Abstract
MiR-216a-5p has been acknowledged as an oncogene and is known to be involved in the progression and metastasis of numerous cancer subtypes. However, the potential role of miR-216a-5p in renal cell carcinoma (RCC) remains to be elucidated. In the present study, reverse transcription-quantitative polymerase chain reaction was performed to detect the expression levels of miR-216a-5p in RCC tissues. Cell counting kit-8, MTT, wound scratch, Transwell and flow cytometric assays were performed to establish the biological functions of miR-216a-5p in RCC. Functional experiments demonstrated that the expression of miR-216a-5p was upregulated in RCC (P<0.05) and miR-216a-5p mimics promoted cellular proliferation, viability and motility, and suppressed apoptosis. Conversely, miR-216a-5p inhibitor suppressed cellular proliferation, viability, motility and induced apoptosis. Based on these findings, it was concluded that miR-216a-5p may function as an oncogene in RCC. MiR-216a-5p target genes need to be explored and the potential of miR-216a-5p to be used as a diagnostic or a prognostic biomarker for RCC needs to be validated by future research.
Keywords: microRNA, miR-216a-5p, renal cell carcinoma, oncogene
Introduction
Renal cell carcinoma (RCC), the most common tumor of the kidney, accounts for about 3% of adult malignancies (1). Approximately 30% of patients are diagnosed with metastatic RCC when the disease is discovered and the 5-year survival rate is estimated to be ~8% (2). Nearly 80% of RCC is clear cell RCC (ccRCC), which is characterized by frequent genetic mutations of the von Hippel Lindau (VHL) (3,4). Patients with metastatic or recurrent RCC are frequently treated by mTOR inhibitors or receptor tyrosine kinase (RTK), which delays the progression of cancer rather than curing cancer (5,6). Surgical therapy is the only definitive treatment when RCC is resistant to chemotherapy (7), besides, approximately 20–40% patients still experience recurrence after the resection (8).
microRNAs (miRNAs, miRs) belong to a group of short, non-coding RNAs that are 19–22 bases long and mediate translational repression and/or RNA degradation by binding to the 3′ untranslated region of the messenger RNA (mRNA) (9). miRNAs are involved in cellular proliferation, differentiation and apoptosis by regulating 60% of the protein-coding genes in the human genome (10). miRNAs are aberrantly expressed in multiple human cancers and stimulate inappropriate cellular programs such as invasion and metastasis (11–13). As aberrantly expressed miRNAs are closely related to tumor initiation and progression (13–15), it is important to elucidate the function of aberrantly expressed miRNAs.
Previous studies have demonstrated that miR-216a-5p is dysregulated in a number of malignancies, such as colorectal cancer, pancreatic cancer, prostate cancer and liver cancer (16–19). However, the expression of miR-216a-5p requires quantification and the role of miR-216a-5p in RCC has not been investigated to date. In the present study, we investigate the expression of miR-216a-5p in RCC tissues and cells lines, and explore the effects of miR-216a-5p on cellular proliferation, viability, motility and apoptosis by wound scratch assay, Transwell assay, MTT assay, CCK-8 assay and flow cytometry assay.
Materials and methods
Sample collection
A total of 24 RCC and matched normal tissues, resected at Peking University Shenzhen Hospital (Shenzhen, China) and reviewed by hematoxylin and eosin staining, were collected for reverse transcription-quantitative polymerase chain reaction (RT-qPCR). After resection, the specimens were immersed in RNA later (Qiagen GmbH, Hilden, Germany) for 30 min and stored at −80°C for further use. Additionally, ethics committees of Peking University Shenzhen Hospital approved this study and written informed consent for participation was provided by all patients. Clinical and pathological characteristics of these twenty-four patients are listed in Table I.
Table I.
Clinicopathological features of patients with renal cell carcinoma.
| Characteristic | Number of patients |
|---|---|
| Mean age, range (years) | 48 (24–63) |
| Males | 8 |
| Females | 16 |
| Histological type | |
| Clear cell | 21 |
| Papillary | 3 |
| pT-stage | |
| T1 | 15 |
| T2 | 7 |
| T3 + T4 | 2 |
| Fuhrmann grade | |
| I | 5 |
| II | 16 |
| III | 2 |
| IV | 1 |
| AJCC clinical stage | |
| I | 7 |
| II | 15 |
| III + IV | 2 |
pT, primary tumor; AJCC, American Joint Committee on Cancer.
Cell culture
The present study used 293T, ACHN and 786-O RCC cell lines, cultured in Dulbecco's modified Eagle's medium (DMEM basic; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 1% penicillin streptomycin (pen strep; Gibco) and 1% glutamine (Gibco) in a humidified incubator containing 5% CO2 at 37°C.
