Abstract
Integrins are transmembrane glycoproteins that consist of an α and a β subunit. Specific integrin heterodimers preferentially bind to distinct extracellular matrix (ECM) proteins to affect the characteristics of cells or the components of the ECM. Among the different integrins, β1 and β3 integrins serve essential roles in the progression of different cancer-associated processes, including the initiation, proliferation, survival, migration and invasion. Furthermore, previous studies have revealed a ratio between these two integrins in cancer cells, which also demonstrated that the functions of these two integrins are paradoxical. This indicated that the proliferation and metastasis of cancer cells are not always parallel and may be considered independently maintained. Additionally, the present review may assist in understanding certain aspects of cancer, and in making clinical decisions in a novel and more comprehensive manner.
Keywords: β1 integrin, β3 integrin, cancer, proliferation, metastasis, combination
1. Introduction
Integrins are transmembrane glycoproteins that consist of an α subunit and a β subunit. A total of eight different β subunits may dimerize, in limited combinations, with 18α subunits to form ≥24 distinct integrins (1,2). Specific integrin heterodimers preferentially bind to distinct extracellular matrix (ECM) proteins. Integrins may bind the ligands in ECM directly, including fibronectin and laminin, to affect the characteristics of cells or the components of ECM. Regarding cancer cells, integrins serve roles in numerous aspects, including proliferation, survival, migration and invasion (1).
Integrins primarily affect cells in two ways, the first is through binding with proteins directly, including talin, vinculin and filamin, which may regulate the actin cytoskeleton of cells (3). The other is by phosphorylating the relative kinases, including focal adhesion kinases (FAKs), proto-oncogene tyrosine-protein kinase (Src)-family kinases (SFKs) and integrin-linked kinase (ILK), to activate or cooperate with the other cell signaling pathways (1,4). Additionally, integrin clustering on the cell surface and trafficking from the endosomes may affect the ligand affinity and quantity of the protein on cell surface (5–7).
Among the different integrins, β1 and β3 integrins serve essential roles in the progression of different types of cancer (1,2). Furthermore, previous studies investigating the association between these two integrins have demonstrated many different perspectives (8–11), together providing a novel and more comprehensive understanding of cancer.
2. Functions of β1 integrin in cancer
In tumors, the β1 subunit of integrin may combine with different α subunits, including α4, α5 and α2, to affect the characteristics of cancer cells, and the progression of tumors (1). The primary function of β1 integrin is to form focal adhesion between cancer cells and ECM. This adhesion is the basis for the survival of cancer cells and is also associated with their migratory and metastatic capabilities (2). There are series of proteins in the cytoplasm, including talin, kindlin and ILK, which can affect the ligand affinity and activation of integrin, subsequently regulating focal adhesion and various characteristics of cancer cells, including invasion and metastasis (1,3,4). Simultaneously, β1 integrin binding with variant ligands in ECM, including laminin-1 and fibronectin, may induce the secretion of certain cytokines and the progression of tumor (12). Furthermore, a previous study demonstrated that β1 integrin may also affect cell-cell junctions (11). β1 integrin may also affect the function of transforming growth factor-β (TGF-β) and regulate microRNA-200/zinc finger E-box-binding homeobox 2 to facilitate the expression of epithelial (E)-cadherin, which forms cell-cell junctions (9,11).
Proliferation and survival
Regarding numerous different types of cancer cells, the expression of β1 integrin may facilitate the growth of tumors. β1 integrin on the surface of the cells, which does not bind with the ECM, induces integrin-mediated death (IMD) of cells (1). However, when combined with their ligands, this integrin promotes the survival of cancer cells by activating different cell signaling proteins, including phosphoinositide 3-kinase/RAC-α serine/threonine protein kinase (AKT), FAKs and SFKs (1,13). While in certain tumors, β1 integrin may also induce anoikis resistance of cancer cells in suspension by phosphorylating FAKs and AKT (14,15). Additionally, β1 integrin may promote the proliferation of cells by phosphorylating FAKs and regulating SRC/mitogen-activated protein kinase (MAPK) to facilitate the expression of v-myc avian myelocytomatosis viral oncogene homolog (c-Myc) and cyclin D1 in cancer cells (16,17). Additionally, although the characteristics of the association between β1 integrin and cancer stem cells are unclear, a previous study has identified that the expression of β1 integrin in cancer stem cells is upregulated (18).
