRIM29 expression level into the TNM staging system may improve risk stratification and render prognosis more accurate in patients with gastric cancer.
Keywords: tripartite motif containing 29, gastric cancer, prognostic value, radical gastrectomy, overall survival
Introduction
Despite advances in surgery, chemoradiotherapy, and molecular-targeted therapy, the prognosis for patients with advanced gastric cancer tends to be poor (1–3). The tumor-node-metastasis (TNM) staging system of the American Joint Committee on Cancer is a useful model for predicting the prognosis of patients with gastric cancer (4,5). However, patients with gastric cancer with the same TNM stage may receive different prognoses, partly due to heterogeneity at the molecular level of the disease; an example of this is the different prognosis of patients with gastric cancer with the same TNM stage in East Asia and Europe (6,7). The identification of specific and sensitive markers to supplement the TNM stage is required to treat patients with gastric cancer more precisely.
Tripartite motif containing 29 (TRIM29), located at chromosome 11q23, was initially identified in a study researching the gene responsible for the genetic disorder ataxia telangiectasia (8). TRIM29 belongs to the TRIM protein family, which is characterized by a Really Interesting New Gene (RING) finger domain, a B-box-type zinc finger domain type (B)1, a B2, and a coiled coil region (CC) (9). Unlike the majority of TRIM proteins, TRIM29 possesses B1-B2-CC domains but lacks the RING finger domain, suggesting that TRIM29 exhibits no E3 ubiquitin ligase activity (9). TRIM29 has been demonstrated to control important cellular processes, including intracellular signaling in innate immunity and viral infection, transcriptional regulation, development, autophagy and carcinogenesis (10). Previous pathological studies have revealed that TRIM29 may be useful in the diagnosis of multiple types of cancer, including breast (11), prostate (12), pancreatic (13), lung (14) and bladder cancer (15), and esophageal squamous cell carcinoma (16). The expression and biological functions of TRIM29 differ between different types of cancer (10). A previous study demonstrated that TRIM29 expression was a marker for lymph node metastasis in gastric cancer (17). However, the function of TRIM29 in the prognosis of patients with gastric cancer remains to be fully understood and requires further study.
The present study assessed the potential function of TRIM29 expression in the prognosis of patients with resectable gastric cancer. TRIM29 expression was evaluated using the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) in gastric cancer tissues. The association between TRIM29 expression and clinical outcomes in patients with gastric cancer was assessed. The results of the present study may assist in evaluating the clinical significance of TRIM29 expression in gastric cancer and provide means for a more precise prognostic system for evaluating outcomes in patients with gastric cancer.
Materials and methods
Clinical specimens
A total of 243 fresh gastric adenocarcinoma and adjacent normal tissues were continuously retrieved from patients who underwent curative surgery (R0 resection and D2 lymphadenectomy) for gastric cancer at the Cancer Hospital of Henan Province (Zhengzhou, China) between January 2005 and December 2011. All surgeries were performed according to the guidelines of the International Gastric Cancer Association and the Japanese Gastric Cancer Association (5). The patients enrolled in the present study provided written informed consent for publication and were divided into two independent sets (all clinicopathological data for the patients is provided in Table I). Tissues from the training set (n=113) were collected between January 2005 and December 2008. Tissues from the validation set (n=130) were collected between January 2009 and December 2011. The fresh cancer tissues were immediately frozen (−180°C) and stored in liquid nitrogen following resection, until further analysis. Pathological examinations were performed and the histological evaluation was reassessed independently by two gastroenterology pathologists of the Cancer Hospital of Henan Province according to the Japanese General Rules for Gastric Cancer Study in Surgery and Pathology (5), and the TNM staging system was applied according to the 2010 International Union Against Cancer TNM classification system (4). Patients were excluded if they had previously been exposed to any radiotherapy, chemotherapy, targeted therapy, or intervention therapy for gastric cancer. The present study was reviewed and approved by the Clinical Research Ethics Committee of the Cancer Hospital of Henan Province.
Table I.