RNA extraction, cDNA synthesis and RT-qPCR
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract total RNA, which was then was purified with the RNeasy Maxi kit (Qiagen GmbH). NanoDrop 2000/2000c (Thermo Fisher Scientific, Inc.) was used to determine the concentration of RNA. To obtain the cDNA templates, 1 µg total RNA of each sample was used for reverse transcription using miScript Reverse Transcription kit (Qiagen GmbH), and the temperature for the reverse transcription reaction was 37°C for 60 min, 95°C for 5 min and storage at 4°C. To detect the expression level of miR-216a-5p, qPCR was performed with miScript SYBR®-Green PCR kit (Qiagen GmbH) on the Roche Lightcycler 480 Real-Time PCR system (Roche Diagnostics, Basel, Switzerland). The temperature for qPCR was 95°C for 1 min, 40 cycles of 95°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec. In addition, the sequences of the primers of miR-216a-5p and internal control (U6), used in PCR, are indicated in Table II. The expression of miR-216-5p was analyzed with the 2−ΔΔCq method (20).
Table II.
Sequences of primers and microRNAs.
| Primer/microRNA | Sequence (5′-3′) |
|---|---|
| miR-216a-5p | |
| F | TAATCTCAGCTGGCAACTGTGA |
| R | Provided by the miScript SYBR®-Green |
| U6 | |
| F | CTCGCTTCGGCAGCACA |
| R | ACGCTTCACGAATTTGCGT |
| miR-216a-5p mimics | |
| F | UAAUCUCAGCUGGCAACUGUGA |
| R | ACAGUUGCCAGCUGAGAUUAUU |
| NC | |
| F | UUCUCCGAACGUGUCACGUTT |
| R | ACGUGACACGUUCGGAGAATT |
| miR-216a-5p inhibitor | UCACAGUUGCCAGCUGAGAUUA |
| NC inhibitor | CAGUACUUUUGUGUAGUACAA |
miR, microRNA; NC, negative control
Cell transfection
The synthesized miR-216a-5p mimics (GenePharma, Shanghai, China) were transfected into the 786-O and ACHN cells to upregulate the expression levels of miR-216a-5p. Similarly, synthesized miR-216a-5p inhibitor (GenePharma) was transfected into the 786-O and ACHN cells to decrease the cellular levels of active miR-216a-5p. Lipofectamine 2000 (Invitrogen), mixed in the Opti-MEM® I Reduced Serum Medium (Gibco), was used for transfection. RT-qPCR was performed to analyze the changes of miR-216a-5p expression after transfection.
Wound scratch assay
The cell migration of 786-O and ACHN cells was assessed by a wound scratch assay. Each well of the 12-well plate was seeded with ~300,000 cells and the cells were incubated for 24 h. Then Lipofectamine® 2000 was used for transfection of cells with 40 pmol of miR-216a-5p mimics, negative control (NC), inhibitors or inhibitor NC. A scratch was introduced in the cell monolayer with a 200 µl pipette tip 6 h after transfection. The initial images of the scratch at 0 h and the residual length of the scratch at 12 h were captured by a digital camera system. At least three pictures were taken for each experiment. Each experiment was performed in triplicates and repeated at least three times.
Transwell assay
The migratory and invasive ability of 786-O and ACHN cells were assessed by Transwell assay. Transwell chamber inserts (BD Biosciences, Franklin Lakes, NJ, USA) with or without Matrigel (for invasion) were used to analyze cell migration and invasive ability respectively. In total, ~1×104 transfected cells were seeded in the upper compartment of the chamber with 200 µl serum-free medium, while medium with 10% FBS was added to the lower chamber. Following migration for 36 h, enough cells migrated to the bottom of chamber, and the invasion time was 48 h. A microscope was used for counting the crystal violet stained migrated cells.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT assay)
The viability of 786-O and ACHN cells were assessed by MTT assay. Each well of the 96-well plate was seeded with ~5,000 cells. The cells were transfected with 5 pmol miR-216a-5p mimics, NC, inhibitors and inhibitor NC. A total of 4 days after transfection, 20 µl MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well and incubated for 4 h. The medium with MTT was replaced by 100 µl dimethyl sulfoxide (Sigma-Aldrich; Merck Millipore). Subsequently, the 96-well plate was shaken using a by reciprocating decolorization shaking table (TSB-108; Qilinbeier, Jiangsu, China) for 10 min in a dark room and the optical density (OD) was determined by an ELISA microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 595 nm. MTT assay was performed in triplicates and repeated at least three times.
