Abstract
Objective
Secreted frizzled related protein 5 (SFRP5) is a novel anti-inflammatory adipokine that is implicated in metabolic and cardiovascular disease (CVD). However, little is known about the relevance of SFRP5 with asymptomatic hypertensive target organ damages (TODs). We aimed to investigate the association between SFRP5 and TOD in a large population.
Clinical trial registration
Methods
A total of 1,745 community-dwelling elderly subjects aged over 65 years from northern Shanghai were recruited in the study. Plasma SFRP5 level was measured by an enzyme-linked immunosorbent assay. Asymptomatic TODs, including left ventricular mass index, peak transmitral pulsed Doppler velocity/early diastolic tissue Doppler velocity, carotid intima–media thickness (CIMT), pulse wave velocity (PWV), estimated glomerular filtration rate, and urinary albumin–creatinine ratio were evaluated.
Results
Plasma SFRP5 level was negatively associated with body mass index, waist/hip ratio, and fasting blood glucose (all P<0.001). Men, compared with women, had lower plasma SFRP5 level (4.19 vs 5.13 ng/mL, P<0.001). Additionally, plasma SFRP5 level was lower in diabetics than in those without diabetes (4.30 vs 4.81 ng/mL, P<0.05). Furthermore, an inverse association was observed between SFRP5 and PWV and CIMT (both P<0.05). Lastly, the multivariate logistic regression analysis showed lower SFRP5 level was significantly associated with increased arterial stiffness in the elderly (odds ratio 0.83, 95% confidence interval 0.71 to 0.99 per 1 standard deviation increase, P<0.05).
Conclusion
Plasma SFRP5 level was inversely correlated with conventional cardiovascular risk factors, and low plasma SFRP5 was also significantly associated with arterial stiffening in the elderly Chinese population.
Keywords: secreted frizzled related protein 5, cardiovascular disease, asymptomatic target organ damage, arterial stiffness
Introduction
Cardiovascular disease (CVD) is highly associated with metabolic diseases such as diabetes, obesity, hyperlipidemia, and so on.1 As an endocrine organ, the adipose tissue affects metabolic homeostasis and plays an important role in metabolic disorders.2,3 Moreover, growing evidence indicates that adipokines secreted from the adipose tissue are significantly associated not only with metabolic disorders but also with CVD.3–5 Additionally, several studies investigated the cross-talk between perivascular adipose tissue and vessels.6–8 Perivascular adipose tissue–derived adipokines regulate vascular function through paracrine and endocrine effects, suggesting adipokines are a new link between metabolic disorders and CVD.9
Secreted frizzled related protein 5 (SFRP5) is an emerging anti-inflammatory adipokine that belongs to the SFRP family,10 and was reported to be implicated in obesity, insulin resistance, and other metabolic syndromes.11–14 Several cross-sectional studies showed an inverse association between SFRP5 and diabetes mellitus (DM),11–13 whereas another study reported the SFRP5 level was increased in DM14 and one study found no association between SFRP5 and DM.15 Similarly, data on SFRP5 and obesity are also controversial in both animal and human studies.10–14,16–18 In addition, SFRP5 was suggested to impair insulin sensitivity19,20 and was diversely associated with insulin resistance.11–14 Some studies found a positive association between SFRP5 and insulin resistance,14 whereas some other studies indicated a negative association.11–13
Notably, SFRP5 was also suggested to be implicated in CVD, including coronary artery disease, vascular calcification, myocardial infraction, myocardial hypertrophy, and so on.21–24 A recent population-based study, which is based on data from the Cooperative Health Research in the Region of Augsburg F4 study, investigated the associations between serum SFRP5 and cardiometabolic risk factors as well as prediabetes/type 2 diabetes in the elderly.25 In the study, SFRP5 was suggested to be associated with CVD risk factors and functions as a novel biomarker for the prevention of cardiometabolic diseases.25
Asymptomatic target organ damages (TODs) are considered to be an intermediate condition in the development of CVD. As a predictor of cardiovascular events, TODs are significantly associated with CVD morbidity and mortality. However, it remained unclear if SFRP5 was significantly associated with TOD. In this study, we focused on the correlation of SFRP5 with TOD in order to better understand the role of SFRP5 in the development of CVD.
