Abstract
Low-load blood flow restricted (BFR) resistance exercise has been suggested to be as effective as moderate and high-load resistance training for increasing muscle size and strength. The purpose of the study was to evaluate the effects of 6 weeks of HL or low-load BFR resistance training on neuromuscular function, strength, and hypertrophy of the knee extensors. Eighteen participants aged 18-22 years old were randomized to one of three training groups: moderate load (ML: 70% of 1 repetition maximum [1-RM]); BFR (20% 1-RM with a vascular restriction set to ~180 mmHg); and a control group (CON) that did not exercise. Participants performed leg extension (LE) and leg press exercises 3 times per week for 6 weeks. Measurements of isometric torque, LE 1-RM, central activation, electrically evoked torque, and muscle volume of the knee extensors were obtained before and after training. Isometric peak torque did not change following the training (p = 0.13). LE 1-RM improved in the ML (34 ± 20%; d = 0.78) and BFR (14 ± 5%; d = 0.67) groups compared to the CON group (0.6 ± 8%; d = 0.09; time x group interaction p = 0.02). Muscle volume increased in the ML (5.6%; d = 0.19) and BFR groups (2.5%; d = 0.09) with no change in the CON group (time x group interaction p = 0.001). There were no changes in central activation and evoked torque in any groups following the training (p > 0.05). Strength and hypertrophy were evident following ML and BFR resistance training programs indicating that both modalities are effective, although ML training appears to be a more potent and efficient. Neuromuscular changes were not evident and warrant more research.
Key points.
This study supports the notion that low-load resistance exercise with a blood flow restriction facilitates increases in muscle size and strength.
The results of this study has implications for elderly and clinical populations who would benefit from gains in muscle size and strength but cannot tolerate the mechanical strain associated with lifting heavy loads.
The central and peripheral neuromuscular variables measured in this study were not enhanced in either group, which indicates that 1) the neuromuscular factors other than those assessed contribute to training adaptations; or 2) the adaptations of the variables assessed were not apparent at the time of testing.
Key words: Strength training, central activation, hypertrophy
Introduction
Low-load resistance training with a blood flow restriction (BFR) has consistently demonstrated favorable training adaptations of improved muscle strength and cross-sectional area of the quadriceps (Ellefsen et al., 2015; Laurentino et al., 2012; Martín-Hernández et al., 2013). These gains in leg extension muscle strength (~20-30%) and quadriceps size (~6-8%) are comparable to those induced by traditional moderate-to-high-load training, albeit using substantially lighter loads (Ellefsen et al., 2015; Karabulut et al., 2010; Kubo et al., 2006; Laurentino et al., 2012; Takarada et al., 2000a). Since there are clinical populations, such as those with osteoarthritis, that are encouraged to avoid heavy weight bearing activities (Chilibeck et al., 2011), implementing low-load BFR training may be a viable option to promote muscular hypertrophy and increased strength. A limitation to this modality of exercise is that there is currently no common consensus among researchers and clinicians on the optimal implementation of BFR exercise. As summarized by Scott et al. (2015), the most frequent use of BFR exercise includes an inflatable cuff applied to the proximal limbs that are set to pressures based on brachial blood pressure or arterial occlusion pressure. Multiple sets of exercise are performed for a designated number of repetitions or to muscular failure. The loads range from 20-40% of one repetition maximum (1-RM) strength and it tends to be the most effective if the cuff regularly remains inflated during rest periods between sets (Cook et al., 2007; Scott et al., 2015).
While it is well established that low-load BFR training can elicit increased muscle size and strength, the mechanisms underpinning these adaptations remain to be fully explained. Gains in strength from traditional moderate-to-high load resistance training are optimized by concomitantly increasing muscle size and enhancing neural function and coordination (Kraemer et al., 1996; Sale, 1988). Since the magnitude of strength gains exceeds the gains in hypertrophy, it is likely that neural adaptations contribute to the strength gains. Although hypertrophy has been observed in numerous studies following low-load BFR training, there is a considerable lack of data to describe whether neural adaptations also lead to the improved strength levels. It is known that a session of BFR exercise results in greater muscular activation than exercise at the same load without BFR (Sundberg, 1994; Takarada et al., 2000b), yet muscle activation during low-load BFR exercise remains substantially lower than that of moderate-to-high load resistance exercise (Cook et al., 2013). This observation suggests that further exploration into the central and peripheral neuromuscular mechanism involved in strength gains is required.
There are apparent differences in neuromuscular activity during acute bouts of BFR and traditional resistance exercise but data regarding the neuromuscular adaptations to BFR resistance training are extremely limited. For example, Moore et al. (2004) and Kubo et al. (2006) reported improvements in strength following BFR training without any changes in the ability to centrally recruit motor units. Together, these studies may suggest that increases in strength following low-load BFR exercise arise predominately from muscular hypertrophy, whereas enhanced strength after moderate-to high load resistance training typically occurs from an interplay between hypertrophic and neuromuscular improvements.