Cell counting kit-8 assay (CCK-8 assay)
The proliferative ability of 786-O and ACHN cells were assessed by CCK-8 (Beyotime Institute of Biotechnology, Haimen, China). The 96-well plate with ~5,000 cells/well was transfected with 5 pmol miR-216a-5p mimics, NC, inhibitors and inhibitor NC after 24 h. At t 0, 24, 48 and 72 h after transfection, 10 µl of CCK-8 was added to each well for 30 min and the OD at a wavelength of 490 nm was recorded using an ELISA microplate reader. CCK-8 assay was performed in triplicates and repeated at least three times.
Flow cytometry assay
The apoptotic rates of 786-O and ACHN cells were assessed by flow cytometry assay. Approximately 3×105 cells transfected with 200 pmol miR-216a-5p mimics, NC, inhibitors or inhibitor NC were harvested from a 6-well plate and the rates of apoptosis were detected by Annexin V-FITC apoptosis detection kit (Invitrogen). The cells were mixed with 100 µl 1X binding buffer and stained with 5 µl Annexin V-fluorescein isothiocyanate and 5 µl propidium iodide in a dark at room temperature. After 15 min, 400 µl 1X binding buffer was added to each cell suspension. The apoptotic rate of each cell suspension was analyzed by flow cytometry (EPICS, Xl-4; Beckman Coulter, Inc., Brea, CA, USA). Flow cytometry assay was performed in triplicates and repeated at least three times.
Statistical analysis
All data for expression levels of miR-216a-5p in matched tumor/normal tissues and different cells were analyzed by paired t-tests. However, relative expression of miR-216a-5p in cells was analyzed by one-way analysis of variance followed by Dunnett's test. All data characterizing the phenotypes of cells were analyzed by Student's t-test using SPSS 19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered as statistically significant difference.
Results
MiR-216a-5p is upregulated in RCC tissues and cell lines
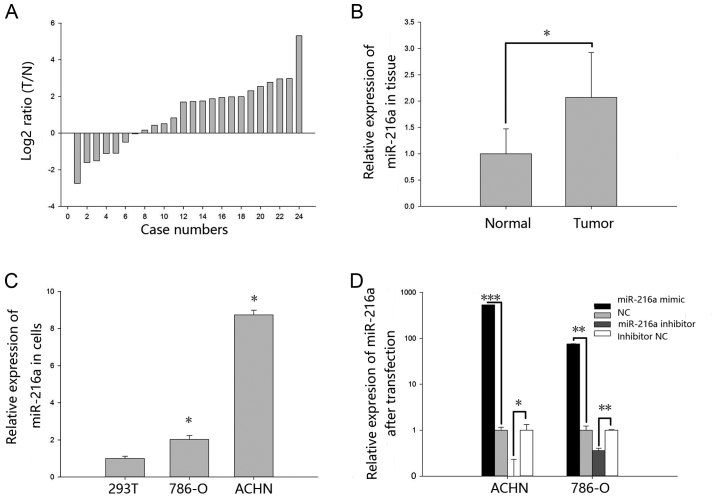
The expression levels of miR-216a-5p in 24 paired RCC tissues and cell lines were explored by RT-qPCR. As shown in Fig. 1A, miR-216a-5p was upregulated in the RCC tissues. Relative expression of miR-216a-5p is shown in Fig. 1B. The mean relative expression of RCC tissues was 2.07 (P<0.05). As demonstrated in Fig. 1C, the expression levels of miR-216a-5p in 293T cell line was significantly lower when compared with RCC cell lines ACHN (P<0.05) and 786-O (P<0.05).
Figure 1.
Expression of miR-216a-5p in 24 paired RCC tissues, adjacent normal tissues and cell lines. (A) Log2 ratios (T/N) of miR-216a-5p in 24 paired tissues. (B) The relative expression of miR-216a-5p in RCC and normal tissues. (C) The relative expression of miR-216a-5p in 293T cell line, ACHN and 786-O RCC cell lines. (D) The relative expression of miR-216a-5p in ACHN and 786-O cells following transfection with miR-216a-5p mimic or NC and miR-216a-5p inhibitor or inhibitor NC. *P<0.05, **P<0.01, ***P<0.001. miR, microRNA; NC, negative control; T, RCC tissues; RCC, renal cell carcinoma; N, normal tissues.
Validation of cell transfection efficiency
The transfection efficiency of miR-216a-5p mimic group and inhibitor group was explored by RT-qPCR. As shown in Fig. 1D, the expression levels of miR-216a-5p were 538.68-fold higher in ACHN cells (P<0.01) after transfection with miR-216a-5p mimic and 75.93-fold higher in 786-O cells (P<0.01) when compared with NC group. The expression levels of miR-216a-5p was 0.09 times in ACHN cells (P<0.001) transfected with miR-216a-5p inhibitor and 0.35 times in 786-O cells (P<0.01) when compared with the inhibitor NC group.
