Abstract
IMPORTANCE
Management of the primary tumor site in patients with metastatic breast cancer remains controversial.
OBJECTIVE
To evaluate the patterns of receipt of initial breast surgery for female patients with stage IV breast cancer in the United States, with particular attention to women who survived at least 10 years.
DESIGN, SETTING, AND PARTICIPANTS
A retrospective cohort study using data from the Surveillance, Epidemiology, and End Results (SEER) program. Female patients diagnosed as having stage IV breast cancer between 1988 and 2011 and who did not receive radiation therapy as part of the first course of treatment were included (N = 21 372). Kaplan-Meier estimates of median survival and descriptive statistics were used to compare patient and tumor characteristics by receipt of breast surgery at diagnosis. A Royston-Parmar survival model and logistic regression analysis assessed demographic and clinical factors associated with survival and prolonged survival (of at least 10 years).
MAIN OUTCOMES AND MEASURES
Differences in survival, particularly survival of at least 10 years, by receipt of initial surgery to the primary tumor.
RESULTS
Among the 21 372 patients, the median survival increased from 20 months (1988–1991) to 26 months (2007–2011). During this time, the rate of surgery declined (odds ratio [OR], 0.16; 95% CI, 0.12–0.21). Even so, receipt of surgery was associated with improved survival in multivariate analysis, which controlled for patient and clinical characteristics, along with time period (hazard ratio, 0.60; 95% CI, 0.57–0.63). For women diagnosed as having cancer before 2002 (n = 7504), survival of at least 10 years was seen in 9.6%(n = 353) and 2.9% (n = 107) of those who did and did not receive surgery, respectively (OR, 3.61; 95% CI, 2.89–4.50). In multivariate analysis, survival of at least 10 years was associated with receipt of surgery (odds ratio, 2.80; 95% CI, 2.08–3.77), hormone receptor–positive disease (OR, 1.76; 95% CI, 1.25–2.48), older age (OR, 0.41; 95% CI, 0.32–0.54), larger tumor size (OR, 0.37; 95% CI, 0.27–0.51), marital status of being separated at the time of diagnosis (OR, 0.67; 95% CI, 0.51–0.88), and more recent year of diagnosis (OR, 1.43; 95% CI, 1.02–1.99).
CONCLUSIONS AND RELEVANCE
Survival in stage IV breast cancer has improved and is increasingly of prolonged duration, particularly for some women undergoing initial breast surgery. As systemic therapy advances provide better control of distant disease in stage IV breast cancer, and as women present with lower distant disease burdens, these findings on initial surgery to the primary tumor may be of importance.
Breast cancer is the most common malignancy in women in the United States and the developed world.1,2 Approximately 5% to 10% of women diagnosed as having breast cancer present with stage IV disease and have an intact primary breast tumor.3 While this represents a small portion of patients with breast cancer, given the prevalence of the disease, management of the primary tumor in stage IV disease remains a common clinical scenario. As with earlier-stage disease, these tumors are heterogeneous with regard to biology and disease burden. The appropriate local management of the primary tumor in stage IV breast cancer, which currently is largely considered incurable, continues to be debated.
This question has also been considered in other solid tumors. Adecade ago, mounting evidence suggested that removal of the primary malignancy offered survival benefit in some advanced stage solid tumors, including renal cell cancer,4 melanoma,5 and colon cancer.6 The effect appeared particularly pronounced in more immunologically driven malignancies. In breast cancer, reports from large retrospective series at that time showed consistent benefit to surgical removal of the primary tumor.7–11 However, these studies also noted that patients undergoing surgery were younger and healthier, likely introducing bias and confounding practical interpretation of these data. Randomized clinical trials are now under way but have been slow to accrue and report. Two randomized trials, here to presented only in abstract form, failed to show an overall survival benefit to primary disease-site surgery.12,13 Prospectively enrolled registries may also further delineate the role of surgery in metastatic breast cancer.14 Current guidelines for the management of stage IV breast cancer consider this a systemic disease and generally reserve primary site treatment for palliation of local symptoms.