Since low-load BFR resistance exercise is suggested as an alternative to traditional resistance training, more novel and innovative studies directly comparing the neuromuscular effects of low-load BFR and moderate load (ML) training are warranted. Understanding how central activation and peripheral neuromuscular factors, such as twitch torque and rate of torque development and relaxation, change after both types of training is needed to describe the overall effects on muscular function. Therefore, the purpose of this study was to compare central and peripheral neuromuscular adaptations and cross-sectional area (CSA) changes following 6 weeks of ML or BFR training on the knee extensor muscles and to evaluate the impact of these training regimens on muscular strength. It was hypothesized that muscle strength and CSA would increase in both types of training and that central neuromuscular adaptations would be more prominent in the HL training regimen.
Methods
Participants
Eighteen healthy, untrained men and women aged 18-22 years participated in this study (Table 1). They were classified as moderately active according to the Lipid Research Clinics Physical Activity Questionnaire (Ainsworth et al., 1993) though they were not currently participating in resistance training of the legs and did not report engaging in structured exercise within the last three months. Participants gave their written informed consent and the study was approved by the local Institutional Review Board. Individuals with orthopedic limitations of the legs, a history of cardiovascular disease, a history or risk of abnormal blood clotting or who were smokers were excluded from participation.
Table 1.
Descriptive statistics of the study sample. Values are means ± (SD).
| ML | BFR | Control | ||||
|---|---|---|---|---|---|---|
| Men (n =3) | Women (n =3) | Men (n =3) | Women (n =3) | Men (n =3) | Women (n =3) | |
| Age (yr) | 19.3 (1.2) | 21 (0.0) | 19.0 (1.0) | 19.7 (1.5) | 19.7 (1.5) | 21 (1.0) |
| Stature (m) | 1.73 (.02) | 1.62 (.10)* | 1.79 (.12) | 1.66 (.07)* | 1.74 (.03) | 163 (.10)* |
| Mass (kg) | 74.5 (9.8) | 54.6 (5.8)* | 74.9 (6.9) | 64.0 (13.1)* | 67.0 (4.9) | 67.9 (9.8)* |
* indicates significant difference between men and women (p < 0.05). ML: moderate-load, BFR: blood flow restricted.
Experimental design
The duration of the study was 8 weeks, with pre- and post-training measurements collected one week prior to and following 6 weeks of resistance training using bilateral leg extension (LE) and leg press exercises. Upon entering into the study, the participants underwent familiarization sessions and baseline measures of leg strength, muscle volume, and neuromuscular function. Using a stratified randomization based on sex, the participants were assigned to one of 3 groups: ML, BFR, or a non-exercising control group (CON). Measurements to quantify neuromuscular adaptations to exercise were obtained at baseline and following the intervention approximately 48 hours post-training.
Training protocols
Resistance training was performed using seated LE and horizontal leg press machines (Nautilus Nitro, Med-Fit Systems, Independence, VA). Considering that several neural adaptations were assessed via isometric knee extensions, the LE was considered the primary training stimulus in this study, while the leg press was used to provide supplementary overload for the knee extensors.
At each training session, participants completed 3 sets of bilateral LE and leg press in a random order, with 30 s of rest between sets and 180 s between exercises. Participants in the ML group trained with a load of 70% 1-RM for 2 sets of 10 repetitions before performing the third set to failure. Participants in the BFR group performed 2 sets of 25 repetitions and a third set to failure using 20% 1-RM. Exercise failure was defined as an inability to maintain the specified rate of contractions (2 s concentric and 2 s eccentric) or an inability to maintain full range of motion for two consecutive contractions. In the BFR condition, participants performed the exercise with specially-designed tourniquet cuffs (54 mm wide; KAATSU Master Mini, Sato Sports Plaza, Tokyo, Japan) applied to the proximal thighs at a suprasystolic pressure. The cuff pressure ranged from 180 mmHg to 200 mmHg. The BFR was maintained continuously throughout each exercise, including inter-set rest periods, but was released between the exercises. The participants in the CON group were asked to not engage in any exercise throughout the duration of the study. This was confirmed through weekly visits with the researchers to ensure no other exercise was being undertaken.
The loads used during training were determined during 1-RM testing (explained in detail below) and remained consistent throughout the training program, with progressive overload achieved via increases in the number of repetitions performed to failure. However, an unforeseen limitation of this exercise protocol was that several participants in the BFR condition did not reach volitional failure in the leg press within 20 minutes, possibly because of contributions from the non-restricted gluteal muscles. To limit potential negative effects associated with prolonged BFR such as bruising, numbness and lightheadedness (Clark et al. 2011), these participants ceased exercise after 20-minutes. Importantly though, all participants did reach fatigue for all sessions with the primary LE exercise.
