Abstract
The clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system has been proven to be an efficient and precise genome editing technology in various organisms. However, the gene editing efficiencies of Cas9 proteins with a nuclear localization signal (NLS) fused to different termini and Cas9 mRNA have not been systematically compared. Here, we compared the ability of Cas9 proteins with NLS fused to the N-, C-, or both the N- and C-termini and N-NLS-Cas9-NLS-C mRNA to target two sites in the tyr gene and two sites in the gol gene related to pigmentation in zebrafish. Phenotypic analysis revealed that all types of Cas9 led to hypopigmentation in similar proportions of injected embryos. Genome analysis by T7 Endonuclease I (T7E1) assays demonstrated that all types of Cas9 similarly induced mutagenesis in four target sites. Sequencing results further confirmed that a high frequency of indels occurred in the target sites (tyr1 > 66%, tyr2 > 73%, gol1 > 50%, and gol2 > 35%), as well as various types (more than six) of indel mutations observed in all four types of Cas9-injected embryos. Furthermore, all types of Cas9 showed efficient targeted mutagenesis on multiplex genome editing, resulting in multiple phenotypes simultaneously. Collectively, we conclude that various NLS-fused Cas9 proteins and Cas9 mRNAs have similar genome editing efficiencies on targeting single or multiple genes, suggesting that the efficiency of CRISPR/Cas9 genome editing is highly dependent on guide RNAs (gRNAs) and gene loci. These findings may help to simplify the selection of Cas9 for gene editing using the CRISPR/Cas9 system.
Keywords: CRISPR/Cas9, Cas9 protein, knockout, efficiency, NLS, zebrafish
The type II CRISPR associated with Cas9 endonuclease (CRISPR/Cas9) system has emerged as a powerful tool for studying gene function (Jinek et al. 2012). The CRISPR/Cas9 system has a high genome editing efficiency, is simple to design, is not time-consuming, and has been successfully applied in a variety of model organisms including the mouse (Mus musculus) (Shen et al. 2013), fruit fly (Drosophila melanogaster) (Bassett et al. 2013), zebrafish (Danio rerio) (Chang et al. 2013; Hwang et al. 2013), nematode (Caenorhabditis elegans) (Tzur et al. 2013), and Arabidopsis (Arabidopsis thaliana) (Fauser et al. 2014).
In the 5 yr since the CRISPR/Cas9 system emerged as a versatile genome editing technology, many researchers have demonstrated that co-injection of in vitro-transcribed capped polyadenylated Cas9 mRNA and gRNA specific to the target genomic locus induces DNA double-strand breaks (DSBs), leading to targeted gene disruption. However, the efficiency of genome editing varies. For instance, Li et al. (2014) designed three gRNAs targeting the fifth exon of the mouse Fumarylacetoacetate hydrolase (Fah) gene and achieved genomic disruption efficiencies of 54.26, 23.15, and 42.05% for gRNA1, 2, and 3, respectively. In zebrafish, Jao et al. (2013) established a highly efficient CRISPR mutagenesis system by modifying the 3′ end of the gRNA with GGAUC, which led to mutation rates >80%.
Injection of Cas9 protein and gRNA can also efficiently induce DSBs. Wu et al. (2017) showed that Cas9 protein/sgRNA resulted in a mutation rate of 30–60% during excision of a point mutation in exon 80 to restore correct localization of collagen VII protein in the mouse. Sung et al. (2014) found C-NLS-Cas9 protein and Cas9 mRNA induced similar gene modification efficiency in zebrafish founder embryos. Consistent with these findings, Gagnon et al. (2014) reported that C-NLS-Cas9 protein and Cas9 mRNA both resulted in a high genome editing frequency at calcium/calmodulin-dependent protein kinase (CaM kinase) II γ 1 (camk2g1) and connective tissue growth factor a (ctgfa) gene target sites in zebrafish, though Cas9 protein induced a higher mutagenesis rate than Cas9 mRNA at the glutamate receptor, ionotropic, AMPA 3a (gria3a), and lysine (K)-specific demethylase 6A, like utx1 loci. Although these initial pioneering results are encouraging, there has been no comprehensive comparison of the gene editing efficiency of Cas9 proteins and Cas9 mRNA.
The CRISPR/Cas9 system is based on a prokaryotic immune mechanism that defends against invasion of nucleic acids (Brouns et al. 2008). As Cas9 is derived from Streptococcus pyogenes, it may be necessary to an attach an NLS so Cas9 can be transported into the nucleus to enable editing of the eukaryotic genome. Cong et al. (2013) found that attaching an NLS to both termini of Cas9 protein improved the efficiency of its transport into the nucleus. Mali et al. (2013) reported that only adding an NLS to the C-terminus of Cas9 protein was required to maintain the gene editing efficiency of the CRISPR/Cas9 system in eukaryotic cells. Moreover, Shen et al. (2013) showed that adding a 32 amino acid linker between the N-terminal NLS and Cas9 protein could improve Cas9 cleavage activity in eukaryotes. However, the gene editing efficiency of Cas9 protein or mRNA with an NLS fused to different termini have not been systematically compared.
