Abstract
Using combined genetic mapping, Illumina sequencing, bioinformatics analyses, and experimental validation, we identified 60 essential genes from 104 lethal mutations in two genomic regions of Caenorhabditis elegans totaling ∼14 Mb on chromosome III(mid) and chromosome V(left). Five of the 60 genes had not previously been shown to have lethal phenotypes by RNA interference depletion. By analyzing the regions around the lethal missense mutations, we identified four putative new protein functional domains. Furthermore, functional characterization of the identified essential genes shows that most are enzymes, including helicases, tRNA synthetases, and kinases in addition to ribosomal proteins. Gene Ontology analysis indicated that essential genes often encode for enzymes that conduct nucleic acid binding activities during fundamental processes, such as intracellular DNA replication, transcription, and translation. Analysis of essential gene shows that they have fewer paralogs, encode proteins that are in protein interaction hubs, and are highly expressed relative to nonessential genes. All these essential gene traits in C. elegans are consistent with those of human disease genes. Most human orthologs (90%) of the essential genes in this study are related to human diseases. Therefore, functional characterization of essential genes underlines their importance as proxies for understanding the biological functions of human disease genes.
Keywords: essential gene, lethal, genetic balancer, whole genome sequencing (WGS), functional characterization
Genes that are required for survival, or genes that strongly contribute to fitness and robust competitive growth are essential genes (Gerdes et al. 2006). In humans, it has been demonstrated that mutations in essential genes contribute to a spectrum of human diseases, from developmental diseases (often resulting in spontaneous abortions) to a range of cancers, including brain and breast cancers (Dickerson et al. 2011). Knowing what essential genes are and how they work will allow researchers to develop a deeper understanding of the molecular nature of such diseases. Identification of essential genes should also help to determine the minimal gene set, which is the smallest group of genes that are necessary and sufficient to sustain a functioning cell or organism under the most favorable conditions [reviewed in Koonin (2000)]. Thus, identifying essential genes will further our understanding of the basics of cell functioning.
Studies of essential genes are ubiquitous in model organisms. In mouse models using individual knockout strains, ∼2000 genes have been classified as essential. The deletion of these genes leads to either lethality before reproduction, or to sterility (Liao and Zhang 2007). In zebrafish, ∼390 genes have been identified through mutagenesis screens as essential for embryonic and early larval development (Amsterdam et al. 2004). In C. elegans, genome-wide functional analysis, using RNAi, indicates that at least 1170 genes are essential (Kamath et al. 2003). Although RNAi is an effective high-throughput technology for identifying essential genes, this technology has limitations that prevent it from being used to identify all essential genes in C. elegans (Kamath et al. 2003).
Genome-wide forward genetic screens are capable of isolating genes that play important roles in given phenotypes, including lethality. For over 35 yr, researchers have taken advantage of genetic balancers and forward genetic screens to isolate lethal mutations and identify essential genes in C. elegans. Using traditional genetic methods, including genetic mapping and transgenic rescue assays, many essential genes have been identified. However, due to the limitations of traditional genetic methods, the process of identifying essential genes is slow.
Here we used whole genome sequencing (WGS) technology to speed up the identification of essential genes in C. elegans mutants isolated from genetic screens (Rosenbluth and Baillie 1981; Rosenbluth et al. 1983, 1985; Johnsen and Baillie 1991; Stewart et al. 1998). Janke et al. (1997) was a harbinger of our work when they used sequenced cosmids to rescue lethal phenotypes on chromosome III and thus molecularly identify essential genes. In C. elegans, WGS is capable of capturing molecular lesions that were induced by ethyl methane sulfonate (EMS) mutagenesis in both homozygous (Sarin et al. 2008; Rose et al. 2010) and heterozygous backgrounds (Chu et al. 2012, 2014). Along with bioinformatics analysis, different types of mutations can be detected, such as base pair alterations, direct repeat sequences, and deletions, as well as insertions of transposable elements (Rose et al. 2010). However, to decide which mutation is responsible for a lethal phenotype, genetic mapping evidence, used for assigning sequenced lethal mutations into individual genetic mapping zones, proved to be indispensable. The mapping zones were defined via complementation tests between sets of overlapping deficiencies and duplications. Once zones were established lethals were complementation tested against those rearrangements to determine which zone each lethal fell into. After that, the lethals were complementation tested against lethals in the same zone to determine if they were allelic or newly identified genes (Rosenbluth and Baillie 1981; Rosenbluth et al. 1988; Johnsen and Baillie 1991; Stewart et al. 1998). Since the breakpoints of several deficiencies on both chromosome III(mid) and chromosome V(left) have been precisely located in a physical map, the boundaries of the corresponding genetic mapping zones are also well defined (Jones et al. 2007, 2009) thus narrowing considerably the molecular regions where the genes must reside. Methods such as single nucleotide polymorphism mapping (Davis et al. 2005) or a recently devised molecular inversion probes–based method (Mok et al. 2017) are necessary when the approximate position of the mutations are not known. In our case, with well-mapped mutations, the bioinformatics analysis needed to discover the exact positions of the recovered lethal lesions is simpler.