MiR-216-5p promotes RCC cell proliferation
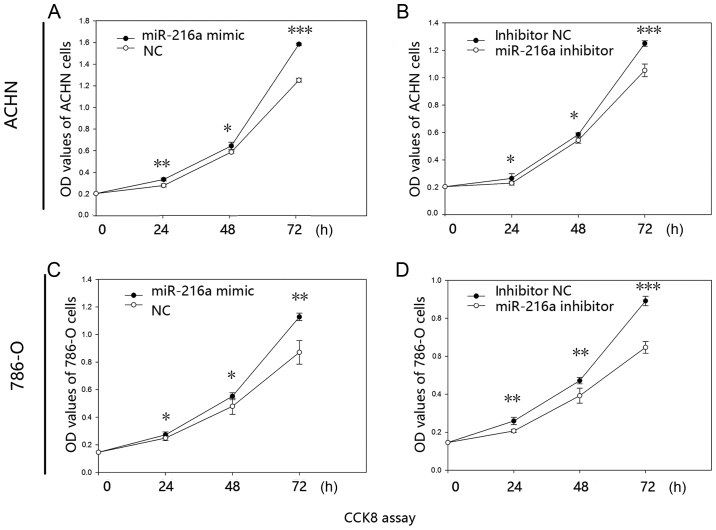
The proliferative ability of miR-216a-5p was validated by CCK-8 assay in vitro. The results revealed that the proliferative ability of mimic group was increased by 19.99% (24 h; P<0.01), 9.72% (48 h; P<0.05) and 26.50% (72 h; P<0.001) in the ACHN cells (Fig. 2A), while the proliferative ability was reduced in the inhibitor group by 12.99% (24 h; P<0.05), 7.13% (48 h; P<0.05) and 15.68% (72 h; P<0.001) (Fig. 2B). Similarly, proliferative ability of mimic group was significantly increased by 9.50% (24 h; P<0.05), 15.20% (48 h; P<0.05) and 29.48% (72 h; P<0.01) in the 786-O cells (Fig. 2C), while the inhibitor group was reduced by 20.19% (24 h; P<0.01), 16.85% (48 h; P<0.01) and 27.42% (72 h; P<0.001) (Fig. 2D).
Figure 2.
Proliferative ability of (A and B) ACHN and (C and D) 786-O cells transfected with (A and C) miR-216a-5p mimic or NC and (B and D) miR-216a-5p inhibitor or inhibitor NC were assessed by CCK-8. *P<0.05, **P<0.01, ***P<0.001. NC, negative control; OD, optical density; CCK-8, Cell Counting kit 8.
miR-216-5p increases the RCC cell viability
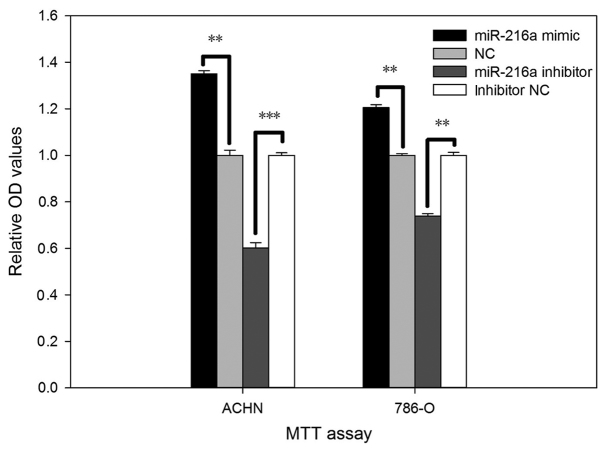
The viability of miR-216a-5p on RCC cell was validated by MTT assay in vitro. As shown in Fig. 3, cell viability after transfection with the miR-216a-5p mimic increased by 35.08% (P<0.01) in ACHN cells and by 20.56% (P<0.01) in 786-O cells. Inversely, viability of cells transfected with the miR-216a-5p inhibitor was reduced by 39.82% (P<0.001) in ACHN cells and by 26.19% (P<0.01) in 786-O cells.
Figure 3.
Viability of ACHN and 786-O cells transfected with miR-216a-5p mimic or NC and miR-216a-5p inhibitor or inhibitor NC were measured using MTT assay. **P<0.01, ***P<0.001. miR, microRNA; NC, negative control.
