Abstract
Although cisplatin (CDDP) is widely used for non-small-cell lung cancer (NSCLC) treatment, resistance remains a major problem that restricts its efficacy. Therefore, identification of drugs that reverse or prevent resistance to CDDP in NSCLC has been a focus of a number of studies. The results of the present study revealed the effect of heat shock protein family A member 12B (HSPA12B) overexpression on chemoresistance in A549 cells in vitro. The effect of HSPA12B overexpression on chemoresistance in mice bearing A549 xenografted tumors was then determined via stable HSPA12B transfection. Finally, the effects of HSPA12B overexpression on the phosphorylation of protein kinase B (Akt) and nuclear factor-κB inhibitor α (IκBα), and the expression of the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) and the pro-apoptotic protein cleaved caspase-3 were determined by western blot analysis. The results demonstrated that HSPA12B overexpression increased resistance to CDDP in NSCLC cells in vivo and in vitro by promoting cell growth and inhibiting CDDP-induced apoptosis. Mechanistically, this effect was mediated by the upregulation of phosphorylated (p-)Akt, p-IκBα and Bcl-2 and the downregulation of cleaved caspase-3. Therefore, the present study provides useful information pertaining to the identification and targeting of a CDDP-resistant population, and the development of potential therapeutics to improve the current treatment modalities in NSCLC.
Keywords: non-small cell lung cancer, heat shock protein family A member 12B, cisplatin, phosphoinositide 3-kinase/protein kinase B/nuclear factor-κB signaling pathway
Introduction
Lung cancer is one of the most frequently diagnosed cancers, and was the leading cause of cancer-associated mortality globally in 2012 (1). Non-small-cell lung cancer (NSCLC), represents ~85% of all newly diagnosed lung cancer, and includes adenocarcinoma (gland-forming), squamous cell carcinoma and large-cell carcinoma histological subtypes (2). Clinically, the majority of patients are not suitable for treatment by surgical resection due to distant metastases and advanced stage; therefore chemotherapeutic agents hold promise for the treatment of NSCLC (3). Cis-diamminedichloroplatinum (cisplatin; CDDP) is one of the most effective chemotherapeutic drugs, exhibiting a wide spectrum of activities against various human cancers, including NSCLC. It produces DNA intra-strand crosslinks between adjacent purines by forming bivalent adducts with nucleophilic sites on purines, thereby exerting its antitumor effects (4).
However, the efficacy of CDDP in cancer treatment is often restricted due to resistance, either intrinsic, as observed in patients with lung, colorectal and prostate cancer, or acquired following CDDP chemotherapy, as often observed in patients with ovarian cancer (5). The course of CDDP resistance appears to be multifactorial, including changes in drug transport resulting in decreased drug accumulation, enhanced drug detoxification, alterations in DNA repair and damage bypass and/or changes in the apoptotic cell death pathways, such as the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/nuclear factor-κB (NF-κB) signaling pathway (6,7). Besides, patients with esophageal cancer that treated with CDDP were reported to experience therapeutic failure or tumor recurrence (8). Accordingly, agents that may elevate the sensitivity to CDDP in human NSCLC are of therapeutic interest, including heat shock protein family A member 12B (HSPA12B).
HSPA12B is a distant member of the mammalian heat shock protein 70 (HSP70) family, because it contains a HSP70 ATPase domain (9). Previous research has indicated the essential role of HSP70 in oncogenesis and chemotherapy resistance (10). For example, a previous study suggested that HSP70 served functional roles in the progression of uterine cervical squamous cell carcinoma (SCC), and that HSP70 knockdown enhanced chemosensitivity to CDDP in cervical SCC cells (11). Notably, several in vitro studies have examined the role of HSPA12B in carcinogenesis and lung cancer progression, and have identified it as a potential therapeutic target (12,13); however, the potential role of HSPA12B in chemosensitivity has not been described.
The present study aimed to investigate the significance of HSPA12B overexpression in CDDP resistance in NSCLC in vivo and in vitro, and to explore the molecular mechanisms underlying the effect of HSPA12B expression.