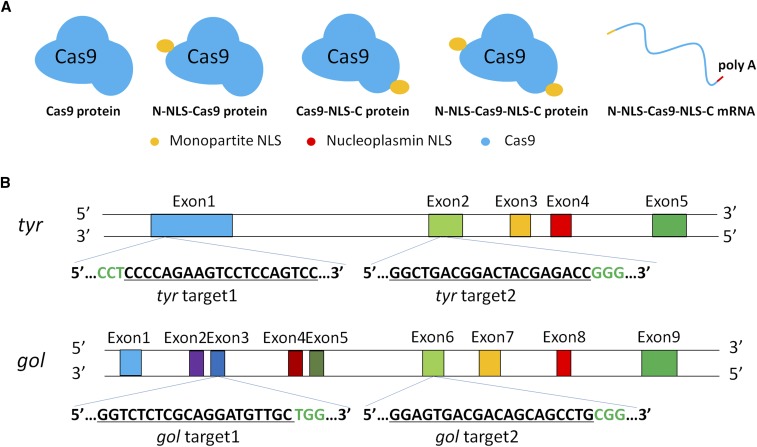
In this study, we used four Cas9 proteins: N-NLS-Cas9 protein (called N-Cas9 protein), Cas9-NLS-C protein (C-Cas9 protein), N-NLS-Cas9-NLS-C protein (N-Cas9-C protein), Cas9 protein without NLS (Cas9 protein) and N-NLS-Cas9-NLS-C mRNA (N-Cas9-C mRNA). We targeted two genes related to body pigmentation to make it easy to visually compare gene editing efficiency by assessing pigmentation defects with two sites of each gene. Each one of the two target sites was designed as reported previously (Jao et al. 2013), and another one was located in the second exon of tyrosinase (tyr) gene and the sixth exon of golden (gol or slc24a5). The Cas9 proteins or Cas9 mRNA were co-injected with synthesized gRNA into single-cell zebrafish embryos to compare gene editing efficiency for two sites in the tyr gene and two sites in the gol gene. Distinct phenotypes were observed, including hypopigmentation of skin melanophores and the retinal pigmented epithelium in injected tyr F0 embryos, and hypopigmentation of the retinal pigmented epithelium in injected gol F0 embryos. Furthermore, we observed that various NLS-fused Cas9 proteins and Cas9 mRNA showed efficient effects on multiplex genome editing in zebrafish. We conclude that the varied NLS-fused Cas9 proteins and Cas9 mRNA tested in this study lead to similarly efficient gene editing efficiencies.
Materials and Methods
Zebrafish husbandry
Zebrafish were maintained in a recirculating system at 28.5° and pH of 7.0–8.0. The circulating water was UV light-treated and aerated. Embryos were collected in purified water and incubated at 28.5°. All handling of fishes was carried out in accordance with the guidelines on the care and use of animals for scientific purposes set up by the Institutional Animal Care and Use Committee (IACUC) of the Shanghai Ocean University (SHOU), Shanghai, China. This research was approved by the IACUC (IACUC 20171009) of SHOU.
Cas9 plasmid and Cas9 proteins
The full-length codon-optimized Cas9 plasmid was obtained from Bo Zhang’s laboratory (Liu et al. 2014). This Cas9 plasmid contains a consensus Kozak sequence at the translational start site, an NLS called a monopartite NLS at the N-terminus whose sequence is PKKKRKV from the SV40 Large T-antigen, and an NLS called a nucleoplasmin NLS at the C-terminus whose sequence is KR[PAATKKAGQA]KKKK. The three Cas9 proteins (N-NLS-Cas9, Cas9-NLS-C, and N-NLS-Cas9-NLS-C) containing the same monopartite NLS, and another Cas9 without an NLS, were obtained from GenScript (Nanjing, China).
RNA synthesis
The codon-optimized Cas9 plasmid was linearized using XbaI and purified using DNA Clean & Concentrator-5 (Zymo Research, Irvine, CA). Capped Cas9 mRNA was synthesized in vitro using the mMESSAGE mMACHINE T7 ULTRA kit (Ambion, New York, NY) as previously described (Liu et al. 2014) and purified using the mirVana miRNA Isolation Kit (Ambion).
To make gRNA, double-stranded DNA was amplified by PCR (TransGen Biotech, Beijing, China) from gRNA using the target site-specific forward primer (5′-TAATACGACTCACTATANNNNNNNNNNNNNNNNNNNNGTTTTAGAGCTAGAAATAGC-3′) and the common reverse primer (5′-AAAAAAAGCACCGACTCGGTGCCAC-3′) (Chang et al. 2013), then gRNA was generated in vitro using the MAXIscript T7 kit (Ambion) and purified using the mirVana miRNA Isolation Kit (Ambion). Each target sequence contains GG at the beginning and NGG as the PAM sequence.
Microinjection
For microinjection 1 nl of a mixture of gRNA (100 pg) and Cas9 protein (800 pg) or Cas9 mRNA (400 pg) was directly co-injected into each single-celled embryo [derived from the wild-type (WT) AB incross], as previously described (Tong et al. 2014). Approximately 300 embryos were injected every experiment. Each experiment was repeated independently three times.
Assessment of mutation efficiency
Two days after microinjection of gRNA and Cas9, genomic DNA was extracted from the four groups of pooled microinjected embryos; five injected embryos were randomly selected for each group in each of the three independent experiments. Genomic DNA was used as template and amplified by PCR using the primer pairs shown in Supplemental Material, Table S1 in File S1. The PCR products were denatured and annealed to enable heteroduplex formation, then treated with T7EI (New England Biolabs, Ipswich, MA) for 45 min at 37°. Mutation efficiency was calculated as previously described (Tong et al. 2014). The digested PCR products were examined by agarose gel (2%) electrophoresis. The remainder of the PCR products were subjected to Sanger sequencing by Sangon Biotech (Shanghai, China). At each target site, PCR products mixed from three independent experiments were ligated into the pMD19-T TA cloning vector (Takara) and transformed into DH5α competent cells (Tiangen) independently. Approximately 20 individual colonies were sequenced for each amplicon as previously described (Zu et al. 2016).
Statistical analysis
Data derived from three independent experiments were evaluated by one-way ANOVA for comparisons between groups using GraphPad Prism 5. P < 0.05 was considered to be statistically significant.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Survival rates of zebrafish injected with various NLS-fused Cas9 proteins and Cas9 mRNA
To investigate the best concentration of Cas9 protein to inject for gene editing, we co-injected 100 pg gol target2 gRNA with 400, 600, and 800 pg of N-Cas9 protein, respectively, into one-cell-stage zebrafish embryos. T7E1 assays demonstrated that the 800 pg Cas9 protein had the highest mutagenesis rate (P < 0.05), and the proportions of mosaic and null-like phenotypes were also higher in the 800 pg Cas9 group (Figure S1 in File S1). Therefore, we chose 800 pg as the concentration of Cas9 protein to perform the following microinjection experiments.