Methods
Study design and population
The Northern Shanghai Study (NSS) was an ongoing community-based prospective study. It was authorized and supported by the Shanghai local government (Grant ID 2013ZYJB0902; 15GWZK1002), and the protocol of the study was published previously.26 From June 2014 to May 2016, adults aged over 65 years from urban communities in the north of Shanghai were enrolled if they were available for long-term follow-up. Subjects were excluded if they had 1) severe cardiac diseases (New York Heart Association IV) or end-stage renal disease (chronic kidney disease >4); 2) known disease (such as lung cancer) with life expectancy <5 years; and 3) stroke history within 3 months. Finally, 1,910 subjects were invited, of whom 1,789 participants were enrolled in the NSS, and 1,745 elderly participated in our analysis after excluding the individuals with missing blood sample(s). The study protocol was approved by the Ethics Committee of Shanghai Tenth People’s Hospital, and written informed consent was obtained from all participants. Clinical trial registration: NCT02368938 (ClinicalTrials.gov).
Social, clinical, and biologic parameters
Medical and family history including gender, age, weight and height, smoking habits, family history of premature CVD, history of DM, hypertension and renal diseases, usage of medications, and so on were obtained using standardized questionnaires. Body weight and body height were measured and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. The waist–hip ratio (WHR) was calculated using the following formula: waist circumference (cm)/hip circumference (cm). Sitting blood pressure (BP) was measured three times after 10 min of rest in sitting position using a semi-automatic oscillometric device (Omron Healthcare, Kyoto, Japan), according to the recommendations of the European Society of Hypertension,27 and an average of the three readings was taken as participants’ BP. Venous blood sample and urine sample were obtained from the subjects after an overnight fast. All examinations including total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides, fasting plasma glucose, plasma creatinine (PCr), urinary creatinine, and microalbumin were assessed by standard methods at the laboratory of Shanghai Tenth People’s Hospital. Level of plasma SFRP5 (Human SFRP-5 DuoSet ELISA, R&D Systems, Inc., Minneapolis, MN, USA) was measured by enzyme-linked immunosorbent assays. Estimated glomerular filtration rate (eGFR) was calculated by the modified Modification of Diet in Renal Disease formula for Chinese: eGFR (mL/min/1.73 m2)=175×PCr−1.234× age−0.179(women×0.79).28 The urine albumin–creatinine ratio (UACR) was defined as the ratio of urinary microalbumin divided by urinary creatinine.
Definition of hypertension, DM, and obesity
Hypertension was defined as a systolic BP (SBP) ≥140 mmHg or a diastolic BP ≥90 mmHg or a previous history of hypertension. DM was defined as a previous self-reported diagnosis of DM by a physician. Individuals with BMI ≥28 were defined as obese.29
Ankle–brachial index
Four-limb BPs were measured automatically with VP-1000 (Omron Healthcare, Kyoto, Japan) by trained staff. The measurement was taken once by one trained physician for each participant. Bilateral ankle–brachial index (ABI) was defined as the ratio of the ankle SBP divided by the higher brachial SBP and could be read from the device. The lower ABI was applied for further analysis.
Ultrasonography
All the ultrasonography measurements were performed by an experienced cardiologist, who was blind to previous results. All measurements were performed with a MyLab 30 CV machine (ESAOTE SpA, Genoa, Italy), according to the American Society of Echocardiography recommendations.30
Carotid ultrasonography was evaluated at the common carotid arteries of both sides using a 7.5 MHz transducer. The common carotid artery intima–media thickness (CIMT) was measured on the left common carotid artery, within 2 cm from the bifurcation, and always performed on plaque-free arterial segments. CIMT was measured as the distance between the lumen–intima interface and the media–adventitia interface. Three measurements of CIMT were taken and the average value was used for further analysis. Simultaneously, the presence or absence of plaques in both the carotid arteries was recorded.
