Abstract
The present study was performed to assess the risk factors for periodontitis in Korean adults with diabetes. Data from the Korean National Health and Nutrition Examination Survey of the Korean population, conducted between January 2012 and December 2014 were used in the investigation. The presence of periodontitis in participants with diabetes in association with demographic variables and the anthropometric characteristics of the participants was investigated. Multiple logistic regression analyses were used to assess the associations between periodontitis and age, sex, diabetic control and duration of diabetes, following adjustment for confounding factors. The odds ratio of periodontitis was higher in individuals ≥65 years old compared with individuals <65 years old (1.152). The odds ratio of periodontitis was significantly higher in males compared with females (1.774). The number of patients with moderate and severe periodontitis differed significantly between different age groups. The present study revealed that age, sex and oral health behavior are risk indicators for periodontitis in patients with diabetes. The present study suggests that that an increased age, being male and engaging in poor oral health behavior increases the risk of periodontitis in participants with diabetes.
Keywords: age, diabetes mellitus, periodontitis, risk factors, sex
Introduction
Diabetes mellitus is a complex metabolic disorder. A key feature of diabetes mellitus is the reduced function of the β cells in the islets of Langerhans in the pancreas. These β cells produce insulin and their reduced function may decrease insulin secretion and lead to increased blood glucose levels (1). The synthesis of advanced glycosylation end-products and the induction of oxidative stress may exacerbate early pathological vascular changes (2). In patients with diabetes, associated complications may contribute to increased morbidity and premature mortality (3). Retinopathy, neuropathy, cardiovascular disease, nephropathy and encephalopathy are considered to be the primary complications of diabetes (4) and periodontal disease has been implicated as the sixth complication of diabetes (5). Periodontitis is a common, chronic, inflammatory disease that induces the gradual destruction of the supporting apparatus of the teeth, causing teeth to become mobile, which may ultimately result in tooth loss (6). It has been reported that the prevalence of periodontal disease in diabetic patients is >85% (27.3% of patients had gingivitis and 59.5% had periodontitis) whereas the prevalence of periodontitis in the general population is 46% (1,7). Diabetes is a known risk factor of periodontal disease and patients with diabetes have an increased prevalence of severe periodontitis compared with healthy adults (8). Indeed, it has been demonstrated that the susceptibility to periodontitis increases by ~3-fold in patients with diabetes (6). Among individuals with chronic periodontitis, patients with diabetes exhibit enhanced lipopolysaccharide-induced immune responsiveness, implicating an exacerbated inflammatory response (9).
It has been suggested that diabetes and periodontitis are biologically linked (10) Hyperglycemia associated with diabetes has been previously studied due to its association with adverse periodontal outcomes (3). However, to the best of our knowledge, the risk factors for periodontitis specifically in patients with diabetes have not yet been identified. Therefore, the present study was performed to assess the risk factors for periodontitis in Korean adults with diabetes using nationally representative data.
Materials and methods
Survey and subjects
The results of the present study were obtained by secondary analysis of data obtained during the Korean National Health and Nutrition Examination Survey (KNHANES) between January 2012 and December 2014. The KNHANES was approved by the Institutional Review Board of the Korea Center for Disease Control and all participants provided written informed consent. The Institutional Review Board at the Catholic University of Korea approved the present study (KC14EISI0636).
A total of 23,626 individuals participated in the KNHANES survey. The number of individuals included in the present study was reduced to 1,630 following the exclusion of participants <30 years old, individuals that had only undergone <8 h fasting prior to examination, participants with missing values, individuals without diabetes mellitus and participants that did not undergo an oral examination (Fig. 1).
Figure 1.
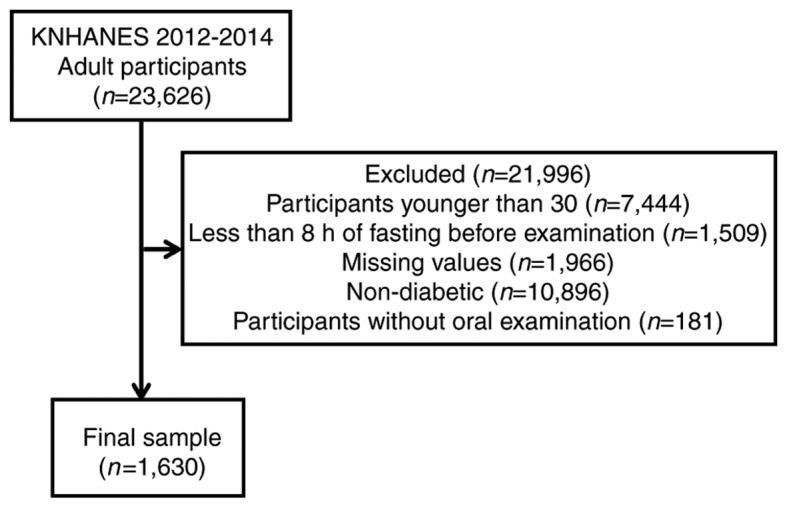
Flow chart of participants included and excluded from the study. KNHANES, Korean National Health and Nutrition Examination Survey.