Association between TRIM29 expression and clinical characteristics in patients with gastric cancer.
| Training set | Validation set | |||||
|---|---|---|---|---|---|---|
| TRIM29 expression | TRIM29 expression | |||||
| Factor | Low (n) | High (n) | P-value | Low (n) | High (n) | P-value |
| All patients | 47 | 66 | 61 | 69 | ||
| Age/yearsa | 0.272 | 0.245 | ||||
| ≤60 | 20 | 35 | 23 | 33 | ||
| >60 | 27 | 31 | 38 | 36 | ||
| Sex | 0.104 | 0.292 | ||||
| Female | 36 | 41 | 38 | 49 | ||
| Male | 11 | 25 | 23 | 20 | ||
| Localization | 0.174 | 0.593 | ||||
| Proximal | 3 | 7 | 10 | 12 | ||
| Middle | 23 | 21 | 18 | 15 | ||
| Distal | 21 | 38 | 33 | 42 | ||
| Differentiation | 0.001 | 0.001 | ||||
| Well | 8 | 1 | 15 | 7 | ||
| Moderately | 25 | 25 | 26 | 17 | ||
| Poorly | 14 | 40 | 20 | 45 | ||
| Lauren classification | 0.459 | 0.202 | ||||
| Intestinal | 31 | 39 | 25 | 36 | ||
| Diffuse | 16 | 27 | 36 | 33 | ||
| Tumor stage | <0.001 | 0.016 | ||||
| 1+2 | 25 | 13 | 16 | 7 | ||
| 3+4 | 22 | 53 | 45 | 62 | ||
| Lymph node metastasis | 0.016 | <0.001 | ||||
| Negative | 19 | 13 | 34 | 14 | ||
| Positive | 28 | 53 | 27 | 55 | ||
| TNM stage | 0.005 | 0.001 | ||||
| I | 18 | 8 | 14 | 6 | ||
| II | 9 | 17 | 22 | 13 | ||
| III | 20 | 41 | 35 | 50 | ||
| Tumor size/cma | 0.157 | 0.316 | ||||
| <4.0 | 23 | 27 | 31 | 29 | ||
| ≥4.0 | 24 | 39 | 30 | 40 | ||
Split at median. TRIM29, tripartite motif containing 29; TNM, tumor-node-metastasis; n, number of patients.
RT-qPCR
TRIM29 expression was evaluated using RT-qPCR, which was performed as previously described (11). Briefly, total RNA containing microRNA (miRNA) was extracted from cultured cells or tissues using the miRNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA), according to manufacturer's protocol. cDNA was synthesized using the miScript Reverse Transcription kit (Qiagen, Inc.) following the manufacturer's protocol. Reverse transcription was performed using 50 ng total RNA with a primer specific for TRIM29, together with the SYBR Green microRNA reverse transcription kit (Applied BioSystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to manufacturer's protocol. miRNAs were quantified using the SYBR Green miRNA RT-qPCR assay (Applied BioSystems; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The qPCR reaction was carried out on a 7500 Fast Real-time System (Applied Biosystems; Thermo Fisher Scientific, Inc.). All RT-qPCRs were performed in triplicate. The data were analyzed using an automated baseline. The threshold cycle (Cq) was defined as the fractional cycle number at which the fluorescence exceeded the given threshold. The data obtained from the RT-qPCR were analyzed using the ΔΔCq method (2ΔΔCq) (11). The primer sequences used for RT-qPCR were as follows: TRIM29 forward, 5′-ACATCATACCAGCCCTCGTC-3′ and reverse, 5′-AGCCTTTCAGGGAGAAGGAG-3′; RNA, U6 small nuclear 1 (U6) forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′. U6 was used as the internal control.
Statistical analysis
Statistical analysis was performed using SPSS Statistics 21.0 software (IBM Corp., Armonk, NY, USA). Pearson's χ2 tests or Fisher's exact tests were applied for categorical variables. Survival curves were constructed using the Kaplan-Meier method and the significance of the difference between survival curves was assessed using the log-rank test. Numbers at risk were calculated for the beginning of each time period. The Cox proportional hazards regression model was used for multivariate analysis. Receiver operating characteristic (ROC) analysis was used to compare the sensitivity and specificity for the prediction of overall survival by the parameters. All P-values were two-sided. P<0.05 was considered to indicate a statistically significant difference.