Metastasis
Regarding metastasis, the effect of β1 integrin is controversial. A previous study suggested that mutant cellular tumor antigen p53 promoted β1 integrin-dependent cell motility and invasion by reusing α5β1 integrin, and epidermal growth factor receptor (EGFR) through recycling endosomes to the tumor cell surface, facilitating the metastasis of cancer cells (19,20). However, this metastasis induced by β1 integrin depends on the expression of EGFR and the phosphorylation of AKT, which is a downstream signaling protein of EGFR (20). Furthermore, in certain types of cancer, the data demonstrated that β1 integrin may activate Src or phosphorylate p38 and AKT to affect urokinase-type plasminogen activator (uPA), and matrix metalloprotease (MMP)-2, promoting the metastasis of cancer cells (21,22). Additionally, due to the lack of vasculature, cancer cells enhance β1 integrin activity to induce vessel cooption by adhering to the vascular basement membrane, thus providing immediate vasculature structures for newly metastatic or locally invasive of cancer cells (23).
In contrast, β1 integrin may cooperate with TGF-β to enhance the expression of E-cadherin and inhibit metastasis in breast cancer (11). Additionally, downregulation of β1 integrin induces not only epithelial-to-mesenchymal transition (EMT) and anokisis resistance in cancer cells, but also the expression of MMP-9, and vascular endothelial growth factor (VEGF), promoting migration and invasion (10).
Prognosis and clinical features
Similar to the controversy concerning metastasis, the associations between β1 integrin and the clinical features of patients are unclear. In certain types of cancer, β1 integrin is associated with poor prognosis or metastasis, including in prostatic cancer (15,24), melanoma (25), gastric carcinoma (26) and hypopharyngeal squamous cell carcinoma (27). However, in other types of cancer, including breast cancer, studies have reported different or contradictory conclusions (28–30). This suggests that additional clinical observations and studies are required to understand this association.
3. Functions of β3 integrin in cancer
Regarding β3 integrin in tumors, the subtype that exhibits the widest range of functions is αvβ3 integrin (1). It is associated with the growth, survival, invasion and metastasis of different cancer cells (10,31–35). Furthermore, in certain types of cancer, it is an indicator of increased lymph node or bone metastasis and decreased patient survival (36–38).
Similar to β1 integrin, β3 integrin may also bind with the components of ECM to form focal adhesions between cancer cells and the ECM. Concomitantly, in suspension, β3 integrin prevents cancer cells from IMD by activating a non-canonical FAK-independent signaling pathway (31). Additionally, cancer cells as well as somatic cells, including endothelial cells, have been demonstrated to affect the growth of tumors by regulating angiogenesis (33).
Tumor growth and initiation
Regarding tumor growth, β3 integrin serves roles in cancer cell survival, tumor initiation and tumor stemness, primarily by regulating cytokines (31,34).
A previous study demonstrated that β3 integrin was associated with cancer stem cells (39). An additional study identified that, mechanistically, αvβ3 integrin in the unbound state recruits GTPase KRas and Ras-related protein Ral-B to the tumor cell plasma membrane, leading to the activation of TANK binding kinase 1 and nuclear factor-κB (34). These two proteins are necessary and sufficient for tumor initiation, anchorage independence, and self-renewal (34). Additionally, in the β3 signaling pathway, the receptor tyrosine kinase (RTK) is unnecessary for the survival of cancer cells; therefore, cancer cells may survive using β3 integrin, without RTK, which will induce resistance to RTK inhibition therapy (34). Furthermore, in a suspension of pancreatic cancer cells, β3 integrin was revealed to activate Src directly without phosphorylating FAKs, to facilitate the survival of tumor cells (31). Simultaneously, β3 integrin in endothelial cells can affect the growth of tumors by regulating angiogenesis (33). A study reported that β3 integrin decreases the expression of VEGF receptor 2 (VEGFR2), thus inhibiting VEGF/VEGFR-induced angiogenesis and tumor growth (33). These results suggest that β3 integrin exhibits the ability to regulate RTK; however, the features of the association remain unclear (40).
