Abstract
Rinsing the mouth with a carbohydrate solution has been shown to improve exercise performance in a manner similar to carbohydrate ingestion. However, the underlying mechanisms behind these ergogenic benefits remain unclear. This study evaluated whether rinsing the mouth with a carbohydrate solution alters plasma insulin and glucose concentration during the initial stages of a 40 km cycling time-trial. Eight trained, competitive cyclists [age (mean ± SEM) = 24 ± 2 y; V̇O2max = 64.5 ± 2.2 ml·kg-1·min-1] completed three simulated 40 km time-trials comprised of a familiarization trial, a carbohydrate condition (CHO) and a placebo mouth rinse condition (PLA). In the two mouth rinse conditions, rinsing was administered prior to onset of exercise and every 5 km throughout exercise. Plasma insulin was collected at 5 km intervals throughout the first 25 km, and glucose samples were collected at 5 km intervals throughout the exercise bout. No change in plasma insulin was detected between conditions (p = 0.638, ES < 0.03) for the first 25 km of the time-trial. Likewise, plasma glucose concentration did not differ between CHO and PLA (p = 0.801, ES < 0.01) and remained relatively stable throughout exercise. Time to complete the 40 km time-trial was significantly faster for CHO (67.1 ± 1.1 min) compared to PLA [67.9 ± 1.0 min; (P = 0.028, ES 0.27)]. Performance time was faster by an average of 1.1% (95% confidence interval range 0.2-2.0%) in the CHO condition. Exercise intensity (% V̇O2max) throughout the trial was similar between conditions (p = 0.846). Respiratory exchange ratio was not significantly different between conditions (0.88 ± 0.01 for PLA, and 0.91 ± 0.01 for GLC; p = 0.081). Performance gains elicited by a carbohydrate mouth rinse occurred independently of changes in plasma insulin concentration.
Key points.
Carbohydrate mouth rinsing has been shown to improve endurance performance for exercise lasting approximately 1 h in duration. The mechanisms responsible for performance gains are yet to be fully understood.
Although 40 km cycling time-trial performance improved when rinsing the mouth with a carbohydrate solution compared to a placebo condition, rinsing the mouth with a carbohydrate solution prior to exercise and every 5 km throughout a 40 km cycling time-trial did not alter plasma insulin concentration.
Blood glucose concentration was not affected when rinsing the mouth with a carbohydrate solution every 5 km throughout a 40 km cycling time-trial.
Rates of macronutrient oxidation did not differ during exercise when rinsing the mouth with a carbohydrate solution compared to a placebo solution.
Key words: Sport nutrition, supplementation, exercise, ergogenic aid
Introduction
Rinsing the mouth with a carbohydrate solution has been shown to improve cycling performance by 1-3% for exercise lasting approximately an hour in duration (Carter et al., 2004; Lane et al., 2012). The mechanisms responsible for the improvement in exercise performance were originally considered to have a metabolic component (Carter et al., 2004), but recent studies have shown that performance improvement is more likely related to an increase in corticomotor excitability and central motor drive to exercising muscle (Bastos-Silva et al., 2016; Chambers et al., 2009; Gant et al., 2010). However, while stimulation of carbohydrate taste receptors within the oral cavity improves performance by increasing central drive to the locomotor muscles, the possibility remains that a cephalic phase insulin response contributes to the improvement in exercise performance. A cephalic insulin response could enhance glucose uptake by the exercising muscles to help maintain high rates of carbohydrate oxidation in a manner similar to that which occurs when carbohydrate is ingested during prolonged exercise (Coggan and Coyle, 1987; Coyle et al., 1986).
A peak in plasma insulin concentration would be expected to occur approximately 60 min post-carbohydrate ingestion (Coulston et al., 1980). However, as a cephalic phase response is not related to absorption of nutrients through the gastrointestinal tract during a carbohydrate mouth rinse, but as a consequence of oral carbohydrate recognition, the insulin response may occur less than 10 min after stimulation (Just et al., 2008). Previously, Carter et al. (2004) reported power output as being significantly higher during the first three quarters of a carbohydrate mouth rinse time-trial compared to a placebo time-trial, with no significant changes in power output observed in the latter portion of the time-trial. Their data indicated that the mechanisms responsible for performance enhancement were most likely prominent in the initial stages of exercise. Thus, it seems reasonable to suggest that cephalic carbohydrate recognition could have an immediate influence on insulin concentration and exercise performance, and that this influence would occur in the initial stages of exercise.
Therefore, the aim of the present study was to investigate the effect of a carbohydrate mouth rinse on plasma insulin and glucose concentrations, as well as carbohydrate oxidation rates, during a 40 km cycling time-trial. We hypothesized that a carbohydrate mouth rinse, compared to a placebo mouth rinse, would increase plasma insulin and lower plasma glucose in the initial stages of exercise, as well as elevate carbohydrate oxidation rates, and improve 40 km time-trial performance.