Results
Association between TRIM29 expression and pathological characteristics
To evaluate whether TRIM29 expression is associated with the development and progression of gastric cancer, the present study assessed TRIM29 expression in 243 patients with gastric cancer using RT-qPCR analysis. Using the results of RT-qPCR, patients were divided into high or low expression groups according to the ratio of the mean expression level of TRIM29 in their normal tissue to that in their cancer tissue (cut-off was 0.5). In the training and validation sets, 58.41 (66/113) and 53.08% (69/130) of the tumors were determined to exhibit high TRIM29 expression, respectively. The characteristics and clinicopathological features of the patients were provided (Table I). TRIM29 expression was significantly associated with tumor cell differentiation (training set, P=0.001; validation set, P=0.001), tumor stage (training set, P<0.001; validation set, P=0.016), lymph node metastasis (training set, P=0.016; validation set, P<0.001), and TNM stage (training set, P=0.005; validation set, P=0.001). Furthermore, the percentage of patients with high TRIM29 expression increased with cancer progression from TNM stage I to III in the training and validation sets (Fig. 1).
Figure 1.
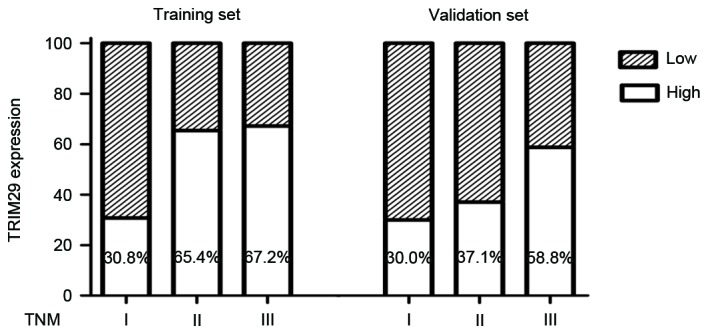
TRIM29 expression in patients with gastric cancer with different TNM stages. The percentage of patients with low TRIM29 expression increased with disease progression from TNM stage I to III in the training and validation sets. TRIM29, tripartite motif containing 29; TNM, tumor-node-metastasis.
Survival analysis to evaluate the prognostic value of TRIM29 in patients with resectable gastric cancer
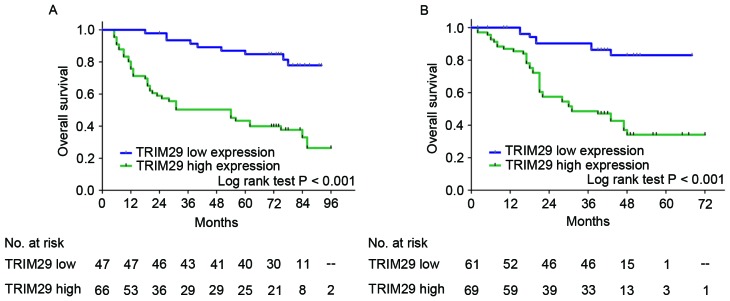
In the training and validation sets, high TRIM29 expression was associated with poorer overall survival than those with low TRIM29 expression (training set, P<0.001; validation set, P<0.001; Fig. 2), which suggested that TRIM29 expression influenced the clinical outcome for patients with resectable gastric cancer. Furthermore, univariate analysis demonstrated that high TRIM29 expression is a significant negative prognostic predictor for patients with gastric cancer in the training [hazard ratio (HR), 5.14; 95% confidence interval (CI), 2.49–10.63; P<0.001] and validation sets (HR, 5.22; 95% CI, 2.44–11.14; P<0.001). Furthermore, tumor cell differentiation (training set, P=0.001; validation set, P=0.010), Lauren classification (training set, P=0.002; validation set, P=0.046), tumor stage (training set, P<0.001; validation set, P=0.007), lymph node metastasis (training set, P=0.001; validation set, P<0.001), and TNM stage (training set, P<0.001; validation set, P<0.001) significantly affected the survival of patients with gastric cancer (Table II). In addition, using multivariate Cox regression analysis, Lauren classification, TNM stage, and TRIM29 expression were identified as independent and significant prognostic parameters in the training (Lauren classification: HR, 2.81; 95% CI, 1.56–5.09; P=0.001; TNM stage: HR, 4.93; 95% CI, 2.32–10.49; P<0.001; TRIM29 expression: HR, 5.68; 95% CI, 2.58–12.51; P<0.001) and validation sets (Lauren classification: HR, 1.73; 95% CI, 1.46–3.19; P=0.047; TNM stage: HR, 8.28; 95% CI, 3.16–21.70; P<0.001; TRIM29 expression: HR, 3.32; 95% CI, 1.50–7.41; P=0.003; Table III).