EMT and metastasis
EMT affects the metastasis of tumors in different ways, including through migration, invasion and adhesion. β3 integrin has been demonstrated to be associated with EMT in cancer cells. The integrin cooperates with TGF-β to form β3 integrin-TGF-β receptor (TβR) type II complexes, which may activate TβR-II through the β3 integrin/SRC signaling pathway and induce EMT by activating MAPKs (32). Furthermore, β3 integrin elevates the expression of MMP-9 and VEGF in cancer cells, contributing to autocrine TGF-β signaling, and activation of EMT processes (10). Additionally, αvβ3 integrin facilitates FAKs in regulating actin cytoskeleton remodeling and the induction of EMT (41). Concomitantly, β3 integrin has the ability to activate canonical FAKs-dependent cytokines to affect cell migration and invasion (35). In addition, β3 integrin is able to contribute to anchorage independence, thus facilitating cancer cell survival and increasing tumor malignancy, including lymph node metastasis (31). However, due to the co-expression of αv and β3 subunits in cancer cells, future studies are warranted to identify which of these factors are essential for specific characteristics of cancer cells.
4. Ratio between β1 and β3 integrins in cancer cells
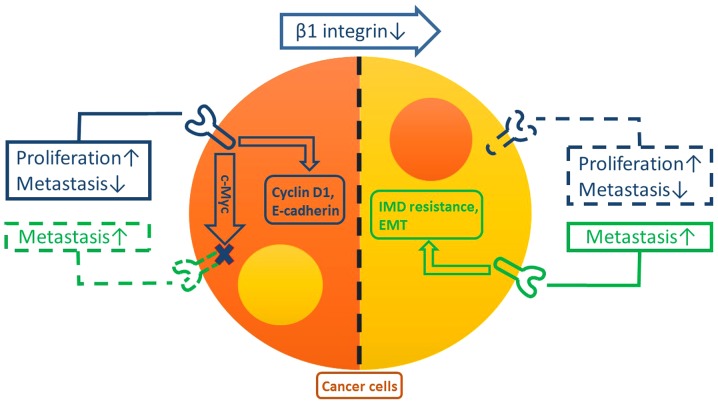
Regarding cancer cells, the expression levels of β1 integrin and β3 integrin are associated; and changes to either integrin exhibit significant effects on cancer cells (9–11) (Fig. 1). In breast cancer, the inactivation of β1 integrin elicits the robust compensatory expression of β3 integrin (10,11). However, the inhibition of β1 integrin cannot induce the compensatory β3 integrin expression in normal mammary epithelial cells (10). Furthermore, this compensatory β3 integrin expression is essential for the growth and metastasis of tumors (10). A previous study demonstrated that when downregulating the expression of β1 and β3 integrins simultaneously, cancer cell survival was reduced (11). This suggests that, perhaps, when β1 integrin is inactivated, the overexpression of β3 integrin is necessary and important to maintain the survival, and characteristics of cancer cells.
Figure 1.
Functions of, and the association between, β1 and β3 integrins in cancer cells. β1 integrin (blue) may facilitate cyclin D1 and E-cadherin in promoting proliferation and reducing the rate of metastasis in cancer cells, respectively. Concurrently, β1 integrin inhibits the expression of β3 integrin (green) in cancer cells, probably by the regulation of c-Myc. However, reductions in levels of β1 integrin induce the compensatory expression of β3 integrin, which promotes EMT and IMD resistance in cancer cells, resulting in metastasis. EMT, epithelial-mesenchymal transition; IMD, integrin-mediated death; E-cadherin, epithelial cadherin; c-Myc, v-myc avian myelocytomatosis viral oncogene homolog.
In addition, this ratio between β1 and β3 integrin activity is not only involved for maintaining the functions of cancer cells, but also for changing the survival and metastasis rates of tumors. In pancreatic cancer, β1 integrin is able facilitate the growth of tumors, and its inhibition reduces the proliferation of cancer cells (11). Additionally, the inhibition of β1 integrin may affect the function of TGF-β, therefore attenuating the expression of E-cadherin to reduce the activity of the cell-cell junctions and enhancing motility and migration (11). Nonetheless, whether β3 integrin is involved in these processes is controversial (10,11). In contrast, β3 integrin can induce EMT (9–11) and activate a non-canonical FAKs-independent signaling pathway, thus preventing cancer cells from undergoing IMD (31), and promoting the metastasis of cancer cells. Therefore, according to these studies, the functions of β1 integrin are the promotion of proliferation and inhibition of metastasis, and the functions of β3 integrin are opposing. This indicates that proliferation and metastasis of cancer cells are not always parallel, and may be considered independently maintained. Furthermore, the association between clinical features, including prognosis, and these two integrins, is complicated and paradoxical.
