Abstract
Purpose
To elicit and evaluate opinions in the diagnosis and management of nonparaneoplastic autoimmune retinopathy (npAIR) among members of the American Uveitis Society (AUS) and to further the development of consensus and criteria in the diagnosis and management of npAIR. We hypothesize that despite lack of any clear guidelines, a general consensus in the clinical diagnosis and treatment of npAIR exists among uveitis experts.
Methods
A literature review was performed and a panel of uveitis experts was consulted to formulate a survey regarding the diagnosis and management of npAIR. An online survey of 10 questions was developed, and a link was distributed through the AUS membership discussion list. We defined “general consensus” as meaning that a majority (>50%) of the respondents provided the same answer to a question.
Results
Fifty-four members of the AUS responded to the survey. Thirty-eight members (70.4%) see one to three AIR patients per year. Greater than 50% consensus was reached on most items, particularly items relating to diagnostic features and tests (up to 96% consensus).
Conclusions
The diagnosis and management of npAIR is challenging, as standardized clinical and laboratory diagnostic criteria have yet to be established. The results of this study support the presence of consensus regarding certain aspects of npAIR, but also indicate the need for developing clear clinical diagnostic criteria and treatment guidelines.
INTRODUCTION
Autoimmune retinopathies (AIRs) are a group of inflammatory-mediated diseases characterized by the presence of antiretinal antibodies (ARAs), visual field deficits, and photoreceptor dysfunction in the setting of progressive unexplained vision loss. Autoimmune retinopathies can be categorized as paraneoplastic AIR (pAIR), which includes cancer-associated retinopathy (CAR) and melanoma-associated retinopathy (MAR), or nonparaneoplastic AIR (npAIR) in the absence of malignancy. Fundus examination may reveal attenuation of retinal vasculature, optic disc pallor, or retinal pigmented epithelium (RPE) abnormality (late disease). However, fundus examination is often unrevealing with minimal to no intraocular inflammation. Patients typically experience photophobia, nyctalopia, dyschromatopsia, or scotomas, and testing often shows abnormal electroretinograms (ERGs) and visual fields.1,2 As AIR lacks standardized diagnostic criteria and shares numerous clinical features with other retinal degenerative diseases, it is probable that AIR may go misdiagnosed and is more prevalent than thought.
The aim of this study is to report the results of a survey among members of the American Uveitis Society (AUS) with the purpose of gauging opinion and consensus in the diagnosis and management of the nonparaneoplastic form of AIR. We hypothesize that results from this survey of AUS members will reflect a general consensus and prompt clinicians and researchers to work toward developing criteria for the diagnosis of npAIR.
HISTORICAL PERSPECTIVE
In 1976, the first cases of CAR were described, in which three female patients with bronchial carcinoma developed vision loss with photoreceptor degeneration.3 Shortly thereafter, ARAs targeting ganglion cells and photoreceptors on immunohistochemistry were identified in patients with CAR,4,5 and in 1987 Thirkill and colleagues identified a 23-kDa retinal antigen targeted by antibodies in the serum of CAR patients, which was later recognized as recoverin.6,7 Subsequently, in 1984, Klingele and colleagues8 coined the term “paraneoplastic retinopathy.” Gass described the first case of MAR,9 which was later classified as a paraneoplastic retinopathy in 1988.10 It was not until 1997 that the first cases of AIR were reported in the absence of malignancy with clinical manifestations similar to CAR.11 Since these initial reports, hundreds of cases of pAIR12–14 and multiple case series of npAIR have been reported.14–16 As might be expected in the spectrum of AIR, the majority of studies and evidence regarding its diagnosis and management involve the paraneoplastic form of AIR. Despite great progress over the last four decades, the diagnosis and management of AIR remains a challenge, as clinical diagnostic criteria and methods for ARA testing have yet to be standardized.
PATHOPHYSIOLOGY
Antiretinal antibodies are central to the pathophysiology of AIR and therefore essential to the diagnosis. Circulating ARAs may be specific to retinal antigens, such as the photoreceptor-specific calcium-binding protein recoverin (23 kDa), or may target antigens also found in nonocular tissues, such as the glycolytic enzyme α-enolase (46 kDa). Recoverin and α-enolase are the most extensively studied antigens and have been identified in paraneoplastic and nonparaneoplastic forms of AIR. Specifically regarding npAIR, consistently identified antigens targeted by ARAs include carbonic anhydrase II (CA II) (30 kDa), arrestin (S-antigen) (48 kDa), interphotoreceptor binding protein (IRBP) (141 kDa), and unknown proteins (22, 34, 35, 37, 40, 68 kDa). Multiple additional antigens have been identified in the pathogenesis of pAIR and have been reviewed elsewhere (Table 1).2,17
TABLE 1.
PROTEINS TARGETED BY ANTIRETINAL ANTIBODIES*
| CAR | Recoverin 23 kDa, α-enolase 46 kDa, Tubby-like protein 1 (TULP-1) 65 kDa, heat shock protein 70 (HSC 70) 70 kDa, unknown proteins (34, 40, 46, 60, 70 kDa) |
| MAR | Transducin 35 kDa, arrestin (S-antigen) 48 kDa |
| npAIR | Recoverin, α-enolase, arrestin (S-antigen), interphotoreceptor binding protein (IRBP) 141 kDa, unknown proteins (22, 34, 35, 37, 40, 68 kDa) |
CAR, cancer-associated retinopathy; MAR, melanoma-associated retinopathy; npAIR, nonparaneoplastic autoimmune retinopathy.
The table includes commonly reported antigens and is not an exhaustive list.