Neuromuscular measurements
Knee extensor peak torque: Maximum isometric torque of the knee extensors on the dominant leg was measured from each subject on a HUMAC Norm dynamometer (CSMI, Stoughton, MA, USA) with analog output that was sampled using the BIOPAC MP150 data acquisition system (AcqKnowledge 4.1 software; BIOPAC Systems Inc., CA, USA). To obtain peak torque, participants were seated in the dynamometer at a hip angle of 85° with the torso secured by a seat belt preventing hip movements during trials. Participants’ knee angle was set at 60° and they were instructed to extend the knee with as much force against an immovable pad of the dynamometer for 3-5 s. This was repeated 3-5 times until the difference in peak torque between two trials was within 5%. Participants rested 1-2 minutes between all attempts. Standardized and consistent verbal encouragement was provided to all subjects.
Electrically evoked torque: Measurements of electrically evoked contraction torques were elicited on the dominant leg by transcutaneous stimulation of the femoral nerve using a hand-held cathode probe placed in the inguinal triangle. When the highest isometric twitch torque produced from the stimulation with the probe was determined, a surface electrode (Ag-AgCl, 36 mm diameter; Kendall MediTrace 200) was placed over the skin and used for subsequent stimulations. An anode was placed on the skin over the greater trochanter (Ag-AgCl, 48 mm diameter; Kendall MediTrace 530). The stimulus consisted of a 1 ms rectangular pulse with 400-V maximum voltage (Digitimer constant current stimulator model DS7AH coupled with the train/delay generator, Hertfordshire, UK). Supramaximal stimulation was achieved by increasing the stimulus intensity 10% beyond that required to elicit peak twitch torque. Doublet (interpulse duration was 6 ms apart) torques and post activation potentiated torque were measured and recorded on the HUMAC Norm dynamometer that was interfaced with the BIOPAC MP150 data acquisition system.
Central activation: Voluntary activation of the knee extensors was assessed using the interpolated twitch technique as previously described (Clark et al., 2007). After evoked torque was measured, participants performed a 4-5 s isometric maximum voluntary contractions. During that time, a supramaximal doublet was delivered to the femoral nerve and again within 1-2 s after the completion of the isometric contraction. The last supramaximal stimulus was the post activation potentiated doublet. The increase in torque following the initial doublet was expressed to the post activation potentiated doublet and expressed as follows:
| %Central Activation = 1 – (doublet torque during isometric contraction ÷ post activation potentiated double torques) x 100 |
Contractile properties: To identify changes in the functional contractile properties of the knee extensors, the torque-time curve from the post activation potentiated torque was evaluated. Peak torque, time to peak torque and time to half-relaxation was calculated.
Muscle size and dynamic strength
Muscle volume: To quantify changes in muscle mass, serial axial plane magnetic resonance imaging scans (10 mm slice thickness, 15 mm apart, 2120 ms repetition time) were acquired from both upper legs using a 1.5 Tesla Siemens Espree Scanner (Siemens Medical Systems, Erlangen, Germany) with a receiver body array coil and a field of view of 420 mm. These scans were obtained between 48 and 72 hours after completion of any exercise and testing sessions. After a 30 minute supine rest period to allow for fluid equilibration, cross-sectional images from the femoral greater trochanter to the patella were obtained. An average of 11 ± 2 slices was used for analysis. The digitized images were transferred to a computer for calculation of muscle volume using the National Institutes of Health ImageJ software (Abramoff et al., 2004). The vasti muscles (lateralis, medialis, and intermedius) and the rectus femoris on the right leg were identified and traced. Slices between the identification of the distal rectus femoris and the appearance of the femoral neck were located and traced to quantify the quadriceps femoris. The same number of slices from the same anatomical locations was analyzed for each participant at pre- and post-training by the same investigator. Volume was calculated as the sum of the measured slices with consideration of interslice gaps.
One repetition maximum (1-RM) testing: Bilateral 1-RM on the seated LE and leg press machines were measured using the procedures described by Baechle and Earle (2008). Two to four minutes of rest was given between attempts and the 1-RM measurements were determined within 4-6 attempts. Six male participants were able to lift the weight stack on the machines at pre-training and/or post-training and in such instances, the number of repetitions performed at the maximum weight of the machine (116 kg) was performed and the 1-RM was then predicted using the Brzycki equation (Brzycki, 1993). The 1-RM scores were used to prescribe exercise load for the training protocols and the LE 1-RM was used to evaluate strength improvements from training.
Statistical analysis
Because of the paucity of data regarding the neuromuscular measurements assessed in this study, the sample size calculation was powered to detect significance between three groups with respect to knee extension strength based on the effect size determined from data presented by Karabulut et al. (2010). The parameters used for this model were an effect size = 0.53, at a power = 0.9 and a specified alpha = 0.05.