5. Targeting therapies
The expression of β1 and β3 integrins in cancer is involved in tumor progression, and various other pathways, which suggests that they are potential therapeutic targets. In preclinical studies, the antagonists of β1 or β3 integrin effectively inhibited tumor growth by affecting tumor cells and tumor-associated host cells (33,40,42–45). These antagonists include monoclonal antibodies and arginylglycylaspartic acid (RGD) peptide mimetics, which mimic the structure of the RGD sequence in the ligands, and inhibit the binding of integrins with their ligands. Furthermore, certain antagonists have been demonstrated to be effective in clinical trials (1).
Cilengitide, a RGD peptide mimetic, inhibits the function of αvβ3 integrin and lengthens the survival time of patients with certain types of cancer with minimal side effects in clinical trials (46,47). Nevertheless, in vivo, specific studies identified that the continuous infusion of low doses of RGD peptides stimulates tumor growth and angiogenesis by increasing VEGFR2 recycling to the endothelial cells membrane, and promoting VEGF-induced migration (33,40). The angiogenesis of tumors may increase the delivery of chemotherapeutic agents to the target areas, which may explain why the combination of cilengitide and chemotherapy is more effective compared with chemotherapy alone (1).
Regarding monoclonal antibodies, etaracizumab, a function-blocking monoclonal antibody of αvβ3 integrin, has demonstrated anti-angiogenic activity, direct inhibition of the tumor cell growth and a reduction in bone metastasis rates in preclinical studies (43,45). In clinical trials, etaracizumab also demonstrated antiangiogenic activity, low toxicity and disease stabilization in patients with certain types of cancer (48,49). In addition, in preclinical and clinical trials, volociximab, a function-blocking monoclonal antibody against integrin α5β1, has exhibited efficacy in inhibiting angiogenesis and tumor growth (42,50).
In specific previous studies, variant peptide antagonists have been developed. For example, ATN-161 is a non-RGD-based peptide inhibitor of integrin α5β1 that inhibits cancer growth and metastasis in vivo (44). It also prolongs disease stabilization in patients with advanced solid tumors (51).
6. Future perspectives
Certain previous studies have demonstrated that the ratio between β1 and β3 integrins activity is associated with specific important cytokines in cancer cells (8,10,17). A previous study has also suggested that bound β1 integrin may activate Src and extracellular signal-related kinase 1/2 MAPK in mammary epithelial cells, which induces the overexpression of c-Myc (17). An additional previous study revealed that MYC repressed transcription of the two subunits of αvβ3 integrin, thus suppressing cancer metastasis in breast cancer cells (8). However, the lack of definitive data makes identifying the association between MYC, and the ratio between β1 and β3 integrins in cancer cells difficult. The details of the association between these two integrins require additional study.
Regarding clinical features, due to the association between these two integrins, relying on one of them to evaluate the status of patient is not comprehensive. The combination of β1 and β3 integrins for prognosis and treatment is necessary. In addition, with reference to previous studies, targeted therapy also requires the combination of these two targets, or a focus on the common downstream cytokines, including FAKs and SFKs, to increase effectiveness.
7. Conclusion
β1 and β3 integrins are essential focal adhesion proteins in various cancer cell types, which may affect the initiation, proliferation, survival and metastasis of tumors. Previous studies have demonstrated a ratio between these two integrins in cancer cells, with contradictory functions. This indicates that perhaps the proliferation and metastasis of cancer cells are not always parallel; therefore, may be considered independently maintained. Furthermore, the association between clinical features and these integrins is more complicated than previously expected. Therefore, there is a requirement for additional clinical and experimental studies to elucidate the role of these notable proteins.
Acknowledgements
The present study was supported by the National High-tech R&D Program (863 Program; grant no. 2014AA020609).