More recent literature has found subsets of women who may benefit from aggressive local management. A randomized clinical trial from Turkey, with more than 20 months of follow-up, suggested that patients with solitary bone metastases had significantly improved survival following complete excision of the primary breast tumor and regional nodes.12 However, interpretation of this work is limited by small patient numbers and lack of a confirmatory biopsy of the presumptive metastatic site. A retrospective study of 300 women found a survival advantage for patients undergoing breast surgery who had bone-only metastases.9 A large meta-analysis found survival benefit in women with small primary tumors, fewer comorbidities, and lower metastatic burden.15
In contrast, the benefit of local control in stage IV breast cancer for palliation is well established.16–18 Previous randomized clinical trials have also reported benefit for primary surgery to the breast in establishing local disease control.12,13 Additionally, at least 1 retrospective series has also suggested that local control, established through early resection of the breast tumor, can be associated with improved survival, although again bias was a concern and randomized clinical trials to address this question were urged.18
Importantly, the spectrum of stage IV breast cancer is also evolving. Precise modern imaging often captures small deposits of metastatic disease. Many women diagnosed as having stage IV disease today have a lower overall disease burden than their counterparts from earlier eras. These lower disease loads likely have survival implications. Several reports, largely institutional series, described prolonged survival in women with limited stage IV disease treated with aggressive multimodality therapy.19–21 With this increasingly common scenario of durable control of stage IV disease, management of the intact primary breast tumor may again be a relevant question.
In this context, we report outcomes by initial treatment to the primary site, by period, for women presenting with stage IV breast cancer in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry from 1988–2011. We also describe treatment trends over time and survival outcomes by clinical parameters. The characteristics of women who experienced prolonged survival (≥10 years) are also presented.
Methods
We obtained population-based data from the SEER program, which currently includes 18 cancer registries and covers approximately 28% of the US population.22 The SEER program collects demographic(eg, patient age at diagnosis, sex, race/ethnicity, and marital status) and clinical (eg, cancer site, histology, stage, tumor size, and hormone receptor status) information, along with local therapy (surgery and/or radiation) and length of survival. The SEER program identifies only the first course of therapy, defined as those recorded in the treatment plan at diagnosis and administered before disease progression or recurrence.
This study was reviewed and approved by the institutional review board at the University of Iowa and determined to not be human participant research; therefore, patient consent was waived.
Between 1988 and 2011, 39 875 women were diagnosed as having microscopically confirmed, adjusted American Joint Committee on Cancer sixth edition stage IV breast cancer. Patients were excluded if they were diagnosed as having disease at autopsy or on death certificate (n = 69), if the index breast cancer was not known to be their first malignant primary (n = 5610), or if their first course of local therapy was unknown (n = 1126). Because SEER does not record the anatomic site of radiation, individuals were also excluded if they received radiation as part of initial therapy, as this could have been delivered to a distant site (n = 11 698). Our final cohort included 21 372 women (eFigure 1 in the Supplement).
Women were categorized by whether they received surgery to the primary site as initial therapy. Tumors were categorized by size as 2 cm or smaller, between 2 and 5 cm, and greater than 5 cm. Hormone receptor(HR) status, available after 1989, was considered positive if either estrogen or progesterone receptors were positive. Women were categorized as white, black, or other race/ethnicity, according to SEER terminology. Age at diagnosis was categorized as younger than 45 years, 45 to 64, and 65 and older. Marital status at the time of diagnosis was categorized as married, single (never married), and separated (including divorced and widowed). Diagnosis year was categorized into 5 periods: 1988–1991, 1992–1996, 1997–2001, 2002–2006, and 2007–2011.
Kaplan-Meier estimates of median survival were calculated by receipt of surgery and compared using log-rank tests; 95% CIs of these differences were calculated via bootstrap. A multivariate survival model was implemented to assess factors associated with overall mortality. Royston-Parmar survival functions were chosen instead of a Cox model because the proportional hazards assumption was violated.23 The baseline survival function was approximated and smoothed by a restricted cubic spline function with 5 df (4 intermediate knots placed at quintiles of the distribution of events and 2 knots at each boundary). This flexible parametric survival model framework is able to account for time-dependent effects using a second spline function (a deviation from the baseline spine) to fit interactions between the covariate and time. We allowed treatment and age effects to vary over time, specifying 3 df for the spline. The underlying time scale was defined as time in months since breast cancer diagnosis.
We also performed additional analyses to assess prolonged survival of 10 or more years. For these sub analyses, only patients diagnosed as having cancer before 2002 were included to allow sufficient follow up (n = 7504). The characteristics of women who survived at least 10 years after diagnosis (n = 460) was compared with those who died within 20 months, the median survival for this sample (n = 4152).A multivariate logistic model predicted prolonged survival, controlling for receipt of surgery, patient characteristics, and period (1988–1991, 1992–1996, and 1997–2001).
Surgery use over the study period was estimated using Joinpoint analyses to allow for changes in trends over time. Log-linear regression models were used to calculate average annual percentage change for each trend segment.
All tests were 2-sided with a significance level set at α = .05. Analyses were conducted using Stata release 12 (StataCorp LP) and Joinpoint version 4.2 (National Cancer Institute).
