Abstract
The clearance of dead cells is a fundamental process in the maintenance of tissue homeostasis. Genetic studies in Drosophila melanogaster, Caenorhabditis elegans, and mammals have identified two evolutionarily conserved signaling pathways that act redundantly to regulate this engulfment process: the ced-1/-6/-7 and ced-2/-5/-12 pathways. Of these engulfment genes, only the ced-7/ABCA1 ortholog remains to be identified in D. melanogaster. Homology searches have revealed a family of putative ced-7/ABCA1 homologs encoding ATP-binding cassette (ABC) transporters in D. melanogaster. To determine which of these genes functions similarly to ced-7/ABCA1, we analyzed mutants for engulfment phenotypes in oogenesis, during which nurse cells (NCs) in each egg chamber undergo programmed cell death (PCD) and are removed by neighboring phagocytic follicle cells (FCs). Our genetic analyses indicate that one of the ABC transporter genes, which we have named Eato (Engulfment ABC Transporter in the ovary), is required for NC clearance in the ovary and acts in the same pathways as drpr, the ced-1 ortholog, and in parallel to Ced-12 in the FCs. Additionally, we show that Eato acts in the FCs to promote accumulation of the transmembrane receptor Drpr, and promote membrane extensions around the NCs for their clearance. Since ABCA class transporters, such as CED-7 and ABCA1, are known to be involved in lipid trafficking, we propose that Eato acts to transport membrane material to the growing phagocytic cup for cell corpse clearance. Our work presented here identifies Eato as the ced-7/ABCA1 ortholog in D. melanogaster, and demonstrates a role for Eato in Drpr accumulation and phagocytic membrane extensions during NC clearance in the ovary.
Keywords: cell death, engulfment, ABC transporter, ced-7, Eato
PCD is a fundamental biological process in animal development and tissue homeostasis. Cells undergoing PCD are selectively cleared by phagocytes in a multi-step engulfment process involving recognition followed by internalization of the dying cell (Arandjelovic and Ravichandran 2015; Green et al. 2016). In some instances, phagocytes can promote the death of their target cells (Reddien et al. 2001; Brown and Neher 2012; Timmons et al. 2016). Abnormal regulation of the engulfment process has been implicated in several human diseases, including developmental malformations, physiological disorders, autoimmunity, neurodegeneration, and cancer (Arandjelovic and Ravichandran 2015; Green et al. 2016).
Engulfment is generally performed by “professional” phagocytes, such as mammalian macrophages, whose primary function is the phagocytosis of cellular debris. In tissues where professional phagocytes have little to no access, resident cells can function as “nonprofessional” phagocytes to remove dead cells (Arandjelovic and Ravichandran 2015; Green et al. 2016). For example, in the Drosophila ovary, a system closed to circulating cells, clearance of dying NCs is accomplished by neighboring epithelial cells called FCs (Giorgi and Deri 1976; Etchegaray et al. 2012). Current evidence suggests that engulfment by professional and nonprofessional phagocytes is regulated similarly (Arandjelovic and Ravichandran 2015; Green et al. 2016).
Extensive genetic studies in Caenorhabditis elegans have identified two parallel but partially redundant signaling pathways, CED-1/-6/-7 and CED-2/-5/-12, which regulate the engulfment process (Ellis et al. 1991; Kinchen et al. 2005). These pathways appear to be conserved in mammals as MEGF10/GULP/ABCA1 and Crk/DOCK180/ELMO, and in Drosophila melanogaster as Drpr/Ced-6 and Crk/Myoblast city/Ced-12, respectively (Mangahas and Zhou 2005). However, the D. melanogaster ortholog for CED-7/ABCA1 has not been identified.
The genes ced-7 and ABCA1 encode members of the ABCA subfamily of ABC transporters (Luciani and Chiminil 1996; Wu and Horvitz 1998). ABC transporters are important in a wide range of physiological processes and can translocate a variety of substrates, including sugars, ions, lipids, and proteins (Rees et al. 2009; ter Beek et al. 2014; Wilkens 2015). Mutations that abolish the ATP-binding function of CED-7 or ABCA1 cause engulfment defects that lead to the accumulation of cell corpses in vivo (Luciani and Chiminil 1996; Wu and Horvitz 1998; Hamon et al. 2000).
In C. elegans, CED-7 has been shown to be required in both the phagocytic and the dying cell for efficient engulfment (Wu and Horvitz 1998). In mammals, ABCA1 is clearly required in phagocytic cells (Hamon et al. 2000), but whether the protein is required in dying cells in vivo has not been determined. In vitro studies in mouse cell culture hemocytes and thymocytes have demonstrated a role for ABCA1 in phosphatidylserine (PtdSer) exposure following apoptotic stimuli (Hamon et al. 2000), suggesting that ABCA1 may act in dying cells to promote cell corpse recognition. In contrast, PtdSer was clearly detected on the surface of cell corpses in vivo in ced-7 mutants (Mapes et al. 2012), indicating that CED-7 is not required for PtdSer exposure in C. elegans.
Multiple reports have speculated whether CED-7/ABCA1 acts as a lipid transporter. Indeed, most ABCA-type transporters appear to be involved in lipid trafficking (Vasiliou et al. 2009; Quazi and Molday 2011). In mammals, ABCA1 has been shown to promote the transport of lipids from the Golgi to the plasma membrane, and the efflux of lipids to form high-density lipoproteins (HDLs) (Hamon et al. 2000; Orsó et al. 2000). In humans, deficiency for ABCA1 is implicated in Tangier disease, a recessive disorder of lipid metabolism characterized by the lack of HDLs due to defective translocation of membrane lipids (Hamon et al. 2000; Orsó et al. 2000; Vasiliou et al. 2009).
In C. elegans, CED-7 has been observed to play a role in both intracellular and extracellular lipid trafficking during engulfment. CED-7 was shown to act with CED-1, CED-6, and DYN-1 to promote the intracellular delivery of vesicles to the phagocytic cup, presumably to provide lipid and protein materials to the growing membrane for pseudopod extensions (Yu et al. 2006). CED-7 has also been shown to be required for the presence of extracellular vesicles and is proposed to mediate the exocytosis of vesicles containing engulfment signals, such as the bridging molecule TTR-52, which facilitates CED-1 recognition of PtdSer (Mapes et al. 2012). However, because CED-7 activity appears to be required in both the phagocytic and dying cells for engulfment in C. elegans (Wu and Horvitz 1998), it has been complicated to determine exactly where CED-7 acts in the signaling pathway.
Downstream of CED-7/ABCA1, a prominent feature observed during engulfment, is the clustering of the transmembrane receptors CED-1/MEGF10 at the phagocytic cup (Zhou et al. 2001). In vivo studies in C. elegans and in vitro studies in mouse cell culture, respectively, show that CED-1/MEGF10 clusters around the cell corpse and facilitates cell clearance in a manner dependent on CED-7/ABCA1 (Zhou et al. 2001; Hamon et al. 2006). Given its putative role in lipid transport, it is tempting to speculate that CED-7/ABCA1 may function at the phagocytic cup to remodel the local lipid composition, and perhaps generate domains such as lipid rafts to which CED-1/MEGF10 can be recruited.
To identify and characterize the CED-7/ABCA1 ortholog in D. melanogaster, we used the D. melanogaster ovary as an in vivo model system to study cell death and engulfment. Two distinct germline PCD events, developmental PCD in late oogenesis and stress-induced PCD in midoogenesis, have been well characterized in the ovary (Jenkins et al. 2013; Peterson et al. 2015). The ovary is comprised of a bundle of 15–20 ovarioles, sheaths of progressively developing egg chambers through 14 stages of oogenesis. Each egg chamber contains 16 interconnected germline-derived cells, composed of a single oocyte and 15 NCs, surrounded by a layer of somatically-derived FCs (King 1970; Spradling. 1993). As each oocyte reaches maturation, the 15 NCs undergo PCD and are cleared by the neighboring FCs. We have found that phagocytosis genes including drpr and Ced-12 are required for NC clearance, as their loss-of-function results in stage 14 egg chambers that exhibit persisting NC nuclei (PN) (Timmons et al. 2016). Additionally, genetically inducing death in a small subset of the phagocytic FCs inhibits the death and removal of the NCs, suggesting that the FCs nonautonomously promote the death and removal of the NCs via phagoptosis.
