Abstract
Background
Bile acids (BAs) are post-prandial hormones that play an important role in glucose and lipid homeostasis as well as energy expenditure. Total and glycine-amidated BAs increase after sleeve gastrectomy (SG) and correlate to improved metabolic disease. No specific bile acid subtype has been shown conclusively to mediate the weight loss effect. Therefore, the objective of this study was to prospectively evaluate the comprehensive changes in meal-stimulated BAs after SG and determine if a specific change in the BA profile correlates to the early weight loss response.
Methods
Patients were prospectively enrolled at the University of Nebraska Medical Center who were undergoing a SG for treatment of morbid obesity. Primary and secondary plasma bile acids and their amidated (glycine, G-, or taurine, T-) subtypes were measured at fasting, 30 and 60 min after a liquid meal performed pre-op, and at 6 and 12 weeks post-op. Area under the curve (AUC) was calculated for the hour meal test for each bile acid subtype. BAs that were significantly increased post-op were correlated to body mass index (BMI) loss.
Results
Total BA AUC was significantly increased at 6 (p<0.01) and 12 weeks post-op (p<0.01) compared to pre-operative values. The increase in total BA AUC was due to a statistically significant increase in G-BAs. Nine different BA AUC subtypes were significantly increased at both 6 and 12 weeks post-op. Increased total and G-chenodeoxycholic acid AUC was significantly correlated to the 6 week BMI loss (p = 0.03). Increased G-hyocholic acid was significantly correlated to increased weight loss at both 6 (p = 0.05) and 12 weeks (p = 0.006).
Conclusions
SG induced an early and persistent post-prandial surge in multiple bile acid subtypes. Increased G-hyocholic consistently correlated with greater early BMI loss. This study provides evidence for a role of BAs in the surgical weight loss response after SG.
Keywords: Bile acids, Sleeve gastrectomy, Hyocholic acid, Deoxycholic acid, Chenodeoxycholic acid, Weight loss
Bariatric surgeries, including the Roux-en-y gastric bypass (RYGB) and sleeve gastrectomy (SG), are the most effective therapeutic options for morbid obesity and many obesity-associated co-morbidities [1]. These metabolic surgeries are successful in comparison to dieting by modifying physiologic signals involved in food intake and energy expenditure, as well as glucose and lipid homeostasis [2]. One of the potential mechanisms mediating sustained weight loss after SG is a change in post-operative bile acids (BAs) and BA signaling due to the physiologic consequences of altering the gastrointestinal anatomy [3, 4].
BAs are steroids that solubilize cholesterol and facilitate in the digestion and absorption of dietary lipids, cholesterol, and fat-soluble vitamins [5]. BAs are now recognized as amphipathic nutrient signaling hormones which activate nuclear receptors including the farsenoid X receptor (FXR), G protein coupled BA receptors (TGR5) and cell signaling pathways [5–7]. FXR regulates glucose and lipid metabolism as well as BA synthesis and transport [7]. The G protein coupled receptor TGR5 increases energy expenditure and stimulates entero-endocrine L cells to release glucagon-like peptide-1 (GLP-1) [8, 9]. Contrastingly, FXR activation in L cells decreases proglucagon expression and thus decreases GLP-1 release highlighting the important overlap in BA-stimulated FXR and TGR5 activities [10]. The collective BA action includes regulation of glucose, lipid, lipoprotein, and energy metabolism which is enhanced in the post-prandial state and an attractive mechanism for weight loss and metabolic improvements after bariatric surgery.
Most studies have found that both fasting and post-prandial total BAs increase after RYGB [3]. This increase includes both primary and secondary BAs, as well as amidated BAs [11–13]. There is less consistency in the literature regarding the effect of SG on fasting BAs. Jahansouz et al. found fasting BAs significantly increased as early as one week after SG, primarily glycine-amidated BAs, and independent from hypocaloric restriction [14]. Conversely, Belgaumkar et al. found no difference in total, fasting bile acids at 6 months after surgery although there were significant changes in the bile acid profile [15]. There are limited clinical studies examining the effect of SG on post-prandial BAs. Initial reports found SG did not impact post-prandial BAs [16]. Recently, Khan et al. published BA changes in ten adolescents after SG. BA levels increased by 143.6% at 30 min after a test meal as early as one month after surgery with a significant decrease in the hydrophobicity of the BA composition. The fasting rise in BA levels after surgery correlated with elevated FGF21 expression but not directly to the weight loss outcome [17].
There is compelling rodent data suggesting a critical role of BAs in both weight loss and changes in glucose homeostasis after SG. SG in mice increases serum BAs and the BA composition which correlates with the maximal surgical weight loss. The elevation in serum BAs after SG was independent of weight loss [18]. Ryan et al. found that lack of FXR signaling prevented post-surgical weight loss and any improvement in glucose homeostasis after SG providing strong evidence for a BA-FXR mechanism mediating surgical weight loss [19].