Results
Patient Characteristics
The full cohort of women from SEER who presented with de novo stage IV disease from 1988–2011 and who did not receive upfront radiation therapy included 21 372 patients (Table 1). Surgery was recorded in the initial treatment plan at diagnosis and delivered to 39.0% of women (n = 8330).Women receiving no surgery were on average 1.9 years older than women who received surgery (64.0 vs 62.1 years; 95% CI of difference, 1.5–2.3). Larger tumor size (>5 cm) correlated with no surgery. Married women represented the largest proportion of women and were more likely to undergo surgery than single or separated women. Although black women make up 12% to 13% of the female population24 and 10.4% of all invasive female breast cancers,25 they comprised 16.1% of the study sample. They were also less likely to undergo surgery. Overall, HR-negative tumors were more likely to receive surgery than HR-positive tumors, although this was not consistently the case until after 2001 (results not shown).
Table 1.
Patient Characteristics
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Full Sample | No Surgery | Surgery | OR (95% CI)a | |
| Patient total | 21 372 (100) | 13 042 (61.0) | 8330 (39.0) | |
| Median age, y | 63 | 64 | 62 | NA |
| Mean age, y | 63.3 | 64.0 | 62.1 | NA |
| Age categories | NA | |||
| <45 y | 2239 (10.5) | 1201 (9.2) | 1038 (12.5) | 1.40 (1.29–1.53) |
| 45–64 y | 9078 (42.5) | 5493 (42.1) | 3585 (43.0) | 1.04 (0.98–1.10) |
| ≥65 y | 10 055 (47.0) | 6348 (48.7) | 3707 (44.5) | 0.85 (0.80–0.89) |
| Tumor size | ||||
| ≤2 cm | 3101 (20.1) | 1617 (19.8) | 1484 (20.3) | 1.03 (0.95–1.12) |
| >2–5 cm | 6468 (41.8) | 3155 (38.7) | 3313 (45.4) | 1.32 (1.24–1.41) |
| >5 cm | 5887 (38.1) | 3387 (41.5) | 2500 (34.3) | 0.73 (0.69–0.78) |
| Marital status | ||||
| Married | 8767 (41.0) | 5030 (38.6) | 3737 (44.9) | 1.30 (1.23–1.37) |
| Single | 3971 (18.6) | 2591 (19.9) | 1380 (16.6) | 0.80 (0.75–0.86) |
| Separated | 7673 (35.9) | 4732 (36.3) | 2941 (35.3) | 0.96 (0.90–1.01) |
| Unknown | 961 (4.5) | 689 (5.3) | 272 (3.3) | 0.61 (0.52–0.70) |
| Race/ethnicity | ||||
| White | 16 567 (77.8) | 9954 (76.7) | 6613 (79.5) | 1.18 (1.10–1.26) |
| Black | 3424 (16.1) | 2228 (17.2) | 1196 (14.4) | 0.81 (0.75–0.87) |
| Other | 1310 (6.1) | 801 (6.2) | 509 (6.1) | 0.99 (0.88–1.11) |
| Hormone receptor status | ||||
| Negative | 4223 (19.8) | 2301 (17.6) | 1922 (23.1) | 1.40 (1.31–1.50) |
| Positive | 11 774 (55.1) | 7000 (53.7) | 4774 (57.3) | 1.16 (1.10–1.22) |
| Unknown | 5375 (25.1) | 3741 (28.7) | 1634 (19.6) | 0.61 (0.57–0.65) |
| Hispanic | 1778 (8.4) | 1071 (8.3) | 707 (8.5) | 1.03 (0.93–1.14) |
| Survived ≥10 yb | 460 (6.2) | 107 (2.9) | 353 (9.6) | 3.61 (2.89–4.50) |
Abbreviations: NA, not applicable; OR, odds ratio.
Odds of receiving surgery for a given characteristic compared with those not in this category.
Sample included women diagnosed as having stage IV breast cancer before 2002.