During midoogenesis, PCD of the germline can occur in response to stress, such as protein starvation. PCD in midoogenesis requires active caspases, including death caspase 1 (Dcp-1), and autophagy genes, suggesting that death is executed via apoptotic and autophagic cell death pathways (Jenkins et al. 2013). As the NCs degenerate, the surrounding FCs synchronously enlarge to engulf the germline debris. Similar to clearance in late oogenesis, this process in midoogenesis is regulated by drpr and Ced-12 (Etchegaray et al. 2012; Meehan et al. 2015a). drpr and Ced-12 mutants produce egg chambers that exhibit dying NCs, with FCs that fail to enlarge or take up the germline material.
The engulfment pathways first defined in C. elegans are highly conserved in D. melanogaster, but a ced-7/ABCA1 ortholog has not been reported. The D. melanogaster genome contains 56 ABC genes, of which 10 encode ABCA type transporters similar to CED-7/ABCA1. Only two of the ABCA genes, CG31731 and CG1718, are expressed at appreciable levels in the ovary (FlyBase). We functionally analyzed these two genes in the D. melanogaster ovary and found that CG31731 mutants show profound defects in NC clearance. Moreover, CG31731 appears to play a similar role to ced-7/ABCA1 in the engulfment process. Thus, CG31731 likely serves a CED-7/ABCA1 role in engulfment in the D. melanogaster ovary, and hereafter will be referred to as Eato.
Materials and Methods
Fly strains and manipulations
All stocks were obtained from the Harvard Transgenic RNAi Project (Perkins et al. 2015) (CG31731HMC06027, CG1718HMS01821, CG1718HMS01796, and Ced-12HM05042), the Bloomington Drosophila Stock Center (BDSC) (CG31731MI14571, CG31731f02254, and CG1718mir-1007-KO), or the Vienna Drosophila Resource Center (CG31731GD1133, CG31731KK104197, CG1718GD3708, and CG1718KK100452), with the exception of the drprΔ5/Δ5 strain (Freeman et al. 2003) provided by Estee Kurant.
The initial EatoMi/CyO strain received from the BDSC was homozygous lethal, but EatoMi/Df(2L)BSC812 flies were viable, so we used the Df(2L)BSC812 strain (which uncovers Eato) to generate the homozygous EatoMi/Mi strain. Specifically, we crossed EatoMi/CyO with Df(2L)BSC812/CyO, and the EatoMi/Df(2L)BSC812 F1 progeny exhibiting straight wings were mated to allow background lethal mutations to recombine off. Since Df(2L)BSC812 is marked by a w+ marker while the EatoMi construct carries no eye pigment marker, F2 progeny exhibiting white eyes were collected to select against the deficiency chromosome and generate the EatoMi/Mi strain.
To make germline clones, we generated an EatoMi FRT 40A stock by recombination and used the FLP ovoD system (Chou and Perrimon 1996). RNAi knockdown lines were generated using the GAL4-UAS binary system, with GAL4 expressed under control of an endogenous tissue-specific enhancer, specifically GR1, which is expressed in all FCs after stage 3 including the stretch FCs (Goentoro et al. 2006), and nanos, which is expressed in the NCs (Rørth 1998).
All strains were reared on standard cornmeal molasses yeast media at 25°. Prior to dissection, adult males and females were transferred to a vial containing fresh media and a teaspoon of yeast paste, and conditioned for ∼2 d. To induce cell death in midstage egg chambers, adults were conditioned with yeast paste for ∼1 d then transferred to apple juice agar vials and starved of yeast for the last 16–20 hr period prior to dissection.
Staining and microscopy
Ovaries were dissected in Grace’s Insect Media (Fisher) and then processed as previously described (Meehan et al. 2015b). Primary antibodies used were: α-Drpr [1:50; Developmental Studies Hybridoma Bank (DSHB)], α-Dlg (1:100; DSHB), and α-cleaved Dcp1 (1:100; Cell Signaling). Secondary antibodies used were: goat-α-rabbit Cy3, goat-α-mouse Cy3, and goat-α-rabbit Alexa Fluor 647 (1:200; Jackson ImmunoResearch). Ovaries were mounted in Vectashield with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Vector Laboratories) and slides were stored at 4°. Egg chambers were imaged on an Olympus BX60 upright fluorescence microscope or an Olympus FV10i confocal microscope, and images were processed in ImageJ.
Quantitative RT-PCR
RNA samples were extracted from pooled ovaries using the QIAGEN RNeasy Mini Kit, and then converted to cDNA using the Thermo Scientific Maxima First Stand cDNA Synthesis Kit. qPCR was performed following the Promega GoTaq qPCR Master Mix protocol with two primer sets, one flanking the fourth and fifth exons and another flanking the 14th and 15th exons of Eato. The results were normalized to Rpl32 as an internal control.
Quantifications
To quantify engulfment, the number of PN in each stage 14 egg chamber was counted. The criteria for a stage 14 egg chamber was fully developed dorsal appendages (Jia et al. 2016). The egg chambers were then grouped into bins of 0 PN, 1–3 PN, 4–6 PN, 7–9 PN, 10–12 PN, or 13–15 PN, and each bin was presented as a percentage of all stage 14 egg chambers quantified per genotype. Alternatively, the average number of PN in stage 14 egg chambers from each genotype was presented. “n” represents the total number of stage 14 egg chambers quantified.
To quantify Drpr accumulation or stretch FC (SFC) membrane extensions around the NCs, using ImageJ, the length around each NC nucleus that was α-Drpr- or GFP-positive was measured as a percentage of the circumference around each NC nucleus. Each NC nucleus was then grouped into bins of 0–10, 11–30, 31–50, 51–70, 71–90, or 91–100%, surrounded by α-Drpr or GFP, and each bin was presented as a percentage of all NC nuclei quantified per genotype. “n” represents the total number of NC nuclei quantified.
All quantifications were performed blind and statistical analyses were performed in Graphpad Prism.
Data availability
All strains and reagents are available upon request.
Results
Eato encodes an ABC transporter similar to ced-7/ABCA1
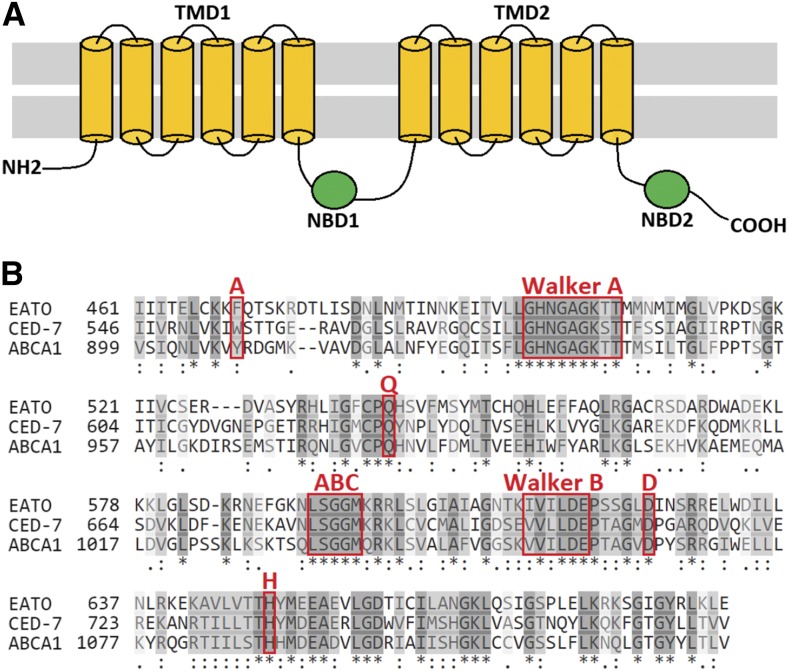
A search of databases [FlyBase, UniProt, and the Drosophila RNAi Screening Center Interactive Ortholog Prediction Tool (DIOPT)] revealed that the predicted amino acid sequence of Eato encodes an ABC transporter of the ABCA subfamily, which includes CED-7 and ABCA1. Like other ABC transporters, Eato encodes a protein with two transmembrane domains (TMDs) and two cytosolic nucleotide-binding domains (NBDs) (Figure 1A) with predicted ATP-binding and catalytic capability. Each NBD was found to contain an “A-loop” (aromatic), “Walker A motif” (GxxGxGKS/T), “Q-loop” (glutamine, Q), “ABC signature motif” (L/YSGGQ/M), “Walker B motif” (φφφφDE), “D-loop” (aspartate, D), and “H-loop” (histidine, H), in highly conserved sequential and spatial organization (Figure 1B), classifying the protein as an ABC transporter (Rees et al. 2009; ter Beek et al. 2014). Additional structural analyses of the predicted amino acid sequence indicated that each Eato TMD contains six hydrophobic α-helical segments, generating a 12-pass transporter. In comparison, both CED-7 and ABCA1 each contain 15 transmembrane segments.
