Abstract
Adult-infant interactions operate simultaneously across multiple domains and at multiple levels – from physiology to behavior. Unpackaging and understanding them, therefore, involves analysis of multiple data streams. In this study, we tested physiological responses and cognitive preferences for infant and adult faces in adult females and males. Infrared thermography was used to assess facial temperature changes as a measure of emotional valence, and we used a behavioral rating system to assess adults’ expressed preferences. We found greater physiological activation in response to infant stimuli in females than males. As for cognitive preferences, we found greater responses to adult stimuli than to infant stimuli, both in males and females. The results are discuss in light of the Life History Theory. Finally, we discuss the importance of integrating the two data streams on our conclusions.
Keywords: Infrared Thermography, Baby Faces, Attitude Toward Infants, Automatic Response, Baby Schema, Emotional Valence, Emotional Representation
Introduction
Dyadic interactions, adult-child relationships included, operate simultaneously across multiple domains – from emotions to cognitions – and at multiple levels – from physiology to behavior (Bornstein, 2013). Unpackaging and understanding them, therefore, involves analysis of multiple data streams. To assess emotional components of interactions, we can ask participants to describe their feelings, but the measurement of physiological responses has proven more challenging. Traditionally, measuring emotional valence has proceded through reactions of peripheral psychophysiological targets, usually heart rate, diastolic blood pressure, and skin conductance (e.g., Cacioppo et al., 2000). However, these measures have not proven to be particularly discerning (Kagan, 1994; Rimm-Kaufman & Kagan, 1996; Weiner, 1992). For example, heart rate shows similar patterns in different emotional states, increasing in both negative/depressed emotional states (see Brosschot & Thayer, 2003) and positive/excited emotional states (see Piira et al., 2013). By contrast, measures of skin temperature, which have been used much less often, show promise of permitting stronger inferences about emotional states. In this study, we measure changes in skin temperature in response to developmentally relevant (infant) stimuli.
Peripheral measurement of body temperature (using external sensors on the fingers) has been employed to dissociate positive versus negative emotional states. For example, Bugental and Cortez (1988) reported larger decreases in skin temperature in adult women, suggesting the presence of fear or anxiety, in reaction to unresponsive children compared to a less stressful, responsive child. Similarly, Hirota and Hirai (1990) observed a greater temperature decrease in response to imagery that elicited negative emotional states compared to neutral imagery. However, past studies that have relied on peripheral measurements of temperature have encountered technical problems because they require participants to wear sensors and to remain still, both of which could influence emotional state. Thanks to technological advances, cameras with infrared thermography now offer suitable novel, non-contact, non-invasive, and low-cost alternative methods to assess skin temperature, and investigate emotional responses.
Infrared thermography (IRT) detects radiation in the infrared range (0.78 to 1000 μm) of the electromagnetic spectrum and produces visible images of that radiation (thermograms). The amount of radiation emitted by an object increases with its temperature. When viewed through a thermal-imaging camera, warm objects stand out against cooler backgrounds. In appearance and operation, thermographic cameras are similar to well-known and widely available commercial videocameras. Infrared thermography has now been used successfully to measure facial skin temperature and its change as a sign of emotional state. Except for few specific situations (i.e., anger blushing), increases in skin temperature are normally associated with positive emotions, and decreases with negative emotions (Kuraoka & Nakamura, 2011; Mizukami et al., 1990; Rimm-Kaufman & Kagan, 1996; Zajonc, Murphy, & Ingelhart, 1989). This technology has also been used in different cultural groups (e.g., in U.S.A.: Rimm-Kaufman & Kagan, 1996; in Japan: Mizukami, et al. 1990), in different age groups (e.g., in infants: Nakanishi & Imai-Matsumura, 2007; in adults: Nhan & Chau, 2010), and in different species (e.g., in macaque monkeys: Kuraoka & Nakamura, 2011; in rats: Vianna & Carrive, 2005; in hens: Moe, Stubsjøen, Bohlin, Flø, & Bakken, 2012).