Methods
Study participants
Subject characteristics are displayed in Table 1. Ten male athletes volunteered to participate in this study. Two participants were excluded from final data analysis due to injury or failure to comply with the dietary protocol. Therefore, eight participants completed the study. All participants were competitive cyclists or triathletes involved in endurance training on a regular basis (≥ 2 sessions per wk). Six participants had recently completed a 50-mile team cycling competition, and one participant was a highly-trained international triathlete familiar with endurance rides. Participants were excluded if they had a current or recent injury, were not engaged in cycling exercise at least twice per week, were unwilling to abstain from intense exercise 24 h prior to testing, or were reluctant to adhere to dietary requirements prior to exercise. Participants were tested at the same time of day for each of the experimental visits and were instructed to arrive at the laboratory having not eaten for at least 5 h and having abstained from alcohol and strenuous exercise for the previous 24 h. All testing procedures and the informed consent statement were approved by the Indiana University Human Subjects Committee. Prior to giving written informed consent, all participants completed a health and medical questionnaire and were informed of possible risks.
Table 1.
Selected characteristics of participants (n = 8). Data are means (±SE).
| Age (yr) | 24(2) |
| Body mass (kg) | 77.5 (1.4) |
| V̇O2max (l·min-1) | 5.0 (.2) |
| V̇O2max (ml·kg-1·min-1) | 64.5 (2.2) |
| Pmax (Watts) | 429.5 (14.7) |
Study design
All participants visited the laboratory on three different occasions, each separated by 6 ± 2 d. All exercise sessions took place on a cycle ergometer. Visit 1 included preliminary testing, and consisted of an incremental exercise to exhaustion to determine maximum oxygen uptake (V̇O2max), and maximum power output (Pmax). Participants with the qualifying V̇O2max (V̇O2max > 55 ml·kg-1·min-1) rested for up to 15 min before completing a 40 km familiarization time-trial. For the experimental trials (Visits 2 and 3), participants were instructed to complete the 40 km time-trial in the shortest time possible. During the experimental trials, participants were given either a 6.4% glucose solution (CHO) or a water solution (PLA) to rinse in the mouth for 10 s prior to exercise, and at every 5 km interval throughout exercise. Additionally, venous blood was drawn prior to mouth rinsing and every 5 km during exercise. Plasma glucose was tracked throughout the 40 km time-trial. Since previous studies have indicated that the ergogenic benefits of mouth rinsing occur most prominently in the initial stages of exercise compared with the final stages of exercise plasma insulin was recorded through the first 25 km of the 40 km time-trial only. Mouth rinse solutions were matched for sweetness, color, and flavor using a non-caloric artificial sweetener free of caffeine or added electrolytes. (Mio Liquid Water Enhancer, Kraft Foods, IL; 1 ml Mio per 25 ml solution). Visits were counterbalanced for order of condition, such that half of the participants performed the PLA condition prior to the CHO condition, and half performed the CHO condition prior to PLA.
Maximal oxygen uptake & familiarization trial
Approximately 1 wk prior to the experimental trials, participants completed a cycling test to exhaustion on an electromagnetically braked cycle ergometer (Velotron Racermate, Seattle, WA) starting at a power output of 100 W and increasing 30 W per min until they could no longer continue or until cadence decreased below 50 rpm (Duke et al., 2014). Maximum oxygen uptake was defined as the highest O2 consumption (V̇O2) attained during any 30 s interval of the test. Participants were considered to have achieved V̇O2max if two of the following criteria were met: 1) a heart rate ≥ 90% of age-predicted maximal heart rate; 2) respiratory exchange ratio (RER) ≥ 1.10; and 3) evidence of a plateau in V̇O2 with an increase in power output, which is defined as ≤ 150 ml increase in V̇O2 with an increase in power. Qualified participants rested for up to 15 min before completing a 40 km familiarization time-trial. During the time-trial participants underwent mouth rinse procedures using only water in order for the subjects to become accustomed to the rinsing protocol. During all exercise procedures participants breathed through a Hans Rudolph mask connected to a flow sensor and computerized gas-analysis system (Vmax, CareFusion, CA) that displayed breath-by-breath V̇O2, CO2 production (V̇CO2), minute ventilation (V̇E), and RER. The facemask remained on the participants throughout the duration of the exercise bouts, except when administering the mouth rinses. Mean power output and heart rate (Polar FT7, Polar Electro, Finland) were recorded continuously throughout all exercise sessions. Prior to each test, calibrations were performed using gases of known O2 and CO2 content.