Demographic variables
The sociodemographic and lifestyle variables of the participants were evaluated by a self-administered questionnaire that assessed education level, household income, smoking, alcohol intake and physical exercise. Regarding smoking status, patients were classified as non-smokers, ex-smokers or current smokers. Non-smokers were those who had never smoked or had smoked <100 cigarettes during their lifetime, whereas ex-smokers were those who had smoked >100 cigarettes in their entire life but were not smoking at the time of the study. Regarding alcohol consumption, patients were categorized as non-drinkers, mild to moderate drinkers (1–30 g/day) or heavy drinkers (>30 g/day). Physical activity was measured using the international physical activity questionnaire as part of the KNHANES. Subjects who exercised for ≥5 occasions per week for 30 min per session, or those who participated in strenuous physical activity for ≥3 occasions per week for 20 min per session, were defined as regular exercisers. Education level was categorized as either high school education (≥10 years schooling) or middle school graduate or lower (<10 years schooling). The number of household members was evaluated. Household income was divided by the number of included family members and categorized into quartiles. The lowest quartile of household income was <USD 1,092.40 per month and patients were categorized as being in the lowest quartile or not. The participants self-reported health status was categorized as good or not good (which consisted of patients reporting their health as average or bad).
Anthropometric measurements
Anthropometric measurements of individuals that participated in the present study were taken by the trained staff members. Participants wore light, indoor clothing without shoes for the measurement of body weight and height, as previously described (11). The narrowest point between the iliac crest and the lower border of the rib cage was used to determine waist circumference. The following formula of weight/height2 (kg/m2) was used to calculate body mass index. A standard mercury sphygmomanometer (Baumanometer; W.A. Baum Co., Inc., Copiague, NY, USA) was used to measure systolic and diastolic blood pressure in the right arm. Blood pressure measurements were performed twice with a 5 min interval and the average of the two measurements was used for analysis. Blood samples were collected from the antecubital vein of each participant to measure the white blood cell count. The white blood cell count was measured using an automated hematology analyzer (XE-2100D; Sysmex Corporation, Kobe, Japan).
Definition of diabetes mellitus and hypertension
A high waist circumference was categorized as ≥90 cm in men and ≥80 cm in women. Hypertension was diagnosed if an individual had a systolic blood pressure of >160 mm Hg or a diastolic blood pressure of >90 mm Hg, or if the individual was using systemic antihypertensive drugs. A fasting blood cholesterol level >240 mg/ml or the use of medication for the condition was considered to indicate the presence of hypercholesterolemia. The level of kidney function was determined by estimating the glomerular filtration rate (eGFR) using the following equations: eGFR (ml/min/1.73 m2)=186.3× (serum creatinine−1.154) × (age−0.203) for males and 186.3× (serum creatinine−1.154) × (age−0.203) ×0.742 for females. Serum creatinine was measured by the colorimetric method using a Hitachi 7600 modular chemistry analyzer (Hitachi, Ltd., Tokyo, Japan). The eGFR was categorized as either ≥60 or not as previously described (12). Cardiovascular disease was considered present if the individual had experienced a stroke or had a congenital heart defect (13).
Diabetes was defined as a fasting blood sugar >126 mg/dl or the individual was currently using anti-diabetic medication (14). The recognition, treatment and control of diabetes was self-evaluated, as well as insulin injection. Glucose and glycated hemoglobin levels were categorized into five levels. Patients were classified into the following 5 groups based on their glycated hemoglobin levels: <6, 6≤x<6.5, 6.5≤x<7, 7≤x<7.9 and ≥8 and into the following 5 groups based on their glucose levels: <100, 100≤x<120, 120≤x<140, 140≤x<160 and >160.
Periodontitis and oral health behaviors
The oral health status of the participants was evaluated using the World Health Organization Community Periodontal Index (CPI) (15,16). The index teeth according to the Federation Dentaire Internationale system were 11, 16, 17, 26, 27, 31, 36, 37, 46 and 47 (17). Periodontitis was defined as CPIs of 3 and 4. The CPI score was 3 in cases of a shallow pocket with a depth of 3.5–5.5 mm and 4 in cases of a deep pocket with a depth ≥5.5 mm (18). Moderate periodontitis was defined as a CPI of 3 and severe periodontitis was defined as a CPI of 4.
The frequency of daily tooth brushing and the use of secondary oral products were used to evaluate oral health behavior. Dental floss, interdental brushes, electric toothbrushes, irrigation devices, end-tufted brushes, tongue cleaners, mouthwash and special devices for dentures were considered to be secondary oral products. The survey also recorded the participants self-reported oral status, the presence of any toothache, chewing discomfort, speech discomfort and whether patients had undergone a dental checkup within that year.