Given the complex composition of the bile acid pool both in the fasting and post-prandial state, it is imperative to clinically investigate both BA profiles rigorously in order to accurately describe the changes in BAs and if there is a relationship of the post-operative BA profile to weight loss after SG. In this study, we hypothesized that SG results in an early increase in post-prandial serum BAs. We further evaluated if the post-operative change in post-prandial serum BAs correlated to the degree of post-surgical weight loss. To test this hypothesis, we prospectively evaluated a comprehensive profile of fasting and meal-stimulated BAs before and 6 and 12 weeks after SG and evaluated significant changes to the weight loss response.
Materials and methods
Subjects
Subjects were adult, morbidly obese patients who had been evaluated in a multi-disciplinary fashion and approved and consented to have SG at the University of Nebraska Medical Center (UNMC). All study procedures for this single-center prospective study were approved by the UNMC Institutional Review Board before implementation. This study was registered in ClinicalTrials.gov (identifier NCT02302677). Patients were approached and offered participation in the study using an IRB approved consent form. Enrollment was offered to consecutive patients that met inclusion criteria with a goal enrollment of 32 participants. Study inclusion criteria included subject age ≥19 and ≤70 years of age at the time of surgery undergoing a primary bariatric surgery or with a history of an adjustable gastric band which was removed two years prior to the date of enrollment. Exclusion criteria included a past medical history of a small bowel resection or right colectomy, history of cirrhosis, and current or planned use of bile acid sequestrants post-operatively. A total of 31 morbidly obese human subjects were prospectively recruited from the UNMC Bariatrics Center during the enrollment period of February 2015–November 2015. Only patients who successfully completed all three study visits were included in data presentation and analysis.
Study visits
Patients were asked to complete a meal challenge pre-operatively and at 6 and 12 weeks post-operatively following SG. All visits were conducted at the Clinical Research Center (CRC) of UNMC. All consenting subjects were instructed to fast 8 h prior a visit. The pre-operative CRC visit occurred prior to initiation of a pre-operative liquid diet. Patients were weighed at each visit at the CRC rather than the Bariatric Center to reduce weight and scale variability. Subjects were given a standardized liquid meal over 10 min (total of 100 ml; Ensure, Abbott Laboratories, Abbott Park, IL). Blood samples were taken at baseline, 30 min, and 1 h after consumption of the test meal. Medical charts of all participants were reviewed for demographics, laboratory data, medical history, and obesity- associated co-morbidities.
Surgery
Patients were placed on a liquid, whey protein diet for two weeks prior to surgery. The SG technique among the two operating bariatric surgeons at UNMC was standardized to include laparoscopic placement of three working ports, a camera port, and a laparoscopic liver retractor. The sleeve was started 6 cm from the pylorus after placement of a 36F bougie and completed with a series of re-enforced linear stapler firings. A drain was placed along the staple line of all patients, and if clinically appropriate, patients were started on a liquid diet on postoperative day #1.
Sample analysis
Serum was isolated and sent to the UNMC clinical laboratory for processing of glucose by a hexokinase/glucose-6-phosphate dehydrogenase assay and insulin by a quantitative ultrafiltration/chemiluminescent immunoassay. For other assays, serum was in a freezer at −80 °C until bile acid analysis was completed. The homeostatic model assessment (HOMA) for insulin resistance (IR) was calculated as (glucose mg/dL × insulin) ÷ 405.
A Waters ACQUITY ultra-performance liquid chromatography (UPLC) system coupled to an Applied Biosystem 4000 Q TRAP quadrupole linear ion trap hybrid mass spectrometer (MS) with an electrospray ionization source was used for BA analysis. The details of the liquid chromatographic, mass spectrometric, and sample preparation conditions as well as method validation were performed as previously published for human BAs [20, 21]. The following bile acids were analyzed including: cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), ursodeoxycholic acid (UDCA), β-muricholic acid (MCA), hyocholic acid (HCA), hyodeoxycholic acid (HDCA), and their taurine (T-) and glycine (G-) amidated sub-fractions. The BA subfractions of muriDCA (MDCA), isoLCA, isoDCA, 7-oxoLCA, 12-oxoLCA, 12-oxoCDCA, 3-dehydroCA, and norDCA were also measured. All BAs concentrations including total and individual BAs refer to the sum of sulfated and unsulfated BAs. The term “total” refers to the sum of amidated (both G and T forms), unamidated, sulfated, and unsulfated fractions for the individual BA measured.