Data are expressed as means and standard deviations. Reliability of the dependent variables has been reported previously with intraclass correlations ranging from 0.7-0.99 (Cook et al. 2014). Box plots were used to examine the data distribution. Multivariate analysis of variance (MANOVA) was used to compare the groups at pre-training for all dependent variables simultaneously. A two-way repeated measures analysis of variance (ANOVA) procedure was used to detect differences in the dependent variables with respect to the within-subjects independent variable (pre-training vs post-testing) and the between subjects factor (ML, BFR, CON). Significant interactions and main effects were followed with appropriate post hoc tests, including Tukey post hoc tests or paired t-tests with Bonferroni adjustments. Cohen’s d were computed and effect sizes were categorized as small (0.10), medium (0.50), or large (0.80) (Cohen, 1988). These values were used to compare the training adaptations between training groups. An alpha level of ≤ 0.05 was required for statistical significance. Statistics were computed using SPSS Statistics version 22.0 (Chicago, IL).
Results
The age, mass and stature of the men and women were similar within the three training groups (p > 0.05), but there was a significant main effect of sex indicating that the males were taller and heavier than the females (Table 1; p < 0.05). There were no significant differences between the groups at pre-training when all dependent variables were simultaneously considered (p = 0.78). There was 100% subject compliance in the training sessions. The participants in the BFR training condition lifted an average load of 68 ± 41 kg on the LE at each session and always performed significantly more repetitions in the exercise session than the participants in the ML training group (average load = 136 ± 50 kg; p < 0.01) (Figure 1). The ML training group significantly increased the average number of repetitions per session performed in each exercise session from week 1 to weeks 4, 5 and 6 (p < 0.01) while the BFR group had greater variability in the average number of repetitions per session completed and only experienced a significant increase from week 1 to week 6 (p < 0.05). Accordingly, LE exercise volume at the end of the study was significantly higher in the BFR group than the ML group (12,250 ± 4971 kg vs 5466 ± 2201 kg, respectively) (p < 0.05). The average duration of training was 11.6 ± 4.6 minutes for the BFR condition and 3.4 ± 0.4 minutes for the ML condition and was significantly different between the groups (p < 0.05).
Figure 1.
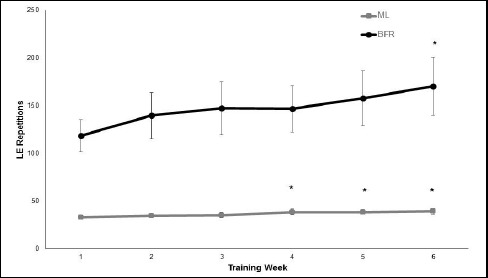
The mean± standard deviation leg extension (LE) repetitions performed at the weekly sessions of moderate-load (ML) and low-load blood flow restricted (BFR) resistance training. * denotes significant increase from Week 1 in the number of repetitions performed (p < 0.05).
Figure 2 depicts box plots of percent change in isometric torque, LE 1-RM and muscle volume following the training regimens and Table 2 depicts corresponding effect sizes. Isometric peak torque of the knee extensors was statistically similar among the three groups prior to training (p = 0.15) and did not significantly change following 6-weeks of resistance training (p = 0.13; d range = 0.09 - 0.44; Fig.2a, Table 2).There was a significant time x group interaction (p = 0.02) in the LE 1-RM that indicated significant improvements in the ML (34%; p = 0.02; d = 0.78) and BFR (13%; p = 0.01; d = 0.67) groups and no change in the CON group (1.5%; p = 0.64; d = 0.07; Figure 2b, Table 2). There was a significant time x group interaction (p = 0.02) in knee extensor volume as there was an increase of 5.6% in the ML group (p = 0.02; d = 0.19) and 2.5% in the BFR group (p = 0.03; d = 0.09) but no change in the CON group (p = 0.96; d = 0.00; Figure 2c, Table 2).
Figure 2.
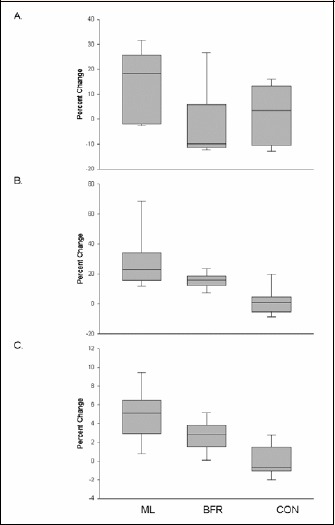
Box plots of percent change following moderate-load (ML), low-load blood flow restricted (BFR) and control (CON) group interventions in a) isometric torque, b) leg extension one-repetition maximum (LE 1-RM) and c) knee extensor muscle volume. The top and bottom lines and the line through the middle of the box represent the 75th percentile (top quartile), 25th percentile (bottom quartile), and 50th percentile (median), respectively. The whiskers on the bottom extend from the 10th percentile (bottom decile) and top 90th percentile (top decile). There are significant time x group interactions for LE 1-RM (p = 0.02) and knee extensor volume (p = 0.02).