Materials and methods
Cell lines
Human lung adenocarcinoma A549 cells were supplied by the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and were cultured at 37°C in a humidified atmosphere containing 5% CO2 in Ham's F12 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with sodium bicarbonate (2.2% w/v), L-glutamine (0.03% w/v), penicillin (100 U/ml), streptomycin (100 g/ml), and 10% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.).
pcDNA3.1-HSPA12B+/+ construction and transfection
Reverse transcription-polymerase chain reaction (RT-PCR) of normal human fibroblast total RNA was conducted using the following primers: Forward, 5′-ATCGCCACCTTCAAAAGGCAA-3′; and reverse, 5′-CTGTGAGGACCACTTCACGA-3′. The cDNA was obtained and cloned into pCR™ II (Invitrogen; Thermo Fisher Scientific, Inc.), and full-length HSPA12B cDNA was then sub-cloned into the pcDNA3.1 plasmid (Invitrogen; Thermo Fisher Scientific, Inc.) to produce the pcDNA3.1-HSPA12B+/+ vector.
A549 cells were plated at a density of 5×105 in 6-cm dishes and transfected using Lipofectamine 2000® according to the manufacturer's instructions (Invitrogen; Thermo Fisher Scientific, Inc.). A549 cells were transfected with endotoxin-free preparations of pcDNA3.1-HSPA12B+/+ or pcDNA3.1 (control) and harvested. After 48 h transfection, the protein expression of HSPA12B was assessed by western blotting to determine the pcDNA3.1-HSPA12B+/+ transfection efficiency in A549 cells. A549/HSPA12B+/+ cells were then exposed to the PI3K inhibitor LY294002 (24 µM; Cell Signaling Technology, Inc., Danvers, MA, USA), the Akt inhibitor Triciribine (30 µM, calbiochem) and the NF-κB inhibitor caffeic acid phenethyl ester (CAPE, 10 µM) (both from Sigma-Aldrich; Merck KGaA) for 1 h.
Subcutaneous implantation of tumor cells
The present study was approved by the Ethics Committee of Jinshan Hospital, Fudan University (Shanghai, China). A549, A549/HSPA12B+/+ and A549/pcDNA3.1 cells were harvested from sub-confluent cultures (50–70% confluence) following a brief exposure to 0.25% trypsin (Sigma-Aldrich; Merck KGaA) and 0.2% EDTA. Trypsinization was halted by adding to the cell suspension 100 ml fresh RPMl-1640 medium (Thermo Fisher Scientific, Inc.) containing 10% FBS. The cells were washed once in serum-free medium and resuspended in PBS. A total of 2×106 cells in 100 µl PBS were injected into the right flanks of 6-week-old BALB/c male nude mice (weighing 18–20 g, n=12 in each group). Mice were purchased from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China) and were housed in polystyrene cages. Two mice were kept per cage with free access to food and water, and a 12/12 h light/dark cycle, with an ambient temperature of 20–25°C.
In vivo model
Tumors were established by subcutaneous injection of 2×106 A549 tumor cells (A549, A549/HSPA12B+/+ and A549/pcDNA3.1) into the right flank of the mice (n=12/group). Tumor volumes were calculated as: π/6 × a2 × b (where a is the short axis and b is the long axis). After 3 weeks, when tumors reached ~100 mm3, the mice in each group (n=12) were randomly divided into two subgroups: Control and CDDP (n=6/subgroup). These mice received daily intravenous injections of either CDDP (4 mg/kg body weight) or the same volume of PBS, respectively. CDDP was administered daily (from 21 days after the initial injection of tumor cells). The treatments lasted for 15 days, during which the size of the tumors was recorded. The mice were euthanized 3 days after the last injection, and tumors were excised. Euthanasia 3 days after the last injection was deemed a humane end-point to minimize pain and distress of experimental mice (14). The present study was approved by the Ethics Committee of Jinshan Hospital, Fudan University, Shanghai, China. All experimental procedures were performed in strict accordance with the guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85–23, revised 1996) (15).