IRT assessment of autonomic mechanisms that may regulate emotions in adult-infant interaction is the main focus of this study. Emotions are shaped by many biological and social characteristics (Bornstein, 2000). Here, we contrasted infant versus adult faces as stimuli to investigate and compare emotional components of female and male adult emotional responsiveness. The current study examines emotional responses to infant faces in females and males across physiological and behavioral data streams. We selected infant faces as stimuli because of their strong emotional valence. The positive emotional response to infant faces was proposed by the ethologist Konrad Lorenz (1943, 1971) who identified a set of physiognomic features in infants known as kindchenschema (large round head, big eyes, small nose and mouth, and chubby cheeks) that automatically elicit affection and nurturance. Neuroimaging and behavioral studies using experimental manipulations of infant schema have supported Lorenz’s hypothesis that infant schema tend to release a set of care behaviors in conspecifics that are immediate, automatic, and unconscious (Kringelbach et al., 2008; Senese et al. 2013) and evolutionarily vital for survival (e.g., Caria et al., 2012; Glocker et al., 2009a, 2009b; Parsons et al., 2011, 2013). Previous studies (Glocker et al., 2009a, 2009b; Caria et al., 2012) have focused on how kindchenschema activate the neural system of both females and males, but they have not explicitly tested or compared emotional responses in the two genders.
Our study extends the literature on gender differences in response to infant stimuli. According to Life History Theory, female versus male attitudes toward infants are conditioned by the trade-off between mating and parenting (Draper & Harpending, 1982; Mascaro, Hackett, & Rilling, 2013). Given that organisms have finite amounts of time, effort, and energy to maximize fitness (the ability to both survive and reproduce), evolution optimizes allocation of these resources in males and females toward one or the other. Women tend to be more interested in infants and caregiving activities than men (Barnard & Solchany, 2002; Calzada, Eyberg, Rich, & Querido, 2004; Kim et al., 2013; Maestripieri & Pelka, 2002; Metsäpelto & Pulkkinen, 2003; Verhoeven et al., 2007). A consequence of the greater investment in parenting in women is reflected in their heightened sensitivity to infant stimuli. For example, De Pisapia and colleagues (2013), using functional MRI, found a sex-dependent modulation of brain responses to infant cries.
Summary of Hypotheses
To compare how females and males respond to infant versus adult facial stimuli at the levels of emotional valence and cognitive representation, we tested the following hypotheses:
Because females show greater sensitivity to infants, emotional valence (physiological change) in response to infant faces should be stronger in females than in males.
Because males show greater investment in mating, emotional valence (physiological change) and cognitive representation (preference) to adult female faces will be stronger in males than in females. We had no empirical or theoretical justification for a specific hypothesis about the interaction effect, and so we explored the interaction between gender and stimulus (adult vs. infant faces). To test these hypotheses, we employed two methodologies. To measure physiology, in specific to assess changes of facial temperature while adults looked at infant and adult faces, we used IRT, and to assess adults’ expressed preferences (ratings) toward infant and adult face we used a behavioral rating scale. Finally, to avoid biases based on caregiving experience, we recruited nonparent female and male adult participants.
Method
Participants
A total of 38 Italian non-parent adults recruited in the urban area of Trento (Italy) participated. Female (n = 19) mean age was 28.92 y (SD = 3.34) and not statistically different from male (n = 19) mean age (M = 29.21; SD = 4.46), t(36) = −.20, ns. All participants were Italian citizens, and their education levels were matched across the female and male participants (all participants had completed undergraduate college courses). Exclusion criteria were parenthood, pregnancy, having an infant in the same household, work or engaging in volunteer activities that involve infants, and neurological or psychiatric disorders, including substance abuse/dependence and psychotropic medication. All participants signed informed consents. In research involving autonomic measures, care is often taken to control participants’ smoking habits, use of prescription medications/illicit drugs, and alcohol consumption. Participants In our experiment were neither habitual smokers nor drinkers nor had they smoked, drank, or taken and medication in the 24 hours preceding the study. In addition, female participants were asked to come to the experimental session without wearing any make-up.