M-mode and two-dimensional echocardiography were performed using the same device with a 3.5 MHz probe. Left ventricular end-diastolic internal diameter (LVIDd), interventricular septal, and posterior wall thickness were measured by M-mode or two-dimensional echocar-diography from the parasternal view and they were used to calculate left ventricular mass (LVM) with the American Society of Echocardiography–recommended formula:31 LVM (g)=0.8×{1.04×[(LVIDd+posterior wall thickness + interventricular septal)3−(LVIDd)3[}+0.6. Left ventricular mass index (LVMI) was defined as LVM divided by body surface area. Left atrial volume (LAV) was calculated using the ellipse model formula: LAV=[π×(SA1×SA2×LA)/6]. In this equation, SA1 is the M-mode left atrial dimension in the parasternal short-axis view and SA2 and LA are measurements of short and long axes in the apical four-chamber view at the ventricular end systole.32
The transmitral early diastolic peak flow (E) was measured by pulse-wave Doppler imaging, and the early diastolic velocity (Ea) was measured by tissue Doppler signals in lateral septum. The ratio of E/Ea in the lateral side was calculated for the evaluation of left ventricular diastolic function.
Pulse wave velocity
Aortic pulse wave velocity (PWV) was measured to assess the arterial stiffness, using applanation tonometry (SphygmoCor; AtCor Medical, Sydney, Australia) with the methods provided by European Expert Consensus on Arterial Stiffness.33 The distances from the sternal notch to the right femoral artery and from the sternal notch to the right carotid artery were measured with an inelastic tape, and the traveling distance was calculated by subtracting them. Carotid–femoral PWV was calculated by dividing the traveled distance by the transit time (PWV=distance/time).
Definition of hypertensive TODs
Asymptomatic TODs include cardiac, arterial, and renal TODs. Left ventricular hypertrophy was defined as LVMI ≥115 g/m2 (male) or LVMI ≥95 g/m2 (female).34 Left ventricular diastolic dysfunction was assessed by E/Ea and other evidence of abnormal left ventricular relaxing and filling, such as enlarged left atrial volume and increased LVM. Specifically, diastolic dysfunction was defined as E/Ea ≥15, or 15>E/Ea>8 with any of the following: LAVI >40 mL/m2 or LVMI >149 g/m2 (men) or LVMI >122 g/m2 (women). As for the arterial TODs, they are defined as increased CIMT (CIMT >900 μm) or peripheral artery disease (ABI <0.9). In addition, arteriosclerosis is defined as PWV >12 m/s and atherosclerosis is defined as ABI <0.9 or the presence of plaque. Chronic kidney diseases (eGFR <60 mL/min/1.73 m2) and microalbuminuria (UACR >30) represented renal TODs.
Statistical analysis
Quantitative and qualitative parameters are presented as means ± standard deviation (SD) and numbers with the percentage in parentheses. Normal distribution of the data was tested using Kolmogorov–Smirnov test. Comparisons between groups were performed with Wilcoxon test. Correlations between SFRP5 and relative variables were assessed using Spearman correlation analyses. Further, multivariate logistic regressions were conducted to investigate the association of hypertensive TODs with SFRP5 in the entire population, in hypertensives, and in diabetics. Statistical analysis was performed using SAS software, V.9.3 (SAS Institute, Cary, NC, USA). P<0.05 was considered statistically significant.
Results
Table 1 shows the characteristics of participants, including conventional cardiovascular risk factors, asymptomatic TOD, as well as diseases and treatment. The mean age of the 1,745 participants was 71.4±6.0 years, and included 779 (44.6%) men, 1,114 (63.9%) hypertensives, and 337 (19.3%) with diabetes. In addition, we investigated the distribution of plasma SFRP5 in the 1,745 participants. The plasma SFRP5 level ranged from 0.40 to 20.3 ng/mL, and the average value was 4.71 ng/mL in this elderly population.
Table 1.