In situ detection of apoptotic cells
Apoptotic cells in the tumor tissues were detected by TUNEL assay, according to the manufacturer's protocol for the In Situ Cell Death Detection kit (cat. no. 11684817910; Roche Diagnostics, GmbH, Mannheim, Germany). Resected tumors were frozen, fixed in 10% formalin solution (pH 6.8–7.2; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 18–20 h at room temperature, embedded in paraffin and sectioned at 5 µm thickness. The 5-µm sections were prepared by the Mayo Clinic Scottsdale Histology Core Facility (Scottsdale, AZ, USA). A TUNEL assay for apoptosis was conducted using an In Situ Cell Death Detection kit (Roche Diagnostics, GmbH) according to the manufacturer's instructions. Sections were deparaffinized in xylene and then treated with a graded series of alcohol (100, 95, 90, 80 and 70% ethanol in double-distilled water) and rehydrated in PBS (pH 7.5). Tissues were then treated with proteinase K solution (2 µg/ml; Roche Diagnostics) for 15 min at room temperature for permeabilization. Endogenous peroxidase was inactivated by 3% H2O2 (Sigma-Aldrich; Merck KGaA) in PBS for 30 min and sections were rinsed with PBS, immersed in citrate buffer (pH=6.0) and then incubated with TdT and digoxigenin dUTP (diluted 1:1) at 37°C for 60 min. Subsequently, the reaction was ceased with 1× TdT stop buffer (17.4 g NaCl and 8.8 g tri-sodium citrate (Na3C6H5O7·2H2O) in 1,000 ml double distilled water) at 37°C for 30 min and anti-digoxigenin peroxidase conjugate was applied for an incubation of 30 min at room temperature. The slides were developed using 0.05% diaminobenzidine substrate for 5 min at room temperature. For the negative control, slides were incubated at 37°C for 60 min with TdT buffer without TdT. As a positive control, slides were treated with DNase (1 µg/ml; Sigma-Aldrich; Merck KGaA) at 37°C for 10 min. Apoptotic cells were imaged under a fluorescence microscope (Nikon Corporation, Tokyo, Japan). The TUNEL-positive cells were counted in 10 randomly selected high-power fields at ×400 magnification. The apoptosis index was calculated as previously described (3).
Cell viability analysis
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) assay was used to quantitatively evaluate cell viability. The cells (A549, A549/HSPA12B+/+ and A549/pcDNA3.1) were seeded onto 96-well plates at a density of 1×104 cells/well for 24 h at room temperature. Cells were exposed to in vitro treatment with 1, 2, 4 and 8 µg/ml CDDP at room temperature for 24, 48 or 72 h. Subsequently, the culture medium was removed, and the cells were washed with PBS; 100 µl Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) and 10 µl CCK-8 solution were then added to each well, and incubated at 37°C for 2.5 h. Following incubation, the optical density at 450 nm was determined using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). Finally, the CCK-8 readings of the treatment group were divided by their corresponding control readings to obtain the ratio of viable cells.
Western blot analysis
Protein was isolated from cells that were lysed in radioimmunoprecipitation buffer (RIPA) containing protease inhibitors at 4°C for 30 min. Cell lysates were prepared with a RIPA lysis buffer kit (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and the protein concentrations were quantified using a Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins (30 µg/lane) were separated on SDS-PAGE (8% gel) and transferred to polyvinylidene difluoride membranes (Amersham; GE Healthcare, Chicago, IL, USA). The membranes were blocked in 5% non-fat milk (Merck KGaA) overnight at 4°C. Membranes were then probed with the following primary antibodies; anti-HSPA12B (cat. no. ab116082; 1:500), anti-AKT (cat. no. ab8932; 1:200), anti-Bcl-2 (cat. no. ab37899; 1:200), anti-cleaved caspase-3 (cat. no. ab13847; 1:500), anti-caspase-3 (cat. no. ab4051; 1:500), anti-GAPDH (cat. no. ab9483; 1:200) and anti-β-actin (cat. no. ab8227; 1:1,000) all purchased from Abcam (Cambridge, MA, USA) and anti-p-AKT (cat. no. 9271S; 1:1,000), anti-IκBα (cat. no. 9242; 1:100), anti-p-IκBα (cat. no. 2859; 1:100) all purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), and incubated overnight at 4°C. Subsequently, protein bands were detected by incubation with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (cat. no. A50-106P; 1:1,000; Origene Technologies, Inc, Beijing, China) at room temperature for 1 h. Signals were detected using an enhanced chemiluminescence kit (Wuhan Boster Biotechnology Co., Ltd., Wuhan, China) and exposed to Kodak X-OMAT film (Kodak, Rochester, NY, USA). Each experiment was performed at least three times and the results were analyzed using Alpha View Analysis Tools (AlphaViewSA software, version 3.2.2; ProteinSimple, Santa Clara, CA, USA).