Homozygous mutations have a 100% allelic ratio, but lethal mutations need to be maintained as heterozygotes, which increases the difficulty of identifying lethal mutations. Furthermore, different balancer systems give rise to different heterozygous allelic ratios. For example, there are two copies of a recessive lethal mutation in a duplication-balanced region and one wild-type allele on the balancer. As a result, the allele frequency is two mutations to one wild type, which should result in a 66.7% variation frequency in the sequencing data. For translocations, only one copy of a lethal allele and one copy of a wild-type allele are present, and the variation frequency should be 50%. It has been demonstrated that duplication balanced essential genes can be identified when there is a 2:1 mutation-to-wild type allele ratio (Chu et al. 2012, 2014); however, when the ratio is 1:1 (using a translocation balancer), the identification becomes more difficult. In this study, we used genetic mapping data, WGS techniques, bioinformatics analyses, and experimental validation, to identify 60 essential genes from 104 lethal mutations in C. elegans. This was followed by functional characterization of those essential genes. We also studied the relationship between gene essentiality and gene duplicability, protein connectivity, gene expression, and showed that compared with nonessential genes, essential genes have fewer paralogs, encode proteins that are in interaction hubs, and are more highly expressed. The essential genes we identified provide a rich resource for future studies. The conserved essential genes should also prove useful for understanding the functions of homologous genes in human, especially disease-related genes. In addition, species-specific essential genes could be good candidates for targeting pathogenic nematodes.
Materials and Methods
C. elegans strains
All the strains that were submitted for sequencing and for complementation tests are included in the Supplemental Material, Table S2, Table S3, and Table S4 in File S1.
WGS and computational analysis
Worms were grown on large (100 by 15 mm) Petri plates until the worms starved or the plates were covered in healthy gravid adults. We rinsed the worms off the plates at room temperature with M9 buffer (6 g Na2HPO4, 3 g KH2PO4, 5 g NaCl, 0.2 g MgSO4 in 1 liter of H20) into 15 ml polypropylene tubes, and pelleted them by centrifuging at 1500 rpm for 2 min at 4°. We then washed them in 12 ml iced M9 buffer, for three times. The worms were placed on a rocker for 2–3 hr at room temperature to digest bacteria. Worms were pelleted at 1500 rpm for 2 min at 4°. Then, the supernatant was removed and the worms were washed and pelleted in 4 ml room temperature M9 buffer. The pellets were frozen at −80°.
The genomic DNA collection for Illumina sequencing was constructed using the QIAGEN DNeasy Blood & Tissue Kit (cat. no. 69504), following the protocol “Purification of Total DNA from Animal Tissues (Spin-Column Protocol).” DNA concentrations were determined using the Qubit dsDNA High Sensitivity Assay Kit performed with the Qubit 2.0 Fluorometer (Life Technologies). The purity of the DNA was assessed using the Nanodrop ND-1000 Spectrophotometer-T* (Thermo Scientific). For each sample, 0.6–2 μg of purified genomic DNA was submitted for sequencing at the British Columbia Cancer Agency Canada’s Michael Smith Genome Sciences Centre using the Illumina PET HiSeq technology.
WGS data analysis
For chromosome III, genomic DNA libraries of 49 strains were prepared and sequenced using Illumina PET HiSeq to generate 100-bp-long paired end reads. BWA (Li and Durbin 2009), GATK (McKenna et al. 2010), and SAMtools (Li et al. 2009) were used to align the reads to the C. elegans reference genome (WS246) and called for variants. For chromosome V, the sequencing reads were first aligned with the C. elegans reference genome using BWA with default settings. WormBase WS249 was used as reference genome for the alignment. This step was followed by filtering out PCR-caused duplicates by using SAMtools (Li et al. 2009), which was also used for analyzing the sequencing depth of each strain. The breakpoints of large deletions, medium insertions, and translocations were detected and viewed using the Integrative Genomics Viewer (Robinson et al. 2011; Thorvaldsdottir et al. 2013). The effect of the variations on each CDS in the genome was analyzed using CooVar (Vergara et al. 2012).
PCR sequencing and the rescue assay
Primers were designed using Primer3 (Koressaar and Remm 2007; Untergasser et al. 2012) to amplify the coding sequences of each candidate essential gene. PCR products were subjected to PCR purification (QIAGEN MinElute PCR Purification Kit, cat. no. 28004) or gel purification (QIAGEN QIAquick Gel Extraction Kit, cat. no. 28704) before they were sequenced using the Sanger technique. The same primers that were used in PCR amplification were also used for Sanger sequencing. The sequencing results were analyzed using the software SeqMan (DNASTAR).
Fosmid DNA was prepared using the Pharmacia Mini-Prep Kit Plus (minus the final column purification step) and was diluted in ddH20. Primers were designed and PCR amplifications were performed to validate the presence of the candidate genes in the tested fosmids and their absence in the negative control fosmids.