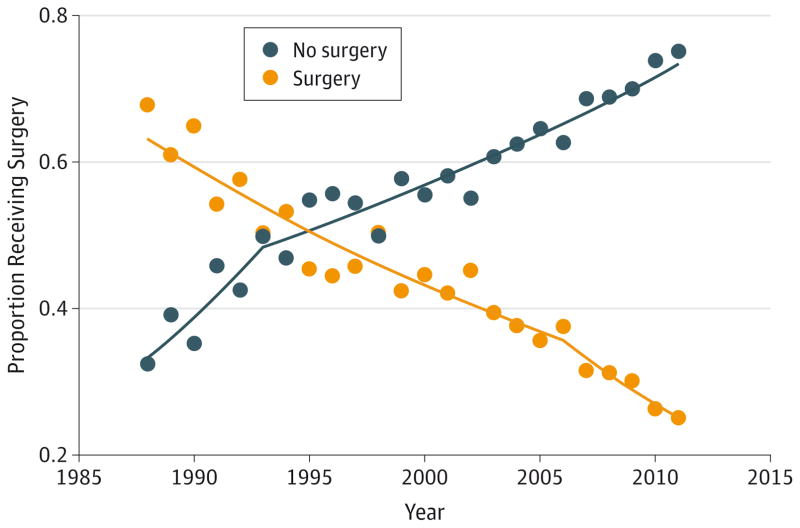
Surgery Over Time
Surgery trends changed significantly over the 24-year period (Figure).Over time, fewer women received surgery, from 67.8% in 1988 to 25.1% in 2011 (odds ratio[OR],0.16;95% CI, 0.12–0.21). The average annual percentage decrease in the proportion of women receiving surgery was 3.2% (95% CI, 2.7%–3.7%) from 1988–2006 and 7.1%(95% CI, 4.9%–9.3%) from 2006–2011.
Figure.
Proportion Receiving Surgery, 1988–2011
Survival by Receipt of Surgery
For the entire cohort, the median survival was 23 months (Table 2). Kaplan-Meier survival curves are shown in eFigure 2 in the Supplement. Those undergoing surgery had longer median survival than those who did not (28 months vs 19 months; 95% CI of difference, 7.6–10.4). Improved survival was associated with surgery, regardless of tumor size. Women with tumors 2 cm or smaller saw an additional improvement in survival of 11 months (95% CI, 6.4–15.6) with surgery. The increase was 7 months for those with tumors greater than 5 cm(95% CI, 4.9–9.0).
Table 2.
Median Survival in Months and Estimated HR by Receipt of Surgery
| Characteristic | Median Survival, mo | HR (95% CI)a | ||
|---|---|---|---|---|
| Full Sample (n = 21 372) | No Surgery n = 13 042) |
Surgery n = 8330) |
||
| Full sample | 23 | 19 | 28 | 0.68 (0.66–0.70) |
| Age, y | ||||
| <45 | 28 | 24 | 34 | 0.71 (0.63–0.78) |
| 45–64 | 27 | 22 | 35 | 0.65 (0.62–0.69) |
| ≥65 | 17 | 14 | 23 | 0.71 (0.68–0.74) |
| Tumor size | ||||
| ≤2 cm | 27 | 23 | 34 | 0.69 (0.63–0.75) |
| >2–5 cm | 27 | 21 | 32 | 0.67 (0.63–0.71) |
| >5 cm | 20 | 17 | 24 | 0.78 (0.73–0.83) |
| Diagnosis year | ||||
| 1988–1991 | 20 | 13 | 24 | 0.63 (0.56–0.71) |
| 1992–1996 | 19 | 14 | 23 | 0.67 (0.61–0.73) |
| 1997–2001 | 20 | 15 | 27 | 0.66 (0.61–0.71) |
| 2002–2006 | 24 | 20 | 30 | 0.67 (0.63–0.71) |
| 2007–2011 | 26 | 22 | 35 | 0.61 (0.57–0.66) |
| Marital status | ||||
| Married | 28 | 23 | 35 | 0.67 (0.63–0.70) |
| Single | 21 | 18 | 28 | 0.72 (0.67–0.78) |
| Separated | 18 | 14 | 23 | 0.68 (0.64–0.71) |
| Unknown | 21 | 19 | 28 | 0.74 (0.62–0.89) |
| Race/ethnicity | ||||
| White | 24 | 20 | 29 | 0.69 (0.66–0.71) |
| Black | 16 | 14 | 20 | 0.68 (0.62–0.73) |
| Other | 25 | 21 | 33 | 0.65 (0.56–0.75) |
| Hispanic | ||||
| No | 23 | 18 | 28 | 0.68 (0.66–0.70) |
| Yes | 24 | 20 | 31 | 0.67 (0.59–0.75) |
| Hormone receptor status | ||||
| Negative | 13 | 11 | 16 | 0.73 (0.68–0.79) |
| Positive | 30 | 26 | 38 | 0.67 (0.64–0.70) |
| Unknown | 16 | 12 | 23 | 0.63 (0.59–0.67) |
Abbreviation: HR, hazard ratio.
Effect of surgery for those with this characteristic compared with those not in this category.