Figure 1.
CG31731/Eato encodes an ATP-binding cassette (ABC) transporter. (A) Schematic of a general ABC transporter, with two transmembrane domains (TMDs) comprised of 12 transmembrane segments and two nucleotide-binding domains (NBDs). (B) The predicted NBD1 amino acid sequences of Eato, ced-7, and ABCA1 aligned using CLUSTALO provided by UniProt. The similarities (light gray) and identities (dark gray) are highlighted. The conserved A-loop, Walker A motif, Q- loop, ABC signature motif, Walker B motif, D-loop, and H-loop, are indicated.
Using Basic Local Alignment Search Tool (BLAST) algorithms to compare the predicted amino acid sequences of Eato with that of CED-7 and ABCA1, a substantial amount of similarity and identity were found throughout their entire length, most notably in the catalytic NBD regions. Overall, Eato was found to be 20% identical to CED-7 and 18% identical to ABCA1. More specifically, the NBDs of Eato were found to be 35% identical and 36% similar to those of CED-7, and 40% identical and 36% similar to those of ABCA1. Additional homology searches using the DIOPT (Hu et al. 2011) revealed that Eato is the best predicted ortholog of ced-7 and also a predicted ortholog of ABCA1. Alignment of the proteins provided by DIOPT revealed a 24% identity and 42% similarity overall between Eato and CED-7, and a 26% identity and 43% similarity between Eato and ABCA1.
Eato mutants have persisting NC corpses in the ovary
To determine whether Eato can act as a functional equivalent for ced-7/ABCA1 during PCD, we obtained several transposon-induced alleles of Eato (Figure 2, A and B) and analyzed them for phenotypes in oogenesis. We focused our initial analysis on late oogenesis because defective clearance is directly quantifiable in late oogenesis compared to midoogenesis. Ovaries from control and Eato mutant strains were dissected and then stained with DAPI to label DNA, and the number of NC nuclei persisting in stage 14 egg chambers were counted. The presence of PN indicated a failure in NC clearance.
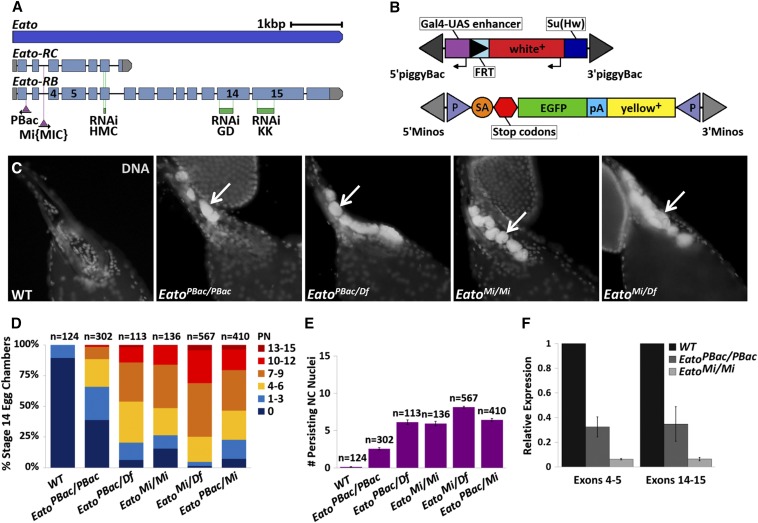
Figure 2.
Eato mutants have persisting nurse cell (NC) nuclei (PN). (A) Schematic of the Eato gene (blue) and the location of transposon insertions and RNA interference (RNAi) target sites. The two mRNA transcripts, Eato-RC and Eato-RB, are illustrated, showing the translated (light blue) and untranslated regions (gray), the insertion sites of the PBac and Mi{MIC} transgenic constructs (purple triangles), and the three RNAi target sites (green), with exons 4, 5, 14, and 15 labeled. The PBac construct is inserted in the reverse orientation while the Mi{MIC} construct is inserted in same orientation as the Eato gene (modified from FlyBase). (B) Schematic of the PBac and Mi{MIC} constructs, modified from Bellen et al. (2011) and Venken et al. (2011). Thin arrows indicate the direction of transcription. Elements are not drawn to scale. (C) Stage 14 egg chambers, stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (white) to label DNA, from wild-type (WT) or Eato allelic combinations. WT (w1118) egg chambers successfully complete programmed cell death and do not have persisting NC nuclei. EatoPBac/PBac and EatoMi/Mi homozygous mutants, and hemizygotes in trans to Df(2L)BSC812 have defects in NC clearance and have PN (arrows). (D) The percentage of egg chambers exhibiting 0 PN, 1–3 PN, 4–6 PN, 7–9 PN, 10–12 PN, and 13–15 PN, of all stage 14 egg chambers quantified per genotype. (E) The average number of persisting NC nuclei in stage 14 egg chambers from each indicated genotype. Error bars indicate ± SEM. (F) Eato transcript levels from WT and mutant whole-ovary mRNA samples quantified by quantitative reverse transcriptase polymerase chain reaction using primers flanking the indicated exons. The relative expression is presented after normalization to Rpl32 as an internal control. Error bars indicate ± SD.
In wild-type (WT) egg chambers, the 15 germline-derived NCs underwent PCD and were cleared normally, leaving only the mature oocyte by stage 14 (Figure 2C). However, in several Eato mutant allelic combinations, we observed significant clearance defects characterized by the presence of PN in stage 14 egg chambers (Figure 2, C–E). Homozygous EatoPBac/ PBac mutants displayed an average of 2–3 PN, while hemizygous EatoPBac/Df, trans-heterozygous EatoPBac/Mi, and homozygous EatoMi/Mi mutants displayed an average of 6 PN per stage 14 egg chamber. The strongest phenotype was observed in hemizygous EatoMi/Df mutants, with stage 14 egg chambers exhibiting an average of 8 PN (Figure 2, D and E). Flies heterozygous for a WT Eato allele did not exhibit any notable defects in NC clearance, suggesting that Eato is not haploinsufficient.
The PBac and Mi{MIC} constructs are inserted at the end of the first coding exon and in the third intron of the Eato gene, respectively (Figure 2A). The weaker phenotype observed in EatoPBac/PBac egg chambers relative to the other alleles suggests that the EatoPBac allele is a weak hypomorph. The Mi{MIC} insertion provides a gene trap and a protein trap (Figure 2B) (Venken et al. 2011), which in theory should generate a null allele. However, the more severe persisting phenotype observed in hemizygous EatoMi/Df mutants compared to homozygous EatoMi/Mi mutants suggests that the EatoMi allele is a strong hypomorph of Eato. RT-qPCR analysis indicated that the EatoMi allele is not a null, but is instead a strong hypomorph with a 16.6-fold decrease in transcript levels, while the EatoPBac allele is a weaker hypomorph with a 3.2-fold decrease in mRNA transcript expression in the ovary relative to WT (w1118) (Figure 2F).
Eato is required in the FCs for NC clearance during developmental PCD
We next wanted to discern in which cell type Eato function is required to facilitate removal of the NCs. Studies in C. elegans showed that CED-7 is required in both the phagocytic cell and the dying cell for efficient engulfment of cell corpses (Wu and Horvitz 1998), while studies in mammals showed that ABCA1 expression in phagocytic cells is sufficient for engulfment (Hamon et al. 2000). To determine in which cell type Eato acts during engulfment of the NCs, we used tissue-specific drivers to express Eato RNAi constructs and knock down Eato expression specifically in the FCs or NCs.