Modified from Ryan SJ, ed. Retina. 5th ed. Vol 2. London: Elsevier; 2013:chap 77
The role of ARAs in the pathogenesis of npAIR has not completely been elucidated, but it is thought that all forms of AIR share a similar mechanism of retinal damage secondary to ARAs. A body of in vitro and in vivo studies has demonstrated that ARAs are cytotoxic to retinal cells through apoptotic mechanisms involving internalization of antibodies, caspase pathways, and calcium influx.14,17–20 In an in vivo experiment, intravitreal injection of human IgG from MAR patients into the eyes of monkeys resulted in similar ERG waveforms characteristic of MAR, a reduced dark-adapted b-wave accompanied by a normal a-wave (also known as a negative ERG), suggesting disruption of ON-bipolar cell signaling.21 Subsequently, transient receptor potential cation channel subfamily M member 1 (TRPM1), expressed by both melanocytes and ON-bipolar cells, was recognized as essential to ON-bipolar cell function and identified as the ARA target in MAR patients.22,23
Whether ARAs actually initiate the pathogenesis of AIR or are the result of retinal degeneration is not clear. In CAR and MAR, it is thought that the expression of tumor antigens, identical to those of the retina, triggers the generation of ARAs, which subsequently cross-react with retinal antigens.24,25 Similarly, it is possible that ARAs in npAIR patients are generated through molecular mimicry between retinal antigens and bacterial or viral proteins. On the other hand, it is conceivable that ARAs are generated as a result of retinal antigen exposure after retinal damage, and the ARAs then propagate the disease.
While studies have suggested that ARAs are central to the pathogenesis of paraneoplastic AIR, their mere presence does not warrant the diagnosis of AIR. Antiretinal antigens have been identified in healthy controls,14 patients with systemic autoimmune diseases,26–28 as well as in uveitides and other retinal disorders.29–31 Moreover, ARAs have been undetectable in some patients with symptoms and findings consistent with AIR. In a cohort of 193 patients, less than half (47.1%) of patients with clinical features of AIR, including painless loss of vision, photopsia, and abnormal ERG, tested positive for ARAs.14 This highlights the complexity of pathogenesis of AIR. To further advance our understanding of AIR, future studies are needed to explore other factors possibly contributing to the pathogenesis of AIR. A study by our group evaluated 24 npAIR patients in an attempt to explore potential genetic associations and identified a significant association of npAIR with HLA alleles, specifically HLA-DRB1-03 and HLA-DRB1-15 (Grange L, et al. Invest Ophthalmol Vis Sci 2013;54:ARVO E-Abstract 2514). As genetic and environmental factors have been implicated in other autoimmune diseases, it is possible these factors play a role in AIR and should be further explored.
DIAGNOSIS: CLINICAL FEATURES AND ANTIRETINAL ANTIBODIES
Diagnosis of AIR is often made by exclusion in the presence of ARAs and a combination of clinical features described below. Though clinical features may vary considerably, commonly recognized manifestations have been identified.2,15,16,32 Symptoms may include photopsia, scotomas (commonly paracentral), dyschromatopsia, photoaversion, and nyctalopia. Symptoms are usually bilateral but may present unilaterally. On examination, the fundus is typically normal-appearing but may show attenuation of retinal vasculature, waxy optic disc pallor, or RPE changes (Figure 1). Intraocular inflammation is typically absent or minimal, which often helps exclude other, more common inflammatory or infectious retinopathies. On testing, visual fields may be constricted with central or paracentral scotomas. Electroretinograms may show abnormalities in scotopic and photopic responses. An extensive workup must be completed to rule out malignancy and pAIR. The workup should begin with extensive history taking and physical examination as well as imaging if warranted.
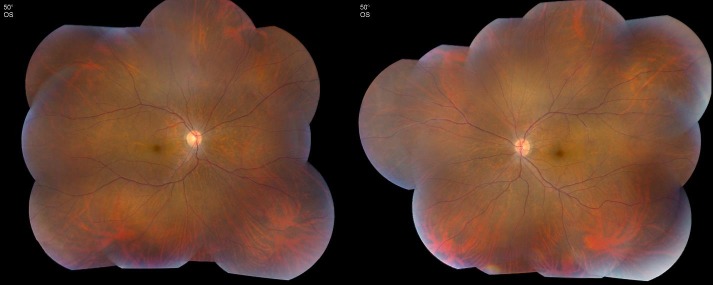
FIGURE 1.
Color fundus photograph of 39-year-old patient with npAIR. Note the mild attenuation of retinal vasculature, poor foveal reflex, mild optic nerve pallor, and an otherwise normal-appearing fundus. Patient’s visual symptoms began 6 years previously with scotomas and floaters in the left eye. Despite treatment with prednisone, mycophenolate mofetil, and cyclosporine, visual acuity gradually declined to 20/320 bilaterally. An electroretinogram shows significantly decreased cone and rod responses.
Other ancillary tests may aid in the diagnosis of AIR, including fluorescein angiography (FA), optical coherence tomography (OCT), and fundus autofluorescence (FAF). In one study, some cases of AIR showed leakage of retinal vasculature on FA and cystoid macular edema (CME) on OCT.16 In addition, reduced macular thickness on OCT was shown to be a predominant characteristic of AIR in a small cohort.33 On FAF, examination of AIR patients showed a parafoveal hyperautofluorescent ring, which showed a disrupted inner-outer segment junction and thinned outer nuclear and photoreceptor layers on spectral domain OCT.34 Similar features on FAF and OCT have been noted in following 24 npAIR patients here at the National Eye Institute (Dalal M, et al. Invest Ophthalmol Vis Sci 2013;54:ARVO E-Abstract 2516) (Figure 2). As technology advances and novel imaging methods are introduced, our ability to further characterize and diagnose AIR will progress. Supporting demographic features and medical history may aid in the diagnosis of AIR as well. As in other autoimmune diseases, AIR more often affects females (63% to 66%) in their fifth or sixth decade of life.14–16 In addition, systemic autoimmune diseases may be associated with AIR. In a cohort of 24 npAIR patients, 16 (66.7%) of the 24 were found to have a personal or family history of autoimmune disease. Interestingly, npAIR has also been reported in patients with autoimmune diseases, such as systemic lupus erythematosus.16,35
FIGURE 2.