References
- 1.Desgrosellier JS, Cheresh DA. Integrins in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howe GA, Addison CL. β1 integrin: An emerging player in the modulation of tumorigenesis and response to therapy. Cell Adh Migr. 2012;6:71–77. doi: 10.4161/cam.20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: Partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legate KR, Montañez E, Kudlacek O, Fässler R. ILK, PINCH and parvin: The tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 5.Ewald AJ, Egeblad M. Cancer: Sugar-coated cell signalling. Nature. 2014;511:298–299. doi: 10.1038/nature13506. [DOI] [PubMed] [Google Scholar]
- 6.Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Hudak JE, Lakins JN, Wijekoon AC, Cassereau L, et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511:319–325. doi: 10.1038/nature13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reverter M, Rentero C, Garcia-Melero A, Hoque M, Vilà de Muga S, Alvarez-Guaita A, Conway JR, Wood P, Cairns R, Lykopoulou L, et al. Cholesterol regulates Syntaxin 6 trafficking at trans-Golgi network endosomal boundaries. Cell Rep. 2014;7:883–897. doi: 10.1016/j.celrep.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Radisky DC, Yang D, Xu R, Radisky ES, Bissell MJ, Bishop JM. MYC suppresses cancer metastasis by direct transcriptional silencing of αv and β3 integrin subunits. Nat Cell Biol. 2012;14:567–574. doi: 10.1038/ncb2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madamanchi A, Zijlstra A, Zutter MM. Flipping the switch: Integrin switching provides metastatic competence. Sci Signal. 2014;7:pe9. doi: 10.1126/scisignal.2005236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parvani JG, Galliher-Beckley AJ, Schiemann BJ, Schiemann WP. Targeted inactivation of β1 integrin induces β3 integrin switching, which drives breast cancer metastasis by TGF-β. Mol Biol Cell. 2013;24:3449–3459. doi: 10.1091/mbc.E12-10-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truong HH, Xiong J, Ghotra VP, Nirmala E, Haazen L, Le Dévédec SE, Balcioğlu HE, He S, Snaar-Jagalska BE, Vreugdenhil E, et al. β1 integrin inhibition elicits a prometastatic switch through the TGFβ-miR-200-ZEB network in E-cadherin-positive triple-negative breast cancer. Sci Signal. 2014;7:ra15. doi: 10.1126/scisignal.2004751. [DOI] [PubMed] [Google Scholar]
- 12.Grzesiak JJ, Smith KC, Burton DW, Deftos LJ, Bouvet M. Integrin-mediated laminin-1 adhesion upregulates CXCR4 and IL-8 expression in pancreatic cancer cells. Surgery. 2007;141:804–814. doi: 10.1016/j.surg.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 14.Jin JK, Tien PC, Cheng CJ, Song JH, Huang C, Lin SH, Gallick GE. Talin1 phosphorylation activates β1 integrins: A novel mechanism to promote prostate cancer bone metastasis. Oncogene. 2015;34:1811–1821. doi: 10.1038/onc.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YC, Jin JK, Cheng CJ, Huang CF, Song JH, Huang M, Brown WS, Zhang S, Yu-Lee LY, Yeh ET, et al. Targeting constitutively activated β1 integrins inhibits prostate cancer metastasis. Mol Cancer Res. 2013;11:405–417. doi: 10.1158/1541-7786.MCR-12-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartolome RA, Barderas R, Torres S, Fernandez-Aceñero MJ, Mendes M, García-Foncillas J, Lopez-Lucendo M, Casal JI. Cadherin-17 interacts with α2β1 integrin to regulate cell proliferation and adhesion in colorectal cancer cells causing liver metastasis. Oncogene. 2014;33:1658–1669. doi: 10.1038/onc.2013.117. [DOI] [PubMed] [Google Scholar]