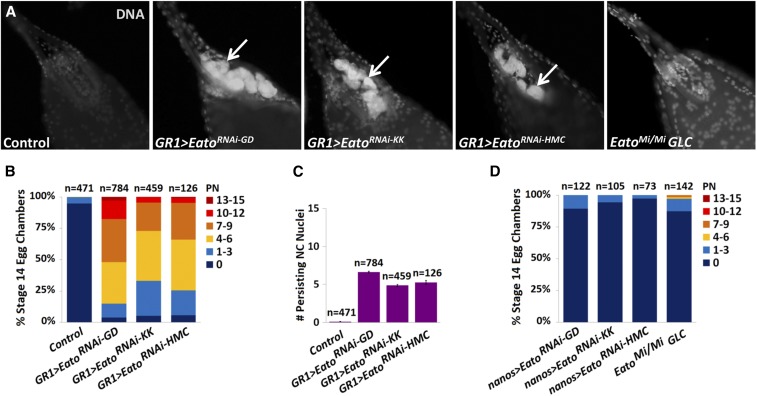
Eato knockdown with three different RNAi constructs (Figure 2A) using a FC-specific driver, GR1-GAL4 (GR1-GAL4 > UAS-EatoRNAi), resulted in stage 14 egg chambers that exhibited PN (Figure 3, A–C). Egg chambers expressing the GD1133, KK104197, or HMC06027 RNAi constructs in FCs displayed an average of 7, 5, and 4–5 PN, respectively (Figure 3C). All three RNAi constructs exhibited stronger phenotypes than the EatoPBac/PBac mutants. The GD1133 RNAi construct also exhibited a stronger phenotype than the EatoMi/Mi mutants, suggesting that PN observed in these mutants can be primarily attributed to loss of Eato function in the FCs. Sibling controls (EatoRNAi/TM6B) containing the RNAi constructs without the driver showed no defects in NC clearance (Figure 3, B and C).
Figure 3.
Eato is required in the follicle cells (FCs) for nurse cell (NC) clearance. (A) Stage 14 egg chambers, stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (white) to label DNA from a sibling control (UAS-EatoRNAi/TM6B), one of three different EatoRNAi constructs (GD1133, KK104197, and HMC06027) expressed specifically in the FCs (GR1-GAL4 > UAS-EatoRNAi), or an EatoMi germline clone. Persisting nuclei (PN) (arrows) are observed when Eato is knocked down specifically in the FCs but not in the germline clones. (B and D) The percentage of egg chambers exhibiting 0 PN, 1–3 PN, 4–6 PN, 7–9 PN, 10–12 PN, and 13–15 PN, of all stage 14 egg chambers quantified per genotype. (C) The average number of persisting NC nuclei in stage 14 egg chambers from each indicated genotype. Control represents combined EatoRNAi/TM6B siblings. Error bars indicate ± SEM.
To determine whether Eato also acts in dying cells, we knocked down Eato specifically in the NCs (nanos-GAL4 > UAS-EatoRNAi). We did not observe a requirement for Eato in the NCs for their clearance. Egg chambers expressing any of the three EatoRNAi constructs specifically in the NCs did not show any engulfment defects and were able to clear all 15 NCs normally (Figure 3D). While GD and KK RNAi libraries generate long hairpin RNA sequences, which are typically ineffective for RNAi-mediated knockdown in the germline (Ni et al. 2011), the HMC06027 allele encodes a short hairpin RNAi sequence that should competently knock down Eato expression in the germline-derived NCs (Ni et al. 2011). The lack of persisting NCs in these Eato RNAi-expressing stage 14 egg chambers suggests that Eato is not required in the dying cells for their clearance. To confirm that Eato was not required in the dying NCs, we generated EatoMi germline clones. Indeed, stage 14 egg chambers from EatoMi germline clones did not show a significant persisting NC phenotype (Figure 3A). However, ∼3% of stage 14 egg chambers exhibited ≥ 4 PN (Figure 3D). We suspect that these egg chambers may have FC clones in addition to germline clones, which can occur in the process of generating germline clones (Peterson and McCall 2013).
Eato acts in parallel to Ced-12, likely in the same pathway as Drpr
In C. elegans, the engulfment mechanism is primarily regulated by two parallel signaling pathways, CED-1/-6/-7 and CED-2/-5/-12 (Ellis et al. 1991; Kinchen et al. 2005). We have shown that, similar to C. elegans, drpr, the ced-1 ortholog, and Ced-12 act in parallel to regulate clearance of the NCs during D. melanogaster oogenesis (Timmons et al. 2016). Double knockdowns expressing Ced-12RNAi and drprRNAi, or the null allele drpr∆5, in the FCs exhibit a significantly more severe persisting NC phenotype compared to either single knockdown alone. In C. elegans and mammals, ced-7/ABCA1 acts in the same pathway as ced-1/MEGF10, in parallel to ced-12/ELMO. The similarities that we have observed between Eato and ced-7/ABCA1 suggest that Eato may act in the same pathway as drpr and in parallel to Ced-12. To ascertain which pathway Eato is involved in during engulfment, we generated Eato+drpr and Eato+Ced-12 double mutants and analyzed the severity of their engulfment defects in NC clearance.
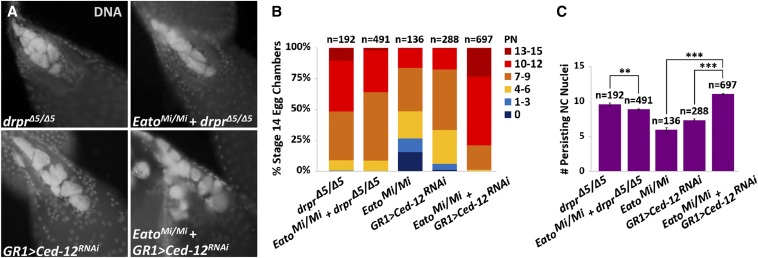
To determine whether Eato acts in the same pathway as drpr, we analyzed EatoMi/Mi; drprΔ5/Δ5 double mutants compared to drprΔ5/Δ5 single mutants. We found that the double mutant did not show a stronger phenotype than the single mutant (Figure 4, A–C), and that both drprΔ5/Δ5 and the double mutants displayed an average of ∼9 PN (Figure 4C). The similar clearance defects between the single and double mutants indicate that Eato acts in the same pathway as drpr.
Figure 4.
Eato acts in the same pathway as drpr and in parallel to Ced-12. (A) Stage 14 egg chambers, stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (white) to label DNA, from drprΔ5/Δ5 and Ced12RNAi expressed specifically in the follicle cells (FCs) (GR1-GAL4 > UAS-Ced12RNAi), single and double mutants with EatoMi/Mi. (B) The percentage of stage 14 egg chambers exhibiting 0 persisting nurse cell (NC) nuclei (PN), 1–3 PN, 4–6 PN, 7–9 PN, 10–12 PN, and 13–15 PN per indicated genotype. (C) The average number of persisting NC nuclei in stage 14 egg chambers from each indicated genotype. Error bars indicate ± SEM. Unpaired t-tests were performed: ** P < 0.001 and *** P < 0.0001.
To clarify whether Eato acts in parallel to Ced-12, we needed to avoid the lethality of Ced-12 mutants and thus we knocked down Ced-12 expression only in the phagocytic FCs (GR1 > Ced-12RNAi). In these Ced-12 knockdowns, stage 14 egg chambers displayed an average of 7 PN. Impressively, in the EatoMi/Mi; GR1 > Ced-12RNAi double mutants, stage 14 egg chambers displayed an average of 11 PN (Figure 4C). The EatoMi/Mi; GR1 > Ced-12RNAi double mutants displayed a much more severe engulfment defect than either single mutant alone, and a stronger phenotype than the EatoMi/Mi; drprΔ5/Δ5 double mutants. Moreover, a considerable percentage of these double mutants completely failed to clear any of the NCs (Figure 4B), indicating that the engulfment machinery had been severely impaired. These findings suggest that Eato acts in parallel to Ced-12, and that its function is important for clearance of the NCs.