Experimental trials
The protocol for the two testing days was identical with the exception of the type of mouth rinse given. To minimize differences in resting muscle and liver glycogen concentration, participants were provided a meal (~800 kcals; 45% carbohydrate, 34% fat, 20% protein) from a commercial sandwich to consume as the last meal prior to testing (~5 h before each experimental visit). Six of the eight participants exercised in the afternoon / evening hours, while two participants required morning exercise; these two participants arrived on a fast > 5 h. Participants consumed the same meal before each of the experimental trials, at the same time of day.
Upon arrival to the laboratory, and preceding any water intake, participants were weighed and adequate hydration was confirmed using a urine refractometer (Pen S.G., Atago, Japan). Previously published data (Oppliger et al., 2005) has established a value of 1.03 as indicative of severe dehydration. In the present study two participants had values greater than 1.02 and were given 500 ml of water to consume before testing. Laboratory environmental conditions (relative humidity ~ 30%, Pbar ~740mmHg, temperature ~ 20.7mC) were kept consistent between trials. As euhydration was confirmed prior to testing, and because laboratory conditions were kept constant, hydration status was not assessed during or following the time-trial.
Prior to exercise, a 20-gauge catheter was inserted into an antecubital vein and secured in place for periodic sampling. Following catheter placement participants completed a 5 min warm up at 40% Pmax prior to starting the 40 km cycling time-trial. During the time-trial, participants received no feedback other than elapsed distance, which was continuously displayed. Regular encouragement was given throughout exercise for both conditions. Seat and handlebar placement were set according to participant preference, and kept consistent between conditions.
Mouth rinse protocol
At the onset of exercise, participants were given a room-temperature, 25 ml bolus of either a glucose or placebo solution, which they rinsed for 10 s before expectorating into a beaker. In accordance with previous studies examining the effect of carbohydrate mouth rinsing on exercise performance (Carter et al., 2004), a 6.4% glucose solution was prepared by mixing a commercial dextrose powder (NOW Foods, Bloomingdale, IL) with tap water. Carbohydrate powder was comprised exclusively of pure dextrose, and free of extraneous ingredients. As a consequence of time conflicts with participant scheduling and investigator availability, the main investigator had to prepare and administer the CHO or PLA solutions on approximately half the occasions. Therefore, blinding was only applied to the participants. Every 5 km interval throughout the 40 km time-trial, participants would temporarily remove the facemask, allowing them to rinse the 25 ml bolus using the same procedure. This protocol has been used previously and established for its efficacy in improving cycling performance of approximately 1 h in duration (Carter et al., 2004). No liquids were ingested during the time-trial, and upon completion of exercise, final volumes of expectorate were examined. Volumes measured post-exercise confirmed that no solution had been consumed.
Blood sampling and analysis
Five milliliters of blood were collected in EDTA-coated tubes upon arrival to the laboratory, as well as immediately prior to each 5 km interval (i.e. directly before mouth rinse administration) during the 40 km time-trial. Following each collection, lines were flushed with 5 ml of saline and IV bags were allowed to drip at a rate of one drop every 3 seconds to ensure catheter lines remained patent. Immediately prior to each collection, 5 ml of sample were drawn and discarded to avoid dilution effects of the saline flush. A slow infusion rate, coupled with the disposal of the initial blood draw, ensured the integrity of glucose and insulin samples. Samples were immediately centrifuged at 1700 x g, 4°C, for 10 min (Beckman Model X-22R, St. Louis, MO). The plasma was pipetted from the blood-collection tube, transferred to microfuge tubes for storage at -80°C, and later measured in duplicate for glucose and lactate (Analox GM7, Analox Instruments Ltd., UK), as well as plasma insulin. Insulin was determined using an enzyme-linked immunosorbent assay [sensitivity: 4μU ml-1; Detection range: 4.0 – 300μU·ml-1; (RayBiotech, Norcross, GA)] as per the manufacturer directions, and analyzed via Powerwave XS™ spectrophotometer (Bio-Tek Instruments, Winooski, VT).
Macronutrient oxidation
Rates of carbohydrate and fat oxidation during exercise were calculated from the V̇O2 and V̇CO2 (l·min-1) values according to updated oxidation formulas (Jeukendrup and Wallis, 2005) established specifically for intense exercise eliciting a V̇O2 response between 50-75% V̇O2max:
| Carbohydrate oxidation (g·min-1) = 4.210 V̇CO2 – 2.962 V̇O2 |
| Fat oxidation (g·min-1) = 1.695 V̇O2 – 1.701 V̇CO2 |
Statistical analyses
Data were analyzed using SPSS 24.0 (IBM Corporation, Chicago, IL, USA) statistical software, and significance was established when P < 0.05. Sphericity of data was assessed using Mauchly’s test. When sphericity was violated, the departure from sphericity (ε) was calculated. When ε < 0.75, the Greenhouse-Geisser correction was applied and when ε > 0.75, the Huynh-Feldt correction factor was applied. Power output, plasma glucose, lactate, and insulin concentrations were analyzed using a two-way, repeated-measures ANOVA (condition x distance) with significant main effects for individual time points further analyzed using paired t-tests and Bonferroni adjustment for multiple comparisons. Performance times are given as mean differences with 95% confidence intervals (95% CI), which displays the likely range of the true value in the sample population. Power analysis (G*Power 3.1, Franz Faul, Germany) showed that a sample size of eight would allow detection of a significant difference in insulin concentration (Robertson et al., 2001), carbohydrate oxidation rate (Fraga et al., 2015), and cycling time-trial performance (Carter et al., 2004) with statistical power of 1 - β = 0.79 and α = 0.05. All data are presented as mean ± SE. Partial eta-squared and Cohen’s d were calculated as measure of effect size (ES).