- 17.Benaud CM, Dickson RB. Regulation of the expression of c-Myc by beta1 integrins in epithelial cells. Oncogene. 2001;20:759–768. doi: 10.1038/sj.onc.1204152. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, He J, Liu Y, Simeone DM, Lubman DM. Identification of glycoprotein markers for pancreatic cancer CD24+CD44+ stem-like cells using nano-LC-MS/MS and tissue microarray. J Proteome Res. 2012;11:2272–2281. doi: 10.1021/pr201059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, et al. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327–1341. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Selivanova G, Ivaska J. Integrins and mutant p53 on the road to metastasis. Cell. 2009;139:1220–1222. doi: 10.1016/j.cell.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Ashour AA, Gurbuz N, Alpay SN, Abdel-Aziz AA, Mansour AM, Huo L, Ozpolat B. Elongation factor-2 kinase regulates TG2/β1 integrin/Src/uPAR pathway and epithelial-mesenchymal transition mediating pancreatic cancer cells invasion. J Cell Mol Med. 2014;18:2235–2251. doi: 10.1111/jcmm.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang WS, Chin CC, Chen CN, Kuo YH, Chen TC, Yu HR, Tung SY, Shen CH, Hsieh YY, Guo SE, et al. Stromal cell-derived factor-1/CXC receptor 4 and β1 integrin interaction regulates urokinase-type plasminogen activator expression in human colorectal cancer cells. J Cell Physiol. 2012;227:1114–1122. doi: 10.1002/jcp.22831. [DOI] [PubMed] [Google Scholar]
- 23.Jahangiri A, Aghi MK, Carbonell WS. β1 integrin: Critical path to antiangiogenic therapy resistance and beyond. Cancer Res. 2014;74:3–7. doi: 10.1158/0008-5472.CAN-13-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pontes-Junior J, Reis ST, Bernardes FS, Oliveira LC, Barros ÉA, Dall'Oglio MF, Timosczuk LM, Ribeiro-Filho LA, Srougi M, Leite KR. Correlation between β1 integrin expression and prognosis in clinically localized prostate cancer. Int Braz J Urol. 2013;39:335–343. doi: 10.1590/S1677-5538.IBJU.2013.03.06. [DOI] [PubMed] [Google Scholar]
- 25.Danen EH, Ten Berge PJ, Van Muijen GN, Van't Hof-Grootenboer AE, Bröcker EB, Ruiter DJ. Emergence of alpha 5 beta 1 fibronectin- and alpha v beta 3 vitronectin-receptor expression in melanocytic tumour progression. Histopathology. 1994;24:249–256. doi: 10.1111/j.1365-2559.1994.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka T, Yashiro M, Nishimura S, Inoue T, Fujihara T, Sawada T, Kato Y, Seki S, Hirakawa-Ys Chung K. Increased expression of alpha2beta1-integrin in the peritoneal dissemination of human gastric carcinoma. Int J Mol Med. 2000;5:21–25. doi: 10.3892/ijmm.5.1.21. [DOI] [PubMed] [Google Scholar]
- 27.Hong YM, Gan WG, Xu ZH. Significance of the expression of integrin β1, VEGF and MVD in hypopharyngeal squamous cell carcinoma. Genet Mol Res. 2014;13:6455–6465. doi: 10.4238/2014.August.25.9. [DOI] [PubMed] [Google Scholar]
- 28.dos Santos PB, Zanetti JS, Ribeiro-Silva A, Beltrão EI. Beta 1 integrin predicts survival in breast cancer: A clinicopathological and immunohistochemical study. Diagn Pathol. 2012;7:104. doi: 10.1186/1746-1596-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez MA, Pinder SE, Wencyk PM, Bell JA, Elston CW, Nicholson RI, Robertson JF, Blamey RW, Ellis IO. An immunohistochemical examination of the expression of E-cadherin, alpha- and beta/gamma-catenins, and alpha2- and beta1-integrins in invasive breast cancer. J Pathol. 1999;187:523–529. doi: 10.1002/(SICI)1096-9896(199904)187:5<523::AID-PATH296>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Restucci B, De Vico G, Maiolino P. Expression of beta 1 integrin in normal, dysplastic and neoplastic canine mammary gland. J Comp Pathol. 1995;113:165–173. doi: 10.1016/S0021-9975(05)80031-3. [DOI] [PubMed] [Google Scholar]
- 31.Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prévost N, Tarin D, Shattil SJ, Cheresh DA. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. 2009;15:1163–1169. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galliher AJ, Schiemann WP. Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 34.Seguin L, Kato S, Franovic A, Camargo MF, Lesperance J, Elliott KC, Yebra M, Mielgo A, Lowy AM, Husain H, et al. An integrin β3 KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol. 2014;16:457–468. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]