Figure 2.
Analysis of overall survival according to TRIM29 expression in patients with gastric cancer. Kaplan-Meier analysis of overall survival according to TRIM29 expression in (A) the training (n=113) and (B) validation sets (n=130). P-values were calculated using the log-rank test. TRIM29, tripartite motif containing 29; n, number of patients.
Table II.
Univariate Cox regression analysis for overall survival in patients with gastric cancer.
| Overall survival | ||||
|---|---|---|---|---|
| Training set | Validation set | |||
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age/yearsa | 0.201 | 0.381 | ||
| ≤60 | 1.00 | 1.00 | ||
| >60 | 1.45 (0.82–2.54) | 1.29 (0.73–2.30) | ||
| Sex | 0.357 | 0.876 | ||
| Female | 1.00 | 1.00 | ||
| Male | 1.24 (0.57–1.69) | 0.95 (0.53–1.73) | ||
| Localization | 0.632 | 0.515 | ||
| Proximal + middle | 1.00 | 1.00 | ||
| Distal | 1.15 (0.66–2.00) | 0.90 (0.65–1.24) | ||
| Differentiation | 0.001 | 0.010 | ||
| Well + moderately | 1.00 | 1.00 | ||
| Poorly | 1.67 (1.24–2.25) | 1.47 (1.10–1.96) | ||
| Lauren classification | 0.002 | 0.046 | ||
| Intestinal | 1.00 | 1.00 | ||
| Diffuse | 2.41 (1.38–4.21) | 1.69 (1.21–3.02) | ||
| Tumor stage | <0.001 | 0.007 | ||
| 1+2 | 1.00 | 1.00 | ||
| 3+4 | 7.66 (3.02–19.43) | 15.25 (2.10–110.62) | ||
| Lymph node metastasis | 0.001 | <0.001 | ||
| Negative | 1.00 | 1.00 | ||
| Positive | 4.04 (1.72–9.51) | 11.42 (4.06–32.13) | ||
| TNM stage | <0.001 | <0.001 | ||
| I+II | 1.00 | 1.00 | ||
| III | 3.82 (2.02–7.23) | 12.30 (4.80–31.55) | ||
| Tumor size, cma | 0.205 | 0.174 | ||
| <4.0 | 1.00 | 1.00 | ||
| ≥4.0 | 1.43 (0.82–2.50) | 1.56 (0.72–3.50) | ||
| TRIM29 expression | <0.001 | <0.001 | ||
| Low | 1.00 | 1.00 | ||
| High | 5.14 (2.49–10.63) | 5.22 (2.44–11.14) | ||
Split at median. HR, hazard ratio; CI, confidence interval; TNM, tumor-node-metastasis; TRIM29, tripartite motif containing 29.
Table III.
Multivariate Cox regression analysis for overall survival in patients with gastric cancer.
| A, Training set | ||||
|---|---|---|---|---|
| Overall survival | ||||
| Patients | Multivariate analysis | |||
| Factor | No. | % | HR (95% CI) | P-value |
| All training set patients | 113 | 100 | ||
| Differentiation | 0.446 | |||
| Well + moderately | 59 | 52.2 | 1.00 | |
| Poorly | 54 | 47.8 | 1.17 (0. 61 to 2.26) | |
| Lauren classification | 0.001 | |||
| Intestinal | 70 | 61.9 | 1.00 | |
| Diffuse | 43 | 38.1 | 2.81 (1.56 to 5.09) | |
| TNM stage | <0.001 | |||
| I+II | 52 | 46.0 | 1.00 | |
| III | 61 | 54.0 | 4.93 (2.32 to 10.49) | |
| TRIM29 expression | <0.001 | |||
| Low | 47 | 41.6 | 1.00 | |
| High | 66 | 58.4 | 5.68 (2.58 to 12.51) | |
| B, Validation set | ||||
| Overall survival | ||||
| Patients | Multivariate analysis | |||
| Factor | No. | % | HR (95% CI) | P-value |
| All validation set patients | 130 | 100 | ||
| Differentiation | 0.264 | |||
| Well + moderately | 65 | 50.0 | 1.00 | |
| Poorly | 65 | 50.0 | 1.18 (0.88 to 1.60) | |
| Lauren classification | 0.047 | |||
| Intestinal | 61 | 46.9 | 1.00 | |
| Diffuse | 69 | 53.1 | 1.73 (1.46 to 3.19) | |
| TNM stage | <0.001 | |||
| I+II | 45 | 34.6 | 1.00 | |
| III | 85 | 65.4 | 8.28 (3.16 to 21.70) | |
| TRIM29 expression | 0.003 | |||
| Low | 61 | 46.9 | 1.00 | |
| High | 69 | 53.1 | 3.32 (1.50 to 7.41) | |
CI, confidence interval; HR, hazard ratio; TNM, tumor-node-metastasis; TRIM29, tripartite motif containing 29.