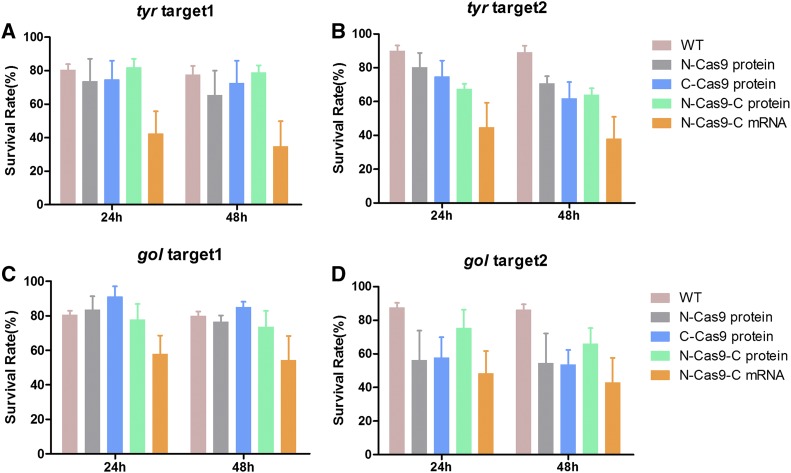
We co-injected 1 nl of 100 pg gRNA and 400 pg N-Cas9-C mRNA, or 800 pg of the three NLS-fused Cas9 proteins (Figure 1A), into single-cell WT zebrafish embryos to target two sites in the tyr gene and two sites in the gol gene (Figure 1B). For every experiment, ∼300 embryos were injected with specific gRNA and different types of Cas9. The numbers of surviving embryos were counted at 24 and 48 hr postfertilization (hpf). The survival rates at 24 and 48 hpf were similar for each site. Moreover, the survival rates of zebrafish embryos injected with the various NLS-fused Cas9 proteins or N-Cas9-C mRNA were not significantly different (Figure 2, A–D, P > 0.05).
Figure 1.
Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9-mediated gene editing in zebrafish. (A) Schematic illustrating the no nuclear localization signal (NLS) Cas9 protein, various NLS-fused Cas9 proteins, and Cas9 mRNA. (B) tyr target 1 and gol target 1 were as previously described, tyr target 2 was located in the second exon of tyr, and gol target 2 in the sixth exon of gol.
Figure 2.
Survival rates of zebrafish embryos after microinjection of various nuclear localization signal (NLS)-fused Cas9 proteins or N-Cas9-C mRNA. Survival rates of zebrafish embryos injected with various NLS-fused Cas9 proteins, or Cas9 mRNA and guide (g)RNA targeting the two target sites in tyr (A and B), and two target sites in gol (C and D) at 24 and 48 hpf (hr postfertilization). WT, wild-type.
Mutation efficiency of the various NLS-fused Cas9 proteins and Cas9 mRNA for two tyr gene sites
The target sites and target region primers were designed using ZIFIT and NCBI programs (Figure 1B and Table S1 in File S1). The various NLS-fused Cas9 proteins or Cas9 mRNA were injected into single-cell zebrafish embryos with tyr gRNA 1 or gRNA 2. For each target site, embryos were injected with four groups of Cas9 and gRNA in three independent experiments. To evaluate the knockout efficiency of genomic disruption, genomic DNA was extracted from three tubes of pooled microinjected embryos at 48 hpf; each tube contained five randomly selected injected embryos and a pool of five randomly selected uninjected WT embryos as controls. The target fragment was amplified by PCR using genomic DNA as template and the rate of mutagenesis quantified by T7E1 assays and PCR Sanger sequencing.
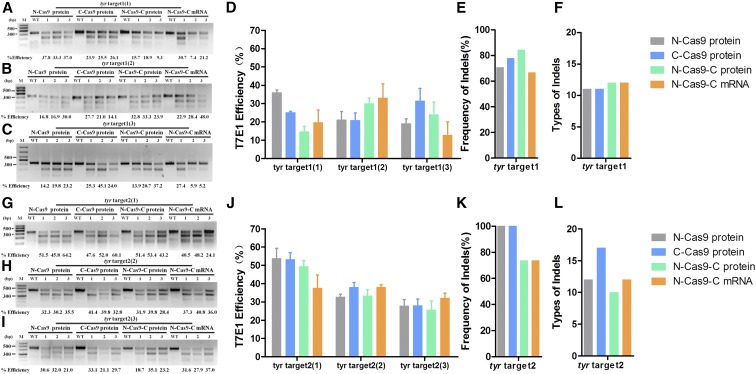
For the tyr target 1, T7E1 assays showed that the rate of mutagenesis ranged from 14.2 to 37.8% for N-Cas9 protein (first experiment: mean ± SD, 36.03 ± 2.40; second experiment: mean ± SD, 21.23 ± 7.59; and third experiment: mean ± SD, 19.07 ± 4.54) in the triplicate experiments, 14.1–45.1% for C-Cas9 protein (first experiment: mean ± SD, 25.17 ± 1.14; second experiment: mean ± SD, 20.93 ± 6.80; and third experiment: mean ± SD, 31.47 ± 11.82), 9.3–37.2% for N-Cas9-C protein (first experiment: mean ± SD, 14.63 ± 4.89; second experiment: mean ± SD, 30.00 ± 5.29; and third experiment: mean ± SD, 29.93 ± 11.98), and 5.2–48.0% for N-Cas9-C mRNA (first experiment: mean ± SD, 19.77 ± 11.72; second experiment: mean ± SD, 33.10 ± 13.19; and third experiment: mean ± SD, 12.83 ± 12.62) (Figure 3, A–D). The corresponding PCR Sanger sequences are shown in Figure S4 in File S1. To further analyze the different types of indels induced by each Cas9 protein and Cas9 mRNA, we sequenced 20 individual colonies of each group and the indel frequencies of all four groups were >66% (Figure 3E and Figure S5 in File S1). There were 11, 11, 12, and 12 types of indel, respectively, documented in the sequences of the N-Cas9 protein-, C-Cas9 protein-, N-Cas9-C protein-, and N-Cas9-C mRNA-targeted embryos (Figure 3F and Figure S5 in File S1). These results indicated that there was no significant difference in knockout efficiency between the various NLS-fused Cas9 proteins and N-Cas9-C mRNA for tyr target 1 (first experiment: P = 0.019, and second and third experiments: P > 0.05).