Stimuli
A total of 10 color pictures of infant and adult Caucasian female faces, 5 for each category, were equated for brightness and color-balance using Adobe Photoshop 9.0.1 (the average luminance value of all pixels fell between 125 and 220 cd/m2). All pictures showed a frontally oriented, neutrally expressive face on a white background; head size was matched across stimuli. Face stimuli came from public domain databases (Nefian et al. 1997; Peer, 2003; Solina, Peer, Batagelj, Juvan, & Kovac, 2003; Van Duuren, Kendell-Scott, & Stark, 2003) or were images taken by a professional photographer and edited by a private graphics company (Fulvia Riccardi). To exclude any potential influence of attractiveness (Parsons et al., 2011; Yamamoto et al., 2009), pictures were selected within a larger database (n = 96 with the same characteristics and sources) and were similarly rated by 40 adults (20 females, M age = 25.19, SD = 2.11; 20 males, M age = 27.88, SD = 3.56) on a 7-point Likert scale assessing attractiveness, t(1, 38) =.63, ns. The stimuli were presented on a laptop (for 5 s each) interleaved in one of two random orders with a 7-point scale ranging from unattractive to attractive. We then selected 10 stimuli for the experiment that were rated as moderately attractive (M = 3.42, SD =1.33).
Stimulus presentation
After stimulus preparation, the 10 picture files were presented twice on a laptop (for 5 s each) in one of two random orders. Adult and infant pictures alternated. The 5-s presentations were interspersed with 10 s of a neutral screen. A pilot study showed that 10 s was adequate to allow face temperature to return to baseline. During the first presentation, participants looked at the stimuli, and their face temperature was recorded. During the second presentation, participants rated the stimuli (see below).
Measurement and environment
A thermal imaging system (Avio TVS-200EX) and its software (Avio thermography studio 2007, version 4.8) were used to measure facial skin temperature. The testing environment was an artificial climate chamber maintained at a constant 25◦C and 55% humidity. The thermocamera was placed 1 m in front of the participant and was set to measure the participant’s overall face skin temperature. Skin temperature of the whole face (from the neck up) was recorded as a thermal image taken at the onset and offset of each visual stimulus (see Figure 1). The tip of the nose was chosen as the focal location for measuring temperature because it is a point on the face that is easy to target and detect reliably across all participants. A video camera, placed next to the thermocamera, was used to record overall behavior and check that participants were looking at the stimuli.
Figure 1.
Examples of responses to the presentation of faces of adults (A-C) and infants (D-F). In the A-C sequence, A is the presented adult stimulus, and B and C show tip of the nose facial temperatures, respectively, at the onset (32.0° C) and at the offset (32.3° C) of picture presentation. The white arrow in B points to the tip of the nose. In the D-F sequence, D is the presented infant stimulus, and E and F show the tip of the nose facial temperatures, respectively, at the onset (31.9° C) and at the offset (33.2° C) of picture presentation.
Behavioral data
Immediately after thermoimaging, participants were presented the stimuli a second time and made ratings on three 7-point scales while reviewing each of the 10 faces. The three scales were: willingness to approach the person, willingness to smile at the person, and willingness to communicate with the person. All scales ranged from not at all to extremely and were selected on the basis of the literature on adult–infant interactions to assess the degree of adults' typical and prominent responses to faces (Beebe et al., 2007; Caria et al., 2012; Feldstein et al., 1993; Stern, 1985; Trevarthen, 2003). Next, correlations among the scales were calculated, (rs = .65 – .68) and converted to an index of Willingness to Interact with the person (Cronbach’s α = .86).
Results
Preliminary analyses
Prior to data analysis, univariate and multivariate distributions of the variables were examined for normality, homogeneity of variance, outliers, and influential cases (Fox, 1997). The variable tip of the nose temperature showed a significantly skewed distribution (p < 0.01); it was therefore treated as an ordinal variable. The scores for willingness to interact were normally distributed. Correlations among the dependent variables (tip of the nose temperature and willingness to interact) ranged from = −.04 to .02, ns.