Table 2.
Pre and post strength values and effect sizes for all training programs. Values are mean ± (SD).
| ML | BFR | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | d | Pre | Post | d | Pre | Post | d | |
| Isometric Torque (Nm) | 203(64) | 238(92) | .44 | 273(58) | 267(55) | .11 | 227(53) | 232(60) | .09 |
| LE 1-RM (kg) | 88(32) | 118(44)* | .78 | 116(21) | 131(24)* | .67 | 124(30) | 126(38) | .07 |
| Muscle Volume (cm3) | 1690(464) | 1785(528)* | .19 | 1952(539) | 2001(531)* | .09 | 1945(384) | 1945(389) | .00 |
* indicates significant difference from Pre (p < 0.05). ML: moderate-load, BFR: blood flow restricted, d: Cohen’s d, LE 1-RM: leg extension one repetition maximum
There were no significant interactions or main effects for electrically evoked isometric twitch and doublet torque, rates of torque development and relaxation and central activation (p > 0.05), but the ML group experienced an increase in doublet torque that was considered a large effect (d = 0.80), while the BFR group had decrements in central activation and half-relaxation time that were considered large effects (d = -0.78 and 0.84, respectively). These data are displayed in Table 3.
Table 3.
Neuromuscular measurements before and after 6-weeks of moderate-load (ML) or low-load blood flow restricted (BFR) resistance training. Values are means (±SD).
| ML | BFR | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | d | Pre | Post | d | Pre | Post | d | |
| Twitch Torque (Nm) | 43(11) | 42(5) | -.35 | 55(20) | 55(21) | .00 | 41(12) | 43(11) | .17 |
| Doublet Torque (Nm) | 55(11) | 63(9) | .80 | 75(26) | 79(33) | .13 | 62(16) | 65(11) | .22 |
| PAP Torque (Nm) | 67(24) | 66(16) | -.05 | 85(31) | 90(38) | .14 | 65(22) | 67(17) | .10 |
| CAR (%) | 86(9) | 89(8) | .35 | 97(2) | 95(3) | -.78 | 93(12) | 93(8) | .00 |
| TPT (ms) | 50(8) | 56(9) | .70 | 52(11) | 54(8) | .21 | 58(16) | 63(20) | .28 |
| HRT (ms) | 139(54) | 137(58) | -.04 | 143(46) | 192(68) | .84 | 125(49) | 104(46) | -.44 |
ML: moderate-load, BFR: blood flow restricted, d: Cohen’s d, CAR: central activation ratio, PAP: post-activation potentiated doublet torque, TPT: time to peak torque, HRT: half-relaxation time. Pre is at 0 weeks of training and post is after 6 weeks of training.
Discussion
This study evaluated the neuromuscular adaptations following 6 weeks of ML or BFR training on the knee extensor muscles. The main findings of this study were that dynamic muscle strength and size improved following ML and BFR resistance training, yet there were no significant alterations in the central and peripheral neuromuscular variables measured in this study.
The present study did not show statistical differences between the magnitude of strength and hypertrophy gains in the ML and BFR training programs, but LE 1-RM strength was almost three times greater following ML training (34% and 13% improvement in 1-RM in the ML and BFR groups, respectively) and yielded larger effect sizes. Increases in muscle strength following moderate-to-high load and BFR resistance training have been reported in other studies (Kubo et al., 2006; Karabulut et al., 2010; Laurentino et al., 2012; Martín-Hernández et al., 2013; Takarada et al., 2000a). For example, Laurentino et al. (2012) assessed the knee extensors after 8-weeks of high-load or BFR leg extension training and found that 1-RM and cross-sectional area improved equally following both programs (36% in 1-RM and 6% in cross-sectional area). Martín-Hernández et al. (2013) reported greater 1-RM strength following high-load training when compared to BFR training (19% vs 7%) despite similar 8% gains in muscle mass. Additionally, muscle volume increases were twofold greater after ML training compared to BFR training (6% and 3%, respectively). The differences in ML and BFR training were apparent in spite of the BFR training being of higher volume and longer duration. Disproportionate gains in muscle mass and strength are typical following moderate-to-high load resistance training as hypertrophy and various neuromuscular adaptations combine to enhance force-generating capacity of muscle (Kraemer et al., 1996). This was evident in the present study as the magnitude of the gains in strength (average of 23.5% for ML and BFR combined) and muscle volume (average of 4.5% for ML and BFR combined) were not congruent. However, these findings are contrary to Kubo et al. (2006) who reported that hypertrophy was the primary mechanism for strength increases following BFR training since the muscle volume and strength improvements were of similar magnitudes.