Figure 3.
Knockout efficiency of various nuclear localization signal (NLS)-fused Cas9 proteins and N-Cas9-C mRNA for two sites of the tyr gene. T7 Endonuclease I (T7E1) assays of the rates of mutagenesis for tyr target 1 (A–D) and tyr target 2 (G–J) in three tubes of pooled embryos microinjected with relevant guide (g)RNA and the various NLS-fused Cas9 proteins and N-Cas9-C mRNA. Each group contained five randomly selected embryos; three independent experiments were performed for each target. The frequency of indels and types of indel of tyr target1 (E and F) and tyr target2 (K and L).
T7E1 assays of the tyr gRNA2 samples at 48 hpf revealed that the mutagenesis rate was 21.0–64.2% for N-Cas9 protein (first experiment: mean ± SD, 53.83 ± 9.42; second experiment: mean ± SD, 32.67 ± 2.67; and third experiment: mean ± SD, 27.87 ± 5.99), 21.1–60.1% for C-Cas9 protein (first experiment: mean ± SD, 53.23 ± 6.34; second experiment: mean ± SD, 37.93 ± 4.54; and third experiment: mean ± SD, 27.97 ± 6.18), 18.7–53.4% for N-Cas9-C protein (first experiment: mean ± SD, 49.33 ± 5.40; second experiment: mean ± SD, 33.37 ± 5.84; and third experiment: mean ± SD, 25.67 ± 8.47), and 24.1–48.2% for N-Cas9-C mRNA (first experiment: mean ± SD, 37.60 ± 12.31; second experiment: mean ± SD, 38.03 ± 2.48; and third experiment: mean ± SD, 32.17 ± 4.58) (Figure 3, G–J). The corresponding PCR Sanger sequences are shown in Figure S4 in File S1. Besides, we sequenced 20 individual colonies of each group as mentioned above and the indel frequencies of the four groups were >73% (Figure 3K and Figure S6 in File S1). There were 12, 17, 10 and 12 types of indels, respectively, documented in the sequences of the N-Cas9 protein-, C-Cas9 protein-, N-Cas9-C protein- and N-Cas9-C mRNA-injected embryos (Figure 3L and Figure S6 in File S1). The results showed that there was no significant difference in knockout efficiency between the various NLS-fused Cas9 proteins and N-Cas9-C mRNA for tyr target 2 (all P > 0.05).
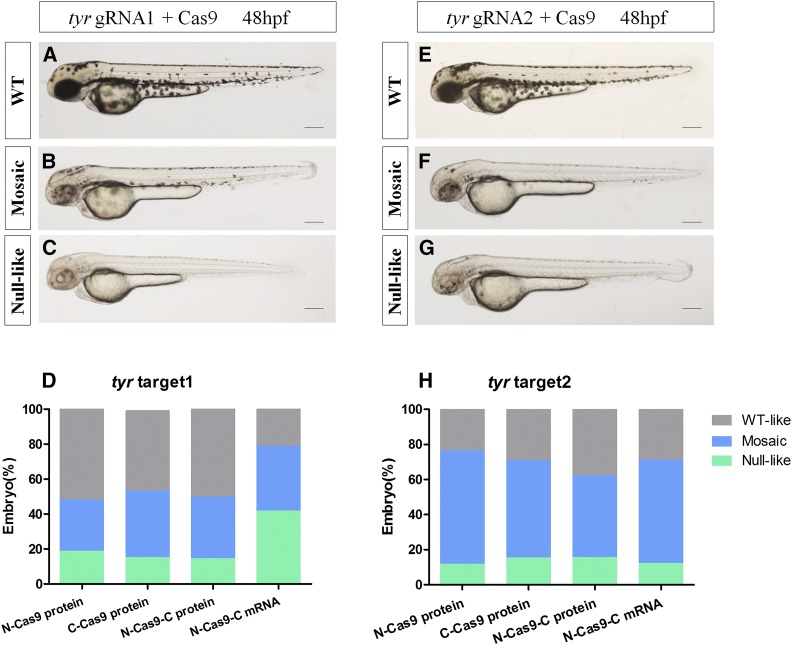
The tyr gene is required for the conversion of tyrosinase into melanin (Page-McCaw et al. 2004). Targeting tyr resulted in three phenotypes at 48 hpf: WT-like (similar to uninjected embryos), mosaic (hypopigmentation on embryo body), and null-like (no melanin on embryo body; Figure 4, A–C and E–G). With over 200 embryos, we assessed the proportions of embryos with each phenotype at 48 hpf to evaluate phenotypic mutation efficiency and specificity at the phenotype level (Figure 4, D and H). N-Cas9-C mRNA induced a high proportion of the null-like phenotype at tyr target 1 (∼40%), while the three Cas9 protein variants induced lower proportions of the null-like phenotype and higher proportions of the WT-like phenotype. N-Cas9-C mRNA and the three Cas9 protein variants led to similar proportions of the mosaic phenotype (Figure 4D). For tyr target 2, N-Cas9-C mRNA and the three Cas9 protein variants generated high proportions of the mosaic and null-like phenotype (62.2–76.5%; Figure 4H).
Figure 4.
Mutagenetic phenotype of various nuclear localization signal (NLS)-fused Cas9 proteins and N-Cas9-C mRNA for two sites of the tyr gene. Lateral views of wild-type (WT) (A and E) and tyr-target 1- (B and C) or tyr-target 2- injected (F and G) embryos at 48 hpf (hr postfertilization). The phenotypes observed were WT (A and E), mosaic hypopigmentation (B and F), and null-like (C and G). Proportions of embryos with each phenotype (D and H). Scale bars: 0.2 mm.