MiR-216-5p promotes RCC cell motility
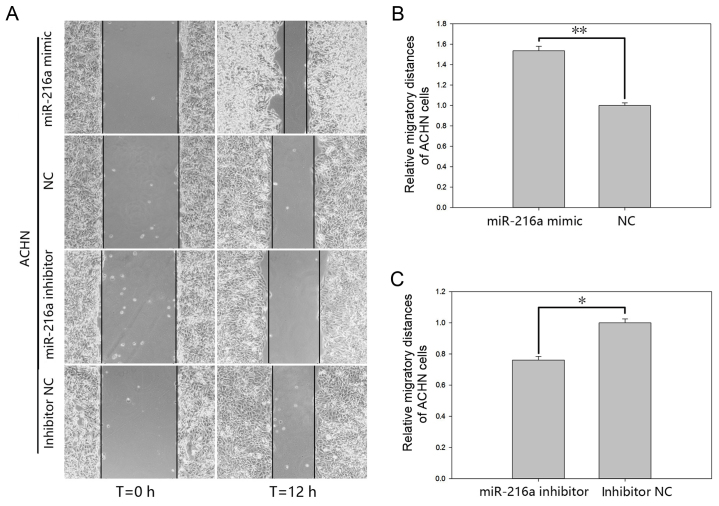
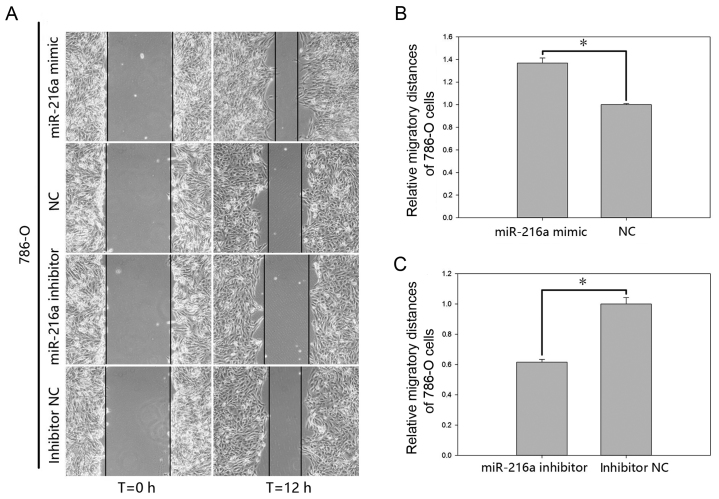
The effect of miR-216a-5p on cell motility of RCC cells was validated by a wound scratch assay and the Transwell assay (Figs. 4 and 5). The results of the wound scratch assay demonstrated that the distance migrated by the cells transfected with miR-216a-5p mimic was increased by 53.49% (P<0.01; Fig. 4B) in ACHN cells and by 36.84% (P<0.05; Fig. 5B) in 786-O cells at 12 h post-transfection. In contrast, the distance migrated by cells transfected with miR-216a-5p inhibitor was reduced by 23.93% (P<0.05; Fig. 4C) in ACHN cells and by 38.42% (P<0.05; Fig. 5C) in 786-O cells at 12 h post-transfection.
Figure 4.
Wound scratch assay of ACHN cells. (A) Migratory images. At least three pictures were taken for each experiment. Data of migratory distances in ACHN cells transfected with (B) miR-216a-5p mimic or NC and (C) miR-216a-5p inhibitor or inhibitor NC. *P<0.05, **P<0.01. miR, microRNA; NC, negative control.
Figure 5.
Wound scratch assay of 786-O cells. (A) Migratory images. At least three pictures were taken for each experiment. Data of migratory distances in 786-O cells transfected with (B) miR-216a-5p mimic or NC and (C) miR-216a-5p inhibitor or inhibitor NC. *P<0.05. miR, microRNA; NC, negative control.
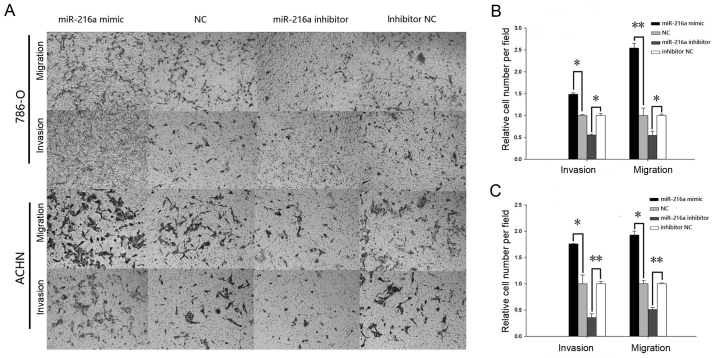
As demonstrated in Fig. 6, the results of the Transwell assay indicated that the cell migration for the mimic group was increased significantly by 48.57% (P<0.05) in the 786-O cells (Fig. 6B) and by 75.81% (P<0.05) in ACHN cells (Fig. 6C), while the cell migration in the inhibitor group was reduced by 44.75% (P<0.05) in 786-O cells (Fig. 6B) and by 64.45% (P<0.01) in ACHN cells (Fig. 6C). Similarly, the invasive ability of mimic group was increased significantly by 154.14% (P<0.01) in the 786-O cells (Fig. 6B) and by 92.8% (P<0.05) in ACHN cells (Fig. 6C), while the invasiveness in the inhibitor group was reduced by 45.61% (P<0.05) in the 786-O cells (Fig. 6B) and by 49.24% (P<0.01) in ACHN cells (Fig. 6C).