Irrespective of surgery, median survival has increased over time, with the largest gains occurring after 2001. Women diagnosed as having breast cancer from 1988–1991 who underwent surgery had a median survival of 24 months. Their counterparts in 2007–2011 had an improvement of 11 months (95% CI, 6.8–15.2).
Estimates from the flexible parametric survival model reveal an association between surgery and longer survival (Table 3). Younger age, smaller tumor, more recent year of diagnosis, being married at the time of diagnosis, white or other race/ethnicity, and HR positivity were also associated with longer survival. Controlling for all other covariates, the largest effect size was associated with HR-positive tumors (hazard ratio, 0.53; 95% CI, 0.50–0.56), followed by receipt of surgery (hazard ratio, 0.60; 95% CI, 0.57–0.63) and then age 65 years and older (hazard ratio, 1.54; 95% CI, 1.46–1.62). Propensity adjusted analyses were conducted ad hoc, as a number of factors were associated with both treatment and survival (eTable 1 in the Supplement). Results from this model found similar survival improvements associated with surgery (OR, 0.63; 95% CI, 0.60–0.66).
Table 3.
HRs for Women with Stage IV Breast Cancer
| Characteristic | HR (95% CI)a | P Value |
|---|---|---|
| Local therapy | ||
| No surgery | 1 [Reference] | NA |
| Surgery | 0.60 (0.57–0.63) | <.001 |
| Age at diagnosis, y | ||
| <45 | 0.77 (0.70–0.85) | <.001 |
| 45–64 | 1 [Reference] | NA |
| ≥65 | 1.54 (1.46–1.62) | <.001 |
| Tumor size | ||
| ≤2 cm | 1 [Reference] | NA |
| >2–5 cm | 1.06 (1.00–1.11) | .045 |
| >5 cm | 1.25 (1.18–1.31) | <.001 |
| Diagnosis year | ||
| 1988–1991 | 1 [Reference] | NA |
| 1992–1996 | 1.03 (0.95–1.13) | .44 |
| 1997–2001 | 1.01 (0.93–1.09) | .83 |
| 2002–2006 | 0.89 (0.82–0.96) | .004 |
| 2007–2011 | 0.81 (0.74–0.88) | <.001 |
| Marital status | ||
| Married | 1 [Reference] | NA |
| Single | 1.19 (1.12–1.25) | <.001 |
| Separated | 1.26 (1.21–1.32) | <.001 |
| Unknown | 1.13 (1.02–1.27) | .03 |
| Race/ethnicity | ||
| White | 1 [Reference] | NA |
| Black | 1.18 (1.12–1.25) | <.001 |
| Other | 0.91 (0.84–0.99) | .03 |
| Hispanic | ||
| No | 1 [Reference] | NA |
| Yes | 1.01 (0.94–1.09) | .77 |
| Hormone receptor status | ||
| Negative | 1 [Reference] | NA |
| Positive | 0.53 (0.50–0.56) | <.001 |
| Unknown | 0.72 (0.68–0.77) | <.001 |
Abbreviations: HR, hazard ratio; NA, not applicable.
HRs estimated from Royston-Parmar flexible parametric survival model.
Prolonged Survival
Women with stage IV breast cancer are increasingly experiencing prolonged survival. For women diagnosed as having stage IV breast cancer before 2002 (n = 7504), 6.2% survived 10 or more years (eTable 2 in the Supplement). Women who received surgery to the intact tumor at diagnosis were more likely to survive at least 10 years than those who did not (9.6% vs 2.9%; OR, 3.61; 95% CI, 2.89–4.50). Of women who underwent surgery as initial local therapy, 13.5% of those 45 to 64 years old, 13.4% of those with tumor size 2 cm or smaller, and 11.1% of those with HR-positive disease survived 10 or more years.
Characteristics of women diagnosed as having stage IV breast cancer before 2002 who survived 10 or more years (n = 460) were compared with those who died within 20 months (n = 4152), the median survival for this group (eTable 3 in the Supplement). Factors associated with prolonged survival in this univariate analysis were similar to those associated with median survival.
On multivariate analysis, survival of at least 10 years was most strongly associated with surgery (Table 4).Women who received surgery were 2.80 times more likely to survive at least 10 years than those who did not (95% CI, 2.08–3.77). Similar significant effects were also seen for 6-and 8-year survival (OR, 2.42; 95% CI, 2.10–2.79 and OR, 2.72; 95% CI, 2.21–3.34, respectively). Additional clinical factors that correlated with prolonged survival were tumor size, HR status, marital status, and year of diagnosis. Propensity-adjusted models were also fitted ad hoc, with similar results (eTable 1 in the Supplement).
Table 4.