Statistics
All values are expressed as mean ± standard deviations. Statistical significance was determined as p ≤ 0.05. SPSS, version 21 (IBM corp.) was used for all statistical analyses. Area under the curve (AUC) was calculated for the three meal times points (0, 30, and 60 min) according to the trapezoidal rule. Percent total weight loss (%TWL) was calculated for each subject as the pre-operative study visit weight minus the measured post-operative body weight at 6 or 12 weeks divided by the initial body weight and multiplied by 100. BMI change was calculated as the post-operative BMI for each time point minus the pre-operative study visit BMI. Statistical significance was evaluated post-operatively by comparison to the pre-operative value using a paired student’s t test. Pearson’s coefficient was calculated comparing BMI change to any significant bile acid change at either 6 or 12 weeks.
Results
Twenty-eight patients completed the pre-operative, 6 and 12 week post-operative study visits (90% of enrolled patients). The average patient age was 45.4 ± 12.9 years with a pre-operative BMI of 45.0 ± 6.8 kg/m2. 82% of patients were female. Type 2 diabetics represented 21% of the patients. There was a significant decrease in body weight, BMI, and %TWL at both 6 and 12 weeks after surgery compared to pre-op (Table 1). We found no significant change in glucose or insulin AUC in response to a liquid test meal after surgery. Insulin resistance as measured by HOMA-IR had significantly decreased from pre-operative values by the 12-week visit (pre-op: 8.88 ± 7.90, 12 weeks: 4.89 ± 4.91, p = 0.03).
Table 1.
Anthropometric and glucose tolerance changes after sleeve gastrectomy
| Pre-op | 6 weeks post-op | 12 weeks post-op | |
|---|---|---|---|
| Weight (kg) | 129.1 ± 22.6 | 116.9 ± 21.0* | 111.6 ± 21.3* |
| BMI (kg/m2) | 45.0 ± 6.8 | 40.7 ± 6.1* | 38.9 ± 6.3* |
| %TWL | 0 | 9.5 ± 3.3* | 13.7 ± 4.7* |
| Glucose AUC (mg* min/dl) | 6113 ± 667 | 6624 ± 1921 | 6692 ± 2450 |
| Insulin AUC (mg* min/dl) | 4024 ± 2083 | 4702 ± 3869 | 3266 ± 2338 |
| HOMA-IR | 8.88 ± 7.90 | 5.35 ± 5.08 | 4.89 ± 4.91* |
Data presented as the mean ± standard deviation
p<0.05 compared to pre-op
As shown in Fig. 1, fasting total bile acids at 6 weeks (5.55 ± 3.35 μM) and 12 weeks (5.59 ± 2.58 μM) post-operatively did not significantly change from pre-operative values (4.75 ± 2.35 μM). Additionally, there was also no difference in the total glycine, taurine, or unamidated fractions. We found no significant change in the fasting concentrations (including the glycine, taurine, or unamidated fractions) of CA or LCA. The total fasting CA concentration pre-operatively was 0.30 ± 0.33 μM compared to 0.34 ± 0.30 μM at 6 weeks (p = 0.60) and 0.28 ± 0.23 μM at 12 weeks post-operatively (p = 0.87). The total fasting LCA concentration pre-operatively was 0.84 ± 0.95 μM compared to 0.69 ± 0.50 μM at 6 weeks (p = 0.46) and 0.94 ± 0.71 μM at 12 weeks post-operatively (p = 0.66).
Fig. 1.
The change in the total fasting bile acid (BA) concentration after sleeve gastrectomy. The total BA is the sum of unamidated and glycine and taurine-amidated subtypes. Data presented as mean ± standard deviation (n = 28). Asterisk denotes statistical significance at week 6 and number sign at week 12 with p<0.05 compared to pre-op
Table 2 highlights all significant changes found in fasting BA concentrations after SG. At 6 weeks, fasting total CDCA (including G-CDCA), total MCA (including unamidated and G-MCA), and total HCA (including unamidated and G-HCA) significantly increased. At 12 weeks, fasting T-UDCA significantly decreased and unamidated DCA significantly increased as well as continued increased total, unamidated, and G-HCA compared to pre-op. Unlike fasting BAs, there was a significant increase in the total BA AUC with the liquid meal test after surgery by 6 weeks post-op (418.7 ± 155.0 μM*min, p<0.01) which remained significantly elevated at 12 weeks post-op (423.0.7 ± 124.3 μM*min, p<0.01) compared to pre-operative values (318.3 ± 123.0 μM*min). The increase in total BA AUC was due to a statistically significant increase in meal-stimulated glycine-amidated BAs (Fig. 2). There was no significant change in the AUC for taurine-amidated BAs or unamidated BAs post-operatively.
Table 2.