Statistical analysis
Data were expressed as mean ± standard deviation. Statistical analyses were performed using SPSS statistical software package standard version 16.0 (SPSS, Inc., Chicago, IL, USA). Experiments were performed in triplicate. Statistical differences among multiple independent groups were determined using a one-way analysis of variance followed by a Dunnett's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
HSPA12B overexpression increased resistance to CDDP in NSCLC cells in vitro
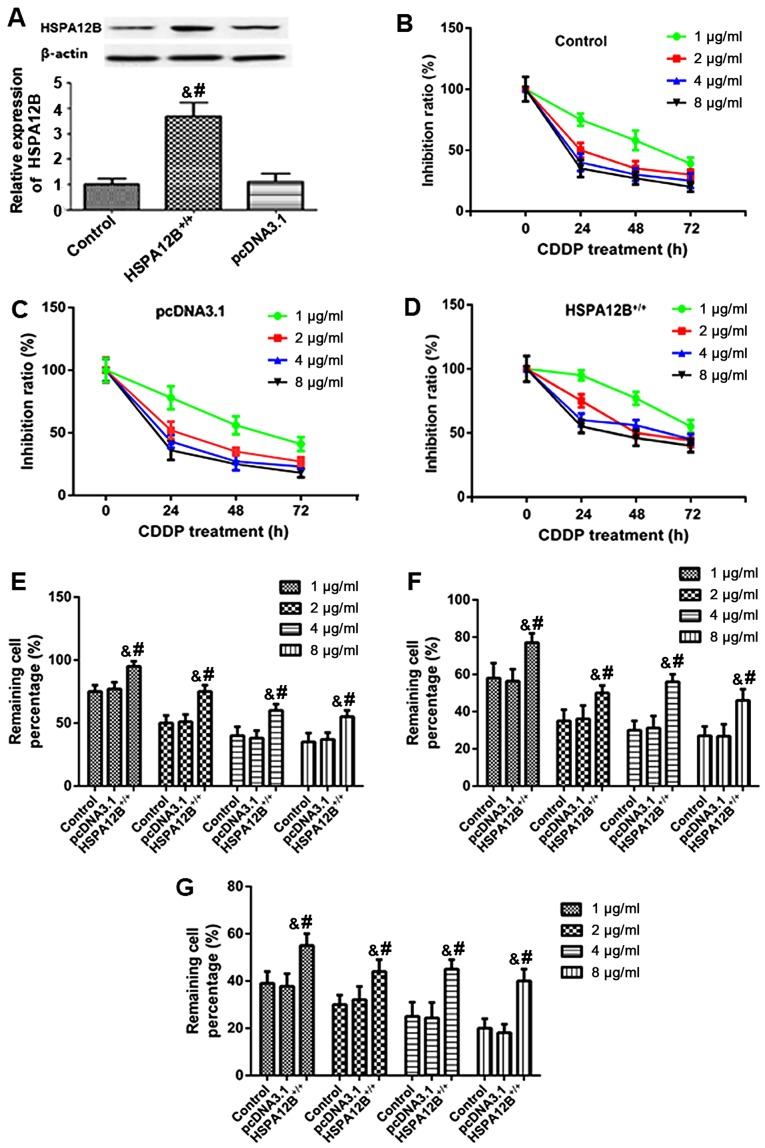
In order to examine whether HSPA12B expression affected CDDP sensitivity in vivo, western blotting was first performed to detect the pcDNA3.1-HSPA12B+/+ transfection efficiency in A549 cells. In the A549/HSPA12B+/+ cells, the protein levels of HSPA12B were significantly higher compared with the HSPA12B levels in the untransfected A549 cells and the control A549/pcDNA3.1 cells (Fig. 1A).
Figure 1.
Increased resistance to chemotherapy in HSPA12B-overexpressing non-small cell lung cancer cells in vitro. (A) Western blot analyses demonstrated that in the pcDNA3.1-HSPA12B+/+-transfected A549 cells, the protein expression of HSPA12B was significantly higher compared with the endogenous HSPA12B levels in untransfected A549 cells and the control pcDNA3.1-transfected A549 cells. (B) Untransfected A549 cells (control), (C) A549 cells transfected with the empty pcDNA3.1 vector and (D) A549 cells overexpressing HSPA12B were exposed to in vitro treatment with 1, 2, 4 or 8 µg/ml CDDP at 24, 48, and 72 h. The remaining cell percentage in (E) untransfected A549 cells (control), (F) A549 cells transfected with the empty pcDNA3.1 vector and (G) A549 cells overexpressing HSPA12B following exposure to in vitro treatment with 1, 2, 4 or 8 µg/ml CDDP at 24, 48, and 72 h was shown. A CCK-8 assay was performed to evaluate cell viability. Data are presented as the mean ± standard deviation. &P<0.05 vs. untransfected control; #P<0.05 vs. empty pcDNA3.1-transfected cells. CDDP, cisplatin; HSPA12B, heat shock protein family A member 12B.