The transgenic strains constructed for this research used the coinjection semidominant marker rol-6(su1006) (Kramer et al. 1990) in plasmid pCes1943 and another co-injected GFP marker Pmyo-2::GFP, which is expressed in the pharyngeal muscle (Dibb et al. 1989). DNA mixtures that contain 2 ng fosmid DNA, 80 ng rol-6(su1006), and 2.5 ng Pmyo-2::GFP were directly injected into the syncytial gonad of young adults of the mutant hermaphrodites (Mello et al. 1991). The presence of DpyUnc F2 progeny with coinjected markers indicated successful rescue.
Interallelic complementation tests
Complementation tests were carried out for genetic validation of the candidate essential genes. For lethals balanced by sDp3, Unc-32 hermaphrodites were crossed to N2 males. Phenotypically wild-type F1 males were then crossed to another lethal with the same genetic background. The presence of fertile DpyUnc F2 hermaphrodites indicates complementation, while the absence of fertile DpyUnc F2 hermaphrodites indicates failure to complement (Stewart et al. 1998). For mutants balanced with eT1, phenotypically wild-type males containing a lethal mutation on chromosome V were crossed to another eT1 balanced lethal. The presence of fertile DpyUnc F1 progeny indicates complementation, while the absence of fertile DpyUnc progeny indicates failure to complement. In some cases, late blocking, sterile, or maternal effect individual F1s were set up in order to observe the terminal phenotypes (Johnsen and Baillie 1991).
Essential genes functional analyses
Protein domain analysis:
A known annotated domain for each protein was searched with InterProScan (Mulder and Apweiler 2007) using the Pfam database (Finn et al. 2014) and visualized with the Perl module FeatureStack (Frech et al. 2012). Identification of orthologs of C. elegans proteins was conducted using InParanoid (Remm et al. 2001) in 25 other nematode species and four model organisms: Homo sapiens, Drosophila melanogaster, Mus musculus, and Danio rerio. This was followed by multiple sequence alignments with ClustalX2 (Thompson et al. 1997; Larkin et al. 2007) between C. elegans proteins and their orthologs. The alignment was examined using Jalview version 2 with the “ClustalX” color scheme.
Gene Ontology analysis:
Gene Ontology (GO) was performed using the PANTHER classification system (Mi et al. 2013) from the website http://pantherdb.org/. Three GO term categories (cellular component, biological process, and molecular function) were examined individually.
Gene duplicability:
An all-against-all BLASTP search was conducted for the whole set of C. elegans proteins (WS250). Only the longest isoform was used if there are multiples transcripts of a gene. This was followed by the computation of the global PID. Only protein pairs that had a PID equal to or higher than 50% were kept and sorted by e-value.
Protein connectivity:
The whole genome protein interactions in C. elegans were downloaded from BioGRID (Stark et al. 2006), which is an interaction repository with data compiled through comprehensive curation efforts. BioGRID currently holds >8037 nonredundant interactions in C. elegans with 3949 unique genes that were derived from 205 publications, including both physical and genetic evidence. This was followed by the filtration of proteins that are from one of four groups.
Gene expression:
Gene expression data were downloaded through the GExplore (version 1.4) expression search interface (Hutter et al. 2009), which includes a series of C. elegans developmental stages from parts of the NHGRI modENCODE project (Hillier et al. 2009; Gerstein et al. 2010). The data were derived from synchronized whole animals from embryonic and postembryonic stages, followed by RNA-seq.
Data availability
Strains are available upon request. Table S1 in File S1 contains detailed information of all identified essential genes and lethal mutations. Table S2 in File S1 includes a total of 86 strains with eT1(III;V) that were sequenced. Table S3 in File S1 contains a total of 49 strains with sDp3 sequenced. Table S4 in File S1 includes all the strains used in the complementation tests. The WGS data of all 135 sequenced strains are publicly available on the NCBI with the BioProject accession number PRJNA416306.