Fasting BAs with statistically significant changes after sleeve gastrectomy
| Bile Acid Subtype | Pre-op (μM) | 6 week (μM) | 12 week (μM) |
|---|---|---|---|
| Total CDCA | 1.28 ± 0.87 | 1.96 ± 1.48* | 1.74 ± 1.19 |
| Glycine-CDCA | 0.87 ± 0.63 | 1.44 ± 1.30* | 1.28 ± 0.98 |
| Unamidated DCA | 0.33 ± .024 | 0.39 ± 0.24 | 0.53 ± 0.36* |
| Total MCA | 0.029 ± 0.048 | 0.056 ± 0.053* | 0.047 ± 0.050 |
| Unamidated MCA | 0.007 ± 0.007 | 0.011 ± 0.10* | 0.010 ± 0.008 |
| Glycine-MCA | 0.009 ± 0.019 | 0.020 ± 0.023* | 0.017 ± 0.012 |
| Total HCA | 0.027 ± 0.038 | 0.061 ± 0.046* | 0.052 ± 0.052* |
| Unamidated HCA | 0.005 ± 0.008 | 0.014 ± 0.011* | 0.014 ± 0.012* |
| Glycine-HCA | 0.09 ± 0.012 | 0.023 ± 0.025* | 0.018 ± 0.016* |
| Taurine-UDCA | 0.034 ± 0.036 | 0.024 ± 0.029 | 0.011 ± 0.007* |
Data presented as the mean ± standard deviation. Total for any individual bile acid is the sum of unamidated and glycine and taurine-amidated subtypes
CDCA chenodoxycholic acid, DCA deoxycholic acid, MCA muricholic acid, HCA hyocholic acid, UDCA ursodeoxycholic acid
p<0.05 compared to pre-op
Fig. 2.
The change in the total bile acid (BA) area under the curve (AUC) from a liquid meal test measured at 0, 30, and 60 min. The total BA AUC is the sum of unamidated and glycine and taurine-amidated subtypes. Data presented as mean ± standard deviation (n = 28). Asterisk denotes statistical significance at week 6 and number sign at week 12 with p<0.05 compared to pre-op
We again found no significant change in post-prandial BA AUC of CA or LCA (including the glycine, taurine, or unamidated fractions). The total post-prandial CA AUC pre-operatively was 23.0 ± 24.0 μM*min compared to 31.4 ± 21.0 μM*min at 6 weeks (p = 0.17) and 29.6 ± 22.6 μM*min at 12 weeks post-operatively (p = 0.29). The total post-prandial LCA AUC pre-operatively was 52.0 ± 56.5 μM*min compared to 48.5 ± 37.6 μM*min at 6 weeks (p = 0.78) and 62.3 ± 41.5 μM*min at 12 weeks post-operatively (p = 0.44).
The significant individual bile acid changes after a 100 cc liquid test meal were very similar to the fasting post-operative changes. Total CDCA, G-CDCA, total DCA, G-DCA, total MCA, G-MCA, total HCA, unamidated HCA, and G-HCA AUC had all significantly increased by six weeks and stayed significantly elevated at 12 weeks after SG from pre-operative values (Table 3). Unamidated CDCA (18.9 ± 21.2, p = 0.009) and unamidated DCA (35.5 ± 22.7, p = 0.03) were significantly increased at 12 weeks after surgery compared to pre-op (8.6 ± 17.2 and 23.7 ± 17.7, respectively). Compared to pre-op, T-UDCA significantly decreased at 12 weeks after SG (2.2 ± 2.2 vs. 0.9 ± 0.7, p = 0.006).
Table 3.