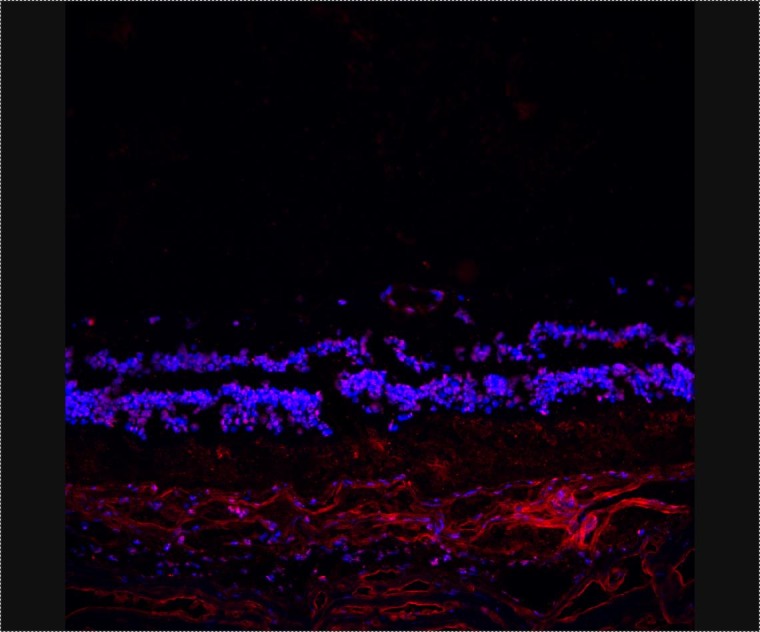
Immunohistochemistry performed using serum of patient with npAIR against fresh-frozen human donor retina shows staining at the outer nuclear layer. (Courtesy of Chi Chao Chan, MD)
As emphasized above, the presence of circulating ARAs is important in the diagnosis of AIR. However, there is no standardization for the detection and measurement of ARAs, which may result in conflicting diagnoses. One study compared the results of ARA detection and measurement between two laboratories and found an overall concordance rate of any antiretinal antibody of 60% and a very poor interobserver agreement (κ=0.13).36
In addition to this variability of testing, multiple laboratory techniques may be used to detect ARAs, including Western blot, immunohistochemistry, and enzyme-linked immunosorbent assay (ELISA), all of which lack standardization. Immunohistochemistry has the advantage of allowing for the localization of ARA binding to cellular structures in whole retinal tissue. However, this is the least sensitive technique, and ARA type cannot be determined. In this technique, serum from patients in whom AIR is suspected is incubated with normal retinal tissue, ideally from human donor, and subsequently with secondary antibodies, to be examined microscopically using fluorescent staining (Figure 3). Western blot allows ARA detection through separation of retinal antigens based on molecular weight using gel electrophoresis. Antiretinal antibodies from patient serum and then secondary antibodies are added to allow binding and visualization. As a result of differentiation based on molecular weight, this technique lacks specificity, as two different antigens, to which ARAs bind, may be similar in size. Finally, ELISA involves the addition of patient serum to wells coated with known retinal antigens and the detection of ARAs using secondary antibodies. ELISA is a highly sensitive technique; however, it requires identification of the antigen of interest prior to testing. As these techniques have advantages and disadvantages, a combination of these tests may be beneficial to maximize sensitivity, specificity, and ARA localization. Nevertheless, it is still not known whether the presence of these antibodies indicates pathogenicity.
FIGURE 3.
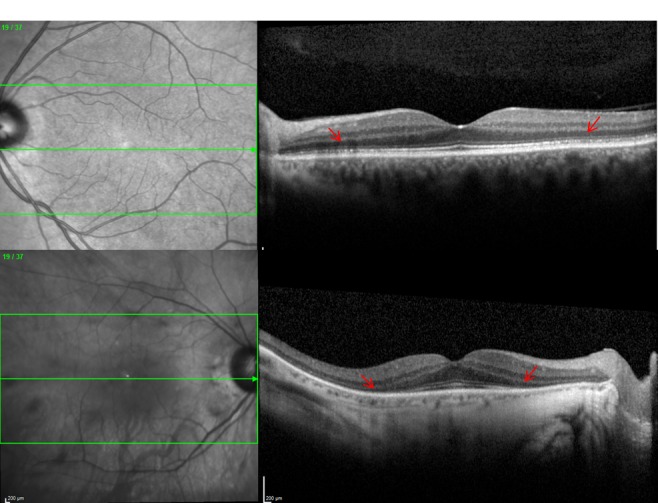
Optical coherence tomography images of patients with npAIR with different severity. Top, Patient 1. Mild disruption at the level of photoreceptors in left eye (arrows). Patient had moderately decreased cone responses (50% to 60%). Bottom, Patient 2. Extensive loss of the photoreceptor layer in right eye of a patient with more severe disease.
Regardless of the technique used, many variables in each technique limit repeatability across laboratories as well as the clinical value of ARAs. Though ARA testing is currently conducted at few institutions in the United States, not all laboratories hold Clinical Laboratory Improvement Amendments (CLIA) certification, which adds further to the variability in ARA testing. To promote collaboration and advance our understanding of AIR, standardization of ARA detection assays is essential.37
MANAGEMENT
Early diagnosis and treatment of AIR is crucial to prevent widespread retinal degeneration and irreversible vision loss; however, this remains a challenge, as the diagnosis is often tentative and made after exclusion. Most evidence in the treatment of AIR relies on reports of pAIR and includes corticosteroids, plasmapheresis, intravenous immunoglobulin (IVIG), and other immunomodulators.12,16,38–41 More recently, biologics targeting lymphocytes or B cells specifically have been reported in multiple cases as a potential treatment option.42–46 As there have been inconsistent results regarding the effectiveness of treatment in AIR, immunosuppression is often empirical due to the presumed autoimmune pathogenesis. A better understanding of AIR and the disease course is needed to validate treatment options with higher-risk profile.