Tip of the nose temperature
For each participant we calculated the amount of change in nose temperature (offset minus onset) while looking at the two types of stimuli (infant vs. adult). Then, we computed an index of the change in nose temperature while looking at the infant faces minus while looking at the adult faces using the formula:
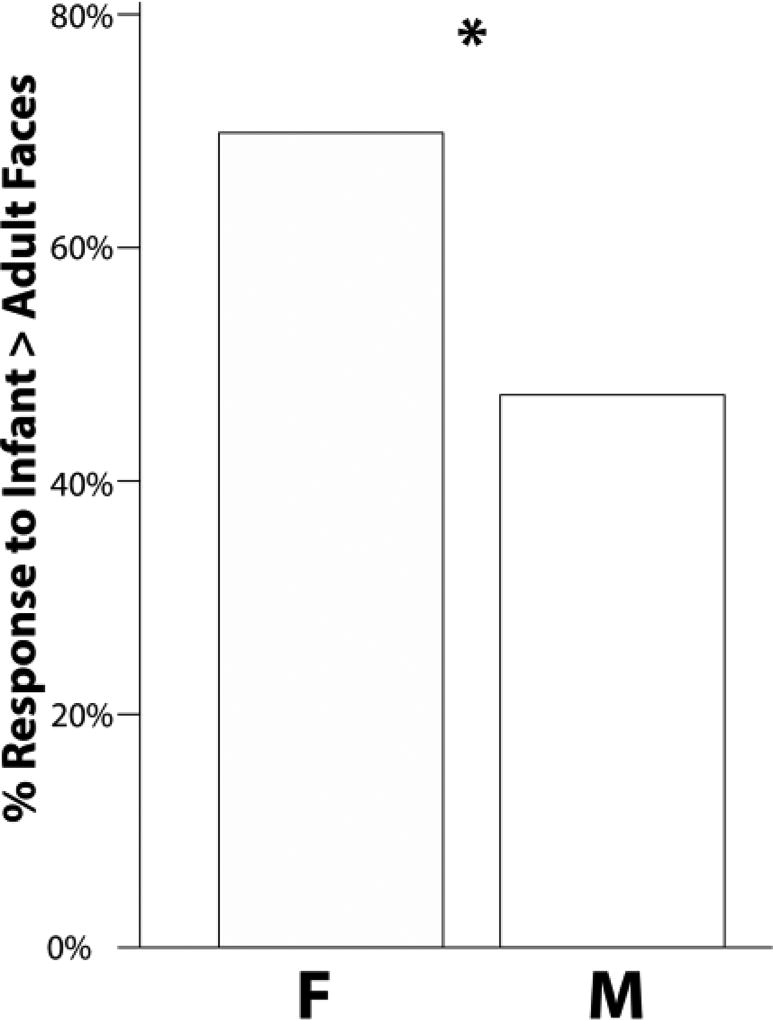
We grouped the participants whose nose temperatures increased more from onset to offset while seeing infant faces vs. participants whose nose temperatures increased more while seeing adult faces. Figure 2 shows the percentages of female and male participants whose nose temperatures increased more while watching infant faces versus adult faces. A chi-square test showed a significant gender effect (F > M), χ2(1) = 8.18, p ≤ .005, Cramer’s φ = .46.
Figure 2.
The percentages of females (F) and males (M) whose tip of the nose facial temperature increased while seeing infant faces than adult faces.