Multivariate Logistic Model Predicting 10 or More Years of Survivala
| Characteristic | OR (95% CI) | P Value |
|---|---|---|
| Local therapy | ||
| No surgery | 1 [Reference] | NA |
| Surgery | 2.80 (2.08–3.77) | <.001 |
| Age at diagnosis, y | ||
| <45 | 0.97 (0.69–1.35) | .85 |
| 45–64 | 1 [Reference] | NA |
| ≥65 | 0.41 (0.32–0.54) | <.001 |
| Tumor size | ||
| ≤2 cm | 1 [Reference] | NA |
| >2–5 cm | 0.76 (0.58–0.98) | .04 |
| >5 cm | 0.37 (0.27–0.51) | <.001 |
| Diagnosis year | ||
| 1988–1991 | 1 [Reference] | NA |
| 1992–1996 | 1.30 (0.92–1.84) | .14 |
| 1997–2001 | 1.43 (1.02–1.99) | .04 |
| Marital status | ||
| Married | 1 [Reference] | NA |
| Single | 0.81 (0.59–1.13) | .22 |
| Separated | 0.67 (0.51–0.88) | .004 |
| Unknown | 0.81 (0.39–1.72) | .59 |
| Race/ethnicity | ||
| White | 1 [Reference] | NA |
| Black | 0.93 (0.66–1.33) | .70 |
| Other | 1.17 (0.75–1.82) | .499 |
| Hispanic | ||
| No | 1 [Reference] | NA |
| Yes | 0.92 (0.59–1.44) | .72 |
| Hormone receptor status | ||
| Negative | 1 [Reference] | NA |
| Positive | 1.76 (1.25–2.48) | .001 |
| Unknown | 1.30 (0.88–1.91) | .19 |
Abbreviations: NA, not applicable; OR, odds ratio.
Sample included women diagnosed as having stage IV breast cancer before 2002.
Discussion
This study provides contemporary information from a large population-based data set on the complex topic of survival for women receiving initial surgery to the primary tumor in stage IV breast cancer. This updates earlier reports, which described outcomes for women diagnosed as having stage IV breast cancer more than a decade ago.7–9 Importantly, the proportion and characteristics of a large cohort of long-term survivors are described. Limited literature has previously been available on this subset of patients with breast cancer.26
During the study, treatment changed significantly, with fewer women who presented with de novo metastatic breast cancer receiving surgery to the primary tumor. This is consistent with the dogma that evolved over this period that metastatic breast cancer is a systemic disease and local therapy would have little impact on outcome. As we move into an era with markedly improved systemic therapies, enhanced radiotherapy, and advanced radiology, which finds ever smaller deposits of distant disease, this is a concept that may need to be revisited. Aggressive local therapy may benefit selectwomen such as those with an already established potential for durable remission, including those with oligometastatic disease or disease that is biologically vulnerable to newer systemic therapies.19–21,27–29 Removal of the primary breast tumor in such cases could improve survival by providing local control, eradicating a potential seed source and possibly a stimulant of distant disease sites, and perhaps also by modulating immune response.8,30
In this population-based study of more than 21 000 patients spanning 24 years, initial surgery to the primary tumor was associated with survival benefit. Women undergoing surgery were younger and more likely to be married. This is consistent with a report describing better cancer outcomes for married patients, as they are generally thought to have stronger support networks.31 However, the multivariate model controlled for these imbalances, as well as year of diagnosis and tumor size, and still found a significant and positive association between surgery and survival. The hazard ratio reported here is similar to those seen in other observational series.7–10 We also found that black women were disproportionately presenting with stage IV disease compared with the general population and invasive breast cancers. They were less likely to undergo surgery and had poorer survival.
The absolute survival benefit observed for women with small primary breast tumors is also consistent with a previous meta-analysis.15 This may support the concept that some women with a lower disease burden could have greater benefit from surgery. Additionally, women with larger tumors received surgery less often. Presumably, many of these cancers were not amenable to surgery, suggesting that these women had a heavier local disease burden. They could have had more challenging local control and may have done poorly on this basis. One observational series has associated local control with survival in the metastatic setting.18 The overview by Clarke and colleagues32 reported that local control in the adjuvant setting impacts overall survival after 15 years. Thus, the notion that local control in the stage IV setting could impact survival is plausible.