In a retrospective review of 24 npAIR patients, patient-tailored combinations of cyclosporine, azathioprine, prednisone, mycophenolate mofetil, and periocular or intravitreal corticosteroid injections were administered, and visual acuity or visual field improved in 15 of the 24 (62.5%). In addition, CME improved in over half (6 of 11) of the npAIR patients with CME.16 In our experience of treating npAIR here at the National Eye Institute, we found no significant difference among npAIR patients who did and did not receive immunomodulatory therapy. Incongruously, npAIR progressed in some patients despite immunomodulatory treatment, whereas a proportion stabilized without treatment (unpublished data).15 While this could be related more to the natural history of the disease and the bias by treatment indication than to the treatment instituted, it highlights the complexity of management in AIR.
Monitoring treatment response and the clinical course of disease can be challenging, as patients often present with rapid progression of vision loss or late in the disease course when treatment is no longer effective. Treatment may be monitored through functional tests such as ERGs and visual fields or imaging such as OCT and FA.2 The utility of following ARA titers after treatment and throughout the clinical course of disease remains unclear.16,31,35,37 As no standardized diagnostic criteria exist, there are, unsurprisingly, no guidelines for the management of AIR. A multicenter registry for prospective natural history studies and randomized controlled trials would be beneficial to understand the disease course and establish effective immunomodulatory therapies in AIR as well as effective tests to monitor treatment.
METHODS
A literature review was performed and survey items were identified based on areas in need of consensus by three uveitis specialists. The survey was aimed at answering the important clinical questions and focusing on the diagnosis and management of npAIR, as these entities are the least characterized in the spectrum of AIR. The survey was kept brief to maximize the response rate yet fully elicit opinions among AUS members on their understanding, diagnosis, and management of npAIR.
The survey consisted of 10 items. The initial question regarding how many patients with AIR were seen within one year’s time was posed to identify and gauge a clinician’s experience in diagnosing and managing AIR. The remaining items were 6 questions about the understanding and diagnosis and 3 questions about management (see Appendix 1).
The survey was created using Web-based software, and a link to the survey was distributed through the AUS website in a way to maintain anonymity among responders. The survey was open for one month, from June 2013 to July 2013, and members were asked and reminded to complete the survey throughout the month. A second survey was conducted in a similar manner from December 2016 to January 2017 to determine any significant difference in responses and to explore additional items within our survey. Comparisons of survey items were made using Fisher’s exact test.
A simple majority consensus (>50%) was used as the general consensus. In addition, we analyzed the responses of clinicians who saw any AIR patients in a year’s time separately to determine if general consensus existed among those clinicians thought to have more experience with AIR.
The study was approved by the Office of Human Subjects Research at the National Institutes of Health and deemed exempt from IRB approval. This study adhered to the Declaration of Helsinki and all federal or state laws.
RESULTS
The AUS is a 237-member society of uveitis specialists. Fifty-four members of the AUS completed this survey on the nonparaneoplastic form of AIR, including the pathophysiology, diagnosis, and management of the disease (see Appendix 1).
We first inquired about the number of AIR patients seen by the members of AUS. The majority reported seeing only 1 to 3 AIR patients a year. Of the 54 members, 3 members reported seeing more than 7 patients with AIR in one year’s time, 3 members see 3 to 7 AIR patients, and 10 answered that they do not manage any AIR patients (see Appendix 2).
In order to obtain consensus on the nomenclature, a question on the categorization of AIR was posed (“2. Do you agree with the categorization of autoimmune retinopathy as: a) paraneoplastic (CAR and MAR) b) nonparaneoplastic autoimmune retinopathy?”). The majority (n=49, 90.7%) agreed with the categorization of AIR as paraneoplastic (CAR and MAR) and npAIR. Antiretinal antibodies, considered an essential part of the diagnosis by most, can be found in many other diseases and healthy controls, though this is not widely realized: 40.7% of the members (n=22) responded that they did not know what proportion of healthy controls may be positive for ARAs, 22.2% selected 0 to 5%, 35.2% selected 5% to 30%, and 1.9% selected >30%. When asked which statement best described their understanding of serum ARAs in AIR, the majority (59.3%, n=32) selected “The detection of serum antiretinal antibodies is necessary but not sufficient for the diagnosis of autoimmune retinopathy.”
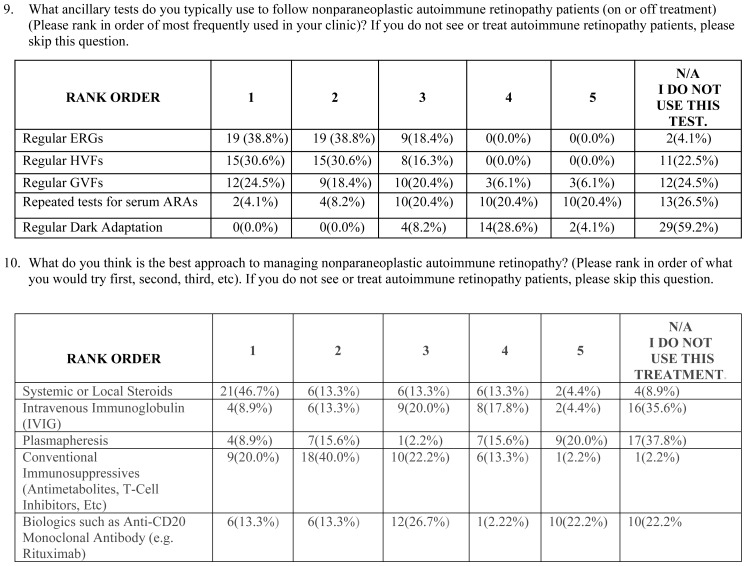
Next, we wanted to understand what the common practice was among specialists for the diagnosis and follow-up of AIR patients. As expected, the majority utilized ERGs (n=52, 96.3%), ARA testing (n=46, 85.2%), FA (n=44, 81.5%), OCT (n=43, 79.6%), FAF (n=34, 63.0%), and Humphrey visual field (HVF) (n=33, 61.1%) when they first suspect AIR in a patient. Similarly, the majority (n=46, 85.2%) initiate a malignancy workup by an appropriate physician in such patients. As for symptoms and findings crucial to diagnosis, presence of symptoms such as photopsias, nyctalopia, photoaversion, or dyschromatopsia in the setting of positive ARAs (n=45, 83.3%), presence of abnormal ERG (decline in photopic or scotopic responses) (n=42, 77.8%), absence of fundus lesions that can explain the visual field or ERG loss (n=38, 70.4%), and absence of degenerative retinal disease such as retinitis pigmentosa (n=30, 55.6%) achieved simple majority or supermajority.