Mutation efficiency of the various NLS-fused Cas9 proteins and Cas9 mRNA for two gol gene sites
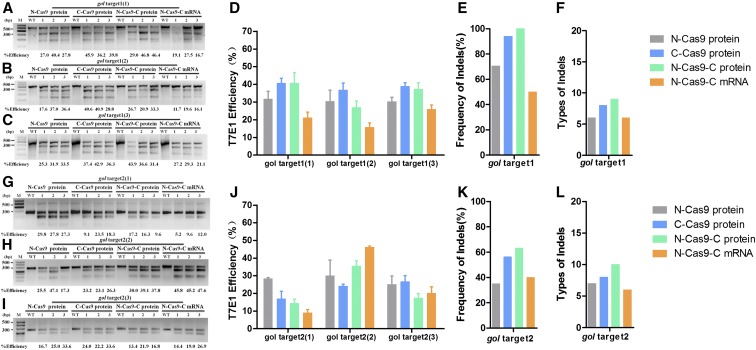
Next, we evaluated the knockout efficiency of the three Cas9 proteins and Cas9 mRNA when targeting another pigmentation-related gene, gol, which encodes the putative cation exchanger Slc24a5 (solute carrier family 24, member 5) (Lamason et al. 2005) (Figure 1B). At gol target 1, T7E1 assays showed that N-Cas9 protein induced an genomic disruption rate of 17.6–40.4% (first experiment: mean ± SD, 31.73 ± 7.52; second experiment: mean ± SD, 30.33 ± 11.03; and third experiment: mean ± SD, 30.23 ± 4.35); C-Cas9 protein, 28.8–45.9% (first experiment: mean ± SD, 40.63 ± 4.90; second experiment: mean ± SD, 36.76 ± 6.90; and third experiment: mean ± SD, 38.87 ± 3.54); N-Cas9-C protein, 20.9–46.8% (first experiment: mean ± SD, 40.73 ± 10.16; second experiment: mean ± SD, 26.97 ± 6.20; and third experiment: mean ± SD, 37.30 ± 6.28); and N-Cas9-C mRNA, 11.7–29.3% (first experiment: mean ± SD, 21.10 ± 5.67; second experiment: mean ± SD, 15.80 ± 3.96; and third experiment: mean ± SD, 25.87 ± 4.26) (Figure 5, A–D). The corresponding sequences are shown in Figure S4 in File S1. To further analyze the different types of indels induced by each Cas9 protein and Cas9 mRNA, 20 individual colonies of each group were sequenced. Indels were detected in each group with frequencies >50% (Figure 5E and Figure S7 in File S1), and the types of indels documented in the sequences of the N-Cas9 protein-, C-Cas9 protein-, N-Cas9-C protein-, and N-Cas9-C mRNA-targeted embryos were 7, 6, 9, and 6, respectively (Figure 5F and Figure S7 in File S1). There were no significant differences in knockout efficiency between the three Cas9 protein variants and Cas9 mRNA for gol target 1 (first experiment: P = 0.035, and second and third experiments: P > 0.05).
Figure 5.
Knockout efficiency of various nuclear localization signal (NLS)-fused Cas9 proteins and N-Cas9-C mRNA for two sites of the gol gene. T7 Endonuclease I (T7E1) assays of the rates of mutagenesis for gol target 1 (A–D) and gol target 2 (G–J) in three tubes of pooled embryos microinjected with guide (g)RNA and the various NLS-fused Cas9 proteins or N-Cas9-C mRNA. Each group contained five randomly selected embryos; three independent experiments were performed for each target. The frequency of indels and types of indel of gol target 1 (E and F) and gol target 2 (K and L) are shown.
For gol target 2, the genomic disruption rate ranged from 16.7 to 47.1% for N-Cas9 protein (first experiment: mean ± SD, 28.3 ± 1.32; second experiment: mean ± SD, 29.97 ± 15.39; and third experiment: mean ± SD, 25.10 ± 8.45), 9.1–33.6% for C-Cas9 protein (first experiment: mean ± SD, 16.97 ± 7.29; second experiment: mean ± SD, 24.20 ± 1.82; and third experiment: mean ± SD, 26.60 ± 6.13), 9.6–39.1% for N-Cas9-C protein (first experiment: mean ± SD, 14.37 ± 4.15; second experiment: mean ± SD, 35.63 ± 4.92; and third experiment: mean ± SD, 17.37 ± 4.28), and 5.2–47.6% for N-Cas9-C mRNA (first experiment: mean ± SD, 8.93 ± 3.45; second experiment: mean ± SD, 46.20 ± 1.25; and third experiment: mean ± SD, 20.10 ± 6.32) (Figure 5, G–J). The corresponding sequences are shown in Figure S4 in File S1. Besides, 20 individual colonies of each group were sequenced as mentioned above and the indel frequency of four groups all exceeded 35% (Figure 5K and Figure S8 in File S1). There were 7, 8, 10, and 6 types of indels, respectively, documented in the sequences of the N-Cas9 protein-, C-Cas9 protein-, N-Cas9-C protein-, and N-Cas9-C mRNA-injected embryos (Figure 5L and Figure S8 in File S1). There was no significant difference in knockout efficiency between the three Cas9 protein variants and Cas9 mRNA for gol target 2 (all P > 0.05).