Willingness to interact
Figure 3 shows scores for the index of willingness to interact. A general linear model (GLM) with one between factor (gender: F vs. M) and one within factor (stimulus: adult vs. infant) and repeated measures (10 trials) was employed. Significant main effects emerged for gender, F(1,36) = 19.76, p ≤ .001, ηp2 = .09, and for stimulus, F(1,340) = 14.21, p ≤ .001, ηp2 = .08. Tukey post-hoc comparisons with Bonferroni correction showed that females had higher ratings than males (M difference (F scores − M scores) = .83; SE = 0.05, p < .01) and that adult faces elicited greater willingness to interact than infant faces (M difference (adult faces − infant faces) = .36, SE = .03, p < .05). The most willingness to interact was expressed by females toward adult faces, followed by infant faces, then by males toward adult faces, followed by infant faces (p ≤ .05). Neither an interaction, F(1,340) = 1.09, ns, nor a repeated effect of trial emerged, F(1,34) = 2.40, ns.
Figure 3.
Behavioral ratings (M±SEM) of females (F) and males (M) to the presentation of adult faces (light grey) and infant faces (white).
Discussion
In this study, we tested the physiological and behavioral reactions to infant and adult faces in females and males. We were interested in a multilevel analysis, investigating at one level emotional valence and at another level cognitive representation elicited by infant and adult faces. Infrared thermography was used to assess facial temperature changes as a measure of emotional valence, and we used a behavioral rating system to assess adults’ expressed preferences toward infant and adult faces.
First, we tested the hypothesis that, because females generally show greater sensitivity to infants, emotional valence (physiological change) in response to infant faces should be stronger in females than in males. In agreement with our hypothesis and with recent literature (e.g. De Pisapia et al., 2013), we found greater physiological activation in response to infant stimuli in females than males. These results might be interpreted in light of the fact that, as evident in everyday situations, and as reported elsewhere (Barnard & Solchany, 2002; Calzada, Eyberg, Rich, & Querido, 2004; Kim et al., 2013; Maestripieri & Pelka, 2002; Metsäpelto & Pulkkinen, 2003; Verhoeven et al., 2007), females tend to be more interested in infants and caregiving activities than males. This result also showed how IRT can be used successfully to differentiate female versus male emotional reactivity.
Second, we tested the hypothesis that, because males theoretically show greater investment in mating, emotional valence (physiological change) and cognitive representation (preference) to adult female faces would be stronger in males than in females. We confirmed that nonparent adult males (compared to females) in our sample showed greater physiological activation toward adult female faces, and this result might be explained by referring the Life History Theory, that evolution motivates males toward mating (Draper & Harpending, 1982; Mascaro, Hackett, & Rilling, 2013). Regarding cognitive representation, we did not find greater preference for adult female faces in males compared to females. However, in general males in our sample tended to show less willingness to interact (cognitive representation) overall (both for adult and infant faces) than females. If we calculate the relative increase in willingness to interact toward adult versus infant faces, we find that males increase their preference slightly more than females (males = 9% vs females = 6%). Although this small difference is not statistically significant, it suggests that even at a behavioural level males prefer adult female faces.
Third, we explored the interaction between gender and stimulus (adult vs. infant faces) even though we did not have empirical or theoretical justification for a specific hypothesis about the interaction . We found no significant interaction at a behavioral level, but emotional representation (cognitive preferences) in response to adult stimuli (faces) was higher in response to infant faces in both males and females. This result may be related to our sample and variable selection. It is possible that adult non-parent females and males tend to orient more to peer social interaction, because having less experience with infants, they believe that infants are less interactive generally.
Taken together, our results suggest, that physiologically and cognitively, non-parent females are more responsive than non-parent males to infants. Animal studies, as well as neuroimaging studies in humans, suggest a hardwired biological mechanism for this sexual dismorphism that probably couples with the effect of cultural environment.
Before concluding, it is useful to acknowledge some limitations of the present study as well as directions where they might lead in the future. This initial study used images of neutral faces. A logical extension and next step could use images of faces that vary in emotional valence and measure change in temperature in response to the same face with different emotional valences (i.e., smiling, grimacing, crying). It is also possible that our results were somehow biased by presenting only adult female faces. It is possible that men react differently to adult female faces than women do because female faces are inherently more attractive to men than women (men may have more positive emotions in response to female faces than women do). Similarly, women may find adult female faces easier to approach than men because there is no issue of attraction for women but there could be for men. To overcome this point, future studies should include adult male faces as stimuli.