Characteristics of participants
| Characteristics | N=1,745 |
|---|---|
| Cardiovascular risk factors | |
| Age, years | 71.4±6.0 |
| Men, n (%) | 779 (44.6) |
| Smoker, n (%) | 238 (13.7) |
| Family history of premature CVD, n (%) | 374 (21.5) |
| Waist circumference, cm | 86.0±9.7 |
| Hip circumference, cm | 97.3±7.2 |
| Body mass index, kg/m2 | 23.9±3.5 |
| Fasting plasma glucose, mmol/L | 5.7±1.7 |
| Total cholesterol, mmol/L | 5.2±1.0 |
| Triglycerides, mmol/L | 1.6±0.9 |
| High-density lipoprotein cholesterol, mmol/L | 1.4±0.4 |
| Low-density lipoprotein cholesterol, mmol/L | 3.2±0.9 |
| Systolic blood pressure, mmHg | 134.3±17.7 |
| Asymptomatic target organ damage | |
| Left ventricular mass index, g/m2 | 90.4±29.0 |
| Carotid intima–media thickness, μm | 612.8±148.0 |
| Plaque in carotid artery, n (%) | 1,171 (67.1) |
| Pulse wave velocity, m/s | 9.4±2.3 |
| Left ankle–brachial index | 1.04±0.13 |
| Estimated glomerular filtration rate, % | 93.3±24.7 |
| Urinary albumin–creatinine ratio, mg/g | 56.0±101.9 |
| Diseases and treatment | |
| Hypertension, n (%) | 1,114 (63.9) |
| Diabetes, n (%) | 337 (19.3) |
| Cardiovascular disease, n (%) | 595 (34.1) |
| Treated hypertension, n (%) | 862 (94.0) |
| Antidiabetic treatment, n (%) | 279 (39.1) |
Note: Data are mean ± standard deviation or number (%).
Abbreviation: CVD, cardiovascular disease.
Figure 1 demonstrates the distribution of plasma SFRP5 in the 1,745 participants. The plasma SFRP5 level ranged from 0.40 to 20.3 ng/mL, and the average value was 4.71 ng/mL in this elderly population.
Figure 1.
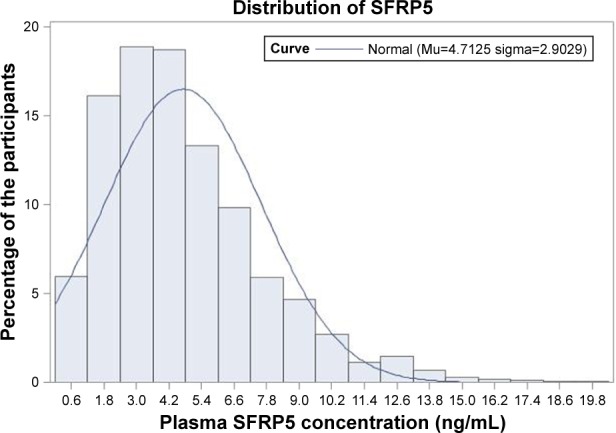
Distribution of plasma SFRP5 level in 1,745 participants.
Abbreviation: SFRP5, secreted frizzled related protein 5.
The relationship of SFRP5 with conventional cardiovascular risk factors, including BMI, WHR, SBP, LDL-C, and fasting plasma glucose, are shown in Table 2 after adjustment for age. Plasma SFRP5 level was negatively and significantly correlated with BMI, WHR, and fasting plasma glucose (all P<0.0001). Additionally, the relationship of SFRP5 with male gender, smoking habits, hypertension, DM, CVD, and obesity was investigated as shown in Figure 2. Plasma SFRP5 level was significantly lower in men than in women (4.19 vs 5.13 ng/mL, P<0.001) and lower in smokers than in nonsmokers (4.34 vs 4.78 ng/mL, P<0.05). Individuals with diabetes had significantly lower plasma SFRP5 level than those without diabetes (4.30 vs 4.81 ng/mL, P<0.05).
Table 2.