Statistical analysis
The results are presented as the mean ± standard error of the mean for continuous variables and as proportions (standard errors) for categorical variables. To assess differences in characteristics as categorized by body mass index, a χ2 test was performed to assess categorical variables and an independent t test was used to assess continuous variables. The risk factors for periodontitis in diabetes were evaluated following adjustments for age, sex, smoking, drinking, number of household members, income, self-reported health, medication, self-reported oral status, tooth pain, frequency of tooth brushing per day and the use of secondary oral products. Multiple logistic regression analyses were used to assess the associations between periodontitis and age, sex, diabetic control and duration of diabetes, following adjustment for confounding factors. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SAS software version 9.2 for Windows (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline characteristics
Table I details the baseline characteristics of the participants included in the study according to the presence of periodontitis. The odds ratio (OR) of periodontitis was higher in individuals aged ≥65 years old compared with individuals aged 30–65 years old (OR 1.152). The OR of periodontitis was significantly higher in males compared with females, with a 95% confidence interval of 1.387–2.269 (P<0.05). Being male, smoking, drinking, have a higher number of household members, being in the lowest income quartile, have an average or bad health status, having problematic self-reported oral status, experiencing tooth pain, experiencing chewing or speech discomfort, having a lower frequency of tooth brushing and not using secondary oral products were significantly associated with the incidence of periodontitis in the diabetic population.
Table I.
Baseline characteristics of study participants with moderate and severe periodontitis.
| Characteristic | No periodontitis, n=838, % (SEM) | Periodontitis, n=792, % (SEM) | P-value | Odds ratio (95% CI) |
|---|---|---|---|---|
| Age (years) | 0.4059 | |||
| 30–64 | 67.4 (1.8) | 65.3 (2.1) | 1 (reference) | |
| ≥65 | 32.6 (1.8) | 34.7 (2.1) | 1.152 (0.916, 1.449) | |
| Sex | <0.0001a | |||
| Male | 49.3 (2.1) | 60.9 (2) | 1.774 (1.387, 2.269) | |
| Female | 50.7 (2.1) | 39.1 (2) | 1 (reference) | |
| Smoking | <0.0001a | |||
| No | 60.4 (2.2) | 43.5 (2) | 1 (reference) | |
| Ex | 21 (1.8) | 24.9 (1.8) | 1.514 (0.993, 2.31) | |
| Current | 18.6 (1.6) | 31.6 (2.1) | 2.503 (1.67, 3.752) | |
| Drinking | 0.0362a | |||
| Non-drinker | 37.3 (1.9) | 30.8 (2) | 1 (reference) | |
| Mild to moderate drinker | 54 (2.1) | 57.1 (2.1) | 1.176 (0.9, 1.535) | |
| Heavy drinker | 8.7 (1.3) | 12.1 (1.4) | 1.388 (0.855, 2.255) | |
| Exercise | 0.3669 | |||
| No | 83.9 (1.8) | 86.1 (1.8) | 1 (reference) | |
| Yes | 16.1 (1.8) | 13.9 (1.8) | 0.832 (0.559, 1.237) | |
| Number of household members | 0.0079a | |||
| 1 | 10.5 (1.2) | 13.6 (1.5) | 1 (reference) | |
| 2 | 30.9 (1.8) | 36.7 (2.2) | 0.793 (0.544, 1.156) | |
| ≥3 | 58.6 (2) | 49.7 (2.5) | 0.626 (0.414, 0.949) | |
| Education | 0.2500 | |||
| Middle-school graduate or lower | 51.2 (2.2) | 54.8 (2.2) | 1 (reference) | |
| High-school education or higher | 48.8 (2.2) | 45.2 (2.2) | 0.924 (0.689, 1.24) | |
| Income (the lowest quartile) | 0.0157a | |||
| No | 76.1 (1.7) | 69.8 (2.2) | 1 (reference) | |
| Yes | 23.9 (1.7) | 30.2 (2.2) | 1.312 (0.979, 1.757) | |
| Self reported health status | 0.0122a | |||
| Good | 17.4 (1.6) | 12.3 (1.3) | 1 (reference) | |
| Average or bad | 82.6 (1.6) | 87.7 (1.3) | 1.67 (1.19, 2.343) | |