In order to ascertain the importance of ARAs in the setting of an alternative clinical diagnosis, the members were asked whether they consider all patients with serum ARAs to have AIR. The majority (n=44, 86.3%) did not consider patients with positive ARAs who already have an alternative clinical diagnosis, such as birdshot chorioretinopathy or acute zonal occult outer retinopathy (AZOOR), as having npAIR.
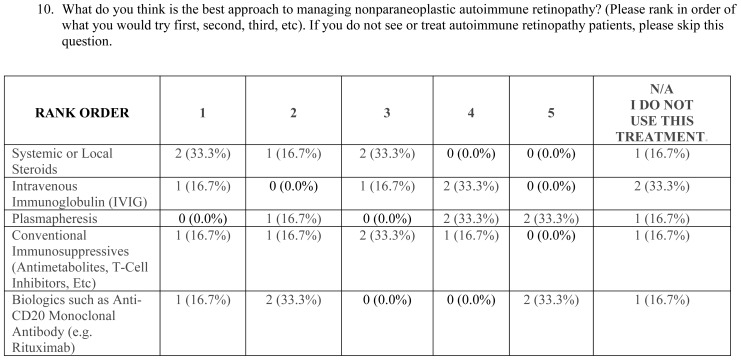
There is no consensus on the best approach or whether treatment is beneficial. To this end, the majority agreed that there is not enough evidence to indicate that npAIR patients should be treated with long-term immunomodulatory therapy but felt obligation to offer treatment to their patients (n=28, 51.9%). On the other hand, 31.5% (n=17) felt there was enough evidence. Of the 45 members who responded to a more detailed question about treatment approaches, 46.7% selected systemic or local corticosteroids as the first step in treatment of npAIR, whereas IVIG (8.9%), plasmapheresis (8.9%), nonbiologic immunosuppressives (20.0%), and biologics (13.3%) were chosen as the first step by a minority. Conventional immunosuppressives were considered the top choice for second-line treatment by 40.0% (n=18). The majority of clinicians (69.4%) followed their npAIR patients (on or off of treatment) using primarily regular ERGs and/or HVFs, whereas regular Goldmann visual field testing (24.5%) and repeated ARA testing (4.1%) were used less frequently.
To determine differences in responses and to explore additional items within our survey, a subsequent survey of similar questions was distributed. A similar number of clinicians responded (49). Though approximately 2½ years passed between surveys, there were few differences between responses from clinicians. A significantly higher proportion of participants saw 3 to 7 patients in the subsequent survey (P=.02). One additional clinician stated that he or she sees more than 7 AIR patients per year. In the survey, a significantly higher proportion of clinicians and a resulting majority stated that the presence of abnormal visual fields (central, paracentral scotoma, or constricted visual fields) (n=37, 75.5%) should be considered essential. In addition, three added findings were surveyed and considered essential to the diagnosis of npAIR: no apparent cause responsible for functional abnormality (n=37, 75.5%), presence of ARAs (n=32, 65.3%), and absence of overt (>+1 grade) intraocular inflammation (n=25, 51.0%).
Regarding ancillary tests used in follow-up of npAIR patients, the choice regular OCTs was added in the subsequent survey and this choice was selected by the majority of clinicians as a preferred test (n=34, 69.4%). Other than the above-mentioned items, there were no significant differences resulting in a change of consensus between surveys.
DISCUSSION
Autoimmune retinopathy remains an ill-defined disorder, and the lack of standardized diagnostic criteria, as well as significant overlap between the clinical features of AIR and other retinal degenerations, complicates the diagnosis and management and likely contributes to an underestimation of its prevalence. The lack of consensus for the diagnosis and treatment of npAIR, as well as the void in understanding of the pathogenic mechanisms of npAIR, motivated this study.
As a result of the survey, there seems to be consensus on the understanding of the clinical features and diagnosis of AIR, which reinforces our previous knowledge even in the absence of standardized diagnostic criteria. From the AUS members surveyed, most specialists (70.4%) answered that they see 1 to 3 AIR patients per year. Of the 54 AUS members who responded to the survey, 44 stated that they see at least one AIR patient in a year’s time. One may expect responses to differ considerably after filtering out those who had not seen an AIR patient in a year’s time. However, the responses of these 44 members did not vary largely from the responses of all 54 members. For all items, the difference in results of both groups varied by less than 6.0%.
The categorization of the spectrum of AIR was agreed upon, which reinforced our previous understanding. The majority (91%) of AUS members agree with the categorization of pAIR, such as CAR and MAR, as a separate entity from npAIR.
The AUS members reached a majority consensus on most items regarding diagnosis. In addition to absence of malignancy, the presence of symptoms such as photopsias, nyctalopia, photoaversion, or dyschromatopsia, as well as ARAs (83%) and abnormal ERGs (78%), the majority agreed that the absence of fundus lesions that could explain visual field or ERG deficits (70%) and the absence of degenerative retinal disease (56%) were essential findings in the diagnosis of AIR. Abnormal visual fields (41%) were not thought to be crucial to the diagnosis. It is important to understand the common practices in diagnosis and follow-up of these patients so that the lack of consensus and related areas of need can be identified.
In addition, AUS members agreed on the ancillary tests utilized to make the diagnosis of AIR. While ERG (96%), malignancy workup by an appropriate physician (85%), and serum ARA testing (85%) were most commonly used in the diagnosis of AIR, FA (82%), OCT (80%), FAF (63%), and HVF (61%) were used by the majority. Goldmann visual field (46%) and dark adaptation testing (9%) were the least utilized tests in the diagnosis of AIR. The utility of other potentially useful tests, such as automated kinetic visual fields, was not investigated in this survey.