Figure 6.
Transwell assay in 786-O and ACHN cells. (A) Migratory and invasive images. The results of migration and invasion in (B) 786-O and (C) ACHN cells transfected with miR-216a-5p mimic or NC and miR-216a-5p inhibitor or inhibitor NC. *P<0.05, **P<0.01. miR, microRNA; NC, negative control.
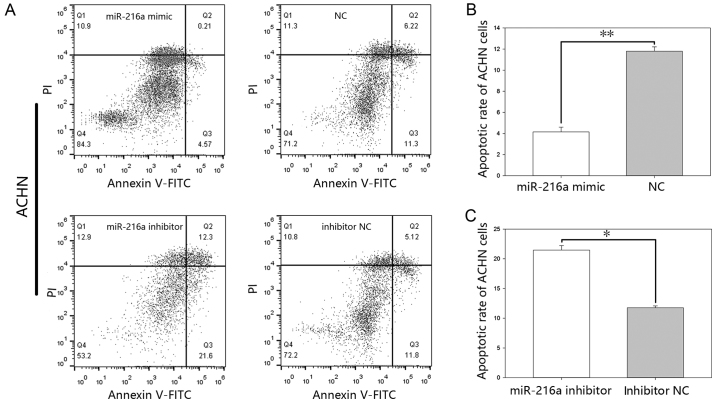
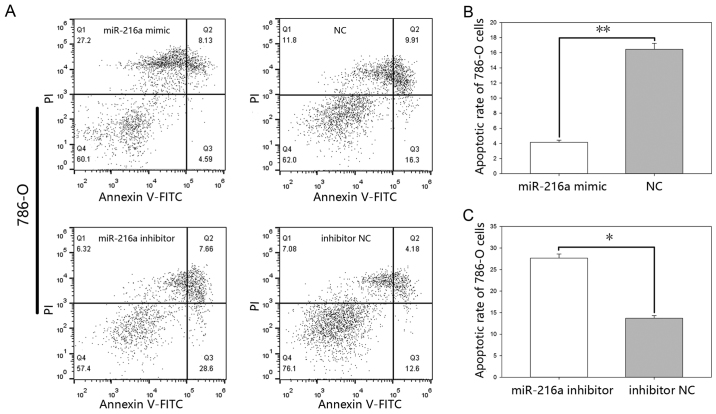
MiR-216-5p suppresses apoptosis. The results of the flow cytometry (Figs. 7 and 8), showed that the early apoptotic rate of the mimic group was 4.14%±0.44 vs. 11.8%±0.40 (P<0.01) in ACHN cells (Fig. 7B) and 4.15%±0.26 vs. 16.46%±0.78 (P<0.01) in 786-O cells (Fig. 8B), while the apoptotic rate in the inhibitor group was 21.46%±0.75 vs. 11.76%±0.31 (P<0.05) in ACHN cells (Fig. 7C) and was 27.66%±0.93 vs. 13.7%±0.58 (P<0.05) in 786-O cells (Fig. 8C).
Figure 7.
(A) Flow cytometry results of apoptosis in ACHN cells. Q3 was used to calculate the apoptotic rates. Cell apoptosis rate (%) of cells following transfection with (B) miR-216a-5p mimic or NC and (C) miR-216a-5p inhibitor or inhibitor NC. *P<0.05, **P<0.01. miR, microRNA; FITC, fluorescein isothiocyanate; NC, negative control.
Figure 8.
(A) Flow cytometry results of apoptosis in 786-O cells. Q3 was used to calculate the apoptotic rates. Cell apoptosis rate (%) of cells following transfection with (B) miR-216a-5p mimic or NC and (C) miR-216a-5p inhibitor or inhibitor NC. *P<0.05, **P<0.01. miR, microRNA; FITC, fluorescein isothiocyanate; NC, negative control.
Discussion
miRNAs play a significant role in tumorigenesis and have been associated with proliferation, differentiation and apoptosis by activating cancer-promoting genes or dysfunction of tumor suppressor genes (21). Available research literature suggests that higher or lower expression of miR-216a-5p occurs in a variety of cancers. Some studies have shown that the expression levels of miR-216a-5p is downregulated in colorectal cancer (16) and pancreatic cancer (17), while others have reported upregulated levels of miR-216a-5p in prostate cancer (18) and liver cancer (19). Intracellular environments of different cells were diversiform, so the signaling pathways of miR-216-5p were described in certain types of tumor. In the present study, miR-216a-5p was implicated in RCC, which was observed to be highly expressed when compared to the expression levels in the normal tissues.