Subsequently, A549/HSPA12B+/+ and A549/pcDNA3.1 cells were exposed to in vitro treatment with 1, 2, 4, and 8 µg/ml CDDP. At 24, 48 and 72 h following treatment, a CCK-8 assay was employed to evaluate cell viability. As demonstrated in Fig. 1B-G, the responses of HSPA12B-overexpressing cells to CDDP treatment were inhibited in a dose- and time-dependent manner after 24, 48 and 72 h. The results also indicated that HSPA12B overexpression led to significant increases in the cell viability of A549 cells in response to CDDP treatment compared with the cells transfected with the empty pcDNA3.1 vector (Fig. 1B-G), suggesting an increased resistance to CDDP in A549 cells overexpressing HSPA12B.
HSPA12B overexpression increased the resistance to CDDP in NSCLC cells in vivo
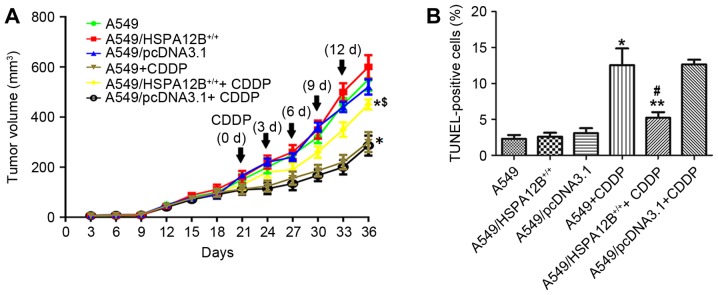
According to the in vitro experiments of HSPA12B in CDDP resistance, whether HSPA12B affects CDDP resistance in vivo was additionally examined. A549, A549/HSPA12B+/+ and A549/pcDNA3.1 cells were injected subcutaneously into the right flanks of nude mice. As indicated in Fig. 2A, it was initially revealed that CDDP treatment significantly inhibited tumor growth in mice injected with A549 as compared with control mice. CDDP treatment significantly inhibited tumor growth in mice injected with A549/pcDNA3.1 cells and untransfected A549 cells compared with mice injected with A549/HSPA12B+/+ cells. In mice treated with CDDP, the A549 cell tumors reached a volume of 300±40 mm3 at 36 days post-treatment, which was significantly smaller compared with the A549/HSPA12B+/+ cell tumors (450±20 mm3) at 36 days after the initial injection of tumor cells. In mice that did not receive CDDP treatment, no significant difference in growth inhibition was observed in the A549/HSPA12B+/+ tumors compared with the control groups.
Figure 2.
HSPA12B overexpression significantly increased resistance to CDDP in non-small cell lung cancer cells in vivo. (A) CDDP significantly inhibited the tumor growth in mice injected with A549/pcDNA3.1 cells and untransfected A549 cells compared with the mice injected with A549/HSPA12B+/+ cells. (B) HSPA12B overexpression inhibited CDDP-induced apoptosis in A549/HSPA12B+/+ cells in vivo. Tumor sections prepared from the groups were stained with the TUNEL agent to detect apoptotic cells. Data are presented as the mean ± standard deviation. *P<0.05 vs. A549; **P<0.05 vs. A549+CDDP; #P<0.05 vs. A549/pcDNA3.1+CDDP. CDDP, cisplatin; HSPA12B, heat shock protein family A member 12B; TUNEL, terminal deoxynucleotidyl-transferase dUTP nick end labeling.
Tumor sections prepared from the groups were stained with the TUNEL agent to detect apoptotic cells. The results in Fig. 2B demonstrated that there were more apoptotic cells in the control group tumors (A549 and A549/pcDNA3.1) treated with CDDP compared with the control tumors without CDDP treatment. However, fewer apoptotic cells were observed in the A549/HSPA12B+/+ tumors treated with CDDP compared with the A549+CDDP and A549/pcDNA3.1+CDDP control tumors. In the groups that did not receive CDDP treatment, no significant differences in the rate of cell apoptosis were observed among the HSPA12B-overexpression and the control groups (A549, A549/pcDNA3.1). Together, these data indicated that HSPA12B overexpression attenuated CDDP-induced apoptosis in NSCLC cells.