Results
Balancer systems
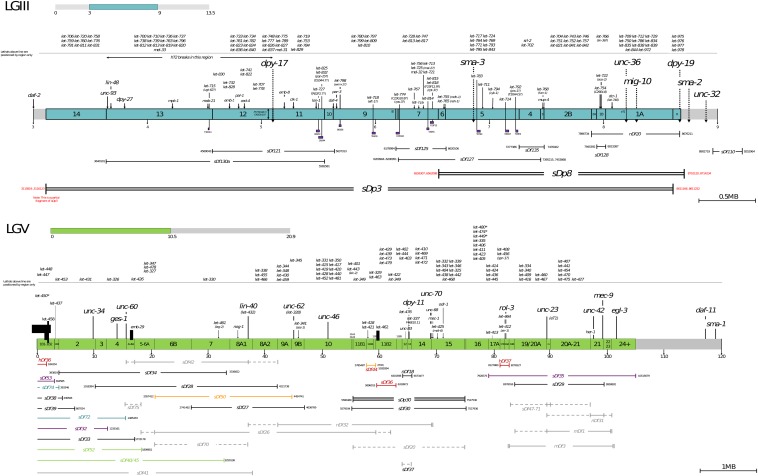
Lethal mutations isolated from two balancers systems were used in our study: dpy-18(e364)/eT1(III); unc-46(e177)/eT1(V) and dpy-17(e164) unc-32(e189)/dpy-17(e164) lin-12(n941) III;sDp3 (III;f). The first, the eT1-system, is a reciprocal translocation between the right half of chromosome III [LGIII(right)] and the left half of chromosome V [LGV(left)], recombinationally balancing those two regions (Rosenbluth and Baillie 1981), which is ∼20% of C. elegans’ genome. LGV(left) contains ∼7% (23 map units) of the recombination distance in the genome and ∼10% of its DNA. LGV(left) has been subdivided into 22 main zones and several additional subzones by sets of overlapping rearrangements (deficiencies and one duplication) (see Figure 1 courtesy of M. Jones) (Johnsen and Baillie 1988, 1991; Rosenbluth et al. 1988; Clark et al. 1990; Stewart et al. 1991). Over 120 nonessential (Edgley and Riddle 1990) and essential genes have been mapped to the zones in LGV(left), making it a genetically well-defined region. A number of different types of mutagens have been characterized in the eT1-system including formaldehyde (Johnsen and Baillie 1988), ultraviolet irradiation (UV) (Stewart et al. 1991), the transposable element Tc1 (Clark et al. 1990), and also EMS, which induces primarily point mutations (Rosenbluth et al. 1983; Johnsen and Baillie 1991), and other mutations that generally induce rearrangements γ-irradiation (Rosenbluth et al. 1985). dpy-18(e364)/eT1(III); unc-46(e177)/eT1(V) is phenotypically wild type; pseudolinked dpy-18; unc-46 have a dumpy uncoordinated (Dpy-18 Unc-46) phenotype while eT1(III) breaks in unc-36, thus giving homozygous eT1 a visible Unc-36 (uncoordinated) phenotype. Screening for the absence of fertile Dpy-18 Unc-46 indicates a lethal mutation in the balanced regions, after which the lethal mutations can be mapped to either LGIII or LGV. Those mutations on LGV can be complementation tested against the set of overlapping rearrangement to determine the zone in which the lethal mutation lies. Further complementation tests against genes in that zone determine which gene the mutation is allelic to.
Figure 1.
Physical deficiency map of LGIII(mid) and LGV(left). The top figure shows the middle region (4.5 Mb) of chromosome III that is balanced by a free duplication (sDp3), which has been subdivided into 14 main zones. The bottom figure shows the left half of chromosome V that is balanced by a reciprocal translocation (eT1), which has been subdivided into 22 main zones and several additional subzones by sets of overlapping rearrangements (deficiencies and one duplication).
The second system (Stewart et al. 1998) uses a free duplication (sDp3) (which does not recombine with the normal LGIII chromosome) to balance a 4.5 Mb region of LGIII (see Figure 1). The sDp3 system consists of dpy-17(e164) unc-32(e189)/dpy-17(e164) lin-12(n941) III;sDp3 (III;f) in which sDp3 covers dpy-17 (which has a dumpy phenotype) (lin-12 is also covered by sDp3 and has a visible phenotype) but not unc-32 (which has an uncoordinated phenotype), so when the duplication is present over dpy-17(e164) unc-32(e189), the phenotype is Unc-32 but when absent it is Dpy-17 Unc-32. dpy-17(e164) unc-32(e189)/dpy-17(e164) lin-12(n941) III;sDp3 (III;f) (which has a wild-type phenotype) was mutagenized and the F2 were screened for the absence of Dpy-17 Unc-32 progeny, indicating a putative lethal mutation in the sDp3 balanced region on the dpy-17(e164) unc-32(e189) marked chromosome, i.e., dpy-17(e164) let-x unc-32(e189)/dpy-17(e164) let-x unc-32(e189) III;sDp3 (III;f). Strains were maintained as homozygotes with sDp3 by picking Unc-32. The sDp3 balanced region has also been divided into zones by a set of overlapping rearrangements. Stewart et al. (1998) reported 112 essential genes in the sDp3 balanced region. A difference between the two systems is that in the eT1-system, the lethals were isolated as heterozygotes whereas in the sDp3-system the lethals were picked up as homozygotes covered by duplication. We did not note any obvious differences in the types of genes isolated by the two systems.
Identification of genomic variations in chromosome III mutations balanced by sDp3
Genomic DNA libraries for 49 lethals (dpy-17(e164) let-x unc-32(e189);sDp3 (III;f)) were prepared and sequenced using Illumina PET HiSeq to generate 100 bp paired end reads. We used BWA (Li and Durbin 2009), GATK (McKenna et al. 2010), and SAMtools (Li et al. 2009) to align the reads to a C. elegans reference genome (WS246) and called for variants. The average coverage for the 49 strains was 25×, with the lowest being 12× and the highest being 38×. The lethal mutations on chromosome III were balanced by sDp3 and thus we expected the variant frequencies to be ∼67%. Single nucleotide variations (SNVs) that occurred with variant frequencies between 40 and 90% were selected from the 49 strains and subjected to three filtration steps. First, mutations found as part of the Million Mutation Project (MMP) are presumptively false positives, since the way MMP was designed should drastically reduce but not eliminate the chances of capturing lethal mutations (Flibotte et al. 2010; Thompson et al. 2013). One possible way the MMP could capture a lethal mutations is to capture a corresponding suppressor, but our initial assumption is “presumptive false positives.” Second, some variations (background variations) could stem from the starting strain that was used for mutagenesis. To be cost-effective, we did not sequence the starting strain because the majority of the sDp3 balanced strains stemmed from it, thus they shared the same genetic background; instead, we assumed all variations that occurred multiple times in the 49 sequenced strained originated in the starting strain and excluded them. Third, we required the variations to be supported by at least eight reads in both forward and reverse directions. After the three steps of filtration the remaining SNVs were subjected to further lethal mutation identification Table 1.