Meal-stimulated area under the curve (AUC) for BAs with statistically significant changes after sleeve gastrectomy
| Bile acid subtype | Pre-op AUC (μM* min) | 6 week AUC (μM* min) | 12 week AUC (μM* min) |
|---|---|---|---|
| Total CDCA | 97.2 ± 53.9 | 161.4 ± 75.6* | 145.6 ± 63.6* |
| Unamidated CDCA | 8.6 ± 17.2 | 14.2 ± 14.6 | 18.9 ± 21.2* |
| Glycine-CDCA | 60.4 ± 40.6 | 111.2 ± 61.7* | 101.3 ± 46.7* |
| Total DCA | 72.0 ± 33.5 | 96.9 ± 50.3* | 107.6 ± 48.0* |
| Unamidated DCA | 23.7 ± 17.7 | 25.5 ± 19.7 | 35.5 ± 22.7* |
| Glycine-DCA | 37.9 ± 23.9 | 60.3 ± 36.5* | 62.7 ± 29.0* |
| Total MCA | 2.2 ± 2.3 | 4.1 ± 3.6* | 3.7 ± 2.7* |
| Glycine-MCA | 0.6 ± 0.8 | 1.5 ± 1.4* | 1.5 ± 1.0* |
| Total HCA | 1.9 ± 2.0 | 4.3 ± 3.0* | 3.8 ± 2.6* |
| Unamidated HCA | 0.3 ± 0.4 | 0.7 ± 0.5* | 0.7 ± 0.5* |
| Glycine-HCA | 0.6 ± 0.6 | 1.8 ± 1.4* | 1.5 ± 1.0* |
| Taurine-UDCA | 2.2 ± 2.2 | 1.6 ± 1.5 | 0.9 ± 0.7* |
Data presented as the means ± standard deviation. Total for any individual bile acid is the sum of unamidated and glycine and taurine-amidated subtypes
CDCA chenodoxycholic acid, DCA deoxycholic acid, MCA muricholic acid, HCA hyocholic acid, UDCA ursodeoxycholic acid
p<0.05 compared to pre-op
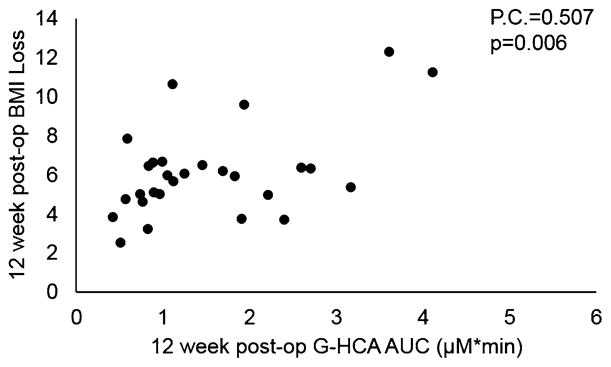
All fasting and meal-stimulated bile acid subtypes that significantly changed as described above were correlated to both the corresponding 6- and 12-week BMI losses. We found CDCA and HCA to significantly correlate to the weight loss response. Increased total post-prandial CDCA AUC was significantly correlated to the 6 week BMI loss (Pearson’s correlation 0.404, p = 0.03). This was primarily due to the increase in G-amidated CDCA AUC as this was also significantly correlated to weight loss at 6 weeks (Pearson’s correlation 0.392, p = 0.04). However, post-prandial CDCA AUC was not significantly correlated to weight loss at 12 weeks post-op. Increased post-prandial G-amidated HCA AUC was significantly correlated to BMI loss at both 6 (Pearson’s correlation 0.379, p = 0.005) and 12 weeks (Pearson’s correlation 0.507, p = 0.006, Fig. 3). At 12 weeks after surgery, elevated fasting G-HCA concentration was also significantly correlated to weight loss (Pearson’s correlation 0.478, p = 0.01).
Fig. 3.
The positive correlation between glycine-amidated HCA (hyocholic acid) area under the curve (AUC) from a liquid test meal and BMI loss at 12 weeks after sleeve gastrectomy
Discussion
In this study, we prospectively evaluated the changes in fasting and meal-stimulated BAs after SG. We found multiple different BA subtypes that changed at 6 or 12 weeks. There was a persistent increase in post-operative fasting total, unamidated and G-HCA. Post-prandial total BA, G-BA, total CDCA, G-CDCA, total DCA, G-DCA, total MCA, G-MCA, total HCA, unamidated HCA, and G-HCA AUC had all significantly increased by six weeks and stayed significantly elevated at 12 weeks after SG. Our results show a novel correlation between a clinical change in BAs after SG and the weight loss outcome. Six week post-prandial total and G-CDCA AUC significantly correlated to the 6 week BMI loss. Further, increased G-amidated HCA significantly correlated to increased weight loss at both 6 and 12 weeks suggesting an early and persistent post-prandial G-HCA change after SG may mediate a component of early surgical weight loss.
While we found multiple significant increases in both fasting BA concentrations and post-pranidal BA AUC, there is inconsistency in the literature regarding the effect of SG on both fasting and post-pranidal BAs. Haluzikova et al. found no difference in fasting total BAs at 6, 12, or 24 months post-operatively [22]. Belgaumkar et al. also found no difference in total fasting bile acids at 6 months after surgery, although similar to our study there were significant changes in the fasting bile acid profile [15]. Jahansouz et al. found fasting total and glycine-amidated BAs significantly increased as early as one week after SG [14]. Although we found no difference in total fasting BAs at 6 weeks, it may be there is a more immediate effect of SG on fasting BAs that changes with increased time post-operatively. From our study, it appears that the critical BA changes after SG associated to weight loss are post-prandial. Steinert et al. found post-prandial total BAs decreased at 1 weeks with no significant changes from baseline at 3 months or 1 year post-operatively however there was no differentiation between amidated and unamidated individual BAs [16]. The extensive post-prandial BA profiling performed by Khan et al. supports our finding of significant increases in total and individual BA fractions, which are primarily hydrophilic. The inconsistency of BA findings post-SG in the literature highlights the importance of evaluating the individual components of the meal-stimulated BA pool in both unamidated and amidated forms to further our understanding of how individual BA changes may mediate post-surgical weight loss and metabolic disease improvement [23].