Correlation of SFRP5 with cardiovascular risk factors
| Cardiovascular risk factors | SFRP5
|
|
|---|---|---|
| r | P-value | |
| Body mass index | −0.13 | <0.0001 |
| Waist/hip circumference | −0.20 | <0.0001 |
| Systolic blood pressure | −0.03 | 0.15 |
| Low-density lipoprotein cholesterol | 0.03 | 0.22 |
| Fasting plasma glucose | −0.15 | <0.0001 |
Note: The model is age adjusted.
Abbreviation: SFRP5, secreted frizzled related protein 5.
Figure 2.
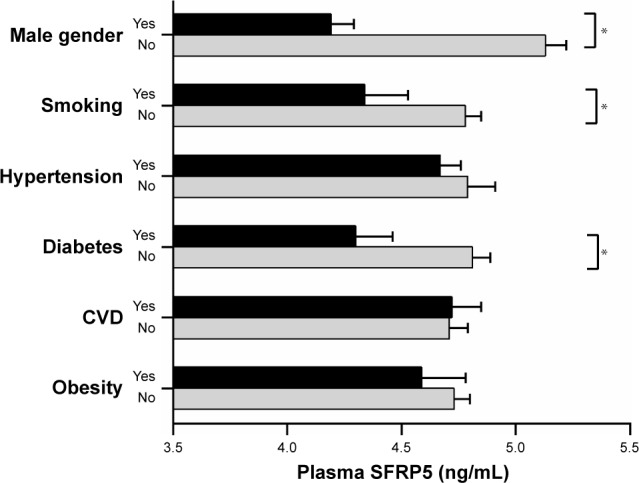
Relationship of SFRP5 with male gender, smoking, hypertension, diabetes, CVD, and obesity.
Note: *P<0.01.
Abbreviations: CVD, cardiovascular disease; SFRP5, secreted frizzled related protein 5.
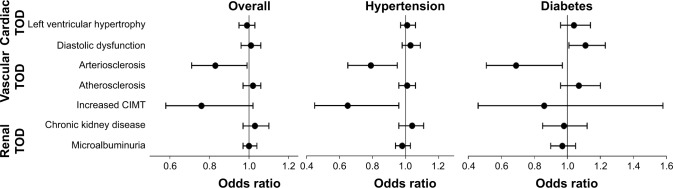
In the correlation analysis of SFRP5 with TOD markers, including LVMI, E/Ea, PWV, CIMT, UACR, and eGFR, it was observed that plasma SFRP5 level was significantly and negatively correlated with CIMT and PWV in an age-adjusted model (both P<0.05; Table 3). Furthermore, the association between SFRP5 and TOD was investigated by multivariate logistic regression analysis after adjustment for potential confounders including age, gender, smoking status, CVD history, sport time per week, LDL-C, and SBP (Figure 3). Low plasma SFRP5 level was associated with increased arterial stiffness in the total population (odds ratio [OR] 0.83, 95% CI 0.71–0.99 per 1 SD increase, P<0.05), in hypertensives (OR 0.79, 95% CI 0.65–0.96 per 1 SD increase, P<0.05), and in diabetics (OR 0.69, 95% CI 0.50–0.97 per 1 SD increase, P<0.05). In addition, low plasma SFRP5 level was also associated with increased CIMT in hypertensives (OR 0.65, 95% CI 0.45–0.96 per 1 SD increase, P<0.05). Of note, there was no significant association with cardiac and renal TOD.
Table 3.
Correlation of SFRP5 with target organ damage
| Target organ damages | SFRP5
|
|
|---|---|---|
| r | P-value | |
| Left ventricular mass index | −0.05 | 0.06 |
| E/Ea | −0.02 | 0.47 |
| Carotid intima–media thickness | −0.06 | 0.01 |
| Pulse wave velocity | −0.06 | 0.01 |
| Urinary albumin–creatinine ratio | −0.01 | 0.81 |
| Estimated glomerular filtration rate | −0.01 | 0.62 |
Notes: E/Ea: peak transmitral pulsed Doppler velocity/early diastolic tissue Doppler velocity. The model is age adjusted.