Results
Plasma insulin, glucose, and lactate concentration
Cycling exercise resulted in no significant (p = 0.807) alteration in insulin concentration from baseline values. Likewise, throughout the first 25 km of the 40 km time-trial, plasma insulin concentration was not significantly different at any time point (p = 0.638, ES < 0.03) between mouth rinse conditions (Figure 1). Mean plasma glucose concentrations for the experimental trials are shown in Figure 2A. Plasma glucose levels measured at baseline were not significantly different (p = 0.150) between conditions. In addition, no significant difference (p = 0.801, ES < 0.01) in plasma glucose concentration was observed between conditions throughout the 40 km time-trial. Plasma lactate concentration was significantly elevated (p = 0.004) at the end of exercise compared to baseline values within conditions, but did not differ between conditions either at the end of the 40 km trial, or throughout the exercise session (Figure 2B).
Figure 1.
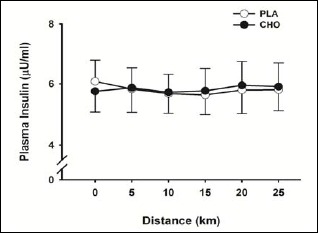
Plasma insulin concentrations for first 25 km of 40 km time-trial. Data are mean ± SE.
Figure 2.
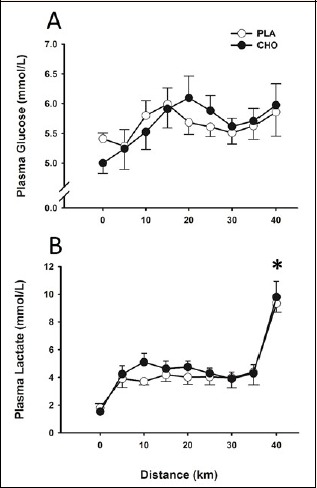
Plasma glucose (A) and plasma lactate (B) concentrations throughout 40 km time-trial. Data are mean ± SE. * p < 0.05 for 40 km mark compared with baseline values.
Cycling time-trial performance and power output
Metabolic and plasma measures, as well as performance outcomes, were similar between the six afternoon subjects and the two subjects that exercised in the morning hours. There was no effect of condition on HR throughout the 40 km time-trial. In both conditions, HR was significantly greater (p = 0.013) at the 40 km mark (172 ± 2 bpm) compared to the 10 km mark (155 ± 3 bpm). Subjects completed the 40 km cycling time-trial significantly faster (p = 0.028, ES 0.27) during the CHO (67.1 ± 1.1 min) compared with the PLA trial (67.9 ± 1.0 min), which represented an average improvement of 1.1% (95% CI 0.2 –2.0%) (Figure 3). Mean power output during the time-trial was significantly higher (1% increase; p = 0.034, ES 0.27) during the CHO trial (244 ± 10 W) compared with the PLA trial (237 ± 8 W). When the time-trial was divided into quarters, no statistical difference in power output was observed (Figure 4). Following completion of the study, participants were unable to correctly identify the CHO rinse.
Figure 3.
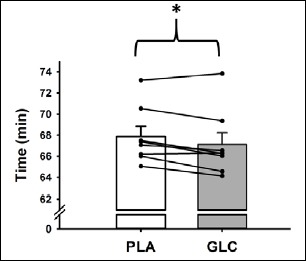
Performance time for PLA and CHO 40 km cycle time-trials. Lines represent individual performances. Bar graphs represent mean ± SE. *p < 0.05 for CHO group mean compared with mean for PLA.
Figure 4.
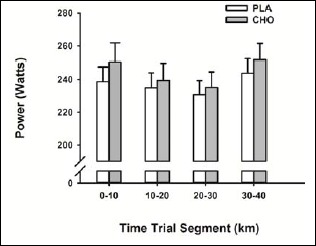
Mean power output for 10 km intervals of 40 km time-trial. Data are mean ± SE.