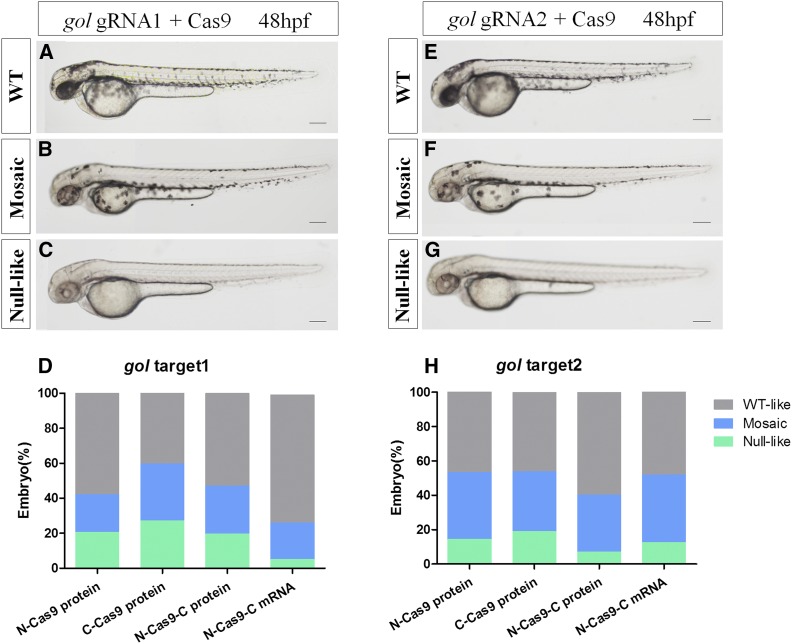
In another way, mutation of gol leads to hypopigmentation of the retinal pigmented epithelium and skin melanophores. Similar to tyr, targeting of gol resulted in three phenotypes: WT-like, mosaic, and null-like. The null-like phenotype had no melanin in the REF or skin melanophores (Figure 6, A–C and E–G). We analyzed the phenotypic proportion at 48 hpf for over 180 embryos; N-Cas9-C mRNA induced the lowest proportion of the null-like phenotype at gol target 1 (∼5%) and a higher proportion of the WT-like phenotype. N-Cas9 protein and N-Cas9-C protein both induced ∼20% of the null-like phenotype at gol target 1, and Cas9-NLS-C protein led to the highest proportions of the null-like and mosaic phenotypes (Figure 6D).
Figure 6.
Mutagenetic phenotype of various nuclear localization signal (NLS)-fused Cas9 proteins and N-Cas9-C mRNA for two sites of the gol gene. (A–C) and (E–G) show lateral views of wild-type (WT) (A and E), gol-target 1 (B and C), and gol-target 2 (F and G) at 48 hpf (hr postfertilization). The phenotypes observed were WT (A and E), mosaic retinal pigmented epithelium (RPE) (B and F), and unpigmented RPE (C and G). (D and H) Proportions of embryos with each phenotype. Scale bars: 0.2 mm.
For gol target 2, N-Cas9-C protein induced the lowest proportion of the null-like phenotype, and all types of Cas9 led to similar proportions of mosaic phenotype (33.2–39.3%; Figure 6H).
Various types of Cas9 with or without NLS-induced efficient multiplex genome mutagenesis resulted in multiple phenotypes
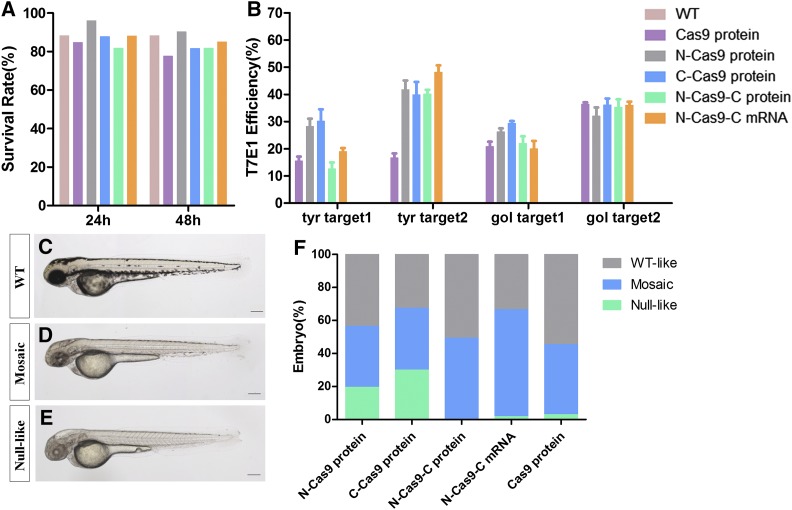
Since various types of NLS-fused Cas9 induced similarly high efficiency in genome editing in zebrafish, we suspected that Cas9 protein may not really require an NLS to assist import into the nucleus. To determine whether Cas9 protein without an NLS has DNA cleavage activity in zebrafish, we used another type of Cas9 protein without an NLS to target the same two sites of the tyr and gol genes under the same conditions. Interestingly, we found that Cas9 protein without an NLS induced hypopigmentation in proportions of 32.0 and 39.5% at tyr target 1 and gol target 2. However, for the tyr target 2 and gol target 1, the efficiency of T7E1 assays were much lower, consistent with a low proportions of mutation phenotypes occurring (Figure S2 in File S1).
To explore whether the five types of Cas9 had effects on multiplex genome editing, we co-injected 400 pg N-Cas9-C mRNA, 800 pg of the three NLS-fused Cas9 proteins, or 800 pg Cas9 protein without an NLS with a mixture of four gRNAs (tyr gRNA1, 16 pg; tyr gRNA2, 100 pg; gol gRNA1, 20 pg; and gol gRNA2, 100 pg) into single-cell-stage zebrafish embryos, expecting to target two genes and four target sites at the same time. The survival rates at 24 and 48 hpf were similar for each group, all >75% (Figure 7A). T7E1 assays showed that NLS-fused Cas9 was induced by 8.7–37.6% at tyr target 1, 31.1–53% at tyr target 2, 15.5–31.0% at gol target 1, and 26–40.7% at gol target 2. The efficiencies of multiplex gene editing were comparable to the efficiencies of previous targeting of single sites (Figure 7B, tyr2 and gol2: P > 0.05, and tyr1 and gol1: P > 0.01). However, genomic disruption rates of no NLS-fused Cas9 protein were lower at two loci of the tyr gene and gol target 1 (tyr1: P = 0.003682; tyr2: P = 0.000258; and gol1: P = 0.0319; gol2: P < 0.05), but were similar high at gol target 2, suggesting that the mutagenesis rates of no NLS-fused Cas9 proteins vary from site to site (Figure 7B). After co-injection, we observed severe pigmentation defects in the embryos, included null-like and mosaic phenotypes (Figure 7, C–F). Phenotypic analysis indicated that these five kinds of Cas9 similarly induce high hypopigmentation (Figure 7G). Collectively, both T7E1 assays and phenotypic analysis indicated that the various types of Cas9 had similar efficiencies on multiplex genome editing in zebrafish, and that the mutagenesis efficiencies of multiplex gene targeting were comparable with those of targeting a single gene.