Abbreviation: SFRP5, secreted frizzled related protein 5.
Figure 3.
Association of SFRP5 with all TODs in the overall population, in hypertensives, and in diabetics.
Note: Odds ratios per standard deviation increase in plasma SFRP5 are presented after adjustments for confounders using logistic regressions.
Abbreviations: CIMT, carotid intima–media thickness; SFRP5, secreted frizzled related protein 5; TOD, target organ damage.
Discussion
In this study, we demonstrated the following: 1) plasma SFRP5 level was inversely associated with multiple CVD risk factors, and 2) low plasma SFRP5 level was consistently and significantly associated with increased arterial stiffness, among asymptomatic TODs.
SFRP5 has been previously linked to metabolic dysfunctions including obesity and diabetes, which were recognized to contribute to the CVD. Studies in humans showed an inverse association between SFRP5 and DM,11–13,25 and Ouchi et al10 also reported a protective role of SFRP5 in type 2 diabetes in mice. Conversely, other studies reported a positive14 or no association15 between SFRP5 and DM. The discrepancies occurred possibly due to the different study designs. Most of the previous studies enrolled hospital-based patients in small sample sizes and the studies varied in their adjustment for potential confounders.11–15 Whereas, our observation was consistent with another large population-based study,25 suggesting a negative correlation between plasma SFRP5 and diabetes. In addition, previous studies found that higher SFRP5 level was associated with lower BMI and lower WHR,11–13 which was in line with our findings. Considering that gender is not only a risk factor for CVD but also may affect adipokine expression, we compared plasma SFRP5 level between genders and found SFRP5 level was lower in men. In contrast, other studies showed no difference in SFRP5 level between different genders.11,15,21 Unlike our large population-based study, these studies were mostly based on relatively small sample sizes. Therefore, we speculated SFRP5 may possess gender–specific activity.
Further, we assessed the association between plasma SFRP5 and asymptomatic TOD. To our knowledge, this is the first study to investigate the relationship between SFRP5 and all asymptomatic TODs in a large population. Asymptomatic TODs are of great importance as a prevalent prodrome in the development of CVD, and the presence of asymptomatic TOD is associated with CVD mortality. In this study, plasma SFRP5 was found to be inversely associated with PWV and CIMT, which represent arterial TOD, and lower plasma SFRP5 level was associated with increased arterial stiffness, especially in hypertensives.
PWV has been widely accepted in clinical practice as a gold standard of arterial stiffness, which was recognized as an important predictor of CVD and mortality.35–37 Additionally, CIMT, another arterial TOD marker, has also been shown to predict cardiovascular risks and influence arterial stiffening.38 In hypertensive individuals, increased BP can increase arterial stiffness.39,40 It was indicated that increased arterial stiffness is related to elastin depletion and collagen deposition in elastic arteries, which can further increase the BP, leading to a vicious cycle.40,41 In literature, hypertension and arterial stiffness are closely associated with age, which is an independent risk factor for CVD.42,43 Therefore, the evaluation of arterial stiffness is of great clinical importance in the elderly.
Our findings about the relevance of SFRP5 with TOD in the elderly suggested that plasma SFRP5 level might have a potential role in predicting arterial stiffness in the elderly. However, there is no other available data on the association between SFRP5 and TOD. More studies are needed to identify the importance of SFRP5 in CVD, especially in arterial stiffness in the elderly.
Limitations
Findings of our study need to be interpreted within the context of its limitations. One main concern is that SFRP5 is an adipokine secreted by the adipose tissue and the possible ethnic difference of SFRP5 secretion should be noted. In addition, NSS is a cross-sectional study and we focused on the association between SFRP5 and TOD, but without any intervention or causality interpretation. Nonetheless, with ongoing follow-up studies, we may provide some prospective data in the near future.
Conclusion
Plasma SFRP5 level was inversely correlated with conventional cardiovascular risk factors, and low plasma SFRP5 was also significantly associated with arterial stiffening in the elderly.