Even so, the biology of breast cancer is heterogeneous. Some patients may have a disease burden or disease biology such that overall control, obtained through systemic therapy, needs to be sought first. Results from a randomized clinical trial, which presented early outcomes, showed that women with aggressive disease consistently do worse with early local therapy.12 Patients with multiple visceral metastases had worse survival with initial surgery when compared with upfront systemic therapy. If these women who may require primary systemic therapy to gain disease control were removed from the analysis, would the benefit to others be still greater? This body of work also leaves open the possibility that some subtypes of breast cancer, perhaps those that are more immunologically driven, may benefit from surgery. This would correspond to the model seen in some other solid tumors.
On multivariate analysis, women who received initial local therapy of surgery were more likely to survive 10 or more years. This was a more powerful predictor than age, tumor size, year of diagnosis, marital status, race/ethnicity, and tumor receptor status. However, SEER cannot account for many variables including use of ever-improving systemic therapies, social support, and access to care. It is possible that receiving upfront surgery is a surrogate for these and other factors that contribute to prolonged disease control.
Further limitations to this work include the caveats inherent in large observational data sets. We excluded a large group of patients who received radiation as initial course of therapy because the anatomic site of radiation is unknown. Additionally, the number and sites of metastases are not reported in SEER, neither is detailed information on treatment and timing of staging. A subset of our cohort was likely upstaged during surgery. For these patients, who presumably had a lower burden of disease, the initial treatment decision was made under the assumption of a lower stage. Information on the extent of surgical margins or regional lymph node surgery is also not available; thus, it is not possible to determine whether the observed benefit of surgery is heterogeneous for these subgroups. Finally, SEER includes palliative surgery as cancer directed surgery when cancer tissue is removed, and some patients may have received palliative surgery for symptoms. The inclusion of palliative surgical procedures would suggest the reported OR associated with surgery is likely a lower bound of the true estimate. Notably, we were unable to account for hormonal therapy, chemotherapy, or targeted therapy, both with regard to their use and timing of delivery. Patients who respond to systemic therapy may be identified as having more controllable disease and subsequently receive surgery. Also, our analysis of prolonged survival included only patients diagnosed as having stage IV breast cancer prior to 2002. Since then, there have been multiple advances in systemic treatment. Any survival benefit of more contemporary therapies is not captured in this long-term survival analysis.
Conclusions
This work will add to the body of evidence on these important concepts in the care of women with advanced breast cancer. Randomized clinical trials and prospectively enrolled registries will be essential to understanding the underlying causal relationship between our observed association of receipt of surgery and improved survival. A large benefit for many women with stage IV breast cancer with surgery to the intact primary tumor is unlikely, especially as an ever-increasing array of more potent and targeted drugs may be able to provide better control or even eradication of systemic disease. However, systemic therapies cannot yet manage all macroscopic disease fully. Hopefully, this time will come. Until then, local therapy with surgery to the primary tumor may offer critical disease control for select patients and could be an essential component of prolonged survival.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported in part by the University of Iowa Holden Comprehensive Cancer Center Population Research Core, which is supported in part by the National Institutes of Health/National Cancer Institute grant P30 CA086862.
Footnotes
Correction: This article was corrected on January 27, 2016, to fix a typographical error in the end matter.
Conflict of Interest Disclosures: None reported.
Author Contributions: Dr Schroeder had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Schroeder.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Thomas, Schroeder.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Schroeder.
Administrative, technical, or material support: Thomas, Chrischilles.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36(3):237–248. doi: 10.1016/j.canep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310(4):389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 4.Flanigan RC, Yonover PM. The role of radical nephrectomy in metastatic renal cell carcinoma. Semin Urol Oncol. 2001;19(2):98–102. [PubMed] [Google Scholar]
- 5.Essner R, Lee JH, Wanek LA, Itakura H, Morton DL. Contemporary surgical treatment of advanced-stage melanoma. Arch Surg. 2004;139(9):961–966. doi: 10.1001/archsurg.139.9.961. [DOI] [PubMed] [Google Scholar]