The central and peripheral neuromuscular adaptations that were evaluated in this study were obtained through the use of the interpolated twitch technique and measures of evoked torque. There were no significant changes in central activation as the ML group demonstrated a 3% (d = 0.35) increase, whereas the BFR group showed a 2% (d = 0.78) reduction. These values are numerically similar to those of Kubo et al. (2006) who reported a significant 3% increase in central activation following high-load training and a non-significant decline of 1.5% after BFR training. The magnitude of change following ML training also aligns with Knight and Kamen (2008) who demonstrated a 2% improvement in central activation following six weeks of high-load resistance training on the knee extensors. These results suggest that we should further assess the involvement of the central nervous system following BFR resistance exercise as strength improvements may not always be of neural origin. It should be noted that these measurements do not encompass all possible neuromuscular adaptations as they provide assessments of central activation and certain aspects of skeletal muscle contractile function. Other adaptations, such as motor cortex and spinal cord excitability and inhibition and muscle architecture, were not assessed in this study. In other BFR research, Clark et al. (2011) found no changes in nerve conduction velocity before and after four weeks of BFR resistance training and resistance training at 80% of maximum strength. Brandner et al. (2015) has recently reported on enhanced corticomotor excitability following one session of BFR training. It is important to consider that the present study was not powered for improvements in central activation and the participants in the study were healthy, moderately-active, untrained, young adults that already possessed high levels of quadriceps muscle activation.
Furthermore, there were no significant changes in peripheral nerve and contractile function observed in our study with evoked torque, time to peak torque, and half-relaxation time remaining constant across all groups. Similar to our findings, Ishida et al. (1990) reported strength improvements following 8-weeks of moderate-to-high load training on the plantar-flexors but could not attribute the strength gains to enhanced contractile function as twitch torque and rates of torque development and relaxation remained constant. There was a tendency for half-relaxation time to slow in the BFR group (34%; d=.84). This is an interesting finding to further explore as the muscle contractile processes depend on Ca2+ sensitivity and efficiency of Ca2+ movement into and out of the sarcoplasmic reticulum. It is known that fast-twitch and slow-twitch muscle fibers differ in the number and size of sarcoplasmic reticulum Ca2+ pumps, such that the slow-twitch fibers have a lower rate of Ca2+ uptake, resulting in slower half-relaxation time (Stephenson et al., 1998). Nielsen et al. (2017) reported a delayed adaptation to BFR exercise as improvements in muscle function were not apparent until twelve days upon completion of a training program. It is possible that a transient impairment in contractile function following BFR exercise persisted through the exercise testing 48 hours after the completion of training.
The current study was unable to replicate the findings of Moore et al. (2004) who noted a depressed resting twitch torque and an augmented post activated potentiated twitch torque following low-load BFR exercise in the biceps. A decline in resting twitch torque following a training regimen would indicate a negative adaptation that the authors speculated could be related to low-frequency fatigue. It is interesting to note that the ML training group showed a non-significant increase in doublet torque accompanied by a high effect size (d = 0.80) perhaps signifying a possible adaptation not evident following BFR training. Moore et al. (2004) also suggested that the increase in post activation potentiated torque following BFR training, which can be considered a positive adaptation that allows muscles to generate more force during submaximal activities, was possibly a compensatory mechanism in response to the depressed twitch torque. In the present study, PAP increased non-significantly with a small effect size (d = 0.14), suggesting more research into this concept.
It is important to note that the central and peripheral neuromuscular measurements in our study were conducted during isometric contractions. The overall strength increases observed in the present study are specific to the training mode of isotonic contractions and the lack of significant change in isometric muscle strength may mask neural adaptations assessed through isometric contractions. To illustrate, Sale et al. (1992) reported increases in 1-RM strength and cross-sectional area following 10 weeks of elbow flexor training despite no changes in isometric strength and twitch torque. They attributed the improvement in 1-RM to neural factors related to coordination and learning, such as a decrease in agonist and antagonist activation, and suggested the possibility that early increases in muscle mass may not necessarily contribute significantly to the enhanced strength.
There are some limitations to our study that should be addressed. Firstly, the sample size for this study was based on effect sizes from knee extension strength improvements of a previous study as the neuromuscular assessments employed in this study have not previously been investigated. Because of the intricacies of measurements, we believe this small-scale study provides substantial insight and direction into future BFR studies. A second limitation to this study is that the training loads were not progressed and it is likely that by the study’s conclusion participants were exercising at lower relative load. However, this was addressed by having all participants exercise to volitional failure, which has been shown as a valid method to induce progressive overload, particularly at light loads (Burd et al., 2012; Cook et al., 2007). A final limitation in this study is that it assesses strength and hypertrophic adaptations following ML and BFR resistance exercise in untrained males and females. While males and females engage in BFR training, the inclusion of mixed-gender groups may have obscured some findings since women have demonstrated greater endurance during BFR exercise than males (LaBarbera, et al., 2013). Also, the magnitude of adaptations apparent in our study may not be as prominent in individuals that already possess high levels of muscle strength and mass due to regular resistance training.