CDCA is a primary BA synthesized from cholesterol in the liver. CDCA is the most potent BA agonist for FXR and an attractive mediator of the effect of SG on FXR activity. The ratio of CDCA:CA after SG increases and is associated with decreased insulin resistance [15]. We found a novel correlation in this study with increased G-CDCA AUC at 6 weeks after SG and BMI loss. Khan et al. also found a significant increase in the proportion of post-prandial CDCA of the BA pool at one month post-operatively after SG in adolescents [17]. There are compelling data in mice studies that FXR plays a critical role in the weight loss outcome after SG. Targeted disruption of FXR in mice disabled the ability of SG to reduce body weight or improve glucose tolerance [19]. Given the correlation of increased meal-stimulated CDCA AUC to BMI loss after SG, our study supports a role of CDCA and likely FXR signaling after SG in the early weight loss response.
Originally identified as a primary BA in pigs, HCA (3α, 6α, 7α-trihydroxy-5β-cholanoic acid) is a minor component of the human BA pool with one of the lowest hydrophobicity indices [24]. HCA has a high affinity for 6-α glucuronidation which markedly enhances urinary excretion and decreases toxicity [25]. HCA can be generated from α-MCA and CDCA by intestinal bacteria or by cytochrome P450 3A4 (CYP3A4) in the liver [26, 27]. HCA levels are elevated in hepatic cirrhosis and cholestasis [28]. Conversion of CDCA to HCA is mediated by enhanced CYP3A4 action in cholestatic disease catalyzing hydroxylation of the BA and increasing hydrophilicity and reducing toxicity [29, 30]. HCA is also cytoprotective against hydrophobic BAs [31]. Very little is published about the affinity of HCA to either FXR or TGR5. However, similar BAs like MCA, with a 6-hydroxyl group, are considered FXR antagonists and have reduced affinity for the TGR5 receptor [32, 33]. Although we found increased HCA consistently correlated with improved post-surgical weight loss, we feel it is plausible that elevated meal-stimulated HCA does not mediate the surgical weight loss but is a sensitive surrogate marker of an augmented BA pool, and in particular increased CDCA levels post-operatively which we hypothesize may mediate post-surgical weight loss.
We found a significant increase in meal-stimulated DCA after SG at both 6 and 12 weeks post-operatively. LCA and DCA are potent agonists of the TGR5 receptor. BA stimulation of TGR5 receptors located on enteroendocrine L-cells stimulates GLP-1 secretion. TGR5 targets are increased after gastric bypass, but not with caloric restriction alone [11]. Further, gastric bypass surgery induced increased post-prandial total BAs that were significantly associated with increased peak GLP-1 but not insulin secretion or energy expenditure [11]. Conversely, Belgaumkar et al. found that SG significantly decreased fasting DCA concentrations at 6 months which correlated with decreased fasting insulin [15]. The different findings between these studies may be related to the time points studied post-operatively or the inclusion in this study of both sulfated and unsulfated BA forms.
This study is a single-center prospective study of a heterogeneous patient population of not only patient gender, age, severity of BMI and ethnicity, but also metabolic disease, all of which are factors that may impact the change in the BA pool post-operatively. Further, we did not have a dietary control group. We cannot distinguish in this study if the effect of a SG independently changes the bile acid pool or if these observed effects are the results of weight loss or post-operative dietary changes. However, Jahansouz et al. found a significant increase in the total BA and amidated BA pool after SG compared to a decrease in the unamidated BA pool after a hypocaloric diet, suggesting that the surgery itself independently affects the BA pool and not dietary or weight loss changes [14]. This study also describes only early weight loss changes. Additional follow-up will determine if the BA changes detected are persistent and continue to correlate to mid-term and long-term weight loss after SG.
In conclusion, in this prospective observational study, we found two changes in BA subtypes after SG which correlate with the early weight loss response. This study presents compelling data that a SG produces a number of significant changes in the BA pool with specific changes in CDCA and HCA correlating to the weight loss response. Given the high affinity of CDCA to FXR and that HCA is an epimerization product of elevated CDCA, it is possible that augmented meal-stimulated CDCA and FXR signaling play a role in the early weight loss after SG. Further clinical and mechanistic studies will be required to determine if the correlation of increased CDCA and HCA after SG to weight loss is mediated by enhanced FXR activity with down-stream effects on glucose and lipid metabolism as well as energy expenditure.