The respondents seemed to overestimate the specificity of ARAs. When asked what percentage of normal, healthy donors might have positive ARAs, a significant proportion either did not know or underestimated the rate of positivity among healthy donors. As previously mentioned, ARAs have been identified in healthy individuals and in many other diseases and disorders. Antiretinal antibodies were detected in 6% to 62% of healthy controls, though it varies depending on the technique used.47–49 However, the majority (59%) did agree that the detection of serum ARAs is necessary but not sufficient for the diagnosis of AIR. Furthermore, the majority (86%) stated that they do not consider all patients with positive serum to have AIR in the presence of alternative diagnoses, such as birdshot chorioretinopathy or AZOOR. It is important to obtain a consensus on the diagnostic criteria and standardization of ARA detection to allow comparison between studies.
To date, reports on the treatment of npAIR are scarce.14,16,50 The survey results suggest that treatment is a challenge for AIR, as might be expected with any disease lacking diagnostic criteria. The majority of the AUS members (52%) agreed that there is not enough evidence to justify treatment of npAIR patients with long-term immunomodulatory therapy. However, they feel an obligation to offer treatment, and therefore offer short-term systemic immunomodulatory therapy. In line with clinical experience, ERG was the most commonly used test for following npAIR patients, along with HVFs. Repeated tests for serum ARAs and dark adaptation were not utilized as much, presumably because they are thought to be less valuable or less accessible and less practical in following up npAIR patients. Regarding specific treatment options, corticosteroids were the preferred treatment choice, followed by conventional immunomodulatory therapy and biologics. Plasmapheresis and IVIG were less preferred when treating npAIR patients. Most of the information we have on treatment of AIR involves CAR and MAR, and few studies focus on npAIR. This lack of evidence and studies likely results from the lack of standardized diagnostic criteria to establish the diagnosis of npAIR.
In the subsequent survey, a similar number of AUS members responded, and there were few significant differences between surveys. This is not unexpected given that there have not been significant advances made in AIR over this time. One difference was the higher number of clinicians who stated that they see a higher number of AIR patients. Eleven clinicians stated that they saw 3 to 7 patients in the subsequent survey, compared to three clinicians in the initial survey (P=.02). There was also one additional clinician who stated that he or she sees more than 7 AIR patients per year. Perhaps this results from an increased awareness of the clinical features of npAIR over the 3 years between surveys. Similarly, there was a significantly higher proportion of clinicians (18.4%) who were aware that greater than 30% of normal, healthy donors may have positive ARAs, as compared to 1.9% responders in the initial survey (P=.01). As indicated in previous studies, ARAs can be found in up to 62% of healthy controls.47–49 With increased awareness, clinicians may become more familiar with the clinical and laboratory features of npAIR.
Medical consensus is useful for synthesizing information to aid in making diagnostic and treatment decisions and resolve inconsistencies when there is insufficient information about a disease, such as a more rare disease. Consensus on clinical criteria and diagnostic tests is crucial to the clinical practice of medicine, as they assist in establishing a diagnosis and treatment. Ferreyra and colleagues16 proposed criteria for the full spectrum of AIR, and more recently we proposed diagnostic criteria for npAIR2 (Table 2). Here, we focus on evaluating and gauging opinions in the diagnosis and management of npAIR among AUS members.
TABLE 2.
PROPOSED DIAGNOSTIC CRITERIA FOR NONPARANEOPLASTIC AIR*
| ESSENTIAL CRITERIA | SUPPORTIVE CRITERIA |
|---|---|
| No evidence of malignancy | Photopsias or scotomas or dyschromatopsia or nyctalopia or photoaversion |
| Positive serum ARAs | |
| RG abnormality | Systemic autoimmune disease |
| Absence of fundus lesions or degenerative retinal disease that can explain the visual field or ERG loss | Acute or subacute vision loss |
| Absence of overt (>+1 grade) intraocular inflammation |
AIR, autoimmune retinopathy; ARA, antiretinal antibodies; ERG, electroretinogram.
The survey included in the current study did not address diagnostic criteria specifically.
The results from these surveys of AUS members likely reflect a general consensus among experts who manage npAIR patients, and this should prompt clinicians and researchers to fully develop criteria for the diagnosis of npAIR in the near future. Indeed, experts in the field of AIR, both clinicians and researchers, have begun to work toward developing standardized criteria for the diagnosis of npAIR as well as standardization for the detection of ARAs.
ACKNOWLEDGMENTS
Funding/Support: This work is supported by the National Eye Institute Intramural Research Program and the National Institutes of Health (NIH) Medical Research Scholars Program. The NIH Medical Research Scholars Program is a public-private partnership supported jointly by the NIH and generous contributions to the foundation for the NIH from Pfizer Inc, Doris Duke Charitable Foundation, Newport Foundation, American Association for Dental Research, Howard Hughes Medical Institute, and Colgate-Palmolive Company, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program.
Financial Disclosures: None.
Author Contributions: Conception and design of the study (H.N.S., L.G.); Conduct of the study (H.N.S., L.G., A.F., M.A.); Collection, management, analysis, and interpretation of the data (H.N.S., L.G., M.A., A.F.); Preparation, review, critical revision, and approval of the manuscript (H.N.S., L.G., A.F., M.A.); Statistical expertise (H.N.S., L.G., A.F.); Obtaining funding (H.N.S., L.G., A.F., M.A.); Literature search (H.N.S., L.G., A.F.); Provision of materials, patients, or resources (H.N.S.); Administrative, technical, or logistic support (H.N.S., L.G., A.F., M.A.).
Other Acknowledgments: The authors would like to thank Chi Chao Chan, MD, for providing Figure 3.