| Body mass index (kg/m2) | 0.2601 | |||
| <25 | 47.8 (2.1) | 51.2 (2.1) | 1 (reference) | |
| ≥25 | 52.2 (2.1) | 48.8 (2.1) | 0.939 (0.735, 1.199) | |
| High waist circumference, ≥90 cm in men and ≥80 cm in women | 0.0793a | |||
| No | 44 (2.1) | 49 (2.1) | 1.091 (0.852, 1.396) | |
| Yes | 56 (2.1) | 51 (2.1) | 1 (reference) | |
| Hypertension | 0.1316 | |||
| No | 47 (2.1) | 42.4 (2.2) | 1 (reference) | |
| Yes | 53 (2.1) | 57.6 (2.2) | 1.052 (0.809, 1.367) | |
| Hypercholesterolemia | 0.8652 | |||
| No | 69.2 (1.9) | 69.7 (1.9) | 1 (reference) | |
| Yes | 30.8 (1.9) | 30.3 (1.9) | 1.04 (0.79, 1.37) | |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | 0.7636 | |||
| ≥60 | 91.7 (1.1) | 92.1 (1) | 1.197 (0.796, 1.801) | |
| <60 | 8.3 (1.1) | 7.9 (1) | 1 (reference) | |
| Cardiovascular disease | 0.9456 | |||
| No | 90.9 (1.1) | 90.8 (1.1) | 1 (reference) | |
| Yes | 9.1 (1.1) | 9.2 (1.1) | 0.912 (0.615, 1.352) | |
| Diabetes (recognition) | 0.3699 | |||
| No | 37.6 (2.2) | 40.2 (2.2) | 1 (reference) | |
| Yes | 62.4 (2.2) | 59.8 (2.2) | 0.785 (0.611, 1.008) | |
| Diabetes (treated) | 0.1644 | |||
| No | 43.4 (2.2) | 47.7 (2.2) | 1 (reference) | |
| Yes | 56.6 (2.2) | 52.3 (2.2) | 0.732 (0.567, 0.944) | |
| Diabetes (controlled) | 0.9387 | |||
| No | 78.2 (1.9) | 78 (1.9) | 1 (reference) | |
| Yes | 21.8 (1.9) | 22 (1.9) | 0.993 (0.728, 1.353) | |
| Insulin injection | 0.4330 | |||
| No | 94.8 (0.8) | 93.8 (1) | 1 (reference) | |
| Yes | 5.2 (0.8) | 6.2 (1) | 1.150 (0.663, 1.994) | |
| Diabetic medication | 0.234 | |||
| No | 45 (2.2) | 48.6 (2.2) | 1 (reference) | |
| Yes | 55 (2.2) | 51.4 (2.2) | 0.758 (0.59, 0.973) | |
| Glucose level | 0.9500 | |||
| <100 | 9.8 (1.1) | 9.4 (1.2) | 1 (reference) | |
| 100≤x<120 | 22.5 (1.7) | 22.2 (1.9) | 1.095 (0.725, 1.655) | |
| 120≤x<140 | 33.9 (2.1) | 32.6 (2.1) | 1.079 (0.721, 1.615) | |
| 140≤x<160 | 15.6 (1.6) | 15.9 (1.4) | 1.146 (0.724, 1.812) | |
| ≥160 | 18.2 (1.7) | 19.9 (1.7) | 1.326 (0.852, 2.065) | |
| Glycated hemoglobin | 0.4800 | |||
| <6 | 5.8 (1.1) | 7.8 (1.1) | 1 (reference) | |
| 6≤x<6.5 | 16 (1.6) | 14.2 (1.6) | 0.701 (0.4, 1.231) | |
| 6.5≤x<7 | 27.6 (1.9) | 28.6 (2.1) | 0.805 (0.468, 1.386) | |
| 7≤x<7.9 | 31.1 (1.9) | 28.1 (1.9) | 0.699 (0.418, 1.168) | |
| ≥8 | 19.5 (1.7) | 21.3 (1.7) | 0.91 (0.526, 1.577) | |
| Dental checkup within a year | 0.2490 | |||
| No | 76.7 (1.7) | 73.8 (1.9) | 1 (reference) | |
| Yes | 23.3 (1.7) | 26.2 (1.9) | 1.184 (0.887, 1.579) | |
| Self-reported oral status | <0.0001a | |||
| Favorable | 14.9 (1.6) | 9.9 (1.2) | 1 (reference) | |
| Average | 36.6 (2.1) | 26.5 (1.7) | 1.179 (0.772, 1.801) | |
| Problematic | 48.6 (2.2) | 63.6 (1.9) | 2.019 (1.346, 3.027) | |
| Tooth pain | <0.0001a | |||
| No | 65.4 (2) | 47.6 (2.1) | 1 (reference) | |
| Yes | 34.6 (2) | 52.4 (2.1) | 2.086 (1.653, 2.632) | |
| Chewing discomfort | <0.0001a | |||
| No | 69.7 (2) | 56.9 (2.1) | 1 (reference) | |
| Yes | 30.3 (2) | 43.1 (2.1) | 1.642 (1.271, 2.12) | |
| Speech discomfort | 0.0339a | |||
| No | 85.5 (1.4) | 81.1 (1.6) | 1 (reference) | |
| Yes | 14.5 (1.4) | 18.9 (1.6) | 1.277 (0.946, 1.724) | |
| Frequency of tooth brushing per day | 0.0019a | |||
| ≤1 | 12.5 (1.4) | 18.5 (1.7) | 1 (reference) | |
| 2 | 42.6 (2.2) | 46.1 (2) | 0.773 (0.534, 1.119) | |
| ≥3 | 44.9 (2.2) | 35.4 (2) | 0.583 (0.393, 0.865) | |
| Use of secondary oral products | 0.0020a | |||
| No | 54.1 (2.2) | 63.6 (2.1) | 1 (reference) | |
| Yes | 45.9 (2.2) | 36.4 (2.1) | 0.757 (0.583, 0.981) |
Data are presented as percentages (standard error of the mean) of the study participants.
P<0.05. CI, confidence interval. SEM, standard error of the mean.