In colorectal cancer, miR-216a-5p is known to decrease migration, invasion and inhibit metastasis by targeting KIAA1199. MiR-216a-5p in colorectal cancer has been shown to be upregulated and is responsible for maintaining the aggressive phenotype of tumor cells (16). Hou et al (22) have demonstrated that miR-216a-5p significantly inhibits cell growth and promotes cell apoptosis by targeting the Janus kinase 2 (JAK2) and transcription 3 (STAT3) in pancreatic cancer (22). In another previous study about pancreatic cancer, miR-216a-5p has been shown to decrease cell viability and induce cell apoptosis by silencing MALAT1 expression (23). In addition, miR-216a-5p is also reported to be upregulated in prostate cancer and could inhibit bicalutamide-mediated growth suppression (18). In hepatocellular carcinoma (HCC), overexpression of miR-216a/217 promotes cell population, migration, and metastatic ability by targeting PTEN and SMAD7 (19). Chen et al (24) also demonstrated that the three target sites at its 3′ untranslated region of TSLC1 were targeted by miR-216a-5p, and miR-216a-5p was observed to be significantly upregulated in cancerous liver tissues (24).
The functional role of miR-216a-5p in RCC has not been reported in previous studies and this is the first study to show that the functional significance of the upregulated miR-216a-5p, which promotes proliferation, viability, mobility and suppresses apoptosis in 786-O and ACHN cells. Conversely, downregulated miR-216a-5p suppressed proliferation, viability, motility and induced apoptosis in 786-O and ACHN cells. Based on these results, we postulate that tumorigenesis was promoted by miR-216a-5p, which acts as an oncogene in RCC.
In addition to being associated with RCC, miR-216a-5p was found to be a potential biomarker for the early identification of severe acute pancreatitis (25). Li et al (26) have also reported that that miR-216a-5p is involved in diabetes and targeted genes including ANGPTL4, ANXA9, CFH, CHI3L2 and INPP4B (26). Mansego et al (27) found that miR-216a-5p might be involved in miRNA mediated epigenetic regulation in childhood obesity and the coding regions of miR-216a-5p were methylated (27). Furthermore, it was suggested that miR-216a-5p had an impact on chronic kidney diseases (28), nephrotic syndrome (29) and end-stage failing hearts (30).
In summary, in the present study, we report for the first time that miR-216a-5p is upregulated in RCC tissues. Also, our data shows that miR-216a-5p is involved in cellular proliferation, viability, cell motility and apoptosis. Furthermore, regulating the expression of miR-216a-5p and further research on the targets of miR-216a-5p may pave the way for development of novel therapeutics against renal cancer in the future. It is important to test the expression of miR-216a-5p in more patients to get more accurate information about the relationship between clinicopathological features and the expression of miR-216a-5p. So, we will explore the clinicopathological features in further study.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81101922), Science and Technology Development Fund Project of Shenzhen (grant nos. JCYJ20160429090753103 and JCYJ20170307111334308), the fund of ‘San-ming’ Project of Medicine in Shenzhen (grant no. SZSM201612066), the Fund of Shenzhen Key Laboratory (grant no. ZDSYS201504301045406) and the fund of Guangdong Key Medical Subject.