- 6.Rosen SA, Buell JF, Yoshida A, et al. Initial presentation with stage IV colorectal cancer: how aggressive should we be? Arch Surg. 2000;135(5):530–534. doi: 10.1001/archsurg.135.5.530. [DOI] [PubMed] [Google Scholar]
- 7.Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery. 2002;132(4):620–626. doi: 10.1067/msy.2002.127544. [DOI] [PubMed] [Google Scholar]
- 8.Gnerlich J, Jeffe DB, Deshpande AD, Beers C, Zander C, Margenthaler JA. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988–2003 SEER data. Ann Surg Oncol. 2007;14(8):2187–2194. doi: 10.1245/s10434-007-9438-0. [DOI] [PubMed] [Google Scholar]
- 9.Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol. 2006;24(18):2743–2749. doi: 10.1200/JCO.2005.04.2226. [DOI] [PubMed] [Google Scholar]
- 10.Ruiterkamp J, Ernst MF, van de Poll-Franse LV, Bosscha K, Tjan-Heijnen VC, Voogd AC. Surgical resection of the primary tumour is associated with improved survival in patients with distant metastatic breast cancer at diagnosis. Eur J Surg Oncol. 2009;35(11):1146–1151. doi: 10.1016/j.ejso.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Dawood S, Broglio K, Gonzalez-Angulo AM, Buzdar AU, Hortobagyi GN, Giordano SH. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;26(30):4891–4898. doi: 10.1200/JCO.2007.14.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soran A, Ozmen V, Ozbas S, et al. Abstract S2–03: Early follow up of a randomized trial evaluating resection of the primary breast tumor in women presenting with de novo stage IV breast cancer: Turkish study (protocol MF07–01) Cancer Res. 2013;73(24 suppl):S2–S03. [Google Scholar]
- 13.Badwe R, Parmar V, Hawaldar R, et al. Abstract S2-02: Surgical removal of primary tumor and axillary lymph nodes in women with metastatic breast cancer at first presentation: a randomized controlled trial. Cancer Res. 2013;73(24 suppl):S2-S02. [Google Scholar]
- 14.King T, Lyman J, Gonen M, et al. Abstract P2-18-09: TBCRC 013: a prospective analysis of the role of surgery in stage IV breast cancer. Cancer Res. 2013;73(24 suppl) P2-18-09. [Google Scholar]
- 15.Harris E, Barry M, Kell MR. Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV breast cancer impacts on survival. Ann Surg Oncol. 2013;20(9):2828–2834. doi: 10.1245/s10434-013-2998-2. [DOI] [PubMed] [Google Scholar]
- 16.Shibasaki S, Jotoku H, Watanabe K, Takahashi M. Does primary tumor resection improve outcomes for patients with incurable advanced breast cancer? Breast. 2011;20(6):543–547. doi: 10.1016/j.breast.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Carmichael AR, Anderson ED, Chetty U, Dixon JM. Does local surgery have a role in the management of stage IV breast cancer? Eur J Surg Oncol. 2003;29(1):17–19. doi: 10.1053/ejso.2002.1339. [DOI] [PubMed] [Google Scholar]
- 18.Hazard HW, Gorla SR, Scholtens D, Kiel K, Gradishar WJ, Khan SA. Surgical resection of the primary tumor, chest wall control, and survival in women with metastatic breast cancer. Cancer. 2008;113(8):2011–2019. doi: 10.1002/cncr.23870. [DOI] [PubMed] [Google Scholar]
- 19.Chua TC, Saxena A, Liauw W, Chu F, Morris DL. Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer. 2011;47(15):2282–2290. doi: 10.1016/j.ejca.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Meimarakis G, Rüttinger D, Stemmler J, et al. Prolonged overall survival after pulmonary metastasectomy in patients with breast cancer. Ann Thorac Surg. 2013;95(4):1170–1180. doi: 10.1016/j.athoracsur.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 21.Hanrahan EO, Broglio KR, Buzdar AU, et al. Combined-modality treatment for isolated recurrences of breast carcinoma: update on 30 years of experience at the University of Texas MD Anderson Cancer Center and assessment of prognostic factors. Cancer. 2005;104(6):1158–1171. doi: 10.1002/cncr.21305. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute. [Accessed February 1, 2015];Surveillance Epidemiology and End Results (SEER) Program. http://seer.cancer.gov/
- 23.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 24.US Census Bureau. [Accessed June 5, 2015]; http://www.census.gov/popest/data/historical/index.html.
- 25.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313(2):165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 26.Pagani O, Senkus E, Wood W, et al. … ESO-MBC Task Force. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102(7):456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman ZU, Frye DK, Smith TL, et al. Results and long term follow-up for 1581 patients with metastatic breast carcinoma treated with standard dose doxorubicin-containing chemotherapy: a reference. Cancer. 1999;85(1):104–111. doi: 10.1002/(sici)1097-0142(19990101)85:1<104::aid-cncr15>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 28.Tait CR, Waterworth A, Loncaster J, Horgan K, Dodwell D. The oligometastatic state in breast cancer: hypothesis or reality. Breast. 2005;14(2):87–93. doi: 10.1016/j.breast.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Jackisch C. HER-2-positive metastatic breast cancer: optimizing trastuzumab-based therapy. Oncologist. 2006;11(suppl 1):34–41. doi: 10.1634/theoncologist.11-90001-34. [DOI] [PubMed] [Google Scholar]
- 30.Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64(6):2205–2211. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 31.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.