- 36.Hosotani R, Kawaguchi M, Masui T, Koshiba T, Ida J, Fujimoto K, Wada M, Doi R, Imamura M. Expression of integrin alphaVbeta3 in pancreatic carcinoma: Relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25:e30–e35. doi: 10.1097/00006676-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 37.McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–6243. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 40.Robinson SD, Hodivala-Dilke KM. The role of β3-integrins in tumor angiogenesis: Context is everything. Curr Opin Cell Biol. 2011;23:630–637. doi: 10.1016/j.ceb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Shah PP, Fong MY, Kakar SS. PTTG induces EMT through integrin αVβ3-focal adhesion kinase signaling in lung cancer cells. Oncogene. 2012;31:3124–3135. doi: 10.1038/onc.2011.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Besse B, Tsao LC, Chao DT, Fang Y, Soria JC, Almokadem S, Belani CP. Phase Ib safety and pharmacokinetic study of volociximab, an anti-α5β1 integrin antibody, in combination with carboplatin and paclitaxel in advanced non-small-cell lung cancer. Ann Oncol. 2013;24:90–96. doi: 10.1093/annonc/mds281. [DOI] [PubMed] [Google Scholar]
- 43.Gramoun A, Shorey S, Bashutski JD, Dixon SJ, Sims SM, Heersche JN, Manolson MF. Effects of Vitaxin, a novel therapeutic in trial for metastatic bone tumors, on osteoclast functions in vitro. J Cell Biochem. 2007;102:341–352. doi: 10.1002/jcb.21296. [DOI] [PubMed] [Google Scholar]
- 44.Khalili P, Arakelian A, Chen G, Plunkett ML, Beck I, Parry GC, Doñate F, Shaw DE, Mazar AP, Rabbani SA. A non-RGD-based integrin binding peptide (ATN-161) blocks breast cancer growth and metastasis in vivo. Mol Cancer Ther. 2006;5:2271–2280. doi: 10.1158/1535-7163.MCT-06-0100. [DOI] [PubMed] [Google Scholar]
- 45.Mulgrew K, Kinneer K, Yao XT, Ward BK, Damschroder MM, Walsh B, Mao SY, Gao C, Kiener PA, Coats S, et al. Direct targeting of alphavbeta3 integrin on tumor cells with a monoclonal antibody, Abegrin. Mol Cancer Ther. 2006;5:3122–3129. doi: 10.1158/1535-7163.MCT-06-0356. [DOI] [PubMed] [Google Scholar]
- 46.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O'Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ, Rich KM, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 47.Vansteenkiste J, Barlesi F, Waller CF, Bennouna J, Gridelli C, Goekkurt E, Verhoeven D, Szczesna A, Feurer M, Milanowski J, et al. Cilengitide combined with cetuximab and platinum-based chemotherapy as first-line treatment in advanced non-small-cell lung cancer (NSCLC) patients: Results of an open-label, randomized, controlled phase II study (CERTO) Ann Oncol. 2015;26:1734–1740. doi: 10.1093/annonc/mdv219. [DOI] [PubMed] [Google Scholar]
- 48.Delbaldo C, Raymond E, Vera K, Hammershaimb L, Kaucic K, Lozahic S, Marty M, Faivre S. Phase I and pharmacokinetic study of etaracizumab (Abegrin), a humanized monoclonal antibody against alphavbeta3 integrin receptor, in patients with advanced solid tumors. Invest new Drugs. 2008;26:35–43. doi: 10.1007/s10637-007-9077-0. [DOI] [PubMed] [Google Scholar]
- 49.Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: A humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- 50.Bhaskar V, Zhang D, Fox M, Seto P, Wong MH, Wales PE, Powers D, Chao DT, Dubridge RB, Ramakrishnan V. A function blocking anti-mouse integrin alpha5beta1 antibody inhibits angiogenesis and impedes tumor growth in vivo. J Transl Med. 2007;5:61. doi: 10.1186/1479-5876-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cianfrocca ME, Kimmel KA, Gallo J, Cardoso T, Brown MM, Hudes G, Lewis N, Weiner L, Lam GN, Brown SC, et al. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. Br J Cancer. 2006;94:1621–1626. doi: 10.1038/sj.bjc.6603171. [DOI] [PMC free article] [PubMed] [Google Scholar]