The odds ratio of periodontitis following adjustments for confounding factors
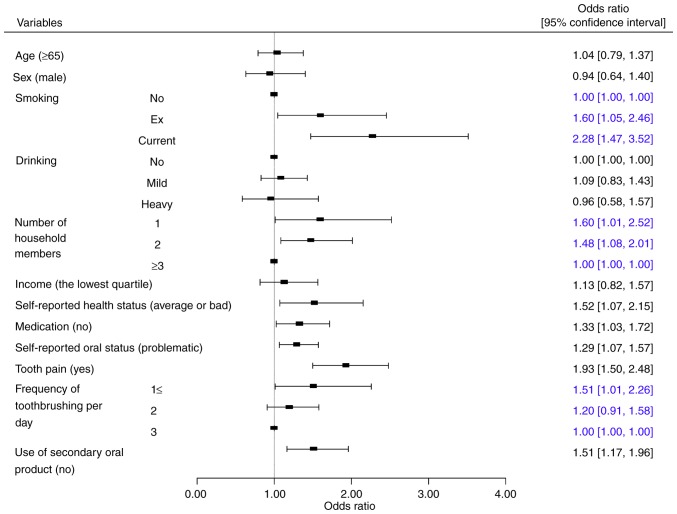
ORs and their 95% confidence intervals for moderate and severe periodontitis categorized by various variables following adjustments for age, sex, smoking, drinking, number of household members, income, self-reported health, medication, self-reported oral status, tooth pain, frequency of tooth brushing per day and use of secondary oral products are presented in Fig. 2. The ORs and 95% confidence intervals of periodontitis were 1.60 (1.05, 2.46) and 2.28 (1.47, 3.52) for ex-smokers and current smokers, respectively when non-smokers were considered as a reference. The ORs and 95% confidence intervals of periodontitis for the individuals were 1.51 (1.01, 2.26) and 1.20 (0.91, 1.58) for the individuals with a toothbrushing frequency of ≤1 and 2, respectively when participants with toothbrushing frequency of 3 was considered as a reference. These results indicate that individuals with current smoking had higher ORs of periodontitis. Similarly, participants with a toothbrushing frequency of ≤1 had higher ORs of periodontitis.
Figure 2.
The odds ratio and their 95% confidence intervals for moderate and severe periodontitis categorized by different variables. Blue text indicates values that were significant.
The percentage of moderate and severe periodontitis categorized by different variables
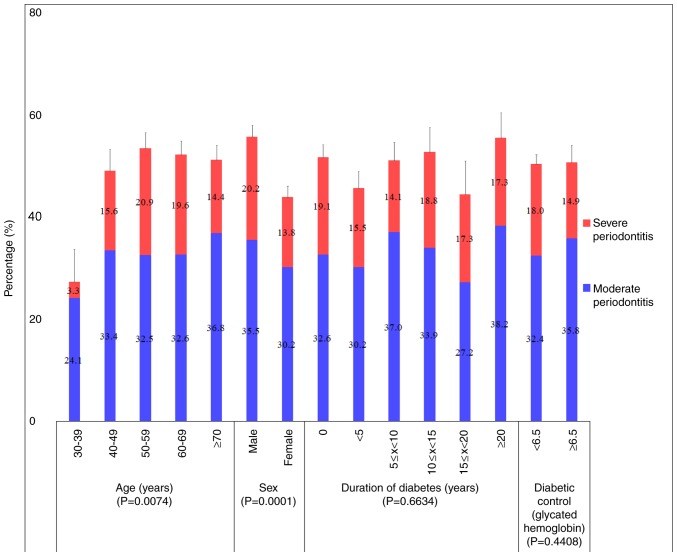
Fig. 3 presents the percentage of moderate and severe periodontitis categorized by age, sex, duration of diabetes and glycated hemoglobin. The percentages of moderate periodontitis in individuals aged 30–39 and ≥70 were 24.1 and 36.8%, respectively. The percentages of moderate periodontitis in male and female were 35.5 and 30.2%, respectively. The percentage of patients with periodontitis differed significantly between different age groups (P=0.0074) and significant differences were also noted between males and females (P<0.0001). There were no significant differences between patients with moderate and severe periodontitis.
Figure 3.
The percentage of the total population of moderate and severe periodontitis categorized by age, sex, duration of diabetes and glycated hemoglobin.
Discussion
The present study used nationally representative data to determine that age, sex and oral health behavior were risk factors for periodontitis in Korean adults with diabetes. The effect of age on the prevalence of periodontitis may partially be explained by the onset of chronic inflammation, which is a common feature of aging and age-associated diseases, including periodontitis (19). It has been previously revealed that the prevalence and severity of periodontal disease increased as age increased (1). Similarly, periodontitis occurred at a higher rate in diabetic participants compared with non-diabetic individuals and this phenomenon was positively correlated with age in individuals with diabetes (19). It has been suggested that patients with diabetes aged ≥35 years old experience more rapid destruction of the periodontium (20). Furthermore, it has been demonstrated that age is significantly associated with the increased prevalence and greater severity of destructive periodontal disease in patients with diabetes (21). The results of present study confirmed the importance of age in determining the prevalence of periodontitis in patients with diabetes.