APPENDIX 1
APPENDIX 2
REFERENCES
- 1.Jacobson DM, Thirkill CE, Tipping SJ. A clinical triad to diagnose paraneoplastic retinopathy. Ann Neurol. 1990;28(2):162–167. doi: 10.1002/ana.410280208. [DOI] [PubMed] [Google Scholar]
- 2.Sen HN, Nussenblatt RB. Autoimmune retinopathies. In: Ryan SJ, editor. Retina. 5th ed. London: Elsevier; 2013. pp. 1381–1389. [Google Scholar]
- 3.Sawyer RA, Selhorst JB, Zimmerman LE, Hoyt WF. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol. 1976;81(5):606–613. doi: 10.1016/0002-9394(76)90125-2. [DOI] [PubMed] [Google Scholar]
- 4.Kornguth SE, Klein R, Appen R, Choate J. Occurrence of anti-retinal ganglion cell antibodies in patients with small cell carcinoma of the lung. Cancer. 1982;50(7):1289–1293. doi: 10.1002/1097-0142(19821001)50:7<1289::aid-cncr2820500711>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Keltner JL, Roth AM, Chang RS. Photoreceptor degeneration. Possible autoimmune disorder. Arch Ophthalmol. 1983;101(4):564–569. doi: 10.1001/archopht.1983.01040010564006. [DOI] [PubMed] [Google Scholar]
- 6.Thirkill CE, Roth AM, Keltner JL. Cancer-associated retinopathy. Arch Ophthalmol. 1987;105:372–375. doi: 10.1001/archopht.1987.01060030092033. [DOI] [PubMed] [Google Scholar]
- 7.Polans AS, Witkowska D, Haley TL, Amundson D, Baizer L, Adamus G. Recoverin, a photoreceptor-specific calcium- binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc Natl Acad Sci U S A. 1995;92(20):9176–9180. doi: 10.1073/pnas.92.20.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klingele TG, Burde RM, Rappazzo JA, Isserman MJ, Burgess D, Kantor O. Paraneoplastic retinopathy. J Clin Neuroophthalmol. 1984;4(4):239–245. [PubMed] [Google Scholar]
- 9.Gass JD. Acute Vogt-Koyonagi-Harada-like syndrome occurring in a patient with metastatic cutaneous melanoma. Paper presented at: Uveitis Update: Proceedings of the First International Symposium on Uveitis; Amsterdam, The Netherlands. 1984. [Google Scholar]
- 10.Berson EL, Lessell S. Paraneoplastic night blindness with malignant melanoma. Am J Ophthalmol. 1988;106:307–311. doi: 10.1016/0002-9394(88)90366-2. [DOI] [PubMed] [Google Scholar]
- 11.Mizener JB, Kimura AE, Adamus G, Thirkill CE, Goeken JA, Kardon RH. Autoimmune retinopathy in the absence of cancer. Am J Ophthalmol. 1997;123(5):607–618. doi: 10.1016/s0002-9394(14)71073-6. [DOI] [PubMed] [Google Scholar]
- 12.Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol. 2001;21(3):173–187. doi: 10.1097/00041327-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev. 2009;8(5):410–414. doi: 10.1016/j.autrev.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol. 2004;4:5. doi: 10.1186/1471-2415-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson TA, Gottlieb CC, Zein WM, et al. Autoimmune retinopathy: prognosis and treatment. Invest Ophthalmol Vis Sci. 2010;51 ARVO E-Abstract 6375. [Google Scholar]
- 16.Ferreyra HA, Jayasundera T, Khan NW, He S, Lu Y, Heckenlively JR. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol. 2009;127(4):390–397. doi: 10.1001/archophthalmol.2009.24. [DOI] [PubMed] [Google Scholar]
- 17.Adamus G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmun Rev. 2003;2(2):63–68. doi: 10.1016/s1568-9972(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 18.Adamus G, Webb S, Shiraga S, Duvoisin RM. Anti-recoverin antibodies induce an increase in intracellular calcium, leading to apoptosis in retinal cells. J Autoimmun. 2006;26(2):146–153. doi: 10.1016/j.jaut.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Magrys A, Anekonda T, Ren G, Adamus G. The role of anti-alpha-enolase autoantibodies in pathogenicity of autoimmune-mediated retinopathy. J Clin Immunol. 2007;27(2):181–192. doi: 10.1007/s10875-006-9065-8. [DOI] [PubMed] [Google Scholar]
- 20.Adamus G, Karren L. Autoimmunity against carbonic anhydrase II affects retinal cell functions in autoimmune retinopathy. J Autoimmun. 2009;32(2):133–139. doi: 10.1016/j.jaut.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei B, Bush RA, Milam AH, Sieving PA. Human melanoma-associated retinopathy (MAR) antibodies alter the retinal ON-response of the monkey ERG in vivo. Invest Ophthalmol Vis Sci. 2000;41(1):262–266. [PubMed] [Google Scholar]
- 22.Xiong WH, Duvoisin RM, Adamus G, Jeffrey BG, Gellman C, Morgans CW. Serum TRPM1 autoantibodies from melanoma associated retinopathy patients enter retinal on-bipolar cells and attenuate the electroretinogram in mice. PLoS One. 2013;8(8):e69506. doi: 10.1371/journal.pone.0069506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalal MD, Morgans CW, Duvoisin RM, et al. Diagnosis of occult melanoma using transient receptor potential melastatin 1 (TRPM1) autoantibody testing: a novel approach. Ophthalmology. 2013;120(12):2560–2564. doi: 10.1016/j.ophtha.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polans AS, Witkowska D, Haley TL, Amundson D, Baizer L, Adamus G. Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc Natl Acad Sci U S A. 1995;92(20):9176–9180. doi: 10.1073/pnas.92.20.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo M, Sanuki R, Ueno S, et al. Identification of autoantibodies against TRPM1 in patients with paraneoplastic retinopathy associated with ON bipolar cell dysfunction. PLoS One. 2011;6(5):e19911. doi: 10.1371/journal.pone.0019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeulen N, Arijs I, Joossens S, et al. Anti-alpha-enolase antibodies in patients with inflammatory bowel disease. Clin Chem. 2008;54(3):534–541. doi: 10.1373/clinchem.2007.098368. [DOI] [PubMed] [Google Scholar]