Table 1. Average number of SNVs per strain with 20–100% variation frequency in the eT1(V) balanced region, before and after the removal of background variations.
| Before Removal of Background Mutations/Strain | After Removal of Background Mutations/Strain | |
|---|---|---|
| Chromosome V eT1 | 1357 | 72 |
| Genetic coding region | 282 | 13 |
Identification of genomic variations in eT1 balanced chromosome V mutations
Before identifying the genomic variations in the eT1 balanced mutations, there was a technical challenge that we needed to address. The progeny of these mutants include both phenotypically wild-type animals, which are dpy-18(e364)/eT1(III); let-x unc-46(e177)/eT1(V), and Unc-36 eT1(III;V) animals (because eT1 breaks in unc-36). These homozygous Unc-36 worms are fertile and contribute to the total sequenced genomic DNA, which makes the allelic ratios of lethal mutations very difficult to predict and effectively impossible to analyze (D. L. Baillie, unpublished data). An important step is to drastically minimize the contribution of eT1 DNA so that the majority of sequenced DNA comes from dpy-18(e364)/eT1(III); let-x unc-46(e177)/eT1(V), allowing the allelic ratio of lethal mutations to approach 50% and thus facilitate the identification of lethal mutations. To do this, we constructed new strains for each mutant using let-500(s2165), which is located on the eT1 balancer resulting in dpy-18(e364)/eT1(III); unc-46(e177) let-x/let-500(s2165) eT1(V). let-500(s2165) blocks development early and thus drastically reduces the contribution of homozygous eT1 DNA to our sequence analyzes. This step proved extremely effective and useful for the identification of heterozygous mutations.
Genomic DNA libraries for 86 mutants were prepared and sequenced using Illumina PET HiSeq to generate 150 bp paired end reads. BWA (Li and Durbin 2009) and SAMtools (Li et al. 2009) were used to align the paired end reads to the WS249 C. elegans reference genome and postalignment processing analysis, respectively. This yielded an average of 31-fold genome coverage per sample (lowest coverage 23-fold, highest coverage 44-fold). We then used VarScan2 (Koboldt et al. 2012) to identify SNVs that occurred with variant frequencies between 20 and 100%, which was followed by three filtration steps as used in the identification of variations in the sDp3 balanced region. After filtration, the numbers of remaining SNVs are summarized in Table 1.
The 86 sequenced strains have four different visible markers. Two (dpy-18(e364)III and unc-46(e177)V) were molecularly identified before and successfully validated in this study. Both those visible markers are heterozygous and balanced by eT1(III;V), and the sequencing data showed average variation frequencies of e364 and e177 are 50 and 51%, respectively. Our analysis also identified the previously unknown mutations in the other two eT1(III;V) balanced visible markers (unc-42(e270)V and unc-60(e677)V). We curated their mutation types and genomic positions: e270 (A->T at position 976,514) causes a valine to glutamic acid change in four isoforms of unc-42; and e677 (G->A at position 1,476,888) causes an amino acid change from glycine to arginine, which inhibits the normal function of the muscle-specific isoform UNC-60B.
Identification of essential genes and experimental validation
Using the identified genomic variations, we performed essential gene identification and experimental validation for both chromosome III(mid) and chromosome V(left).
For the chromosome III(mid) dataset, 49 mutants with 49 lethal mutations representing 43 genes were sequenced. Six genes had two sequenced alleles while 37 genes had single alleles sequenced. The 49 mutations had previously been assigned to 14 genetic mapping zones (Table S3 in File S1). The 86 mutants from chromosome V(left) had previously been mapped to 23 zones (Table S2 in File S1), representing 46 genes. Two alleles were sequenced for 40 of the 46 genes while only six genes had a single allele sequenced. In total, there are 89 essential genes from chromosome III(mid) and chromosome V(left) that need to be identified molecularly.