Acknowledgments
We thank all the investigators and subjects who participated in the Northern Shanghai Study. Hongwei Ji and Ximin Fan provided writing assistance, while Jing Xiong and Jun Zhang provided technical help. This study was authorized and financially supported by the Shanghai municipal government (grant IDs: 2013ZYJB0902; 15GWZK1002). Dr Yi Zhang was supported by the National Nature Science Foundation of China (grant IDs: 81300239; 81670377).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006;64(4):355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 3.Northcott JM, Yeganeh A, Taylor CG, Zahradka P, Wigle JT. Adipokines and the cardiovascular system: mechanisms mediating health and disease. Can J Physiol Pharmacol. 2012;90(8):1029–1059. doi: 10.1139/y2012-053. [DOI] [PubMed] [Google Scholar]
- 4.Yamawaki H. Vascular effects of novel adipocytokines: focus on vascular contractility and inflammatory responses. Biol Pharm Bull. 2011;34(3):307–310. doi: 10.1248/bpb.34.307. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;2010:535918. doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10(2):191–196. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown NK, Zhou Z, Zhang J, et al. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34(8):1621–1630. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oriowo MA. Perivascular adipose tissue, vascular reactivity and hypertension. Med Princ Pract. 2015;24(Suppl 1):29–37. doi: 10.1159/000356380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14(9):2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouchi N, Higuchi A, Ohashi K, et al. Sfrp5 is an anti-inflammatory adi-pokine that modulates metabolic dysfunction in obesity. Science. 2010;329(5990):454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Z, Deng H, Qu H. Plasma SFRP5 levels are decreased in Chinese subjects with obesity and type 2 diabetes and negatively correlated with parameters of insulin resistance. Diabetes Res Clin Pract. 2013;99(3):391–395. doi: 10.1016/j.diabres.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Hu W, Li L, Yang M, et al. Circulating Sfrp5 is a signature of obesity-related metabolic disorders and is regulated by glucose and liraglutide in humans. J Clin Endocrinol Metab. 2013;98(1):290–298. doi: 10.1210/jc.2012-2466. [DOI] [PubMed] [Google Scholar]
- 13.Xu Q, Wang H, Li Y, et al. Plasma Sfrp5 levels correlate with determinants of the metabolic syndrome in Chinese adults. Diabetes Metab Res Rev. 2017 Apr 18; doi: 10.1002/dmrr.2896. Epub. [DOI] [PubMed] [Google Scholar]
- 14.Carstensen M, Herder C, Kempf K, et al. Sfrp5 correlates with insulin resistance and oxidative stress. Eur J Clin Invest. 2013;43(4):350–357. doi: 10.1111/eci.12052. [DOI] [PubMed] [Google Scholar]
- 15.Canivell S, Rebuffat S, Ruano EG, et al. Circulating SFRP5 levels are elevated in drug-naive recently diagnosed type 2 diabetic patients as compared with prediabetic subjects and controls. Diabetes Metab Res Rev. 2015;31(2):212–219. doi: 10.1002/dmrr.2599. [DOI] [PubMed] [Google Scholar]
- 16.Schulte DM, Muller N, Neumann K, et al. Pro-inflammatory wnt5a and anti-inflammatory sFRP5 are differentially regulated by nutritional factors in obese human subjects. PLoS One. 2012;7(2):e32437. doi: 10.1371/journal.pone.0032437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori H, Prestwich TC, Reid MA, et al. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest. 2012;122(7):2405–2416. doi: 10.1172/JCI63604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koza RA, Nikonova L, Hogan J, et al. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006;2(5):e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carstensen M, Wiza C, Röhrig K, et al. Effect of Sfrp5 on cytokine release and insulin action in primary human adipocytes and skeletal muscle cells. PLos One. 2014;9(1):e85906. doi: 10.1371/journal.pone.0085906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carstensen-Kirberg M, Hatziagelaki E, Tsiavou A, et al. Sfrp5 associates with beta-cell function in humans. Eur J Clin Invest. 2016;46(6):535–543. doi: 10.1111/eci.12629. [DOI] [PubMed] [Google Scholar]