Increased HSPA12B induced chemoresistance in NSCLC cells by modulating the PI3K/Akt/NF-κB signaling pathway and apoptosis-associated proteins
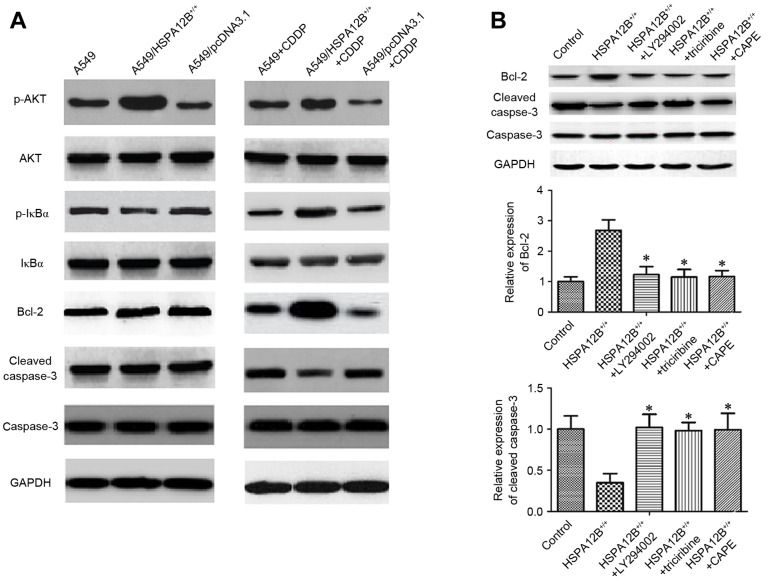
The mechanism responsible for CDDP resistance is associated with the inhibition of the propagation of the DNA damage signal to the apoptotic machinery, including the activation of the PI3K/Akt and its downstream NF-κB pathways, and the overexpression of anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) and interference in caspase activation (5,16). In order to additionally explore the mechanism underlying the inhibitory role of HSPA12B in CDDP-induced apoptosis, associated proteins were investigated by western blotting (Fig. 3A). In the CDDP-treated A549/HSPA12B+/+ solid tumors, the levels of phosphorylated (p-)NF-κB inhibitor α (IκBα), p-Akt and Bcl-2 were all significantly increased, whereas the levels of cleaved caspase-3 were significantly decreased compared with CDDP-treated A549 solid tumors, indicating that HSPA12B overexpression diminished the effect of CDDP on A549 cells. No notable changes in the levels of IκBα, Akt and caspase-3 were observed (Fig. 3A).
Figure 3.
Increased HSPA12B induces chemoresistance in NSCLC cells by modulating PI3K/Akt/NF-κB signaling pathway and apoptosis-associated proteins. (A) In the CDDP-treated A549/HSPA12B+/+ solid tumors, the expression of p-IκBα, p-Akt and Bcl-2 were significantly increased while cleaved caspase-3 expression was significantly decreased compared with CDDP-treated A549 tumors. (B) The PI3K inhibitor LY294002, Akt inhibitor triciribine and NF-κB inhibitor CAPE significantly increased cleaved caspase-3 expression and inhibited the Bcl-2 level in HSPA12B+/+ cells compared with the control groups. Data are presented as the mean ± standard deviation. *P<0.05 vs. HSPA12B+/+. CDDP, cisplatin; HSPA12B, heat shock protein family A member 12B; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; NF-κB nuclear factor-κB; p-, phosphorylated; IκBz, nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor, α; CAPE, caffeic acid phenethyl ester; Bcl-2, B-cell lymphoma 2.
Finally, to verify the mechanism of HSPA12B-induced CDDP resistance, A549/HSPA12B+/+ cells were exposed to the PI3K inhibitor LY294002, the Akt inhibitor Triciribine and the NF-κB inhibitor caffeic acid phenethyl ester (CAPE), and the expression levels of Bcl-2, caspase-3 and cleaved caspase-3 were analyzed. As demonstrated in Fig. 3B, the levels of caspase-3 did not differ significantly amongst all the groups, but LY94002, triciribine and CAPE significantly increased the levels of cleaved caspase-3 and decreased the levels of Bcl-2. These data implicate the PI3K/Akt/NF-κB signaling pathway and apoptosis-associated proteins in HSPA12B-induced CDDP resistance in NSCLC cells.
Discussion
Despite significant advances in oncology over previous decades, lung cancer still has a high mortality rate (1). CDDP is a platinum chemotherapeutic agent and is widely used for the treatment of lung cancer (17). DNA is the primary target of CDDP; CDDP-induced DNA damage results in characteristic cellular changes, including the inhibition of DNA synthesis, suppression of RNA transcription, effects on the cell cycle, and the therapeutically beneficial process of apoptosis (16–18).