Metabolic response and macronutrient oxidation rates
There was no effect of condition on V̇O2, V̇E, or RER during the 40 km time-trial (Table 2). A main effect for distance was observed for V̇E, where V̇E was significantly higher (p = 0.021) at the end of exercise (109.0 ± 3.7 l·min-1) compared to the V̇E at 10 km (91.7 ± 4.0 l·min-1). Additionally, a main effect for time was found for carbohydrate oxidation rates, where rates were significantly higher at the 10 km and 40 km marks compared to the 20 km and 30 km intervals (Table 2). There was no effect of condition on carbohydrate oxidation rate (p = 0.062, ES 0.41) or fat oxidation rate (p = 0.103, ES 0.33) during the 40 km time-trial.
Table 2.
Metabolic responses and substrate oxidation during 40 km time-trial. Data are mean (±SE).
| 10 km | 20 km | 30 km | 40 km | ||
|---|---|---|---|---|---|
| HR (bpm) | PLA | 158(2) | 160(3) | 163(4) | 170(3)* |
| CHO | 157(2) | 160(3) | 160(2) | 173(3)* | |
| V̇O2 (l·min-1) | PLA | 3.9 (.1) | 3.9 (.1) | 4.0 (.1) | 4.2 (.1) |
| CHO | 3.9 (.1) | 3.9 (.1) | 4.0 (.2) | 4.4 (.2) | |
| V̇E (l·min-1) | PLA | 89.6 (4.1) | 89.6 (4.4) | 92.8 (3.7) | 104.2 (2.3)† |
| CHO | 93.9 (4.1) | 93.9 (4.1) | 94.9 (5.2) | 113.8 (5.6)* | |
| RER | PLA | .90 (.01) | .87 (.01)‡ | .87 (.01) | .89 (.01) |
| CHO | .92 (.02) | .90 (.01)‡ | .89 (.02)§ | .92 (.02) | |
| Carb. oxidation (g·min-1) | PLA | 3.27 (.23) | 2.77 (.20)‡ | 2.75 (.20) | 3.33 (.29) |
| CHO | 3.68 (.27) | 3.28 (.23) | 3.15 (.33)¶ | 4.06 (.43) | |
| Fat oxidation (g·min-1) | PLA | .64 (.09) | .82 (.05) | .86 (.05) | .76 (.08) |
| CHO | .47 (.12) | .64 (.09) | .73(10) | .57 (.12) |
No differences (p > 0.05) were observed between conditions for overall mean values.
* Significantly different from 10 km, 20 km, and 30 km values
† Significantly different from 20 km and 30 km values
‡ Significantly different from 10 km value
§ Significantly different from 10 km, 20 km, and 40 km values
¶ Significantly different from 40 km value
Discussion
The novel finding of the present study was that mouth rinsing with a 6.4% glucose solution improved 40 km cycling time-trial performance without altering plasma insulin or glucose concentration. Therefore, the data from the present study suggest that the improvement in exercise performance is unlikely to be mediated by insulin-induced glucose uptake into exercising skeletal muscle.
The observed improvement in 40 km time-trial performance in the present study is in agreement with several studies that have shown benefits of a carbohydrate mouth rinse on power output and exercise performance (Carter et al., 2004; Chambers et al., 2009; Pottier et al., 2010; Phillips et al., 2014). The difference in time to completion between the CHO and PLA conditions in the present study was slightly greater than 1%, which is consistent with previously reported findings of a 1-3% improvement in exercise performance, of approximately 1 h in duration (Chambers et al., 2009; Rollo et al., 2010).
The present study tracked changes in insulin concentration over the first 25 km of the 40 km cycling time-trial. As mentioned in the introduction, the rationale as to why the present study only measured plasma insulin concentration for the first 25 km of the time-trial is based on data from Carter et al. (2004) study, which reported power output as being significantly higher during the first three quarters of a carbohydrate mouth rinse time-trial compared to a PLA time-trial, with no significant changes in power output observed in the latter portion (~10-km) of the time-trial. Their data indicated that the mechanisms responsible for performance enhancement were most likely prominent in the initial stages of exercise, and thus, it was for this reason, that the present study chose to only measure plasma insulin concentration during the first 25 km of the 40 km cycling time-trial.
In the present study repeated rinsing of the mouth for 10 s with glucose did not elicit a change in plasma insulin concentration in the initial stages (25 km) of a 40 km cycling time-trial. While the current study is the first to show that plasma insulin concentration does not change as a result of a carbohydrate mouth rinse during exercise, it has previously been shown that chewing nutrients without swallowing can elicit a rise in insulin by as much as 250% above baseline concentrations (Robertson et al., 2001). Robertson et al. (2001) demonstrated a peak insulin response 16 min following expectoration. A number of other studies have estimated the cephalic insulin response to occur within 10 min of stimulation (Bellisle et al., 1983, 1985, Teff et al., 1991, 1995; Teff and Engelman, 1996). Indeed, Just et al. (2008) found peak concentrations 5 min after mouth rinsing, with a return to baseline at min 7. Differences in the time course of the plasma insulin response may be due to variations in rinse time, or the volume and nature of the administered carbohydrate load. In the present study, blood samples were taken every ~8.5 min. It is possible, that the peak insulin level occurred prior to sampling. However, based on the aforementioned time span of < 10 min, and potential peak values at ~16 min, the sampling window used in the present study should have been sufficient to capture any insulin response.