We used a multilevel assessment to detect different aspects of reactivity (physiological and behavioral) to infant and adult faces. We believe that integration of the two data streams had a great impact on our conclusions and strengthened the results because it provided a wider perspective of analysis. Although research has failed to link autonomic activity with emotional valence consistently, a considerable literature points to lateralized patterns in frontal EEG activation that reflect positive versus negative emotional valence (see, e.g., Heller, 1993). Future testing might well couple IT assessments of facial temperature with existing well-validated measures of EEG and or other neuroimaging techniques, for example, to gain deeper insights into the neural mechanisms that regulate reactivity to infant and adult faces.
Acknowledgments
All participants in this study are gratefully acknowledged. The authors also thank Peter Peer (University of Ljubiana), Paola Venuti, Nicola Chiste`, Nadia Zanella, Patrizia Villotti, Maria Rostagno and Giulia Castelletti (University of Trento), and Fulvia Riccardi and Fabio Esposito for their assistance. This research was supported by the ST fellowship from Japan Society for the Promotion of Science (JSPS PE09064), Grant-in-aid for Scientific Research from Japan Society for the Promotion of Science (Projects #22530694 and # 24730563), the FPR Program (RIKEN Brain Science Institute), and the Intramural Research Program of the NIH, NICHD (USA).
References
- Beebe B, Jaffe J, Buck K, Chen H, Cohen P, Blatt S, Kaminer T, Feldstein S, Andrews H. Six-week postpartum maternal self-criticism and dependency and 4-month mother–infant self- and interactive contingencies. Developmental Psychology. 2007;43:1360–1376. doi: 10.1037/0012-1649.43.6.1360. [DOI] [PubMed] [Google Scholar]
- Bornstein MH. Mother-infant attunement: A multilevel approach via body, brain, and behavior. In: Legerstee M, Haley DW, Bornstein MH, editors. The infant mind: Origins of the social brain. New York: Guilford; 2013. pp. 266–298. [Google Scholar]
- Bornstein MH. Infancy: Emotions and temperament. In: Kazdin AE, editor. The Encyclopedia of Psychology. Vol. 2. New York: American Psychological Association and Oxford University Press; 2000. pp. 278–284. [Google Scholar]
- Bornstein R. Exposure and effect: Overview and meta-analysis of research 1968 – 1987. Psychological Bulletin. 1989;106:265–289. [Google Scholar]
- Bradley MM. Natural selective attention: Orienting and emotion. Psychophysiology. 2008;46(1):1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer MB. In-group bias in the minimal intergroup situation: A cognitive-motivational analysis. Psychological Bullettin. 1979;86(2):307–324. [Google Scholar]
- Brosschot JF, Thayer JF. Heart rate response is longer after negative emotions than after positive emotions. International Journal of Psychophysiology. 2003;50(3):181–187. doi: 10.1016/s0167-8760(03)00146-6. [DOI] [PubMed] [Google Scholar]
- Bugental DB, Cortez VL. Physiological reactivity to responsive and unresponsive children as moderated by perceived control. Child Development. 1988:686–693. [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, Ito TA. The psychophysiology of emotion. Handbook of emotions. 2000;2:173–191. [Google Scholar]
- Caria A, de Falco S, Venuti P, Lee S, Esposito G, Rigo P, Birbaumer N, Bornstein MH. Species-specific response to human infant faces in the premotor cortex. Neuroimage. 2012;60:884–893. doi: 10.1016/j.neuroimage.2011.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Væth M, Wenzel A. Thermographic imaging of facial skin—gender differences and temperature changes over time in healthy subjects. Dentomaxillofacial Radiology. 2012;41(8):662–667. doi: 10.1259/dmfr/55922484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham Y, Chen EE, Banaji MR. Two Signatures of Implicit Intergroup Attitudes Developmental Invariance and Early Enculturation. Psychological Science. 2013;24(6):860–868. doi: 10.1177/0956797612463081. [DOI] [PubMed] [Google Scholar]
- Feldstein S, Jaffe J, Beebe B, Crown CL, Jasnow M, Fox H, Gordon S. Coordinated interpersonal timing in adult–infant vocal interactions: a cross-site replication. Infant Behavior and Development. 1993;16:455–470. [Google Scholar]