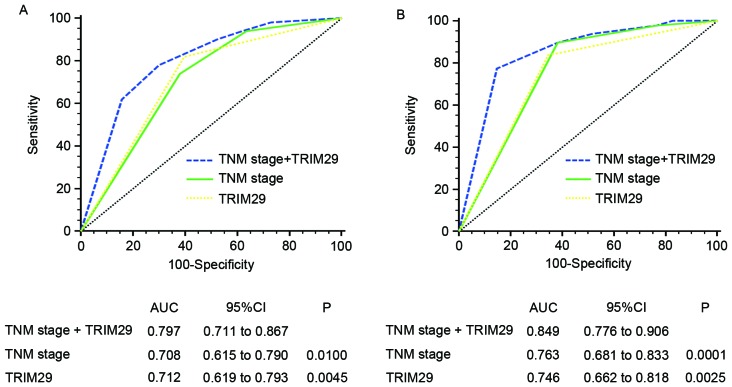
To develop a more sensitive predictive method for patients with gastric cancer, a novel prognostic model combining TNM stage and TRIM29 expression was constructed. ROC analysis was performed to compare its prognostic value against TNM stage or TRIM29 expression alone. The prognostic model that included TNM stage and TRIM29 expression [training set, area under the curve (AUC)=0.797; validation set, AUC=0.849] was associated with increased prognostic value compared with those that included TNM stage (training set, AUC=0.708; P=0.0100; validation set, AUC=0.763; P=0.0001) or TRIM29 expression (training set, AUC=0.712; P=0.0045; validation set, AUC=0.746; P=0.0025) alone for the training and validation sets (Fig. 3).
Figure 3.
ROC analysis for predicting overall survival in patients with gastric cancer. ROC analysis of the prognostic sensitivity and specificity for overall survival of the TNM stage/TRIM29 expression, TNM stage, and TRIM29 expression models in (A) the training (n=113) and (B) validation sets (n=130). ROC, receiver operating characteristic; TNM, tumor-node-metastasis; TRIM29, tripartite motif containing 29; AUC, area under the curve; CI, confidence interval; n, number of patients.
Discussion
Although the incidence of gastric cancer has decreased during previous decades in numerous industrialized countries, the incidence in China remains one of the highest globally, and the disease has already reached an advanced stage in the majority of patients by the time of diagnosis (1–3,5). The TNM staging system remains an important factor for predicting the prognosis of patients with gastric cancer (4). However, patients with gastric cancer with the same TNM stage may receive different prognoses, partly due to the heterogeneity of the disease at the molecular level (6,7). Identifying specific and sensitive markers to supplement the TNM stage is required to treat patients with gastric cancer more precisely. The present study demonstrated that TRIM29 expression in gastric cancer tissues represents a promising, independent predictor for the survival of patients with gastric cancer. High TRIM29 expression was associated with a decreased overall survival time following surgical resection. Furthermore, incorporating the TRIM29 expression level into the TNM staging system increased the prognostic value of the latter. The results of the present study suggested that TRIM29 expression may possess discriminatory power as a supplementary risk factor in patients with gastric cancer, and facilitate an increase in classification accuracy under the TNM staging system. However, the function of TRIM29 in the prognosis of patients with gastric cancer remains to be fully understood and requires further study.