The molecular identification of essential genes on chromosome III(mid) and chromosome V(left) was determined by using five lines of evidence. First, previous genetic mapping data narrowed down the location of the lethal mutation for each strain (Rosenbluth and Baillie 1981; Rosenbluth et al. 1988; Johnsen and Baillie 1991; Stewart et al. 1998). Second, bioinformatics analyses identified a limited number of variations in the genetically determined regions (Table 1). Third, there are two independent sequenced alleles for most of the genes analyzed, which not only makes genomic identification easier but the result for each allele provides validation for the other allele. Fourth, we used WormBase information about lethal phenotypes, including those supported by RNAi (www.wormbase.org), to further narrow our search for variations in our sequences. Last, the MMP dataset was used to help verify possible essential genes. In MMP, 2007 strains were subjected to mutagenesis and >800,000 different mutations were recovered in 20,115 genes. The large number of variations provides an unprecedented genetic resource for C. elegans research. However, during the MMP genetic screens only mutants with nonlethal phenotypes were isolated, and so most chain-terminating mutations such as the majority of nonsense mutations or splicing mutations in essential genes would be selected against (Thompson et al. 2013). In general, only missense mutations that do not cause malfunctioning proteins would be recovered. An essential gene example is the ribosomal large subunit rpl-22, for which MMP recovers only missense mutations. Using this hypothesis, we checked candidate genes by searching the MMP database for lack of chain-terminating mutations. With the above methodology we identified, with high confidence, 62 out of 89 sequenced essential genes in the chromosome III and chromosome V balanced regions. The majority of genes with only one allele identified on chromosome III had secondary support by lethal alleles from other laboratories, published RNAi analysis, or PCR results (Table 2 and Table S1 in File S1). In the case of let-786, sequencing led to two candidate genes: prp-8 and unc-116. Two alleles of let-712 (s2439 and s2598) are in prp-8. Both let-712 alleles complement let-786(s2631), therefore let-786 is not prp-8. unc-116(rh24) is lethal, therefore unc-116 is a lethal gene and we concluded that unc-116 and let-786 are likely the same gene.
Table 2. The number of essential genes identified in this study.
| Essential Genes Sequenced | Allele Sequenced | Genes Identified | Total Essential Genes Identified |
|---|---|---|---|
| Chromosome III (mid/sDp3): 43 genes | 37 genes with single allele sequenced | 21 of 37 genes identified | 27 of 43 genes |
| Six genes with two alleles sequenced | Six of six genes identified | ||
| Chromosome V (left/eT1): 46 genes | Six genes with single allele sequenced | Five of six genes identified | 35 of 46 genes |
| 40 genes with two alleles sequenced | 30 of 40 genes identified | ||
| Total: 89 genes | 62 (60 mapped molecularly) |
Fourteen genes were confirmed by experiments using PCR sequencing, rescue assay, and complementation tests. In the chromosome III and chromosome V balanced regions, 37 and 4, respectively, have only one Illumina sequenced allele. For five genes on III and four on V, there were multiple variations in different candidate genes in the zones they were mapped to. To test the candidate genes, we took advantage of the second alleles of those genes (Johnsen and Baillie 1991; Stewart et al. 1998). We PCR-amplified the candidate genes using genomic DNA of a strain with the second allele, which was followed by Sanger sequencing both ends of the PCR fragments. Using this method, we were able to identify five genes on chromosome III and four on chromosome V.
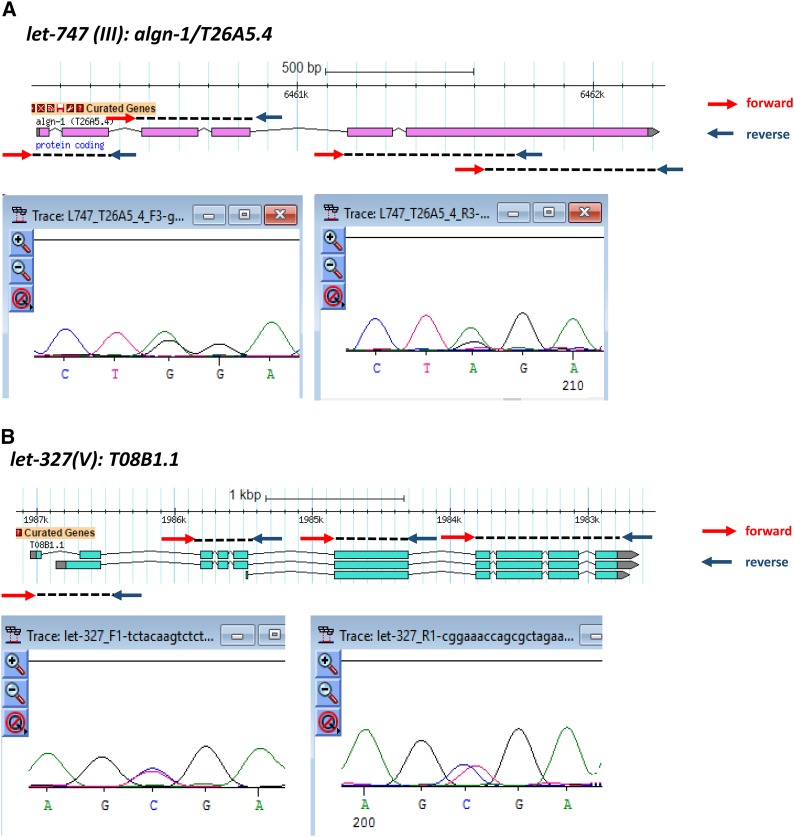
Figure 2 includes two examples of validated genes, one from chromosome III and the other from chromosome V, which were successfully validated. Due to the heterozygosity of the lethal mutation balanced by duplications or translocations, one would expect to see overlapping sequencing peaks for both the wild-type allele and mutation allele at the same position. Because duplication balancers cover two lethal alleles over one wild-type allele, we see the height of the sequence peak of the mutant allele is slightly higher than the wild-type allele. However, the displayed nucleotide can be the wild-type allele or the mutant allele when Sanger sequencing was performed from both ends of the PCR products (Figure 2A). For translocations, the ratio of mutant allele to wild-type allele is 1:1, therefore the sequencing peaks from the two alleles have identical heights. However, the displayed nucleotide for let-327 is the wild-type allele when sequenced from both strands (Figure 2B). In either case, considering that the relative abundances of the wild-type allele and mutated allele are not easily distinguishable in heterozygotes, the lethal mutations could be easily missed.