Acknowledgments
This study was supported by a SAGES Research Grant, a Center for Clinical and Translational Research Pilot Grant from the University of Nebraska Medical Center and funding support by DK100314 (R.K.).
Funding Sages Research Grant (T.K.), Translational Research Pilot Grant from the University of Nebraska Medical Center (T.K.) NIH DK100314 (R.K.)
Footnotes
Presented at the SAGES 2017Annual Meeting, March 22–25, 2017, Houston, Texas.
Compliance with ethical standards
Disclosures Dr. Kohli discloses investigator initiated research with Raptor Pharmaceuticals, DSMB/research agreements with Shire, Galectin Therapeutics and Takeda Pharmaceutical Company and consultant/speaking compensation for Intercept Pharmaceuticals and Alexion Pharmaceuticals. Drs. Kindel, Krause, McBride, Oleynikov, Kothari, and Alnouti as well as Ms. Helm, Mr. Thakare and Mr. Alamoudi have no conflicts of interest or financial ties to disclose.
References
- 1.Peterli R, Wolnerhanssen BK, Vetter D, Nett P, Gass M, Borbely Y, Peters T, Schiesser M, Schultes B, Beglinger C, Drewe J, Bueter M. Laparoscopic sleeve gastrectomy versus Roux-Y-gastric bypass for morbid obesity-3-year outcomes of the prospective randomized Swiss Multicenter Bypass or Sleeve Study (SM-BOSS) Ann Surg. 2017;265:466–473. doi: 10.1097/SLA.0000000000001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evers SS, Sandoval DA, Seeley RJ. The physiology and molecular underpinnings of the effects of bariatric surgery on obesity and diabetes. Annu Rev Physiol. 2017;79:313–334. doi: 10.1146/annurev-physiol-022516-034423. [DOI] [PubMed] [Google Scholar]
- 3.Fouladi F, Mitchell JE, Wonderlich JA, Steffen KJ. The contributing role of bile acids to metabolic improvements after obesity and metabolic surgery. Obes Surg. 2016;26:2492–2502. doi: 10.1007/s11695-016-2272-3. [DOI] [PubMed] [Google Scholar]
- 4.Escalona A, Munoz R, Irribarra V, Solari S, Allende F, Francisco Miquel J. Bile acids synthesis decreases after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:763–769. doi: 10.1016/j.soard.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Zhou H, Hylemon PB. Bile acids are nutrient signaling hormones. Steroids. 2014;86:62–68. doi: 10.1016/j.steroids.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 7.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 9.Kumar DP, Rajagopal S, Mahavadi S, Mirshahi F, Grider JR, Murthy KS, Sanyal AJ. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem Biophys Res Commun. 2012;427:600–605. doi: 10.1016/j.bbrc.2012.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trabelsi MS, Daoudi M, Prawitt J, Ducastel S, Touche V, Sayin SI, Perino A, Brighton CA, Sebti Y, Kluza J, Briand O, Dehondt H, Vallez E, Dorchies E, Baud G, Spinelli V, Hennuyer N, Caron S, Bantubungi K, Caiazzo R, Reimann F, Marchetti P, Lefebvre P, Bäckhed F, Gribble FM, Schoonjans K, Pattou F, Tailleux A, Staels B, Lestavel S. Farnesoid X receptor inhibits glucagon- like peptide-1 production by enteroendocrine L cells. Nat Commun. 2015;6:7629. doi: 10.1038/ncomms8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–E712. doi: 10.1210/jc.2012-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Giorgi S, Campos V, Egli L, Toepel U, Carrel G, Cariou B, Rainteau D, Schneiter P, Tappy L, Giusti V. Long-term effects of Roux-en-Y gastric bypass on postprandial plasma lipid and bile acid kinetics in female non diabetic subjects: a cross-sectional pilot study. Clin Nutr. 2015;34:911–917. doi: 10.1016/j.clnu.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Werling M, Vincent RP, Cross GF, Marschall HU, Fandriks L, Lonroth H, Taylor DR, Alaghband-Zadeh J, Olbers T, Le Roux CW. Enhanced fasting and post-prandial plasma bile acid responses after Roux-en-Y gastric bypass surgery. Scand J Gastroenterol. 2013;48:1257–1264. doi: 10.3109/00365521.2013.833647. [DOI] [PubMed] [Google Scholar]
- 14.Jahansouz C, Xu H, Hertzel AV, Serrot FJ, Kvalheim N, Cole A, Abraham A, Luthra G, Ewing K, Leslie DB, Bernlohr DA, Ikramuddin S. Bile acids increase independently from hypocaloric restriction after bariatric surgery. Ann Surg. 2016;264:1022–1028. doi: 10.1097/SLA.0000000000001552. [DOI] [PubMed] [Google Scholar]