- Fox J. Applied regression analysis, linear models and related methods. Thousand Oaks, CA: SAGE; 1997. [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Gur RC, Sachser N. Baby schema in infant faces induces cuteness perception and motivation for caretaking in adults. Ethology. 2009a;115(3):257–263. doi: 10.1111/j.1439-0310.2008.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, et al. Baby schema modulates the brain reward system in nulliparous women. Proceedings of the National Academy of Sciences of the United States of America. 2009b;106(22):9115–9119. doi: 10.1073/pnas.0811620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Jones LE, Gustafson GE. Perception of Cries by Parents and Nonparents: Relation to Cry Acoustics. Developmental Psychology. 1987;23(3):370–382. [Google Scholar]
- Hirota A, Hirai H. Effects of relaxation-response- or arousal-response-oriented training on psychologicalresponses during fear imagery. Japanese Psychological Research. 1990;32:26–35. [Google Scholar]
- Hrdy SB. Mothers and others. Cambridge, Massachusetts: Harvard University Press; 2009. [Google Scholar]
- Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, et al. A specific and rapid neural signature for parental instinct. PLoS One. 2008;3(2):e1664. doi: 10.1371/journal.pone.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka K, Nakamura K. The use of nasal skin temperature measurements in studying emotion in macaque monkeys. Physiology & Behavior. 2011;102(3):347–355. doi: 10.1016/j.physbeh.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Little AC, Jones BC, DeBruine LM. Facial attractiveness: evolutionary based research. Philosophical Transactions of the Royal Society B. 2011;366:1638–59. doi: 10.1098/rstb.2010.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K. Die angeborenen Formen möglicher Erfahrung. (Innate form of potential experience) 13. Zeitschrift für Tierpsychologie. 1943;5:235–309. doi: 10.1111/j.1439-0310.1943.tb00655.x. [DOI] [Google Scholar]
- Lorenz K. Studies in animal and human behavior. II. London: Methuen; 1971. [Google Scholar]
- Mizukami K, Kobayashi N, Ishii T, Iwata H. First selective attachment begins in early infancy: A study using telethermography. Infant Behavior and Development. 1990;13(3):257–271. [Google Scholar]
- Moe RO, Stubsjøen SM, Bohlin J, Flø A, Bakken M. Peripheral temperature drop in response to anticipation and consumption of a signaled palatable reward in laying hens (Gallus domesticus) Physiological Behavior. 2012;106:527–33. doi: 10.1016/j.physbeh.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Nakanishi R, Imai-Matsumura K. Facial skin temperature decreases in infants with joyful expression. Infant Behavior and Development. 2008;31(1):137–144. doi: 10.1016/j.infbeh.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Nefian AV, Khosravi M, Hayes MH., III Real-time human face detection from uncontrolled environments. SPIE Visual Communications on Image Processing. 1997 http://www.anefian.com/research/face_reco.htm.
- Nhan BR, Chau T. Classifying affective states using thermal infrared imaging of the human face. IEEE Transactions on Biomedical Engineering. 2010;57(4):979–987. doi: 10.1109/TBME.2009.2035926. [DOI] [PubMed] [Google Scholar]
- Ochs E. Culture and language development: Language acquisition and language socialization in a Samoan village. Cambridge, England: Cambridge University Press; 1988. [Google Scholar]
- Panksepp J. Affective neuroscience. New York: Oxford University Press; 1998. [Google Scholar]
- Parsons CE, Young KS, Kumari N, Stein A, Kringelbach ML. The motivational salience of infant faces is similar for men and women. PLoS One. 2011;6(5):e20632. doi: 10.1371/journal.pone.0020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CE, Young KS, Mohseni H, Woolrich MW, Thomsen KR, Joensson M, et al. Minor structural abnormalities in the infant face disrupt neural processing: A unique window into early caregiving responses. Social Neuroscience. 2013;8(4):268–274. doi: 10.1080/17470919.2013.795189. [DOI] [PubMed] [Google Scholar]
- Peer P. CVL Face Database. 2003 http://www.lrv.fri.uni-lj.si/facedb.html.