References
- 1.White NM, Yousef GM. MicroRNAs: Exploring a new dimension in the pathogenesis of kidney cancer. BMC Med. 2010;8:65. doi: 10.1186/1741-7015-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlesinger-Raab A, Treiber U, Zaak D, Hölzel D, Engel J. Metastatic renal cell carcinoma: Results of a population-based study with 25 years follow-up. Eur J Cancer. 2008;44:2485–2495. doi: 10.1016/j.ejca.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 3.Moch H. An overview of renal cell cancer: Pathology and genetics. Semin Cancer Biol. 2013;23:3–9. doi: 10.1016/j.semcancer.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Hadoux J, Vignot S, De La Motte Rouge T. Renal cell carcinoma: Focus on safety and efficacy of temsirolimus. Clin Med Insights Oncol. 2010;4:143–154. doi: 10.4137/CMO.S4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naito S, Tomita Y, Rha SY, Uemura H, Oya M, Song HZ, Zhong LH, Wahid MI. Kidney Cancer Working Group report. Jpn J Clin Oncol. 2010;40(Suppl 1):i51–i56. doi: 10.1093/jjco/hyq127. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow TF, Youssef YM, Lianidou E, Romaschin AD, Honey RJ, Stewart R, Pace KT, Yousef GM. Differential expression profiling of microRNAs and their potential involvement in renal cell carcinoma pathogenesis. Clin Biochem. 2010;43:150–158. doi: 10.1016/j.clinbiochem.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. doi: 10.1016/S0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 9.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Nelson KM, Weiss GJ. MicroRNAs and cancer: Past, present, and potential future. Mol Cancer Ther. 2008;7:3655–3660. doi: 10.1158/1535-7163.MCT-08-0586. [DOI] [PubMed] [Google Scholar]
- 12.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 14.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 15.Negrini M, Ferracin M, Sabbioni S, Croce CM. MicroRNAs in human cancer: From research to therapy. J Cell Sci. 2007;120:1833–1840. doi: 10.1242/jcs.03450. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Zhao L, Shen Q, Lv Q, Jin M, Ma H, Nie X, Zheng X, Huang S, Zhou P, et al. Down-regulation of KIAA1199/CEMIP by miR-216a suppresses tumor invasion and metastasis in colorectal cancer. Int J Cancer. 2017;140:2298–2309. doi: 10.1002/ijc.30656. [DOI] [PubMed] [Google Scholar]
- 17.Link A, Becker V, Goel A, Wex T, Malfertheiner P. Feasibility of fecal microRNAs as novel biomarkers for pancreatic cancer. PLoS One. 2012;7:e42933. doi: 10.1371/journal.pone.0042933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazaki T, Ikeda K, Sato W, Horie-Inoue K, Okamoto K, Inoue S. MicroRNA library-based functional screening identified androgen-sensitive miR-216a as a player in bicalutamide resistance in prostate cancer. J Clin Med. 2015;21:1853–1865. doi: 10.3390/jcm4101853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia H, Ooi LL, Hui KM. MicroRNA-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology. 2013;58:629–641. doi: 10.1002/hep.26369. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Palanichamy JK, Rao DS. miRNA dysregulation in cancer: Towards a mechanistic understanding. Front Genet. 2014;5:54. doi: 10.3389/fgene.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou BH, Jian ZX, Cui P, Li SJ, Tian RQ, Ou JR. miR-216a may inhibit pancreatic tumor growth by targeting JAK2. FEBS Lett. 2015;589:2224–2232. doi: 10.1016/j.febslet.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Tang X, Shi M, Wen C, Shen B. MiR-216a decreases MALAT1 expression, induces G2/M arrest and apoptosis in pancreatic cancer cells. Biochem Biophys Res Commun. 2017;483:816–822. doi: 10.1016/j.bbrc.2016.12.167. [DOI] [PubMed] [Google Scholar]
- 24.Chen PJ, Yeh SH, Liu WH, Lin CC, Huang HC, Chen CL, Chen DS, Chen PJ. Androgen pathway stimulates microRNA-216a transcription to suppress the tumor suppressor in lung cancer-1 gene in early hepatocarcinogenesis. Hepatology. 2012;56:632–643. doi: 10.1002/hep.25695. [DOI] [PubMed] [Google Scholar]
- 25.Zhang XX, Deng LH, Chen WW, Shi N, Jin T, Lin ZQ, Ma Y, Jiang K, Yang XN, Xia Q. Circulating microRNA 216 as a marker for the early identification of severe acute pancreatitis. Am J Med Sci. 2017;353:178–186. doi: 10.1016/j.amjms.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Ma W, Xie C, Zhang M, Yin X, Wang F, Xu J, Shi B. Identification of genes and signaling pathways associated with diabetic neuropathy using a weighted correlation network analysis: A consort study. Medicine (Baltimore) 2016;95:e5443. doi: 10.1097/MD.0000000000005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansego ML, Garcia-Lacarte M, Milagro FI, Marti A, Martinez JA. DNA methylation of miRNA coding sequences putatively associated with childhood obesity. Pediatr Obes. 2017;12:19–27. doi: 10.1111/ijpo.12101. [DOI] [PubMed] [Google Scholar]
- 28.Szeto CC, Ching-Ha KB, Ka-Bik L, Mac-Moune LF, Cheung-Lung CP, Gang W, Kai-Ming C, Kam-Tao LP. Micro-RNA expression in the urinary sediment of patients with chronic kidney diseases. Dis Markers. 2012;33:137–144. doi: 10.1155/2012/842764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szeto CC. Urine miRNA in nephrotic syndrome. Clin Chim Acta. 2014;436:308–313. doi: 10.1016/j.cca.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Barsanti C, Trivella MG, D'Aurizio R, El Baroudi M, Baumgart M, Groth M, Caruso R, Verde A, Botta L, Cozzi L, Pitto L. Differential regulation of microRNAs in end-stage failing hearts is associated with left ventricular assist device unloading. Biomed Res Int. 2015;2015:592512. doi: 10.1155/2015/592512. [DOI] [PMC free article] [PubMed] [Google Scholar]