Figure 2.
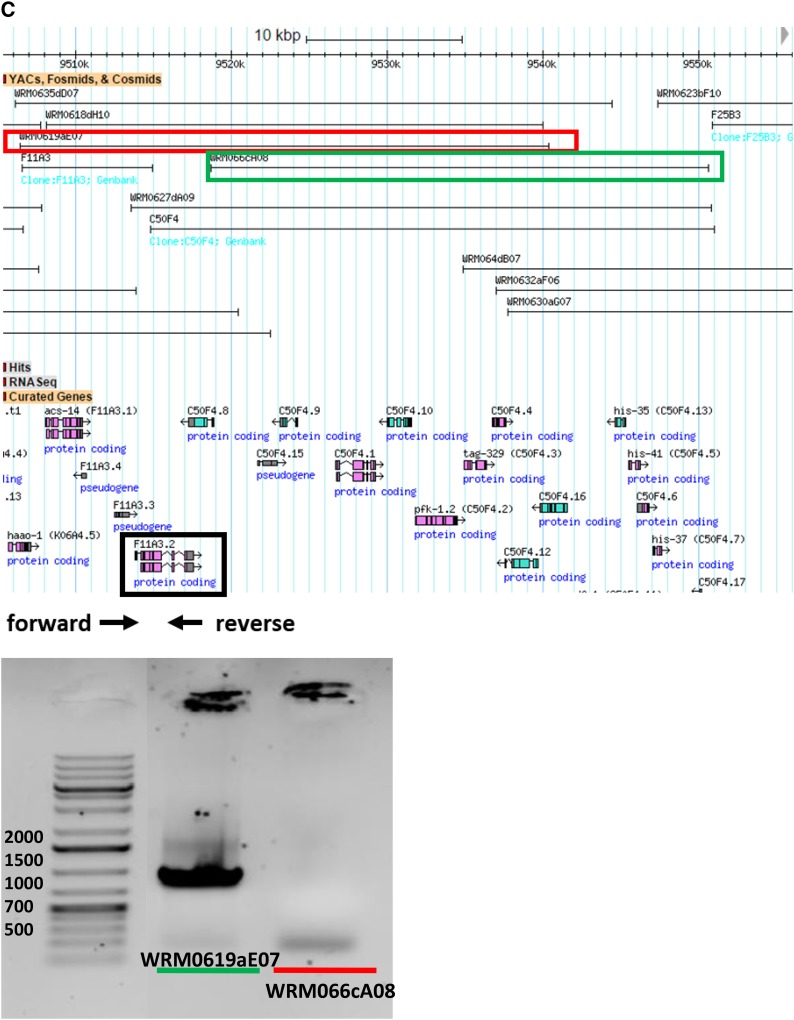
Three examples of PCR sequencing validation and rescue assay. (A) Gene validation of algn-1/T26AA5.4 for let-747 on chromosome III. (B) Gene validation of T08B1.1 for let-327 on chromosome V. The gene model in each figure shows that the designed primers cover all coding exons of each gene. The red arrows are forward primers while the arrows in dark blue are reverse primers. Under each gene model, there are partials of Sanger sequencing results using forward and reverse PCR primers as sequencing primers. For let-747/algn-1/T26AA5.4, a point mutation (G->A) was found in the last exon causing a premature stop codon. A C->T point mutation was found in the second exon of T08B1.1a and the first exon of T08B1.1b. A premature stop codon occurred in both T08B1.1 transcripts because of this mutation. (C) Fosmids used for the rescue assay. The fosmid (WRM0619aE07) containing the candidate gene, F11A3.2, is in the red box while WRM066cA08 without F11A3.2 is in the green box. F11A3.2 is in the black box with the designed forward primer and reverse primer underneath. The gel image shows the presence of F11A3.2 in WRM0619aE07 with a target band of 794 bp and the absence of F11A3.2 in WRM066cA08 without the 794 bp PCR product. The weak band of ∼200 bp might be a nonspecific band because it can be found in both products using two fosmids as template.