- Piira OP, Miettinen JA, Hautala AJ, Huikuri HV, Tulppo MP. Physiological responses to emotional excitement in healthy subjects and patients with coronary artery disease. Autonomic Neuroscience. 2013;177(2):280–285. doi: 10.1016/j.autneu.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Rimm-Kaufman SE, Kagan J. The psychological significance of changes in skin temperature. Motivation and Emotion. 1996;20(1):63–78. [Google Scholar]
- Senese VP, De Falco S, Bornstein MH, Caria A, Buffolino S, Venuti P. Human Infant Faces Provoke Implicit Positive Affective Responses in Parents and Non-Parents. PLoS One. 2013;8(11):e80379. doi: 10.1371/journal.pone.0080379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN. Higher nervous functions: The orienting reflex. Annual Review of Physiology. 1963;25(1):545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Solina F, Peer P, Batagelj B, Juvan S, Kovac J. Color-based face detection in the 15 seconds of fame art installation. Proceedings of Mirage INRIA Rocquencourt France. 2003:38–47. [Google Scholar]
- Sprengelmeyer R, Perrett DI, Fagan EC, Cornwell RE, Lobmaier JS, Sprengelmeyer A, et al. The Cutest Little Baby Face A Hormonal Link to Sensitivity to Cuteness in Infant Faces. Psychological Science. 2009;20(2):149–154. doi: 10.1111/j.1467-9280.2009.02272.x. [DOI] [PubMed] [Google Scholar]
- Stern D. The Interpersonal World of the Infant. New York: Basic Books; 1985. [Google Scholar]
- Trevarthen C. Conversations with a two month-old. In: Raphael-Leff J, editor. Parent–infant Psychodynamics: Wild things, Mirrors and Ghosts. Philadelphia: Whurr Publishers; 2003. pp. 25–34. [Google Scholar]
- Van Duuren M, Kendell-Scott L, Stark N. Early aesthetic choices: Infant preferences for attractive premature infant faces. International Journal of Behavioral Development. 2003;27:212–219. [Google Scholar]
- Vianna DM, Carrive P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. European Journal of Neuroscience. 2005;21:2505–2512. doi: 10.1111/j.1460-9568.2005.04073.x. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brammer MJ, Skerrett D, Lagopolous J, Rennie C, Kozek K, et al. The neural correlates of orienting: An integration of fMRI and skin conductance orienting. Neuroreport. 2000;11(13):3011–3015. doi: 10.1097/00001756-200009110-00037. [DOI] [PubMed] [Google Scholar]
- Winecoff A, Clithero JA, Carter RM, Bergman SR, Wang L, Huettel SA. Ventromedial Prefrontal Cortex Encodes Emotional Value. The Journal of Neuroscience. 2013;33(27):11032–11039. doi: 10.1523/JNEUROSCI.4317-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Ariely D, Chi W, Langleben DD, Elman I. Gender differences in the motivational processing of babies are determined by their facial attractiveness. PLoS One. 2009;4(6):e6042. doi: 10.1371/journal.pone.0006042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc RB, Murphy ST, Inglehart M. Feeling and facial efference: implications of the vascular theory of emotion. Psychological Review. 1989;96(3):395–416. doi: 10.1037/0033-295x.96.3.395. [DOI] [PubMed] [Google Scholar]
- Zajonc RB. Mere exposure: A gateway to the subliminal. Current Directions in Psychological Science. 2001;10:224–228. [Google Scholar]