Conclusions
Our results demonstrate significant gains in muscular size and strength following six weeks of ML and low-load BFR training, with ML training appearing to show greater magnitudes of adaptation in a more efficient and timely manner in each exercise session. There were no significant changes in central and peripheral neuromuscular function yet moderate-to-large effect sizes in central activation and evoked torque suggest that further study on the neuromuscular variables is necessary. This study has implications for several clinical populations who would benefit from gains in muscle size and strength but cannot tolerate the mechanical strain associated with lifting heavy loads. BFR resistance training may be a suitable modality of resistance exercise until moderate-to-high load resistance training is achievable.
Acknowledgements
This work was supported by the American College of Sports Medicine Foundation for KAATSU Research. This study was approved by the University of New Hampshire Institutional Review Board (IRB #4738) and conforms to the Declaration of Helsinki. The experiments comply with the current laws of the country in which they were performed. The authors have no conflict of interest to declare.
Biographies

Summer B. COOK
Employment
Associate Professor, Department of Kinesiology, University of New Hampshire
Degree
PhD
Research interest
Neuromuscular function, aging and blood flow restricted exercise.
E-mail: summer.cook@unh.edu

Brendan R. SCOTT
Employment
Lecturer in Strength and Conditioning, School of Psychology and Exercise Science, Murdoch University, AUS.
Degree
PhD
Research interest
Resistance training methods, athlete monitoring
E-mail: Brendan.Scott@murdoch.edu.au

Katherine L. HAYES
Employment
Postdoctoral associate at the University of Massachusetts Medical School
Degree
PhD
Research interest
Effect of exercise on stem cells in healthy and diseased conditions
E-mail: klabhayes@gmail.com

Bethany G. MURPHY
Employment
Clinical Instructor at the Birchtree Center, Portsmouth, NH
Degree
BSc
Research interest
Exercise and health
E-mail: bgmurphy52@yahoo.com
References
- Abramoff M.D., Magelhaes P.J., Ram S.J. (2004) Image processing with ImageJ. Biophotonics International 11, 36-42. [Google Scholar]
- Ainsworth B.E., Jacobs D.R., Leon A.S. (1993) Validity and reliability of self-reported physical activity status: the Lipids Research Clinics questionnaire. Medicine and Science in Sports and Exercise 25, 92-98. [DOI] [PubMed] [Google Scholar]
- Baechle T.R., Earle R.W. (2008) Essentials of Strength Training and Conditioning. 3rd edition Human Kinetics, Champaign, IL; p.396. [Google Scholar]
- Brzycki M. (1993) Strength testing: predicting a one-rep max from repetitions to fatigue. Journal of Physical Education, Recreation and Dance 64, 88-90. [Google Scholar]
- Brandner C.R., Warmington S.A., Kidgell D.J. (2015) Corticomotor excitability is increased following an acute bout of blood flow restriction resistance exercise. Frontiers in Human Neuroscience 9, 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd N.A., Mitchell C.J., Churchward-Venne T.A., Phillips S.M. (2012) Bigger weights may not beget bigger muscles: evidence from acute muscle protein responses after resistance exercise. Applied Physiology, Nutrition, Metabolism 37(3), 551-554. [DOI] [PubMed] [Google Scholar]
- Chilibeck P.D., Vatanparast H., Cornish S.M., Charlesworth S. (2011) Evidence-based risk assessment and recommendations for physical activity: arthritis, osteoporosis, and low back pain. Applied Physiology, Nutrition, Metabolism 36(Suppl 1), S49-70. [DOI] [PubMed] [Google Scholar]
- Clark B.C., Cook S.B., Ploutz-Snyder L.L. (2007) Reliability of techniques to assess human neuromuscular function in vivo. Journal of Electromyography and Kinesiology 17, 90-101 [DOI] [PubMed] [Google Scholar]
- Clark B.C., Manini T.M., Hoffman R.L., Williams P.S., Guiler M.K., Knutson M.J., McGlynn M.L., Kushnick M.R. (2011) Relative safety of 4 weeks of blood flow-restricted resistance exercise in young, healthy adults. Scandinavian Journal of Medicine and Science in Sports 21, 653-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis for the behavioral sciences. 2nd edition New Jersey: Lawrence Erlbaum. [Google Scholar]
- Cook S.B., Clark B.C., Ploutz-Snyder L.L. (2007) Effects of exercise load and blood flow restriction on skeletal muscle function. Medicine and Science in Sports and Exercise 39, 1708-1713. [DOI] [PubMed] [Google Scholar]
- Cook S.B., Murphy B.G., Labarbera K.E. (2013) Neuromuscular function following a bout of low-load blood flow restricted exercise. Medicine and Science in Sports and Exercise 45(1), 67-74. [DOI] [PubMed] [Google Scholar]