- 27.Forooghian F, Adamus G, Sproule M, Westall C, O’Connor P. Enolase autoantibodies and retinal function in multiple sclerosis patients. Graefes Arch Clin Exp Ophthalmol. 2007;245(8):1077–1084. doi: 10.1007/s00417-006-0527-8. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Cho SB, Bang D, et al. Human anti-alpha-enolase antibody in sera from patients with Behcet’s disease and rheumatologic disorders. Clin Exp Rheumatol. 2009;27(2 Suppl 53):S63–S66. [PubMed] [Google Scholar]
- 29.Patel N, Ohbayashi M, Nugent AK, et al. Circulating anti- retinal antibodies as immune markers in age-related macular degeneration. Immunology. 2005;115(3):422–430. doi: 10.1111/j.1365-2567.2005.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu X, Meer SG, Miyagi M, et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age- related macular degeneration. J Biol Chem. 2003;278(43):42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 31.Hooks JJ, Tso MO, Detrick B. Retinopathies associated with antiretinal antibodies. Clin Diagn Lab Immunol. 2001;8(5):853–858. doi: 10.1128/CDLI.8.5.853-858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braithwaite T, Holder GE, Lee RWJ, Plant G, Tufail A. Diagnostic features of the autoimmune retinopathies. Autoimmun Rev. 2014;13(4–5):534–538. doi: 10.1016/j.autrev.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 33.Abazari A, Allam SS, Adamus G, Ghazi NG. Optical coherence tomography findings in autoimmune retinopathy. Am J Ophthalmol. 2012;153(4):750–756. doi: 10.1016/j.ajo.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lima LH, Greenberg JP, Greenstein VC, et al. Hyperautofluorescent ring in autoimmune retinopathy. Retina. 2012;32(7):1385–1394. doi: 10.1097/IAE.0b013e3182398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao X, Bishop RJ, Forooghian F, Cho Y, Fariss RN, Chan CC. Autoimmune retinopathy in systemic lupus erythematosus: histopathologic features. Open Ophthalmol J. 2009;3:20–25. doi: 10.2174/1874364100903010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faez S, Loewenstein J, Sobrin L. Concordance of antiretinal antibody testing results between laboratories in autoimmune retinopathy. JAMA Ophthalmol. 2013;131(1):113–115. doi: 10.1001/jamaophthalmol.2013.574. [DOI] [PubMed] [Google Scholar]
- 37.Forooghian F, Macdonald IM, Heckenlively JR, et al. The need for standardization of antiretinal antibody detection and measurement. Am J Ophthalmol. 2008;146(4):489–495. doi: 10.1016/j.ajo.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Arch Ophthalmol. 1999;117(4):471–477. doi: 10.1001/archopht.117.4.471. [DOI] [PubMed] [Google Scholar]
- 39.Chan J. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol. 2003;48(1):12–38. doi: 10.1016/s0039-6257(02)00416-2. [DOI] [PubMed] [Google Scholar]
- 40.Whitcup SM, Vistica BP, Milam AH, Nussenblatt RB, Gery I. Recoverin-associated retinopathy: a clinically and immunologically distinctive disease. Am J Ophthalmol. 1998;126(2):230–237. doi: 10.1016/s0002-9394(98)00149-4. [DOI] [PubMed] [Google Scholar]
- 41.Weleber RG, Watzke RC, Shults WT, et al. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol. 2005;139(5):780–794. doi: 10.1016/j.ajo.2004.12.104. [DOI] [PubMed] [Google Scholar]
- 42.Sen HN, Chan CC, Caruso RC, Fariss RN, Nussenblatt RB, Buggage RR. Waldenstrom’s macroglobulinemia-associated retinopathy. Ophthalmology. 2004;111(3):535–539. doi: 10.1016/j.ophtha.2003.05.036. [DOI] [PubMed] [Google Scholar]
- 43.Mahdi N, Faia LJ, Goodwin J, Nussenblatt RB, Sen HN. A case of autoimmune retinopathy associated with thyroid carcinoma. Ocul Immunol Inflamm. 2010;18(4):322–323. doi: 10.3109/09273941003802379. [DOI] [PubMed] [Google Scholar]
- 44.Or C, Collins DR, Merkur AB, Wang Y, Chan CC, Forooghian F. Intravenous rituximab for the treatment of cancer-associated retinopathy. Can J Ophthalmol. 2013;48(2):35–38. doi: 10.1016/j.jcjo.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Dy I, Chintapatla R, Preeshagul I, Becker D. Treatment of cancer-associated retinopathy with rituximab. J Natl Compr Canc Netw. 2013;11(11):1320–1324. doi: 10.6004/jnccn.2013.0156. [DOI] [PubMed] [Google Scholar]
- 46.Espandar L, O’Brien S, Thirkill C, Lubecki LA, Esmaeli B. Successful treatment of cancer- associated retinopathy with alemtuzumab. J Neurooncol. 2007;83(3):295–302. doi: 10.1007/s11060-006-9326-7. [DOI] [PubMed] [Google Scholar]
- 47.Heckenlively JR, Jordan BL, Aptsiauri N. Association of antiretinal antibodies and cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 1999;127(5):565–573. doi: 10.1016/s0002-9394(98)00446-2. [DOI] [PubMed] [Google Scholar]
- 48.Ko AC, Brinton JP, Mahajan VB, et al. Seroreactivity against aqueous-soluble and detergent-soluble retinal proteins in posterior uveitis. Arch Ophthalmol. 2011;129(4):415–420. doi: 10.1001/archophthalmol.2011.65. [DOI] [PubMed] [Google Scholar]
- 49.Shimazaki K, Jirawuthiworavong GV, Heckenlively JR, Gordon LK. Frequency of anti-retinal antibodies in normal human serum. J Neuroophthalmol. 2008;28(1):5–11. doi: 10.1097/WNO.0b013e318167549f. [DOI] [PubMed] [Google Scholar]
- 50.Bursztyn LL, Belrose JC, Coupland SG, Fraser JA, Proulx AA. Remission of nonparaneoplastic autoimmune retinopathy after minimal steroid treatment. Retin Cases Brief Rep. 2015;9(2):173–176. doi: 10.1097/ICB.0000000000000131. [DOI] [PubMed] [Google Scholar]