Differences between the two sexes have also been noted in previous studies. A previous report revealed that diabetic males had worse periodontal conditions compared with diabetic females (22). A previous study also revealed statistically significant associations between males and severe periodontitis (23). These results therefore suggest that there is a greater need for the regular periodontal evaluation and effective oral hygiene care among males with diabetes than in females with diabetes to decrease the risk of developing periodontitis and progression of periodontitis into a more severe form (24). The present study revealed that the percentage of patients with moderate and severe periodontitis did not significantly differ between different duration of diabetes or diabetic control. In a previous study, diabetic status was associated with an increased severity and prevalence of destructive periodontal disease in the diabetic population (21). Furthermore, glycemic status influences the prevalence and severity of periodontal disease in diabetic participants (1). Individuals with poorly controlled diabetes and periodontitis are more likely to suffer from gingival bleeding than those with good or moderate control (25). Conversely, no significant correlation was observed between periodontitis and glycated hemoglobin levels in non-diabetic subjects (26). Similarly, glycemic control, defined by fasting plasma glucose (140 mg/dl) and glycohemoglobin value (6.5%), was not significantly associated with periodontal status (27). Diabetic control did not reveal any differences in the periodontal condition (20). A previous report demonstrated that the glycated hemoglobin levels were not significantly correlated with periodontal values in diabetic participants (28), which was similar to trends observed in the present study. The association between the duration of diabetes and periodontitis was evaluated in the present study; however no significant association was observed. The association between the duration of diabetes and periodontitis is controversial as some reports have demonstrated that the two factors were associated, while others have reported no association (19,20,25,27,28). A previous study reported that the number of years since the diagnosis of diabetes was more significant than age for predicting the severity of periodontal disease in diabetic patients (29). A previous study observed that the duration of diabetes mellitus had an association with the prevalence and severity of periodontal disease (1). It should be noted that the number of participants in these previous reports were relatively small, being composed of only 100 patients (46 males and 54 females) (29) and 1,500 patients (751 males and 749 females), respectively (1). However, no correlation was observed between periodontitis in individuals with diabetes and the length of time from the diagnosis of diabetes (19). Several previous studies have reported that the duration of diabetes was not associated with the periodontal condition (20,27,28). A previous report revealed that no significant correlation was observed between diabetic duration and gingival bleeding (25). An advantage of the present study is that it was based on a nationally representative sample of Korean patients with diabetes, which allows for an effective investigation into whether age and sex are associated with periodontitis. The sampling units were based on the population and housing census conducted by the National Census Registry in Korea and survey sample weights were adjusted for participation and response rates (30). To the best of our knowledge, the present study is the first to demonstrate the importance of age and sex as risk indicators for the prevalence of periodontitis in individuals with diabetes (31). The present study assessed the effects of various risk indicators by subgroup analysis, including age, sex, control of diabetes and duration of diabetes. However, the present study did have certain limitations. Firstly, as it was a cross-sectional study it was not possible to identify a cause-and-effect association. Secondly, the present study used partial-mouth recording protocols of CPI, which may underestimate the prevalence of periodontal disease. To overcome these limitations a prospective study using full-mouth recording may be designed, however this type of study requires far greater resources to complete.
A bidirectional association has been reported between diabetes and periodontitis (3). Patients with diabetes have a higher predisposition to periodontitis (9) and periodontitis is a risk factor in a number of systemic diseases, including diabetes and cardiovascular pathologies (2). In diabetic patients, the elimination of periodontal infections and a reduction in periodontal inflammation produces a noticeable short-term reduction in glycated hemoglobin (32). Individuals with periodontitis had an increased rate of microvascular complications ketoacidosis and of hospitalizations associated with hyperglycemia with odds ratio and 95% confidence intervals of [2.43 (1.74–3.40)], [2.72 (1.53–4.80)] and [2.76 (1.72–4.42)], respectively (33). Treatment of periodontitis reduces the risk of cardiovascular disease in diabetic patients (34). Therefore, periodontitis may stimulate inflammatory changes in adipose tissue and this may create a self-generating cycle of morbidity that links diabetes and periodontal disease (35).
Dental management has a beneficial effect on the health of patients with diabetes (34). Maintaining oral health helps prevent oral chronic diseases and ameliorate the consequences of chronic inflammatory processes (36). Patients with diabetes are likely to visit a physician more frequently than they visit a dentist and it is the physician's responsibility to educate and motivate the patient to seek dental treatment (37).
In conclusion, the results of the present study indicate that age, sex and oral health behavior are risk indicators of periodontitis in Korean adults with diabetes. The present study suggests that that an increased age, being male and engaging in poor oral health behavior increases the risk of periodontitis in participants with diabetes. Further prospective studies involving a larger sample size of diabetic subjects over a longer period of time are required to evaluate the cause-and-effect association. The present study suggests that individuals of a certain age with diabetes should be recommended for regular periodontal evaluation, particularly males.
Acknowledgements
The authors thank the Korea Centers for Disease Control and Prevention for providing the data.
Funding
The present study was supported by Research Fund of Seoul St. Mary's Hospital, The Catholic University of Korea (2017). This work was partly supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (grant no. NRF-2017R1A1A1A05001307; Daejeon, Republic of Korea).