Figure 7.
Multiplex knockout efficiency of various nuclear localization signal (NLS)-fused Cas9 proteins and N-Cas9-C mRNA. Survival rates of zebrafish embryos at 24 and 48 hr postfertilization (hpf) (A) and T7 Endonuclease I (T7E1) assays of the rates of mutagenesis for each site (B). The phenotypes observed were wild-type (WT) (C), severe mosaic hypopigmentation (D), and null-like (E). (F) Proportions of embryos with each phenotype. Scale bars: 0.2 mm.
Discussion
A previous study of CRISPR/Cas9 technology indicated that C-NLS-Cas9 protein and Cas9 mRNA induce similar rates of genome modifications in zebrafish founder embryos, though Cas9 protein induced a higher rate of mutagenesis than Cas9 mRNA at the gria3a and utx1 loci (Gagnon et al. 2014). Therefore, we sought to directly compare the targeting efficiency of Cas9 protein and Cas9 mRNA. Our results showed that all three NLS-fused Cas9 proteins and Cas9 mRNA resulted in high knockout efficiency for the two sites in the tyr and two sites in the gol genes. Overall, we conjecture that the knockout efficiency of gRNA/Cas9-encoding proteins or mRNA in zebrafish varies for different gRNAs.
Similarly, the survival rates of the zebrafish embryos were not significantly different for the various Cas9 proteins and Cas9 mRNA at each of the four target sites, although the survival rates were lower for gol target 2 than the other three sites. Likewise, previous studies have demonstrated zinc-finger nucleases, transcription activator-like effector nucleases, and CRISPR/Cas9 targeting rates vary depending on the loci (Foley et al. 2009; Cade et al. 2012; Hwang et al. 2013). At the phenotypic level, targeting two different sites in tyr and gol resulted in similar pigmentation defects. We conclude that different gRNAs targeting the same gene will generate the same phenotype, as previously described (Jao et al. 2013), but that the rate of mutagenesis (at the genomic level) may not be equivalent (Li et al. 2014).
Targeting the tyr or gol genes using the CRISPR/Cas9 system in WT zebrafish embryos led to hypopigmentation and genomic disruption; T7E1 assays and sequencing further confirmed that all of the NLS-fused Cas9 proteins resulted in successful gene editing. In contrast, Shen et al. (2013) reported that Cas9 with either an N- or C-terminal-fused NLS or three N-terminal-fused NLS could not lead to functional cleavage of the genome in zebrafish embryos, and that only addition of an NLS containing a 32 amino acid linker to the N-terminus of Cas9 resulted in gene editing. Moreover, two other publications reported that Cas9 mRNA with a C-terminal NLS and or both N- and C-terminal NLS led to gene editing in HEK293T cells (Cong et al. 2013; Mali et al. 2013). Our results indicate that the position of the NLS on Cas9 protein or mRNA does not affect the gene editing function of the CRISPR/Cas9 system in zebrafish. Hence, we hypothesized that Cas9 protein may not require an NLS to assist import into the nucleus. The Cas9 protein without an NLS induced comparable high targeted mutagenesis at gol target 2, partially supporting our hypothesis. In future studies, it would be interesting to examine the mechanism by which Cas9 protein without an NLS imports into the nucleus in eukaryotic organisms. Additionally, to improve the mutation rates for other gene loci, future studies should focus on the specific structures of Cas9 and gRNA that affect knockout efficiency.
T7E1 assays and the phenotypic mutation rates revealed no significant differences in the targeting rates of the three NLS-fused Cas9 proteins tested in this study. Similarly, Guilinger et al. (2014) fused FokI to either the N- or C-termini of an inactive variant Cas9 protein (dCas9), and varied the location of the NLS from either terminus to between the two domains; the FokI-dCas9 fusion variants led to similar frequencies. Additionally, a recent publication demonstrated that adding an NLS to the N-terminal or both the N- and C-termini of dCas9 did not improve transposition activity or gene targeting in human cells (Luo et al. 2017). Therefore, our results verify that the location and numbers of NLS do not affect knockout efficiency in zebrafish as the N-Cas9, C-Cas9, and N-Cas9-C proteins had similarly high knockout efficiencies.
Disruption of the endogenous genes tyr and gol led to the expected phenotypes in the F0 generation. When targeting tyr target 1, N-Cas9-C mRNA led to a higher proportion of the null-like phenotype (≤ 40%), though T7E1 assays showed that the rate of mutagenesis was highest for N-Cas9-C protein and tyr gRNA1 (Figure 3D). When targeting gol target 1, C-Cas9 protein induced the highest proportions of the null-like and mosaic phenotypes, though T7E1 assays showed that the mutagenesis rate was highest for N-Cas9-C protein and gRNA2. Similarly, we suggest that a high phenotypic mutation rate may not always reflect a high mutagenesis rate in the T7E1 assay. We speculate that this phenomenon occurs for two reasons. First, as these endogenous genes related to body pigmentation are dominant, cleavage of both alleles will result in an obvious hypopigmentation phenotype. Second, T7E1 assays cannot detect mutations if single mutated DNA alleles anneal together (Kim et al. 2014). Therefore, the mutation rate determined by the T7E1 assay may be lower than the actual mutagenesis rate.
Overall, sequencing results showed high frequencies of indels observed in the target sites (tyr1 > 66%, tyr2 > 73%, gol1 > 50%, and gol2 > 35%). Both T7E1 assays and phenotypic analysis indicated that the various NLS-fused Cas9 proteins and Cas9 mRNA had efficient effects on multiplex genome editing in zebrafish, and that the mutagenesis efficiencies of multiplex genes targeting were comparable with that of a single gene. In summary, these results indicate that gRNA/Cas9-encoding proteins lead to a high indel frequency, regardless of the position of the NLS fusion. Furthermore, Cas9 protein and Cas9 mRNA result in similarly high knockout efficiencies with single gRNA or multiple gRNAs. These data may help researchers to select various NLS-fused Cas9 when using the CRISPR/Cas9 gene editing system.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.300359/-/DC1.