- Cook S.B., Kanaley J.A., Ploutz-Snyder L.L. (2014). Neuromuscular function following unloading and blood flow restricted exercise. European Journal of Applied Physiology 114(7), 1357-1365. [DOI] [PubMed] [Google Scholar]
- Ellefsen S., Hammarström D., Strand T.A., Zacharoff E., Whist J.E., Rauk I., Nygaard H., Vegge G., Hanestadhaugen M., Wernbom M., Cumming K.T., Rønning R., Raastad T., Rønnestad B.R. (2015) Blood flow-restricted strength training displays high functional and biological efficacy in women: a within-subject comparison with high-load strength training. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 309(7), R767-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K., Moritani T., Itoh K. (1990) Changes in voluntary and electrically induced contractions during strength training and detraining. European Journal of Applied Physiology 60, 244-248. [DOI] [PubMed] [Google Scholar]
- Karabulut M., Abe T., Sato Y., Bemben M.G. (2010) The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. European Journal of Applied Physiology 108(1), 147-155. [DOI] [PubMed] [Google Scholar]
- Knight C.A., Kamen G. (2008) Relationships between voluntary activation and motor unit firing rate during maximal voluntary contractions in young and older adults. European Journal of Applied Physiology 103, 625-630. [DOI] [PubMed] [Google Scholar]
- Kraemer W.J., Fleck S.J., Evans W.J. (1996) Strength and power training: Physiological mechanisms of adaptation. Exercise and Sport Sciences Reviews 24, 363-397. [PubMed] [Google Scholar]
- Kubo K., Komuro T., Ishiguro N., Tsunoda N., Sato Y., Ishii N., Kanehisa H., Fukunaga T. (2006) Effects of low-load resistance training with vascular occlusion on the mechanical properties of muscle and tendon. Journal of Applied Biomechanics 22, 112-119. [DOI] [PubMed] [Google Scholar]
- LaBarbera K.E., Murphy B.G., LaRoche D.P., Cook S.B. (2013) Sex differences in blood flow restricted isotonic knee extensions to fatigue. Journal of Sports Medicine and Physical Fitness 53(4), 444-452. [PubMed] [Google Scholar]
- Laurentino G.C., Ugrinowitsch C., Roschel H., Aoki M.S., Soares A.G., Neves M., Aihara A.Y., Frenandes A.R., Tricoli V. (2012) Strength training with blood flow restriction diminishes myostatin gene expression. Medicine and Science in Sports and Exercise 44, 406-412. [DOI] [PubMed] [Google Scholar]
- Martín-Hernández J., Marín P.J., Menéndez H., Ferrero C., Loenneke J.P., Herrero A.J. (2013) Muscular adaptations after two different volumes of blood flow-restricted training. Scand Journal of Medicine and Science in Sports 23, 114-120. [DOI] [PubMed] [Google Scholar]
- Moore D.R., Burgomaster K.A., Schofield L.M., Gibala M.J., Sale D.G., Phillips S.M. (2004). Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. European Journal of Applied Physiology 92, 399-406. [DOI] [PubMed] [Google Scholar]
- Nielsen J.L., Frandsen U., Prokhorova T., Bech R.D., Nygaard T., Suetta C., Aagaard P. (2017) Delayed effect of blood flow-restricted resistance training on rapid force capacity. Medicine and Science in Sports and Exercise 49(6), 1157-1167. [DOI] [PubMed] [Google Scholar]
- Scott B.R., Loenneke J.P., Slattery K.M., Dascombe B.J. (2015). Exercise with blood flow restriction: An updated evidence-based approach for enhanced muscular development. Sports Medicine 45(3), 313-325. [DOI] [PubMed] [Google Scholar]
- Sale D.G. (1988) Neural adaptation to resistance training. Medicine and Science in Sports and Exercise 20(5), S135-145. [DOI] [PubMed] [Google Scholar]
- Sale D.G, Martin J.E., Moroz D.E. (1992) Hypertrophy without increased isometric strength after weight training. European Journal of Applied Physiology 64, 51-55. [DOI] [PubMed] [Google Scholar]
- Stephenson D.G., Lamb D.G., Stephenson G.M. (1998) Events of the excitation-contraction-relaxation (E-C-R) cycle in fast- and slow-twitch mammalian muscle fibres relevant to muscle fatigue. Acta Physiologica Scandinavica 162, 229-245. [DOI] [PubMed] [Google Scholar]
- Sundberg C.J. (1994) Exercise and training during graded leg ischemia in health man with special reference to effects on skeletal muscle. Acta Physiologica Scandinavica Suppl 615, 1-50. [PubMed] [Google Scholar]
- Takarada Y.H., Takazawa H., Sato Y., Takebayashi S., Tanaka Y., Ishii N. (2000a) Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. Journal of Applied Physiology 88(6), 2097-2106. [DOI] [PubMed] [Google Scholar]
- Takarada Y., Nakamura Y., Aruga S., Orida T., Miyazaki S., Ishii N. (2000b) Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. Journal of Applied Physiology 88(1), 61-65. [DOI] [PubMed] [Google Scholar]