Availability of data and materials
All data generated or analyzed during this study are included in the published article.
Authors' contributions
KH and JP designed the research, analyzed the data and wrote the manuscript. Both authors reviewed the final manuscript.
Ethics approval and consent to participate
The KNHANES was approved by the Institutional Review Board of the Korea Center for Disease Control and all participants provided written informed consent. The Institutional Review Board at the Catholic University of Korea approved the present study (KC14EISI0636).
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing interests.
References
- 1.Rajhans NS, Kohad RM, Chaudhari VG, Mhaske NH. A clinical study of the relationship between diabetes mellitus and periodontal disease. J Indian Soc Periodontol. 2011;15:388–392. doi: 10.4103/0972-124X.92576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Napora M, Grabowska E, Górska R. Prospective analysis of the relationship between the state of periodontal tissues and changes in selected cardiovascular parameters in patients with type 2 diabetes. Adv Clin Exp Med. 2016;25:879–886. doi: 10.17219/acem/34206. [DOI] [PubMed] [Google Scholar]
- 3.Chapple IL, Genco R. working group 2 of the joint EFP/AAP workshop: Diabetes and periodontal diseases: Consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol. 2013;84(4 Suppl):S106–S112. doi: 10.1902/jop.2013.1340011. [DOI] [PubMed] [Google Scholar]
- 4.Khanuja PK, Narula SC, Rajput R, Sharma RK, Tewari S. Association of periodontal disease with glycemic control in patients with type 2 diabetes in Indian population. Front Med. 2017;11:110–119. doi: 10.1007/s11684-016-0484-5. [DOI] [PubMed] [Google Scholar]
- 5.Saini R, Saini S, Sugandha R. Periodontal disease: The sixth complication of diabetes. J Family Community Med. 2011;18:31. doi: 10.4103/1319-1683.78636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preshaw PM, Bissett SM. Periodontitis: Oral complication of diabetes. Endocrinol Metab Clin North Am. 2013;42:849–867. doi: 10.1016/j.ecl.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakschevitz F, Aboodi G, Tenenbaum H, Glogauer M. Diabetes and periodontal diseases: Interplay and links. Curr Diabetes Rev. 2011;7:433–439. doi: 10.2174/157339911797579205. [DOI] [PubMed] [Google Scholar]
- 9.Mesia R, Gholami F, Huang H, Clare-Salzler M, Aukhil I, Wallet SM, Shaddox LM. Systemic inflammatory responses in patients with type 2 diabetes with chronic periodontitis. BMJ Open Diabetes Res Care. 2016;4:e000260. doi: 10.1136/bmjdrc-2016-000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shetty A, Bhandary R, Thomas B, Ramesh A. A comparative evaluation of serum magnesium in diabetes mellitus type 2 patients with and without periodontitis-a clinico-biochemical study. J Clin Diagn Res. 2016;10:ZC59–ZC61. doi: 10.7860/JCDR/2016/21063.9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nam GE, Kim DH, Cho KH, Park YG, Han KD, Choi YS, Kim SM, Ko BJ, Kim YH, Lee KS. Estimate of a predictive cut-off value for serum 25-hydroxyvitamin D reflecting abdominal obesity in Korean adolescents. Nutr Res. 2012;32:395–402. doi: 10.1016/j.nutres.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang MS, Kim CH, Jeong SJ, Park TS. Dietary sodium intake in people with diabetes in Korea: The Korean national health and nutrition examination survey for 2008 to 2010. Diabetes Metab J. 2016;40:290–296. doi: 10.4093/dmj.2016.40.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeon JY, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, Song KH, Won JC, Lim S, Choi SH, et al. Prevalence of diabetes and prediabetes according to fasting plasma glucose and HbA1c. Diabetes Metab J. 2013;37:349–357. doi: 10.4093/dmj.2013.37.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingman A, Susin C, Albandar JM. Effect of partial recording protocols on severity estimates of periodontal disease. J Clin Periodontol. 2008;35:659–667. doi: 10.1111/j.1600-051X.2008.01243.x. [DOI] [PubMed] [Google Scholar]
- 16.Park JB, Han K, Park YG, Ko Y. Association between alcohol consumption and periodontal disease: The 2008 to 2010 Korea national health and nutrition examination survey. J Periodontol. 2014;85:1521–1528. doi: 10.1902/jop.2014.130782. [DOI] [PubMed] [Google Scholar]
- 17.Blinkhorn AS, Choi CL, Paget HE. An investigation into the use of the FDI tooth notation system by dental schools in the UK. Eur J Dent Educ. 1998;2:39–41. doi: 10.1111/j.1600-0579.1998.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 18.Islam SA, Seo M, Lee YS, Moon SS. Association of periodontitis with insulin resistance, β-cell function, and impaired fasting glucose before onset of diabetes. Endocr J. 2015;62:981–989. doi: 10.1507/endocrj.EJ15-0350. [DOI] [PubMed] [Google Scholar]