Acknowledgments
We thank Bo Zhang (School of Life Sciences, Peking University) for providing the ZCas9 plasmid, Jingwei Xiong (Institute of Molecular Medicine, Peking University) for providing the pUC19-scaffold plasmid for gRNA synthesis, Jing Xie for zebrafish management, Xiangli Meng for technical assistance, and Joao Cardoso (Universidade do Algarve) for proofreading the article. This work was supported by the National Natural Science Foundation of China (grant number 31501166), the Shanghai Sailing Program of Science and Technology Commission of Shanghai Municipality (grant number 15YF1405000), the Chenguang Program of the Shanghai Municipal Education Commission (grant number 14CG49), and the Development Fund for Shanghai Talents (grant number 201568).
Author contributions: Y.Z. conceived the project and designed the experiments. P.H. synthesized the Cas9 mRNA and gRNAs, performed the microinjections and T7E1 assays, analyzed the phenotypes, and captured the images. Y.Z. and P.H. analyzed the data. P.H., X.Z., Q.Z., W.L., and Y.Z. wrote the article. The authors declare no competing financial interests.
Footnotes
Communicating editor: B. Andrews
Literature Cited
- Bassett A. R., Tibbit C., Ponting C. P., Liu J. L., 2013. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 4: 220–228 (erratum: Cell Rep. 6: 1178–1179).23827738 [Google Scholar]
- Brouns S. J., Jore M. M., Lundgren M., Westra E. R., Slijkhuis R. J., et al. , 2008. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321: 960–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade L., Reyon D., Hwang W. Y., Tsai S. Q., Patel S., et al. , 2012. Highly efficient generation of heritable zebrafish gene mutations using homo- and heterodimeric TALENs. Nucleic Acids Res. 40: 8001–8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N., Sun C., Gao L., Zhu D., Xu X., et al. , 2013. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res. 23: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L., Ran F. A., Cox D., Lin S., Barretto R., et al. , 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser F., Schiml S., Puchta H., 2014. Both CRISPR/Cas-based nucleases and nickases can be used efficiently for genome engineering in Arabidopsis thaliana. Plant J. 79: 348–359. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Yeh J. R., Maeder M. L., Reyon D., Sander J. D., et al. , 2009. Rapid mutation of endogenous zebrafish genes using zinc finger nucleases made by Oligomerized Pool ENgineering (OPEN). PLoS One 4: e4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon J. A., Valen E., Thyme S. B., Huang P., Akhmetova L., et al. , 2014. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS One 9: e98186 (erratum: PLoS One 9: e106396). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilinger J. P., Thompson D. B., Liu D. R., 2014. Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat. Biotechnol. 32: 577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W. Y., Fu Y., Reyon D., Maeder M. L., Tsai S. Q., et al. , 2013. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31: 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao L. E., Wente S. R., Chen W., 2013. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 110: 13904–13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., et al. , 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. M., Kim D., Kim S., Kim J. S., 2014. Genotyping with CRISPR-Cas-derived RNA-guided endonucleases. Nat. Commun. 5: 3157. [DOI] [PubMed] [Google Scholar]
- Lamason R. L., Mohideen M. A., Mest J. R., Wong A. C., Norton H. L., et al. , 2005. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 310: 1782–1786. [DOI] [PubMed] [Google Scholar]
- Li F., Cowley D. O., Banner D., Holle E., Zhang L., et al. , 2014. Efficient genetic manipulation of the NOD-Rag1−/−IL2RgammaC-null mouse by combining in vitro fertilization and CRISPR/Cas9 technology. Sci. Rep. 4: 5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Wang Z., Xiao A., Zhang Y., Li W., et al. , 2014. Efficient gene targeting in zebrafish mediated by a zebrafish-codon-optimized cas9 and evaluation of off-targeting effect. J. Genet. Genomics 41: 43–46. [DOI] [PubMed] [Google Scholar]
- Luo W., Galvan D. L., Woodard L. E., Dorset D., Levy S., et al. , 2017. Comparative analysis of chimeric ZFP-, TALE- and Cas9-piggyBac transposases for integration into a single locus in human cells. Nucleic Acids Res. 45: 8411–8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P., Yang L., Esvelt K. M., Aach J., Guell M., et al. , 2013. RNA-guided human genome engineering via Cas9. Science 339: 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw P. S., Chung S. C., Muto A., Roeser T., Staub W., et al. , 2004. Retinal network adaptation to bright light requires tyrosinase. Nat. Neurosci. 7: 1329–1336. [DOI] [PubMed] [Google Scholar]
- Shen B., Zhang J., Wu H., Wang J., Ma K., et al. , 2013. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 23: 720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Y. H., Kim J. M., Kim H. T., Lee J., Jeon J., et al. , 2014. Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome Res. 24: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X., Zu Y., Li Z., Li W., Ying L., et al. , 2014. Kctd10 regulates heart morphogenesis by repressing the transcriptional activity of Tbx5a in zebrafish. Nat. Commun. 5: 3153. [DOI] [PubMed] [Google Scholar]
- Tzur Y. B., Friedland A. E., Nadarajan S., Church G. M., Calarco J. A., et al. , 2013. Heritable custom genomic modifications in Caenorhabditis elegans via a CRISPR-Cas9 system. Genetics 195: 1181–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Lu Z., Li F., Wang W., Qian N., et al. , 2017. Efficient in vivo gene editing using ribonucleoproteins in skin stem cells of recessive dystrophic epidermolysis bullosa mouse model. Proc. Natl. Acad. Sci. USA 114: 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Y., Zhang X., Ren J., Dong X., Zhu Z., et al. , 2016. Biallelic editing of a lamprey genome using the CRISPR/Cas9 system. Sci. Rep. 6: 23496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.