Eato promotes Drpr enrichment and stretch FC membrane extensions surrounding the NCs
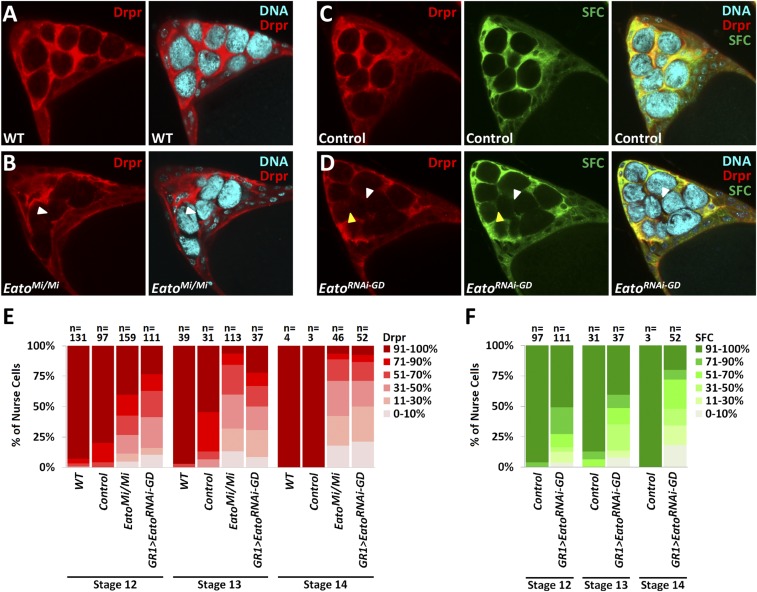
During engulfment, CED-1/MEGF10 has been shown to accumulate at the phagocytic cup (Hamon et al. 2000; Zhou et al. 2001). In vivo and in vitro studies in C. elegans and mouse cell culture, respectively, show that the uniform clustering of CED-1/MEGF10 around cell corpses occurs in a manner dependent on CED-7/ABCA1 activity. To examine whether Eato may function orthologously to CED-7/ABCA1 and be required for Drpr accumulation around the NCs, we analyzed late-stage egg chambers from WT and EatoMi/Mi mutants with DAPI to label DNA and anti-Drpr antibody to label Drpr.
In late-stage egg chambers, a subset of anterior FCs, known as the SFCs, associate with the NCs as a squamous epithelium (Wu et al. 2008). In WT stage 12–14 egg chambers, Drpr staining becomes enriched in the SFCs and clearly surrounds each NC (Figure 5A) (Timmons et al. 2016). However, in EatoMi/Mi egg chambers, Drpr staining appeared unevenly enriched and scattered around the NCs. Strikingly, in some areas, Drpr staining was completely absent in the SFCs surrounding the NCs (Figure 5, B and E), indicating that Drpr failed to properly accumulate. These observations suggest a requirement for Eato in Drpr accumulation during NC clearance.
Figure 5.
Eato is required for Drpr accumulation and follicle cell (FC) membrane extensions around the dying nurse cells (NCs). (A–D) Stage 12 egg chambers, stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (cyan), anti-Drpr (red), and expressing green fluorescent protein (GFP) (green) specifically in the SFC membrane (GR1 > mCD8-GFP), from wild-type (WT) (w1118), control (GR1 > mCD8-GFP), EatoMi/Mi and Eato knockdown (GR1 > mCD8-GFP, EatoRNAi-GD) mutants. Areas lacking Drpr staining or GFP expression (white arrowheads) and areas lacking Drpr staining but exhibiting GFP expression (yellow arrowheads) are observed in Eato mutants. (E and F) The percentage of NCs exhibiting 0–10, 11–30, 31–50, 51–70, 71–90, and 91–100% Drpr enrichment or GFP in the stretch FC (SFC) membrane around each NC per indicated stage and genotype. In WT and control egg chambers, NCs are normally cleared by stage 14, resulting in an n < 10.
We considered two possible mechanisms that could produce the lack of Drpr staining: either Drpr fails to accumulate at the SFC membrane or the SFC membrane fails to extend and surround the NC. To observe any defects in SFC membrane extensions, we expressed the mCD8-GFP transgene, which encodes a membrane-tethered GFP fusion protein, to label FC membranes. In both control (GR1-GAL4 > UAS-mCD8-GFP) and Eato knockdown egg chambers (GR1-GAL4 > UAS-mCD8-GFP, UAS-EatoRNAi-GD) we observed an overlap of the presence or absence of GFP expression and Drpr staining in the SFCs (Figure 5, C and D). However, in Eato knockdown egg chambers, some areas exhibiting GFP did not exhibit Drpr accumulation (Figure 5, D–F). Altogether, these observations indicate a requirement for Eato in both SFC membrane extension and Drpr enrichment around the NCs.
CG1718 may act with Eato in the FCs for NC clearance during developmental PCD
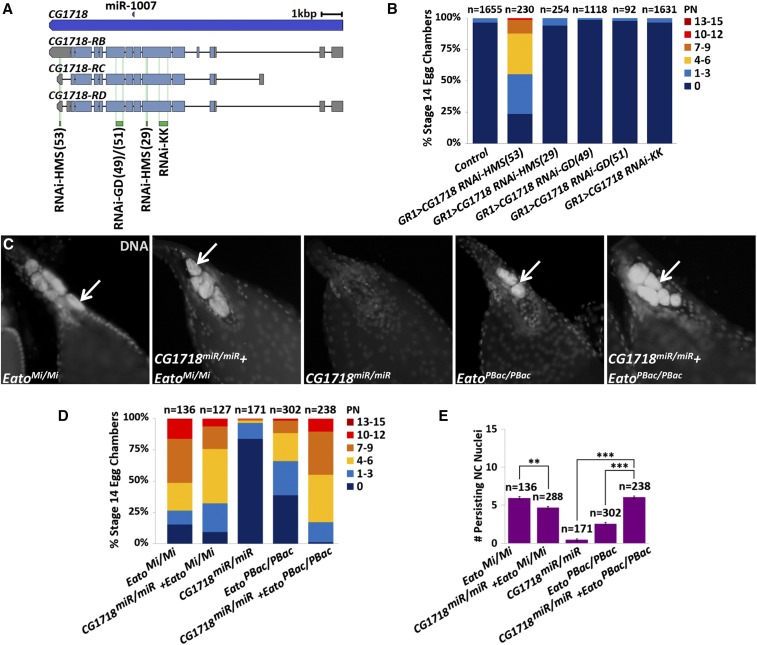
We also examined the engulfment function of CG1718 in NC clearance during oogenesis. CG1718 encodes another ABCA protein that is expressed in the ovary, and has been proposed as the D. melanogaster homolog of ABCA1 for its role in lipid and cholesterol homeostasis (Bujold et al. 2010), and the homolog of ced-7 in cell clearance (Ziegenfuss 2012; Nainu et al. 2017). Additionally, while studies in engulfing glia did not find a role for CG1718 in neuronal corpse or axonal debris clearance (Ziegenfuss 2012), pan-neuronal-specific knockdown of CG1718 resulted in synaptic bouton overgrowth at the neuromuscular junction (Ueoka et al. 2018). To determine whether CG1718 plays a role in NC clearance, we quantified PN in CG1718 knockdowns and mutants.
Egg chambers expressing one of five CG1718RNAi constructs (Figure 6A) specifically in the NCs (nanos > CG1718RNAi) did not exhibit any engulfment defects (data not shown). Intriguingly, while egg chambers expressing any one of four CG1718RNAi constructs specifically in the FCs (GR1 > CG1718RNAi) did not exhibit any engulfment defects, expression of the HMS01821 RNAi construct specifically in the FCs resulted in moderate engulfment defects as 45% of these stage 14 egg chambers exhibited ≥ 4 PN (Figure 6B). Unique from the other CG1718 RNAi constructs, the HMS01821 RNAi sequence targets the 3′ untranslated region (3′UTR) of CG1718 transcripts (Figure 6A).
Figure 6.
CG1718 may act with Eato in the follicle cells (FCs) for nurse cell (NC) clearance. (A) Schematic of the CG1718 gene and the three mRNA transcripts, CG1718-RB, CG1718-RC, and CG1718-RD, with the locations of the RNA interference (RNAi) target sites (green). The miR-1007 gene (dark blue) is also shown (figure modified from FlyBase). (B and D) The percentage of stage 14 egg chambers exhibiting 0 persisting NC nuclei (PN), 1–3 PN, 4–6 PN, 7–9 PN, 10–12 PN, and 13–15 PN per indicated genotype. (C) Stage 14 egg chambers, stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (white) to label DNA, from EatoMi/Mi mutants, EatoPBac/ PBac mutants, CG1718miR/miR mutants, or double mutant combinations. PN are shown (arrows). (E) The average number of PN in stage 14 egg chambers from each indicated genotype. Error bars indicate ± SEM. Unpaired t-tests were performed: ** P < 0.001 and *** P < 0.0001.