One possible explanation for why plasma insulin concentration was not altered during the cycling time-trial may be related to mouth rinse duration. Just et al. (2008) demonstrated that insulin is released when carbohydrate is rinsed in the mouth for 45 s. However, cyclists in the present study rinsed for only 10 s prior to expectorating. While it has been shown that a 10 s rinse can be effective in improving exercise performance (Sinclair et al., 2014), it may be too short to elicit a significant rise in insulin levels. Further research on the influence of rinse time on the cephalic phase insulin response is warranted. In addition to the rinsing protocol, the exercise per se may have influenced oral carbohydrate recognition. Cephalic phase insulin release has previously been observed when participants were in the rested state (Just et al., 2008; Robertson et al., 2001), and thus it is possible that the addition of exercise may alter this response. Exercise stimulates epinephrine release, which subsequently inhibits insulin release from the pancreas. Intense exercise has also been shown to impact hepatic glucose release (Kjaer, 1998). If epinephrine influenced pancreatic insulin release, one might also have expected glucose output from the liver to be impacted. No change in blood glucose was observed, however, and the flux between glucose uptake in the skeletal muscle and hepatic glucose release is difficult to discern. For the aforementioned reasons it is possible that the intensity and duration of the cycling time-trial would prevent an appreciable rise in plasma insulin concentration, even while cyclists were rinsing their mouths with a glucose solution during the 40 km time-trial.
In the present study plasma glucose concentration did not differ between the CHO and PLA mouth rinse conditions during the 40 km time-trial. The few studies that have characterized the blood glucose response to carbohydrate mouth rinsing throughout exercise have observed either no difference in blood glucose (Ispoglou et al., 2015; Pottier et al., 2010), or a tendency for a carbohydrate mouth rinse to elicit a higher plasma glucose concentration (Ataide-Silva et al., 2016; Watson et al., 2013), potentially via an upregulation in catecholamine release and liver glycogenolysis brought on by an elevation in exercise intensity. Thus, our data suggest that while the carbohydrate mouth rinse has a positive effect on 40 km time-trial performance, the mechanism responsible for the improvement is not metabolic in nature.
An alternative metabolic explanation as to how a carbohydrate mouth rinse may improve exercise performance is via a potential increase in carbohydrate oxidation in exercising skeletal muscle. Although, in the present study, a ~15% difference in the rates of carbohydrate oxidation between conditions was approaching significance, in light of the small effect size, alterations in substrate utilization seem unlikely. Non-metabolic events may also explain the observed exercise performance improvement. Hagger and Chatzisarantis (2013) have demonstrated that alterations in self-control are associated with carbohydrate rinsing. Due to the inability of participants to accurately distinguish the CHO condition from the PLA condition, it is unlikely that the nearly unanimous improvement in time-trial performance was the result of conscious motivation. Data from the present study lend support to previous work (Chambers et al., 2009) demonstrating a neural mechanism for performance improvement with a CHO mouth rinse.
We acknowledge that a limitation of the present study was the low sample size. However, based on previous studies a sample size of eight was sufficient to detect changes in both exercise performance (Carter et al., 2004), and plasma insulin concentration, due to the mouth rinse (Robertson et al., 2001). Fasting insulin values were approximately 5.9 ± 0.7 μU·ml-1 (Figure 3). These values correspond well with previously published data displaying fasting insulin values of roughly 5-8 μU·ml-1 in nondiabetic individuals (Manolio et al., 1991; Haffner et al., 1996; Johnson et al., 2009). In the present study, power calculations were performed based on data from Robertson et al. (2001), which reported an increase in plasma insulin concentration of nearly 12 μU·ml-1. However, Just et al. (2008) found that carbohydrate mouth rinsing significantly elevated insulin concentration by only a fraction of that which was observed in the Robertson paper; approximately 2 μU·ml-1. Although the present study found insulin to remain relatively stable throughout the 25 km sampling period (Figure 1), if the effect size of mouth rinsing during exercise more closely resembles the findings from Just et al., our sample size may have limited detection of these smaller adjustments in plasma insulin concentrations. While the current sample size was sufficient to detect large differences in insulin concentration, post-hoc power analysis has demonstrated the need for a larger sample size to detect the presence of subtle differences between conditions.
Conclusion
In conclusion, the present study has shown that while rinsing the mouth with a carbohydrate-containing solution during a 40 km cycling time-trial improved performance compared with a placebo rinse, plasma insulin concentration remained unaltered during the 25 km sampling period. While postulated mechanisms underpinning performance improvement previously included both central activation as well as a cephalic phase insulin response (Carter et al., 2004), it appears that performance gains elicited by a carbohydrate mouth rinse are not the result of a change in plasma insulin concentration during the first two-thirds of a 40 km time-trial.