A previous study identified that TRIM29 was associated with increased aggressiveness in multiple types of cancer (10). TRIM29 antagonizes multiple processes in a number of different cancer cells by regulating certain genes and influencing their functions in numerous cellular signaling pathways (11–16). Another study reported that TRIM29 expression increased as normal pancreatic ductal epithelia progressed to infiltrating cancer, which suggested that upregulating TRIM29 promoted the development of invasive pancreatic cancer (18). However, the underexpression of TRIM29 in breast and prostate cancer has also been reported using serial analysis of gene expression and DNA microarray analysis (19,20). TRIM29 may induce a malignant phenotype to revert to a non-malignant phenotype in osteosarcoma and breast cancer cell lines (21). Zhou et al (22) indicated that upregulating TRIM29 expression promoted proliferation and metastasis in nasopharyngeal carcinoma via the phosphatase and tensin homolog/protein kinase B/mechanistic target of rapamycin signaling pathway. Kosaka et al (17) reported that TRIM29 expression was positively associated with a poorer histological grade, increased tumor size and invasion, and lymph node metastasis in gastric cancer. In addition, Qiu et al (23) demonstrated that TRIM29 functioned as an oncogene in gastric cancer and was regulated by microRNA-185. However, the association between TRIM29 expression and the prognosis of patients with gastric cancer has not yet been established. The results of the present study revealed that TRIM29 expression was significantly associated with tumor cell differentiation, tumor stage, lymph node metastasis, and TNM stage. Furthermore, an increased percentage of patients with high TRIM29 expression was associated with cancer progression from TNM stage I to III in the training and validation sets. However, elucidating the mechanism underlying the dysregulation of TRIM29 expression in gastric cancer tissues requires further study.
In the present study, all samples were collected from patients at the Cancer Hospital of Henan Province and all surgeries were performed according to the guidelines of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. Immediately following resection of the gastric cancer specimens from the patients, the fresh cancer tissues were frozen and stored in liquid nitrogen until analysis. Prior to RT-qPCR analysis, the specimens were evaluated independently by two gastroenterology pathologists blind to clinicopathological patient data. In addition, the experiment was repeated three times with each gastric cancer tissue. However, the present study is limited in certain respects. To begin, the present study is retrospective, and selection bias may not be entirely eliminated. Secondly, data on disease-free survival were not included in the present study and should be collected in subsequent studies. Finally, few patients were enrolled in the present study, confirming the results of which requires large, randomized controlled trials.
To conclude, the results of the present study revealed that TRIM29 expression was associated with unfavorable prognosis and may be adopted as a novel prognostic indicator in patients with resectable gastric cancer. Furthermore, incorporating TRIM29 expression level into the TNM staging system increased the prognostic value of the latter for patients with resectable gastric cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic and Advanced Technology Research Project of Science and Technology Department of Henan Province (grant no. 142300410276).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
CW and YZ acquired, analyzed and interpreted the data, performed statistical analysis and drafted the manuscript. BC and WY offered technical and material support. JH designed the study, analyzed and interpreted the data, drafted the manuscript, obtained funding and supervised the study.
Ethics approval and consent to participate
The present study was reviewed and approved by the Clinical Research Ethics Committee of the Cancer Hospital of Henan Province. All patients provided written informed consent to participate.
Consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen WQ, Zheng RS, Zhang SW, Zeng HM, Zou XN. The incidences and mortalities of major cancers in China, 2010. Chin J Cancer. 2014;33:402–405. doi: 10.5732/cjc.014.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Washington K. 7th edition of the AJCC cancer staging manual: Stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 5.Thrumurthy SG, Chaudry MA, Hochhauser D, Mughal M. The diagnosis and management of gastric cancer. BMJ. 2013;347:f6367. doi: 10.1136/bmj.f6367. [DOI] [PubMed] [Google Scholar]
- 6.Stadtländer CT, Waterbor JW. Molecular epidemiology, pathogenesis and prevention of gastric cancer. Carcinogenesis. 1999;20:2195–2208. doi: 10.1093/carcin/20.12.2195. [DOI] [PubMed] [Google Scholar]
- 7.Figueiredo C, Garcia-Gonzalez MA, Machado JC. Molecular pathogenesis of gastric cancer. Helicobacter. 2013;18(Suppl 1):S28–S33. doi: 10.1111/hel.12083. [DOI] [PubMed] [Google Scholar]
- 8.Kapp LN, Painter RB, Yu LC, van Loon N, Richard CW, III, James MR, Cox DR, Murnane JP. Cloning of a candidate gene for ataxia-telangiectasia group D. Am J Hum Genet. 1992;51:45–54. [PMC free article] [PubMed] [Google Scholar]
- 9.Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, et al. The tripartite motif family identifies cell compartments. EMBO J. 2001;20:2140–2151. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatakeyama S. Early evidence for the role of TRIM29 in multiple cancer models. Expert Opin Ther Targets. 2016;20:767–770. doi: 10.1517/14728222.2016.1148687. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–428. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Kanno Y, Watanabe M, Kimura T, Nonomura K, Tanaka S, Hatakeyama S. TRIM29 as a novel prostate basal cell marker for diagnosis of prostate cancer. Acta Histochem. 2014;116:708–712. doi: 10.1016/j.acthis.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Dai X, Han B. TRIM29 as a novel biomarker in pancreatic adenocarcinoma. Dis Markers. 2014;2014:317817. doi: 10.1155/2014/317817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou ZY, Yang GY, Zhou J, Yu MH. Significance of TRIM29 and β-catenin expression in non-small-cell lung cancer. J Chin Med Assoc. 2012;75:269–274. doi: 10.1016/j.jcma.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Fristrup N, Birkenkamp-Demtröder K, Reinert T, Sanchez-Carbayo M, Segersten U, Malmström PU, Palou J, Alvarez-Múgica M, Pan CC, Ulhøi BP, et al. Multicenter validation of cyclin D1, MCM7, TRIM29, and UBE2C as prognostic protein markers in non-muscle-invasive bladder cancer. Am J Pathol. 2013;182:339–349. doi: 10.1016/j.ajpath.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Lai W, Zheng X, Huang Q, Wu X, Yang M. Down-regulating ATDC inhibits the proliferation of esophageal carcinoma cells. Eur Rev Med Pharmacol Sci. 2014;18:3511–3516. [PubMed] [Google Scholar]
- 17.Kosaka Y, Inoue H, Ohmachi T, Yokoe T, Matsumoto T, Mimori K, Tanaka F, Watanabe M, Mori M. Tripartite motif-containing 29 (TRIM29) is a novel marker for lymph node metastasis in gastric cancer. Ann Surg Oncol. 2007;14:2543–2549. doi: 10.1245/s10434-007-9461-1. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Heidt DG, Lee CJ, Yang H, Logsdon CD, Zhang L, Fearon ER, Ljungman M, Simeone DM. Oncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilization. Cancer Cell. 2009;15:207–219. doi: 10.1016/j.ccr.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernst T, Hergenhahn M, Kenzelmann M, Cohen CD, Bonrouhi M, Weninger A, Klären R, Gröne EF, Wiesel M, Güdemann C, et al. Decrease and gain of gene expression are equally discriminatory markers for prostate carcinoma: A gene expression analysis on total and microdissected prostate tissue. Am J Pathol. 2002;160:2169–2180. doi: 10.1016/S0002-9440(10)61165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nacht M, Ferguson AT, Zhang W, Petroziello JM, Cook BP, Gao YH, Maguire S, Riley D, Coppola G, Landes GM, et al. Combining serial analysis of gene expression and array technologies to identify genes differentially expressed in breast cancer. Cancer Res. 1999;59:5464–5470. [PubMed] [Google Scholar]
- 21.Hosoi Y, Kapp LN, Murnane JP, Matsumoto Y, Enomoto A, Ono T, Miyagawa K. Suppression of anchorage-independent growth by expression of the ataxia-telangiectasia group D complementing gene, ATDC. Biochem Biophys Res Commun. 2006;348:728–734. doi: 10.1016/j.bbrc.2006.07.115. [DOI] [PubMed] [Google Scholar]
- 22.Zhou XM, Sun R, Luo DH, Sun J, Zhang MY, Wang MH, Yang Y, Wang HY, Mai SJ. Upregulated TRIM29 promotes proliferation and metastasis of nasopharyngeal carcinoma via PTEN/AKT/mTOR signal pathway. Oncotarget. 2016;7:13634–13650. doi: 10.18632/oncotarget.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu F, Xiong JP, Deng J, Xiang XJ. TRIM29 functions as an oncogene in gastric cancer and is regulated by miR-185. Int J Clin Exp Pathol. 2015;8:5053–5061. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.