We also examined a miR-1007 deletion strain (CG1718miR/miR), in which the expression of CG1718 was reported to be reduced (Chen et al. 2014). The miR-1007 gene is nestled in one of the introns of CG1718 (Figure 6A), and a systematic study of Drosophila microRNA (miR) functions previously generated the CG1718miR/miR strain. Stage 14 egg chambers from these mutants did not exhibit any pronounced NC clearance defects (Figure 6, C–E).
ABC transporters of the ABCA family share a common function in lipid transport (Vasiliou et al. 2009; Quazi and Molday 2011), and thus we wondered whether CG1718 and Eato could provide compensatory or redundant roles for each other in NC clearance. We generated CG1718+Eato double mutants carrying homozygous CG1718miR/miR and EatoMi/Mi, or EatoPBac/PBac, hypomorphic alleles. Interestingly, egg chambers from CG1718miR/miR; EatoMi/Mi double mutants exhibited a less severe clearance defect than EatoMi/Mi mutants alone, with an average of 4–5 PN. However, egg chambers from CG1718miR/miR; EatoPBac/PBac double mutants exhibited a much more severe PN phenotype than either single mutant alone, with an average of 6 PN per stage 14 egg chamber (Figure 6E). While the less severe phenotype observed in CG1718miR/miR;EatoMi/Mi double mutants remains bewildering, the much more severe phenotype observed in CG1718miR/miR; EatoPBac/PBac double mutants suggests a functional relationship between the two ABCA transporters. In mammals, ABCA1 and ABCA7 have been demonstrated to provide redundant functions (Wang et al. 2003; Abe-Dohmae et al. 2004; Kim et al. 2005). Perhaps in D. melanogaster, CG1718 could provide a compensatory or redundant function for Eato.
Eato is required in the FCs for engulfment of the germline during stress-induced PCD
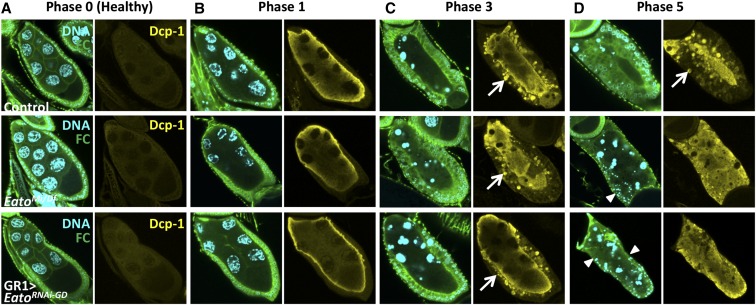
To determine if Eato is required for clearance in other forms of cell death, we investigated whether Eato plays a role during starvation-induced PCD in midoogenesis. In response to starvation, NCs die via apoptosis during midoogenesis (Jenkins et al. 2013) and are engulfed by surrounding epithelial FCs. The cell death of the NCs in midoogenesis proceeds through five morphologically distinct phases defined by changes in the NC chromatin (Etchegaray et al. 2012). In phase 0 healthy egg chambers, the 15 NCs exhibit dispersed chromatin; in phase 1 dying egg chambers, the NC chromatin becomes disordered and begins to condense; by phase 3 the NC chromatin becomes highly condensed in single large balls and the FCs appear enlarged; and by phase 5 the FCs have phagocytosed the germline material and constitute almost the entire egg chamber.
From our studies in late oogenesis, we selected the Eato mutants that exhibited the most severe phenotypes and looked for engulfment defects in midoogenesis. We selected EatoMi/Df and FC knockdown EatoRNAi-GD mutants and starved the adults for a 16–20 hr period to induce PCD in midstage egg chambers. Subsequently, we dissected and stained the ovaries with DAPI to label DNA, anti-Dlg to label FC membranes, and anti-cleaved Dcp-1 to label active caspases and apoptotic germline debris. We analyzed dying egg chambers from each phase, sorted by the changes in NC chromatin, and compared the progression of engulfment to control dying egg chambers.
Similar to the results in late oogenesis, Eato mutants exhibited engulfment defects and showed FCs that failed to enlarge normally but also died prematurely, leaving behind egg chambers with unengulfed germline debris. In both EatoMi/Df and GR1 > EatoRNAi-GD mutants, healthy phase 0 egg chambers resembled the control, suggesting that Eato mutant egg chambers develop normally through midoogenesis (Figure 7A). Phase 1 dying egg chambers also resembled the control, appearing to activate caspases and initiate PCD normally (Figure 7B). While phase 3 dying egg chambers exhibited enlarged FCs, they appeared to show fewer engulfed Dcp-1-positive vesicles present inside the FCs compared to control (Figure 7C). By phase 5, Eato mutant dying egg chambers exhibited severe engulfment defects and FC death. In phase 5 dying egg chambers, the majority of the FCs exhibited pyknotic nuclei without any membrane markers or disappeared, leaving behind egg chambers with completely unengulfed germline material (Figure 7D), resembling drpr mutants (Etchegaray et al. 2012). Many of the dying egg chambers found in the ovaries of Eato mutants were these phase 5 egg chambers, indicating a pronounced defect in removal of these egg chambers. These observations signify severe impairments in the engulfment machinery in the later steps of engulfment, and illustrate an important role for Eato in the FCs for engulfment of the germline debris during midstage apoptotic death.
Figure 7.
Eato is required in the follicle cells (FCs) for engulfment of nurse cells (NCs) during starvation-induced programmed cell death. Egg chambers stained with anti-Dlg antibody (green) to label FC membranes, anti-cleaved Dcp-1 antibody (yellow) to label active caspases and apoptotic germline debris, and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (cyan) to label DNA. (A) Phase 0 healthy egg chambers. (B) Phase 1 dying egg chambers of EatoMi/Df mutants, or when EatoRNAi-GD1133 is expressed specifically in the FCs (GR1-GAL4 > UAS-EatoRNAi-GD1133), resemble the control (UAS-EatoRNAi-GD1133/TM6B) dying egg chambers. (C) Phase 3 dying egg chambers of Eato mutants exhibit enlarged FCs; however, they show fewer Dcp-1-positive vesicles inside the FCs (arrows). (D) Phase 5 dying egg chambers of Eato mutants show severe engulfment defects with completely unengulfed germline and pyknotic FC nuclei (arrowheads) without membrane markers.
We did not observe any obvious engulfment defects in dying egg chambers when EatoRNAi-GD or EatoRNAi-HMC was expressed only in the germline (data not shown), suggesting that, as in late oogenesis, Eato is primarily required in the phagocytic FCs during engulfment of the dying germline material.
Discussion
Here, we report the characterization of Eato, which encodes an ABC transporter that is structurally and functionally similar to ced-7/ABCA1. Like ced-7/ABCA1, Eato encodes an ABCA type transporter and presents substantial sequence similarity and identity to that of CED-7 and ABCA1. Moreover, our genetic analyses identify a role for Eato in cell clearance during PCD, demonstrating functional conservation. To our knowledge, prior to this study there have been no reports of a D. melanogaster functional equivalent for CED-7/ABCA1.
Using the D. melanogaster ovary as an in vivo model system, we observed cell clearance defects in Eato mutants in both developmental PCD, which proceeds via phagoptosis (Timmons et al. 2016), and starvation-induced PCD, which proceeds via apoptosis (Jenkins et al. 2013). Unlike in C. elegans, where CED-7 was reported to be required both in the dying cell and the phagocytic cell for corpse clearance (Wu and Horvitz 1998), our investigation indicates a requirement for Eato only in the phagocytic FCs and not in the dying NCs. Genetically knocking down Eato specifically in the phagocytic FCs resulted in engulfment defects, while knocking down Eato expression specifically in the germline did not affect clearance. These findings demonstrate a conserved role for Eato in two distinct PCD modalities, specifically in the phagocytic cells for cell clearance.
In C. elegans and mammals, ced-7/ABCA1 acts in the same pathway as ced-1/MEGF10, in parallel to ced-12/ELMO (Ellis et al. 1991; Kinchen et al. 2005; Mangahas and Zhou 2005). Correspondingly, our double mutant analyses of EatoMi/Mi with drprΔ5/Δ5or Ced-12RNAi show that Eato acts in the same pathway as drpr and in parallel to Ced-12, strongly suggesting that Eato provides a ced-7/ABCA1-like role in this conserved engulfment mechanism. Indeed, we observed a requirement for Eato in Drpr enrichment around the NCs, similar to the requirement for CED-7/ABCA1 in CED-1/MEGF10 clustering at the phagocytic cup (Zhou et al. 2001; Hamon et al. 2006).