Acknowledgements
The authors would like to thank the participants of this study. The experiments comply with the current laws of the country in which they were performed. The authors did not receive funding for this project and have no conflicts of interest to declare.
Biographies

Kevin O. MURRAY
Employment
Graduate Student
Degree
Master of Science (Kinesiology)
Research interests
Muscle metabolism, exercise physiology
E-mail: kevmurra@ufl.edu

Hunter L. PARIS
Employment
Graduate Student
Degree
Master of Science (Health & Exercise Science)
Research interests
Metabolism, exercise physiology
E-mail: hlparis@indiana.edu

Alyce D. FLY
Employment
Department of Applied Health Science, Indiana University
Degree
PhD
Research interests
Interventions for childhood obesity, peripheral vascular health
E-mail: afly@indiana.edu

Robert F. CHAPMAN
Employment
Department of Kinesiology, Indiana University
Degree
PhD
Research interests
Exercise physiology, human performance
E-mail: rfchapma@indiana.edu

Timothy D. MICKLEBOROUGH
Employment
Department of Kinesiology, Indiana University
Degree
PhD
Research interests
Exercise physiology, human performance
E-mail: tmickleb@indiana.edu
References
- Ataide-Silva T., Ghiarone T., Bertuzzi R., Stathis C.G., Leandro C.G., Lima-Silva A.E. (2016) CHO mouth rinse ameliorates neuromuscular response with lower endogenous CHO stores. Medicine and Science in Sports and Exercise 48, 1810-1820. [DOI] [PubMed] [Google Scholar]
- Bastos-Silva V.J., Melo A. de A., Lima-Silva A.E., Moura F.A., Bertuzzi R., de Araujo G.G. (2016) Carbohydrate mouth rinse maintains muscle electromyographic activity and increases time to exhaustion during moderate but not high-intensity cycling exercise. Nutrients 8, 49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellisle F., Louis-Sylvestre J., Demozay F., Blazy D., Le Magnen J. (1985) Cephalic phase of insulin secretion and food stimulation in humans: a new perspective. American Journal of Physiology-Endocrinology and Metabolism 249, E639-E645. [DOI] [PubMed] [Google Scholar]
- Bellisle F., Louis-Sylvestre J., Demozay F., Blazy D., Le Magnen J. (1983) Reflex insulin response associated to food intake in human subjects. Physiology & Behavior 31, 515-521. [DOI] [PubMed] [Google Scholar]
- Carter J.M., Jeukendrup A.E., Jones D.A. (2004) The effect of carbohydrate mouth rinse on 1-h cycle time trial performance. Medicine and Science in Sports and Exercise 36, 2107-2111. [DOI] [PubMed] [Google Scholar]
- Chambers E.S., Bridge M.W., Jones D.A. (2009) Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. The Journal of Physiology 587, 1779-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan A.R., Coyle E.F. (1987) Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. Journal of Applied Physiology 63, 2388-2395. [DOI] [PubMed] [Google Scholar]
- Coulston A., Greenfield M., Kraemer F., Tobey T., Reaven G. (1980) Effect of source of dietary carbohydrate on plasma glucose and insulin responses to test meals in normal subjects. The American Journal of Clinical Nutrition 33, 1279–1282. [DOI] [PubMed] [Google Scholar]
- Coyle E.F., Coggan A.R., Hemmert M., Ivy J.L. (1986) Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. Journal of Applied Physiology 61, 165-172. [DOI] [PubMed] [Google Scholar]
- Duke J.W., Stickford J.L., Weavil J.C., Chapman R.F., Stager J.M., Mickleborough T.D. (2014) Operating lung volumes are affected by exercise mode but not trunk and hip angle during maximal exercise. European Journal of Applied Physiology 114, 2387-2397. [DOI] [PubMed] [Google Scholar]
- Fraga C., Velasques B., Koch A.J., Machado M., Paulucio D., Ribeiro P., Pompeu F.A.M.S. (2015) Carbohydrate mouth rinse enhances time to exhaustion during treadmill exercise. Clinical Physiology and Functional Imaging 37, 17-22. [DOI] [PubMed] [Google Scholar]
- Gant N., Stinear C.M., Byblow W.D. (2010) Carbohydrate in the mouth immediately facilitates motor output. Brain Research 1350, 151-158. [DOI] [PubMed] [Google Scholar]
- Haffner S.M., Miettinen H., Stern M.P. (1996) Nondiabetic Mexican-Americans do not have reduced insulin responses relative to nondiabetic non-Hispanic whites. Diabetes Care 19, 67-69. [DOI] [PubMed] [Google Scholar]