- 19.Albrecht M, Bánóczy J, Tamás G., Jr Dental and oral symptoms of diabetes mellitus. Community Dent Oral Epidemiol. 1988;16:378–380. doi: 10.1111/j.1600-0528.1988.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 20.Bacić M, Plancak D, Granić M. CPITN assessment of periodontal disease in diabetic patients. J Periodontol. 1988;59:816–822. doi: 10.1902/jop.1988.59.12.816. [DOI] [PubMed] [Google Scholar]
- 21.Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991;62:123–131. doi: 10.1902/jop.1991.62.2.123. [DOI] [PubMed] [Google Scholar]
- 22.Schulze A, Busse M. Gender differences in periodontal status and oral hygiene of non-diabetic and type 2 diabetic patients. Open Dent J. 2016;10:287–297. doi: 10.2174/1874210601610010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kongstad J, Enevold C, Christensen LB, Fiehn NE, Holmstrup P. Impact of periodontitis case criteria: A cross-sectional study of lifestyle. J Periodontol. 2017;88:602–609. doi: 10.1902/jop.2017.160426. [DOI] [PubMed] [Google Scholar]
- 24.Strauss SM, Stefanou LB. Interdental cleaning among persons with diabetes: Relationships with individual characteristics. Int J Dent Hyg. 2014;12:127–132. doi: 10.1111/idh.12037. [DOI] [PubMed] [Google Scholar]
- 25.Ervasti T, Knuuttila M, Pohjamo L, Haukipuro K. Relation between control of diabetes and gingival bleeding. J Periodontol. 1985;56:154–157. doi: 10.1902/jop.1985.56.3.154. [DOI] [PubMed] [Google Scholar]
- 26.Wahi S, Tripathi A, Wahi S, Mishra VD, Singh AP, Sinha N. Assessment of levels of glycosylated hemoglobin in patients with periodontal pathologies: A comparative study. J Contemp Dent Pract. 2017;18:506–509. doi: 10.5005/jp-journals-10024-2074. [DOI] [PubMed] [Google Scholar]
- 27.Bridges RB, Anderson JW, Saxe SR, Gregory K, Bridges SR. Periodontal status of diabetic and non-diabetic men: Effects of smoking, glycemic control, and socioeconomic factors. J Periodontol. 1996;67:1185–1192. doi: 10.1902/jop.1996.67.11.1185. [DOI] [PubMed] [Google Scholar]
- 28.Rylander H, Ramberg P, Blohme G, Lindhe J. Prevalence of periodontal disease in young diabetics. J Clin Periodontol. 1987;14:38–43. doi: 10.1111/j.1600-051X.1987.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 29.Cerda J, Vázquez de la Torre C, Malacara JM, Nava LE. Periodontal disease in non-insulin dependent diabetes mellitus (NIDDM). The effect of age and time since diagnosis. J Periodontol. 1994;65:991–995. doi: 10.1902/jop.1994.65.11.991. [DOI] [PubMed] [Google Scholar]
- 30.Han K, Park JB. Association between oral health behavior and periodontal disease among Korean adults: The Korea national health and nutrition examination survey. Medicine (Baltimore) 2017;96:e6176. doi: 10.1097/MD.0000000000006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song IS, Han K, Park YM, Ji S, Jun SH, Ryu JJ, Park JB. Severe periodontitis is associated with insulin resistance in non-abdominal obese adults. J Clin Endocrinol Metab. 2016;101:4251–4259. doi: 10.1210/jc.2016-2061. [DOI] [PubMed] [Google Scholar]
- 32.Grossi SG, Skrepcinski FB, DeCaro T, Zambon JJ, Cummins D, Genco RJ. Response to periodontal therapy in diabetics and smokers. J Periodontol. 1996;67(10 Suppl):S1094–S1102. doi: 10.1902/jop.1996.67.10s.1094. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira LS, Lira-Junior R, Figueredo CM, Gomes MB, Fischer RG. Self-reported periodontitis and complications in type 1 diabetes patients: A Brazilian nationwide survey. Braz Dent J. 2016;27:599–603. doi: 10.1590/0103-6440201601054. [DOI] [PubMed] [Google Scholar]
- 34.Peng CH, Yang YS, Chan KC, Kornelius E, Chiou JY, Huang CN. Periodontal treatment and the risks of cardiovascular disease in patients with type 2 diabetes: A retrospective cohort study. Intern Med. 2017;56:1015–1021. doi: 10.2169/internalmedicine.56.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine RS. Obesity, diabetes and periodontitis-a triangular relationship? Br Dent J. 2013;215:35–39. doi: 10.1038/sj.bdj.2013.627. [DOI] [PubMed] [Google Scholar]
- 36.Lifshitz F, Casavalle PL, Bordoni N, Rodriguez PN, Friedman SM. Oral health in children with obesity or diabetes mellitus. Pediatr Endocrinol Rev. 2016;14:159–167. doi: 10.17458/PER.2016.LCB.Oralhealth. [DOI] [PubMed] [Google Scholar]
- 37.Ummadisetty T, Chava VK, Bhumanapalli VR. Diabetes and periodontitis: How well are the patients aware about an established relation? J Indian Soc Periodontol. 2016;20:472–475. doi: 10.4103/0972-124X.184035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the published article.