Multiple investigations in C. elegans and mammals have reported a role for CED-7/ABCA1 in lipid transport (Hamon et al. 2000; Wang et al. 2010; Mapes et al. 2012). Importantly, CED-7 has been implicated in intracellular vesicle delivery to the phagocytic cup to provide membrane material (Yu et al. 2006). Our investigation showed that in Eato mutants, phagocytic membrane extensions around the NCs are disrupted. In both late-stage and dying midstage egg chambers from Eato mutants, the FCs fail to extend and complete engulfment of the germline debris. Thus, we speculate that Eato may function as a lipid transporter to deliver vesicles containing membrane material and other proteins, such as Drpr, to the growing FC membrane. This would explain the defects observed in SFC membrane extensions and Drpr accumulation in late oogenesis, and the FC enlargement and germline uptake in midoogenesis in Eato mutants.
Transporters of the ABCA family are commonly involved in lipid trafficking, though the specific substrates transported by CED-7/ABCA1 remain to be identified. Cell culture experiments have suggested that ABCA1 may act as a translocase to translocate the “eat-me signal” PtdSer from the inner leaflet to the outer leaflet (Hamon et al. 2000; Smith et al. 2002; Albrecht et al. 2005). However, in vivo PtdSer exposure on the surface of dying cells does not require CED-7 in C. elegans. Instead, CED-7 was shown to be required for the transfer of PtdSer-containing vesicles from the surface of dying cells to the surface of phagocytic cells (Mapes et al. 2012), suggesting that CED-7 can efflux PtdSer and potentially other phospholipids. In the context of HDL formation, ABCA1 has also been speculated to preferentially transport phosphatidylcholine (Takahashi et al. 2006). Our identification of Eato provides another system to elucidate the transport activities of this unique class of proteins in engulfment.
In mammals, ABCA1 and ABCA7 have been observed to provide homologous functions, especially in lipid homeostasis (Wang et al. 2003; Abe-Dohmae et al. 2004; Hamon et al. 2006). Like ABCA1, ABCA7 has been demonstrated to mediate phospholipid and cholesterol release to form HDLs, and even compensate for ABCA1 in certain conditions (Wang et al. 2003; Abe-Dohmae et al. 2004; Kim et al. 2005). Similarly, we observed a role for another ABCA-encoding gene, CG1718, in NC clearance. Most noticeably, in an EatoPBac/PBac hypomorphic background, CG1718 may provide a compensatory or redundant function for Eato during NC clearance. Since both genes encode ABC transporters of the same family, which are known to share a functional relationship in lipid trafficking, the proteins may be able to provide similar if not the same functions. Thus, as in the case of ABCA1 and ABCA7, CG1718 may share redundant functions or possibly provide compensatory mechanisms in the absence of Eato.
CG1718 pan-neuronal knockdown flies were recently established as a model for autism spectrum disorder. These flies exhibited behavioral characteristics similar to those observed in human autism spectrum disorder patients, and showed excessive synaptic satellite bouton outgrowths (Ueoka et al. 2018), similar to those in Fmr1 mutants (Zhang et al. 2001). The Fmr1 gene has been reported to play a role in glial phagocytosis of neuronal and axonal debris, and in hemocyte phagocytosis of bacteria (Logan 2017; O’Connor et al. 2017), and thus, by analogy, CG1718 may similarly play a phagocytic role in cell clearance, though likely not in engulfing glia (Ziegenfuss 2012).
We also looked for a role for Eato in glial phagocytosis of neuronal debris. EatoMi/Mi mutants did not show any accumulation of uncleared neuronal corpses, suggesting that Eato does not play a prominent role during corpse clearance in the brain. We speculate that perhaps there is another ABCA gene that provides a ced-7-/ABCA1-like role during PCD events in engulfing glia, perhaps CG34120, which appears to be the most appreciably expressed ABCA transporter in the head (FlyBase).
Eato may also be involved in salivary gland clearance during development. Eato is expressed at high levels in the salivary glands (FlyBase), which undergo autophagic cell death and are cleared during larval development. E93 mutants, which exhibit persisting salivary glands (Lee and Baehrecke 2001), show decreased expression of Eato (Dutta 2008). Additionally, Drpr was also found to be enriched in the salivary glands and to be required for salivary gland clearance (McPhee and Baehrecke 2010). As our study indicates a relationship between drpr and Eato, it seems likely that Eato may have a role in degradation and clearance of the salivary glands.
In conclusion, our findings provide insight into the molecular activities that occur during engulfment in PCD, with specific attention to the role of ABCA transporters. We have identified Eato, a ced-7/ABCA1-like ABCA transporter gene that is required during engulfment in the D. melanogaster ovary. To our knowledge, this is the first report of a role for ABCA transporters in PCD in Drosophila. Further characterization of this ced-7/ABCA1 ortholog in D. melanogaster will help elucidate the functions and mechanisms of this unique class of transporters during PCD.
Acknowledgments
We thank members of our laboratory for helpful comments and assistance, especially Albert Mondragon, Alla Yalonetskaya, Johnny Elguero, and Victoria Jenkins, and Todd Blute for assistance with microscopy. This work was supported by National institutes of Health grants R01 GM-060574 and R01 GM-094452 to K.M. T.M.C. was supported by National Science Foundation-Research Experiences for Undergraduates (BIO-1659605) and C.V.T. was supported by Boston University’s Undergraduate Research Opportunities Program.
Author contributions: C.S.S., T.L.M., and K.M. designed the research; T.L.M. and C.V.T. performed the preliminary experiments that identified CG31731 and CG1718 as putative ced-7 orthologs; J.S.P. and T.M.C. generated the EatoMi/Mi germline clones; C.S.S. performed all other experiments and the data analyses presented here; C.S.S. and K.M. wrote the manuscript; and K.M. supervised and coordinated the research project, and acquired the funding to support the project.