- Hagger M.S., Chatzisarantis N.L. (2013) The sweet taste of success: the presence of glucose in the oral cavity moderates the depletion of self-control resources. Personality and Social Psychology Bulletin 39, 28-42. [DOI] [PubMed] [Google Scholar]
- Ispoglou T., O’Kelly D., Angelopoulou A., Bargh M., O’Hara J.P., Duckworth L.C. (2015) Mouth rinsing with carbohydrate solutions at the postprandial state fail to improve performance during simulated cycling time trials. The Journal of Strength & Conditioning Research 29, 2316-2325. [DOI] [PubMed] [Google Scholar]
- Jeukendrup A., Wallis G. (2005) Measurement of substrate oxidation during exercise by means of gas exchange measurements. International Journal of Sports Medicine 26, S28-S37. [DOI] [PubMed] [Google Scholar]
- Johnson J., Duick D., Chui M., Aldasouqi S. (2009) Identifying prediabetes using fasting insulin levels. Endocrine Practice 16, 47-52. [DOI] [PubMed] [Google Scholar]
- Just T., Pau H.W., Engel U., Hummel T. (2008) Cephalic phase insulin release in healthy humans after taste stimulation? Appetite 51, 622-627. [DOI] [PubMed] [Google Scholar]
- Kjaer M. (1998) Hepatic glucose production during exercise. In: Skeletal Muscle Metabolism in Exercise and Diabetes. Ed: Richter E.A., Kiens B., Galbo H., Saltin B. Boston: Springer; 117-127. [Google Scholar]
- Lane S.C., Bird S.R., Burke L.M., Hawley J.A. (2012) Effect of a carbohydrate mouth rinse on simulated cycling time-trial performance commenced in a fed or fasted state. Applied Physiology, Nutrition, and Metabolism 38, 134-139. [DOI] [PubMed] [Google Scholar]
- Manolio T.A., Savage P.J., Burke G.L., Hilner J.E., Liu K., Orchard T.J., Sidney S., Oberman A. (1991) Correlates of fasting insulin levels in young adults: the CARDIA study. Journal of Clinical Epidemiology 44, 571-578. [DOI] [PubMed] [Google Scholar]
- Oppliger R.A., Magnes S.A., Popowski L.A., Gisolfi C.V. (2005) Accuracy of urine specific gravity and osmolality as indicators of hydration status. International Journal of Sport Nutrition and Exercise Metabolism 15, 236-251. [DOI] [PubMed] [Google Scholar]
- Phillips S.M., Findlay S., Kavaliauskas M., Grant M.C. (2014) The influence of serial carbohydrate mouth rinsing on power output during a cycle sprint. Journal of Sports Science & Medicine 13, 252-258. [PMC free article] [PubMed] [Google Scholar]
- Pottier A., Bouckaert J., Gilis W., Roels T., Derave W. (2010) Mouth rinse but not ingestion of a carbohydrate solution improves 1-h cycle time trial performance. Scandinavian Journal of Medicine & Science in Sports 20, 105-111. [DOI] [PubMed] [Google Scholar]
- Robertson M.D., Jackson K.G., Williams C.M., Fielding B.A., Frayn K.N. (2001) Prolonged effects of modified sham feeding on energy substrate mobilization. The American Journal of Clinical Nutrition 73, 111-117. [DOI] [PubMed] [Google Scholar]
- Rollo I., Cole M., Miller R., Williams C. (2010) Influence of mouth rinsing a carbohydrate solution on 1-h running performance. Medicine and Science in Sports and Exercise 42, 798-804. [DOI] [PubMed] [Google Scholar]
- Sinclair J., Bottoms L., Flynn C., Bradley E., Alexander G., McCullagh S., Finn T., Hurst H.T. (2014) The effect of different durations of carbohydrate mouth rinse on cycling performance. European Journal of Sport Science 14, 259-264. [DOI] [PubMed] [Google Scholar]
- Teff K.L., Devine J., Engelman K. (1995) Sweet taste: effect on cephalic phase insulin release in men. Physiology & Behavior 57, 1089-1095. [DOI] [PubMed] [Google Scholar]
- Teff K.L., Engelman K. (1996) Palatability and dietary restraint: effect on cephalic phase insulin release in women. Physiology & Behavior 60, 567-573. [DOI] [PubMed] [Google Scholar]
- Teff K.L., Mattes R.D., Engelman K. (1991) Cephalic phase insulin release in normal weight males: verification and reliability. American Journal of Physiology-Endocrinology and Metabolism 261, E430-E436. [DOI] [PubMed] [Google Scholar]
- Watson P., Nichols D., Cordery P. (2013) Mouth rinsing with a carbohydrate solution does not influence cycle time trial performance in the heat. Applied Physiology, Nutrition, and Metabolism 39, 1064-1069 [DOI] [PubMed] [Google Scholar]