Drug resistance to CDDP is a critical problem in the context of cancer treatment, and the mechanism appears to be complex. Cancer cells can develop CDDP resistance through alterations in drug transport systems that lead to decreased intracellular CDDP accumulation; through increased drug detoxification activity due to the elevated levels of intracellular scavengers such as glutathione and/or metallothioneins; through alterations in DNA repair involving increased nucleotide excision repair, inter-strand crosslink repair or loss of mismatch repair; through alterations in DNA damage tolerance mechanisms; and through changes in the apoptotic cell death pathways (6,7). Therefore, novel methods or molecules that may enhance chemosensitivity to CDDP and enable development of novel therapeutic methods to treat NSCLC are required.
The present study investigated the significance of HSPA12B overexpression in CDDP chemosensitivity in the A549 cell line, and the molecular mechanisms underlying the effects of HSPA12B overexpression. Firstly, HSPA12B overexpression was suggested to contribute to CDDP resistance in vitro. CDDP treatment alone significantly inhibited cell growth, whereas CDDP treatment of HSPA12B-overexpressing resulted in significantly attenuated growth inhibition.
Following this, mice with A549/HSPA12B+/+ tumors received doses of CDDP as described. The tumor volumes were monitored during the study period at least twice a week. HSPA12B-overexpression alone was not associated with any significant changes in tumor volume or apoptotic cell numbers compared with the control tumors. Furthermore, significantly decreased tumor volumes and increased levels of apoptotic cells were identified following CDDP treatment. However, HSPA12B overexpression led to a significant attenuation of CDDP-induced inhibition of tumor growth inhibition and CDDP-induced apoptosis.
To the best of our knowledge, the data from the present study represent the first evidence that HSPA12B serves an essential role in CDDP resistance. HSPA12B belongs to the HSP70 family, which is involved in modulating chemosensitivity in various types of cancer cells (10,11). Further investigation in the present study found that HSPA12B overexpression contributes to CDDP resistance via p-IκBα, p-Akt and Bcl-2 upregulation and cleaved caspase-3 downregulation. Consistent with this observation, increased levels of cleaved caspase-3, and decreased Bcl-2 levels were identified in the present study following treatment with PI3K/Akt/NF-κB signaling pathway inhibitors in HSPA12B-expressing cells.
The PI3K/Akt/NF-κB signaling pathway is known to be involved in promoting tumor cell survival, invasive behavior, and chemosensitivity in various malignancies (19). A previous study has demonstrated that CDDP activates p-Akt in A549 cells, and that blockage of the PI3K/Akt pathway with chemical inhibitors moderately sensitizes A549 cells to CDDP-induced apoptosis, and reduces cell viability (20). An additional study indicated that NF-κB was a downstream target of the PI3K/Akt pathway in triptolide-induced apoptosis in MM.1 cells, and that PI3K/Akt may serve a central role in the effect of triptolide on dexamethasone-resistant and -sensitive multiple myeloma cell lines (21). In addition, the anti-apoptotic protein Bcl-2 is well-known to be transcriptionally regulated by NF-κB, and to regulate mitochondria-mediated apoptosis (22). Therefore, it raises the possibility that CDDP primarily modulates the PI3K/Akt pathway, which regulates the NF-κB pathway, which affects Bcl-2 expression, and subsequently the expression of the apoptotic protein cleaved caspase-3 is altered. These alterations thereby regulate NSCLC cell growth and apoptosis.
Strategies for overcoming CDDP resistance include combined treatment with CDDP plus drugs that specifically target cancer cells, combinations of CDDP with compounds that target effectors involved in CDDP resistance, and the development of novel platinating drugs (5). HSPA12B siRNA may be effective in modulating the PI3K/Akt/NF-κB signaling pathway involved in CDDP resistance, providing a potential treatment for NSCLC. However, future clinical trials are required to confirm this conclusion.