Footnotes
Communicating editor: B. Reed
Literature Cited
- Abe-Dohmae S., Ikeda Y., Matsuo M., Hayashi M., Okuhira K. I., et al. , 2004. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J. Biol. Chem. 279: 604–611. [DOI] [PubMed] [Google Scholar]
- Albrecht C., McVey J. H., Elliott J. I., Sardini A., Kasza I., et al. , 2005. A novel missense mutation in ABCA1 results in altered protein trafficking and reduced phosphatidylserine translocation in a patient with Scott syndrome. Blood 106: 542–549. [DOI] [PubMed] [Google Scholar]
- Arandjelovic S., Ravichandran K. S., 2015. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 16: 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen H. J., Levis R. W., He Y., Carlson J. W., Evans-Holm M., et al. , 2011. The Drosophila gene disruption project: progress using transposons with distinctive site specificities. Genetics 188: 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C., Neher J. J., 2012. Eaten alive! Cell death by primary phagocytosis: “phagoptosis.” Trends Biochem. Sci. 37: 325–332. [DOI] [PubMed] [Google Scholar]
- Bujold M., Gopalakrishnan A., Nally E., King-Jones K., 2010. Nuclear receptor DHR96 acts as a sentinel for low cholesterol concentrations in Drosophila melanogaster. Mol. Cell. Biol. 30: 793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. W., Song S., Weng R., Verma P., Kugler J. M., et al. , 2014. Systematic study of Drosophila MicroRNA functions using a collection of targeted knockout mutations. Dev. Cell 31: 784–800. [DOI] [PubMed] [Google Scholar]
- Chou T. B., Perrimon N., 1996. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144: 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, S., 2008 Genetic regulation of autophagic cell death in Drosophila melanogaster. Ph.D. Thesis. University of Massachusetts Medical School. [Google Scholar]
- Ellis R. E., Jacobson D. M., Horvitz H. R., 1991. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics 129: 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray J. I., Timmons A. K., Klein A. P., Pritchett T. L., Welch E., et al. , 2012. Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells. Development 139: 4029–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. R., Delrow J., Kim J., Johnson E., Doe C. Q., 2003. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron 38: 567–580. [DOI] [PubMed] [Google Scholar]
- Giorgi F., Deri P., 1976. Cell death in ovarian chambers of Drosophila melanogaster. J. Embryol. Exp. Morphol. 35: 521–533. [PubMed] [Google Scholar]
- Goentoro L. A., Yakoby N., Goodhouse J., Schüpbach T., Shvartsman S. Y., 2006. Quantitative analysis of the GAL4/UAS system in Drosophila oogenesis. Genesis 44: 66–74. [DOI] [PubMed] [Google Scholar]
- Green D. R., Oguin T. H., Martinez J., 2016. The clearance of dying cells: table for two. Cell Death Differ. 23: 915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon Y., Broccardo C., Chambenoit O., Luciani M. F., Toti F., et al. , 2000. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2: 399–406. [DOI] [PubMed] [Google Scholar]
- Hamon Y., Trompier D., Ma Z., Venegas V., Pophillat M., et al. , 2006. Cooperation between engulfment receptors: the case of ABCA1 and MEGF10. PLoS One 1: e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Flockhart I., Vinayagam A., Bergwitz C., Berger B., et al. , 2011. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinformatics 12: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins V. K., Timmons A. K., McCall K., 2013. Diversity of cell death pathways: insight from the fly ovary. Trends Cell Biol. 23: 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D., Xu Q., Xie Q., Mio W., Deng W., 2016. Automatic stage identification of Drosophila egg chamber based on DAPI images. Sci. Rep. 6: 18850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. S., Fitzgerald M. L., Kang K., Okuhira K. I., Bell S. A., et al. , 2005. Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J. Biol. Chem. 280: 3989–3995. [DOI] [PubMed] [Google Scholar]
- Kinchen J. M., Cabello J., Klingele D., Wong K., Feichtinger R., et al. , 2005. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature 434: 93–99. [DOI] [PubMed] [Google Scholar]
- King R. C., 1970. Ovarian Development in Drosophila melanogaster. Academic Press, New York. [Google Scholar]
- Lee C. Y., Baehrecke E. H., 2001. Steroid regulation of autophagic programmed cell death during development. Cell Death Differ. 128: 1443–1455. [DOI] [PubMed] [Google Scholar]
- Logan M. A., 2017. Fragile phagocytes: FMRP positively regulates engulfment activity. J. Cell Biol. 216: 531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani M. F., Chiminil G., 1996. The ATP binding cassette transporter ABC1, is required for the engulfment of corpses generated by apoptotic cell death. EMBO J. 15: 226–235. [PMC free article] [PubMed] [Google Scholar]
- Mangahas P. M., Zhou Z., 2005. Clearance of apoptotic cells in Caenorhabditis elegans. Semin. Cell Dev. Biol. 16: 295–306. [DOI] [PubMed] [Google Scholar]
- Mapes J., Chen Y. Z., Kim A., Mitani S., Kang B. H., et al. , 2012. CED-1, CED-7, and TTR-52 regulate surface phosphatidylserine expression on apoptotic and phagocytic cells. Curr. Biol. 22: 1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee C. K., Baehrecke E. H., 2010. The engulfment receptor Draper is required for autophagy during cell death. Autophagy 6: 1192–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan T. L., Kleinsorge S. E., Timmons A. K., Taylor J. D., McCall K., 2015a Polarization of the epithelial layer and apical localization of integrins are required for engulfment of apoptotic cells. Dis. Model. Mech. 8: 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan T. L., Yalonetskaya A., Joudi T. F., McCall K., 2015b Detection of cell death and phagocytosis in the Drosophila ovary. Methods Mol. Biol. 1328: 191–206. [DOI] [PubMed] [Google Scholar]
- Nainu F., Shiratsuchi A., Nakanishi Y., 2017. Induction of apoptosis and subsequent phagocytosis of virus-infected cells as an antiviral mechanism. Front. Immunol. 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J. Q., Zhou R., Czech B., Liu L. P., Holderbaum L., et al. , 2011. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8: 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor R. M., Stone E. F., Wayne C. R., Marcinkevicius E. V., Ulgherait M., et al. , 2017. A Drosophila model of Fragile X syndrome exhibits defects in phagocytosis by innate immune cells. J. Cell Biol. 216: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsó E., Broccardo C., Kaminski W. E., Böttcher A., Liebisch G., et al. , 2000. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat. Genet. 24: 192–196. [DOI] [PubMed] [Google Scholar]
- Perkins L. A., Holderbaum L., Tao R., Hu Y., Sopko R., et al. , 2015. The transgenic RNAi project at Harvard medical school: Resources and validation. Genetics 201: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. S., McCall K., 2013. Combined inhibition of autophagy and caspases fails to prevent developmental nurse cell death in the Drosophila melanogaster ovary. PLoS One 8: e76046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. S., Timmons A. K., Mondragon A. A., McCall K., 2015. The end of the beginning: cell death in the germline. Curr. Top. Dev. Biol. 114: 93–119. [DOI] [PubMed] [Google Scholar]
- Quazi F., Molday R. S., 2011. Lipid transport by mammalian ABC proteins. Essays Biochem. 50: 265–290. [DOI] [PubMed] [Google Scholar]
- Reddien P. W., Cameron S., Horvitz H. R., 2001. Phagocytosis promotes programmed cell death in C. elegans. Nature 412: 198–202. [DOI] [PubMed] [Google Scholar]
- Rees D. C., Johnson E., Lewinson O., 2009. ABC transporters: the power to change. Nat. Rev. Mol. Cell Biol. 10: 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rørth P., 1998. GAL4 in the Drosophila female germline. Mech. Dev. 78: 113–118. [DOI] [PubMed] [Google Scholar]
- Smith J. D., Waelde C., Horwitz A., Zheng P., 2002. Evaluation of the role of phosphatidylserine translocase activity in ABCA1-mediated lipid efflux. J. Biol. Chem. 277: 17797–17803. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., 1993. Developmental genetics of oogenesis, pp. 1–70 in The Development of Drosophila melanogaster, edited by Bate M., Martinez Arias A. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Takahashi K., Kimura Y., Kioka N., Matsuo M., Ueda K., 2006. Purification and ATPase activity of human ABCA1. J. Biol. Chem. 281: 10760–10768. [DOI] [PubMed] [Google Scholar]
- ter Beek J., Guskov A., Slotboom D., 2014. Review structural diversity of ABC transporters. J. Gen. Physiol. 143: 419–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons A. K., Mondragon A. A., Schenkel C. E., Yalonetskaya A., Taylor J. D., et al. , 2016. Phagocytosis genes nonautonomously promote developmental cell death in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 113: E1246–E1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueoka I., Kawashima H., Konishi A., Aoki M., Tanaka R., et al. , 2018. Novel Drosophila model for psychiatric disorders including autism spectrum disorder by targeting of ATP-binding cassette protein A. Exp. Neurol. 300: 51–59. [DOI] [PubMed] [Google Scholar]
- Vasiliou V., Vasiliou K., Nebert D. W., 2009. Human ATP-binding cassette (ABC) transporter family. Hum. Genomics 3: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken K. J. T., Schulze K. L., Haelterman N. A., Pan H., He Y., et al. , 2011. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods 8: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Lan D., Gerbod-Giannone M., Linsel-Nitschke P., Jehle A. W., et al. , 2003. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J. Biol. Chem. 278: 42906–42912. [DOI] [PubMed] [Google Scholar]
- Wang X., Li W., Zhao D., Liu B., Shi Y., et al. , 2010. Caenorhabditis elegans transthyretin-like protein TTR-52 mediates recognition of apoptotic cells by the CED-1 phagocyte receptor. Nat. Cell Biol. 12: 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens S., 2015. Structure and mechanism of ABC transporters. F1000Prime Rep. 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Tanwar P. S., Raftery L. A., 2008. Drosophila follicle cells: morphogenesis in an eggshell. Semin. Cell Dev. Biol. 19: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. C., Horvitz H. R., 1998. The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell 93: 951–960. [DOI] [PubMed] [Google Scholar]
- Yu X., Odera S., Chuang C. H., Lu N., Zhou Z., 2006. C. elegans dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells. Dev. Cell 10: 743–757. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Q., Bailey A. M., Matthies H. J. G., Renden R. B., Smith M. A., et al. , 2001. Drosophila fragile x-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 107: 591–603. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Hartwieg E., Horvitz H. R., 2001. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 104: 43–56. [DOI] [PubMed] [Google Scholar]
- Ziegenfuss, J. S., 2012 Eaters of the dead: how glial cells respond to and engulf degenerating axons in the CNS. Ph.D. Thesis, University of Massachusetts Medical School. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All strains and reagents are available upon request.