- 21.Miyoshi T, Doi M, Usui S, et al. Low serum level of secreted frizzled-related protein 5, an anti-inflammatory adipokine, is associated with coronary artery disease. Atherosclerosis. 2014;233(2):454–459. doi: 10.1016/j.atherosclerosis.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Deng D, Diao Z, Han X, Liu W. Secreted frizzled-related protein 5 attenuates high phosphate-induced calcification in vascular smooth muscle cells by inhibiting the Wnt/ss-catenin pathway. Calcif Tissue Int. 2016;99(1):66–75. doi: 10.1007/s00223-016-0117-7. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Sano S, Fuster JJ, et al. Secreted frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia/reperfusion injury. J Biol Chem. 2016;291(6):2566–2575. doi: 10.1074/jbc.M115.693937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin X, Guo B, Yan J, et al. Angiotensin II increases secreted frizzled-related protein 5 (sFRP5) expression through AT1 receptor/Rho/ROCK1/JNK signaling in cardiomyocytes. Mol Cell Biochem. 2015;408(1–2):215–222. doi: 10.1007/s11010-015-2497-9. [DOI] [PubMed] [Google Scholar]
- 25.Carstensen-Kirberg M, Kannenberg JM, Huth C, et al. Inverse associations between serum levels of secreted frizzled-related protein-5 (SFRP5) and multiple cardiometabolic risk factors: KORA F4 study. Cardiovasc Diabetol. 2017;16(1):109. doi: 10.1186/s12933-017-0591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji H, Xiong J, Yu S, et al. Northern Shanghai Study: cardiovascular risk and its associated factors in the Chinese elderly-a study protocol of a prospective study design. BMJ Open. 2017;7(3):e013880. doi: 10.1136/bmjopen-2016-013880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23(4):697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- 28.Ma YC, Zuo L, Chen JH, et al. Chinese eGFR Investigation Collaboration Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney Int. 2007;72(12):1535–1542. doi: 10.1038/sj.ki.5002566. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Lu FC, Department of Disease Control Ministry of Health, PR China The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36. [PubMed] [Google Scholar]
- 30.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Li Y, Liu M, Sheng CS, Huang QF, Wang JG. Cardiac structure and function in relation to cardiovascular risk factors in Chinese. BMC Cardiovasc Disord. 2012;12:86. doi: 10.1186/1471-2261-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Kollias G, Argyris AA, et al. Association of left ventricular diastolic dysfunction with 24-h aortic ambulatory blood pressure: the SAFAR study. J Hum Hypertens. 2015;29(7):442–448. doi: 10.1038/jhh.2014.101. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Agnoletti D, Protogerou AD, et al. Characteristics of pulse wave velocity in elastic and muscular arteries: a mismatch beyond age. J Hypertens. 2013;31(3):554–559. doi: 10.1097/HJH.0b013e32835d4aec. discussion 559. [DOI] [PubMed] [Google Scholar]
- 38.Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension. 2005;45(6):1050–1055. doi: 10.1161/01.HYP.0000164580.39991.3d. [DOI] [PubMed] [Google Scholar]
- 39.Aatola H, Hutri-Kahonen N, Juonala M, et al. Lifetime risk factors and arterial pulse wave velocity in adulthood: the cardiovascular risk in young Finns study. Hypertension. 2010;55(3):806–811. doi: 10.1161/HYPERTENSIONAHA.109.145102. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Huang B, Liu M, Li X. Effects of different types of antihypertensive agents on arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. J Thorac Dis. 2015;7(12):2339–2347. doi: 10.3978/j.issn.2072-1439.2015.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: emerging concepts. Hypertension. 2010;55(1):9–14. doi: 10.1161/HYPERTENSIONAHA.107.090464. [DOI] [PubMed] [Google Scholar]
- 42.Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65(2):252–256. doi: 10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.AlGhatrif M, Strait JB, Morrell CH, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62(5):934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]