- 15.Belgaumkar AP, Vincent RP, Carswell KA, Hughes RD, Alaghband-Zadeh J, Mitry RR, le Roux CW, Patel AG. Changes in bile acid profile after laparoscopic sleeve gastrectomy are associated with improvements in metabolic profile and fatty liver disease. Obes Surg. 2016;26:1195–1202. doi: 10.1007/s11695-015-1878-1. [DOI] [PubMed] [Google Scholar]
- 16.Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T, Beglinger C. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity. 2013;21:E660–E668. doi: 10.1002/oby.20522. [DOI] [PubMed] [Google Scholar]
- 17.Khan FH, Shaw L, Zhang W, Salazar Gonzalez RM, Mowery S, Oehrle M, Zhao X, Jenkins T, Setchell KD, Inge TH, Kohli R. Fibroblast growth factor 21 correlates with weight loss after vertical sleeve gastrectomy in adolescents. Obesity. 2016;24:2377–2383. doi: 10.1002/oby.21658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, Kohli R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity. 2014;22:390–400. doi: 10.1002/oby.20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Perez HE, Sandoval DA, Kohli R, Backhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bathena SP, Mukherjee S, Olivera M, Alnouti Y. The profile of bile acids and their sulfate metabolites in human urine and serum. J Chromatogr B. 2013;942–943:53–62. doi: 10.1016/j.jchromb.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Bathena SP, Cxanaky IL, Alnouti Y. Simultaneous characterization of bile acids and their sulfate metabolites in mouse liver, plasma, bile and urine using LC-MS/MS. J Pharm Biomed Anal. 2011;55:1111–1119. doi: 10.1016/j.jpba.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 22.Haluzikova D, Lacinova Z, Kavalkova P, Drapalova J, Krizova J, Bartlova M, Mraz M, Petr T, Vitek L, Kasalicky M, Haluzik M. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity. 2013;21:1335–1342. doi: 10.1002/oby.20208. [DOI] [PubMed] [Google Scholar]
- 23.Khan FH, Kohli R. Bariatric surgery: the rise and fall of bile acids. Surg Obes Relat Dis. 2016;12:770–771. doi: 10.1016/j.soard.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 24.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–730. [PubMed] [Google Scholar]
- 25.Radominska-Pyrek A, Zimniak P, Irshaid YM, Lester R, Tephly TR, St Pyrek J. Glucuronidation of 6 alpa-hydroxy bile acids by human liver microsomes. J Clin Invest. 1987;80:234–241. doi: 10.1172/JCI113053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap–bile acids in metabolic control. Nat Rev Endocrinol. 2014;10:488–498. doi: 10.1038/nrendo.2014.60. [DOI] [PubMed] [Google Scholar]
- 27.Bodin K, Lindbom U, Diczfalusy U. Novel pathways of bile acid metabolism involving CYP3A4. Biochim Biophys Acta. 2005;1687:84–93. doi: 10.1016/j.bbalip.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Kano M, Matsumoto M, Kamano T, Tsurumaru M. ELISA determination of serum hyocholic acid concentrations in humans and their possible clinical significance. Hepatogastroenterology. 1999;46:983–984. [PubMed] [Google Scholar]
- 29.Chen J, Zhao KN, Chen C. The role of CYP3A4 in the biotransformation of bile acids and therapeutic implication for cholestasis. Ann Transl Med. 2014;2:7. doi: 10.3978/j.issn.2305-5839.2013.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stedman C, Robertson G, Coulter S, Liddle C. Feed-forward regulation of bile acid detoxification by CYP3A4: studies in humanized transgenic mice. J Biol Chem. 2004;279:11336–11343. doi: 10.1074/jbc.M310258200. [DOI] [PubMed] [Google Scholar]
- 31.Araki Y, Andoh A, Bamba H, Yoshikawa K, Doi H, Komai Y, Higuchi A, Fujiyama Y. The cytotoxicity of hydrophobic bile acids is ameliorated by more hydrophilic bile acids in intestinal cell lines IEC-6 and Caco-2. Oncol Rep. 2003;10:1931–1936. [PubMed] [Google Scholar]
- 32.Sato H, Macchiarulo A, Thomas C, Gioiello A, Une M, Hofmann AF, Saladin R, Schoonjans K, Pellicciari R, Auwerx J. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem. 2008;51:1831–1841. doi: 10.1021/jm7015864. [DOI] [PubMed] [Google Scholar]
- 33.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]