To conclude, these data demonstrate that HSPA12B overexpression enhances CDDP resistance through the regulation of p-Akt and p-IκBα in the PI3K/Akt/NF-κB signaling pathway in NSCLC cells. These experimental data support the development of targeted strategies employing HSPA12B siRNA complementary to conventional cytotoxic therapies for NSCLC, which contributes to the formulation of potential therapeutics for improving the current treatment modalities for patients with NSCLC.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: Relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31:992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 3.Ma G, Cai H, Gao L, Wang M, Wang H. sCLU regulates cisplatin chemosensitivity of lung cancer cells in vivo. World J Surg Oncol. 2015;13:80. doi: 10.1186/s12957-015-0501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan JM, Li XD, Liu ZY, Hou GQ, Kang JH, Huang DY, Du SX. Cisplatin induces apoptosis via upregulating Wrap53 in U-2OS osteosarcoma cells. Asian Pac J Cancer Prev. 2011;12:3465–3469. [PubMed] [Google Scholar]
- 5.Koberle B, Tomicic MT, Usanova S, Kaina B. Cisplatin resistance: Preclinical findings and clinical implications. Biochim Biophys Acta. 2010;1806:172–182. doi: 10.1016/j.bbcan.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001;478:23–43. doi: 10.1016/S0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 7.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law S, Wong J. The current management of esophageal cancer. Adv Surg. 2007;41:93–119. doi: 10.1016/j.yasu.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Han Z, Truong QA, Park S, Breslow JL. Two Hsp70 family members expressed in atherosclerotic lesions; Proc Natl Acad Sci USA; 2003; pp. 1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren A, Yan G, You B, Sun J. Down-regulation of mammalian sterile 20-like kinase 1 by heat shock protein 70 mediates cisplatin resistance in prostate cancer cells. Cancer Res. 2008;68:2266–2274. doi: 10.1158/0008-5472.CAN-07-6248. [DOI] [PubMed] [Google Scholar]
- 11.Yoshidomi K, Murakami A, Yakabe K, Sueoka K, Nawata S, Sugino N. Heat shock protein 70 is involved in malignant behaviors and chemosensitivities to cisplatin in cervical squamous cell carcinoma cells. J Obstet Gynaecol Res. 2014;40:1188–1196. doi: 10.1111/jog.12325. [DOI] [PubMed] [Google Scholar]
- 12.Steagall RJ, Hua F, Thirunazukarasu M, Zhan L, Li C, Maulik N, Han Z. Abstract 3600: HspA12B promotes angiogenesis through suppressing AKAP12 and up-regulating VEGF pathway. Circulation. 2008;118(Suppl 18):S449. [Google Scholar]
- 13.Ma H, Lu T, Zhang X, Li C, Xiong J, Huang L, Liu P, Li Y, Liu L, Ding Z. HSPA12B: A novel facilitator of lung tumor growth. Oncotarget. 2015;6:9924–9936. doi: 10.18632/oncotarget.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Liu ZM, Hao FG, Wang M. siRNA-directed clusterin silencing promotes cisplatin antitumor activity in human non-small cell lung cancer xenografts in immunodeficient mice. Eur Rev Med Pharmacol Sci. 2014;18:1595–1601. [PubMed] [Google Scholar]
- 15.Bayne K. Revised guide for the care and use of laboratory animals available. American physiological society. Physiologist. 1996;39:199–208. 111. [PubMed] [Google Scholar]
- 16.Siddik ZH. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 17.Su J, Wu S, Tang W, Qian H, Zhou H, Guo T. Reduced SLC27A2 induces cisplatin resistance in lung cancer stem cells by negatively regulating Bmi1-ABCG2 signaling. Mol Carcinog. 2016;55:1822–1832. doi: 10.1002/mc.22430. [DOI] [PubMed] [Google Scholar]
- 18.Lippard SJ, editor. Platinum, Gold, and Other Metal Chemotherapeutic Agents. Vol. 209. American Chemical Society; Washington, DC: 1983. doi: 10.1021/bk-1983-0209. [DOI] [Google Scholar]
- 19.Azijli K, Weyhenmeyer B, Peters GJ, de Jong S, Kruyt FA. Non-canonical kinase signaling by the death ligand TRAIL in cancer cells: discord in the death receptor family. Cell Death Differ. 2013;20:858–868. doi: 10.1038/cdd.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Liu ZM, Li XC, Yao YT, Yin ZX. Activation of ERK1/2 and Akt is associated with cisplatin resistance in human lung cancer cells. J Chemother. 2013;25:162–169. doi: 10.1179/1973947812Y.0000000056. [DOI] [PubMed] [Google Scholar]
- 21.Yang M, Huang J, Pan HZ, Jin J. Triptolide overcomes dexamethasone resistance and enhanced PS-341-induced apoptosis via PI3k/Akt/NF-kappaB pathways in human multiple myeloma cells. Int J Mol Med. 2008;22:489–496. [PubMed] [Google Scholar]
- 22.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]