No mutation was identified in the zones containing let-470(s1581) and let-470(s1629). However, let-470(s1581) has only F11A3.2 as a candidate essential gene, with a splicing acceptor mutation besides the zone boundary. To test if F11A3.2 is let-470(s1629), we performed rescue assay using two different fosmids. The result showed that the fosmids containing the wild-type copy of F11A3.2 rescues the lethal phenotype of let-470(s1629) while the fosmids without the candidate gene did not rescue the lethal phenotype. Figure 2C shows the genomic locations of two fosmids that were used for the rescue assay. PCR was also conducted to confirm the presence or absence of the candidate gene in the two fosmids.
Complementation tests were carried out on four genes. let-459(s1615) contains a mutation that changes the splicing signal, causing a frame shift and premature stop codon in hpo-18/F32D1.2. WGS analysis did not find a corroborating mutation in the let-459(s1432) sequence. A knockout allele hpo-18(ok3436) is lethal. We did complementation tests between hpo-18(ok3436) and both let-459(s1432) and let-459(1615). Both tests showed failure to complement, which confirmed that hpo-18 and let-459 are the same gene. Complementation tests also showed that let-447 and egl-8 are the same gene. The phenotype of egl-8(md1971) includes reduced locomotion, reduced body flexion at rest, and loopy backing. Both let-447(s1664)/egl-8(md1971) and let-447(s1457)/egl-8(md1971) have the egl-8(md1971) phenotype, and therefore failure to complement. Strains used for complementation tests are summarized in Table S4 in File S1.
Complementation tests were used to recheck genes whose mutations were found in genes with different “let” names. let-439 has two sequenced alleles and both of them have mutations in M03F8.3, showing that it is let-439. However, let-443(s1417) had previously been mapped to a different zone than let-439, but contained a nonsense mutation in M03F8.3. We complementation-tested let-443(s1417) against both alleles of let-439 and they failed to complement, which supported the sequencing result; therefore, we renamed let-443(s1417) let-439(s1417). Complementation tests also showed that both let-757(s2460) and let-757(s2867) fail to complement let-821(s2804) and sequence analysis showed them to be fbn-1 (ZK783.1). These two results reduce the number of genes from 62 to 60, so 60 identified genes have been mapped to their molecular counterpart (Table 2). In addition, a total of 104 lethal mutations (from 135 sequenced strains) were molecularly discovered in 60 essential genes with 41 missense mutations and 39 nonsense mutations (Table S1 in File S1). We did not identify any lethal mutations in the other 31 sequenced strains.
All these cases show the value of combined genetic and sequencing analyses because they not only help with narrowing down the search region for lethal mutations, but also aid in correcting and improving the quality of genetic mapping.
We checked the 60 genes on WormBase for lethal alleles (including gk, tm, and ok alleles) that were presented in previous publications. We summarized the genes into three categories and details of each gene are listed in the last column of Table S1 in File S1. The first category is genes published with lethal alleles (n = 12; two with only tm alleles, these are highlighted in green). Five of those genes, unc-116, lin-13, egl-8, sos-1, and mig-6, were published prior to Johnsen and Baillie (1991) and Stewart et al. (1998). See Stewart et al. (1998) for let-786 and let-752 and Johnsen and Baillie (1991) for let-447, let-344 and let-423. Therefore, let-786 is unc-116, let-752 is lin-13, let-447 is egl-8, let-344 is sos-1, and let-423 is mig-6. The other seven were first published in Stewart et al. (1998) and Johnsen and Baillie (1991) and therefore the “let” names in those publications take precedent. The second category is genes that have known lethal alleles that have not been curated and characterized (n = 18; all of the alleles listed are gk, tm, or ok; these are black in the table. Their correct names were first published in Stewart et al. (1998) or Johnsen and Baillie (1991). The third category is genes that have no alleles in WormBase (n = 30; highlighted in red in the table). Their gene names are published in Stewart et al. (1998) or Johnsen and Baillie (1991).
Functions of the identified 60 essential genes
We looked at the functions of the 60 essential genes that we identified. Most of these genes, based on orthologous relationships, have biological functions that have been predicted in other organisms. Among the 60 essential genes, 22 encode enzymes, including helicases, kinases, and tRNA synthetases. There are also structure proteins including ribosomal proteins and transcription factors, as well as mRNA splicing factors. Note that seven of our essential genes have no known functions. Five of these seven genes have orthologs in nematodes but no other organisms.
We took advantage of WormBase to investigate the evolutionary conservation of each gene by looking for orthologs in Nematodes (N), Invertebrate (D. melanogaster) (I), Mammals (mouse as well as human) (M), and single-cell Fungi (Saccharomycetaceae) (F), as shown in Table 3.
Table 3. Biological functions of the 60 essential genes.
| let-Name | Chr | Gene Name | Protein Function | Pathway | Evolutionary Conservation |
|---|---|---|---|---|---|
| let-727 | III | R02F2.7 | Unknown | Unknown | N |
| let-728 | III | C23G10.8 | Unknown | Unknown | N |
| let-752 | III | lin-13 | Zinc-regulated transcription factor | In C. elegans, LIN-13 is involved in the tumor suppressor Rb-mediated transcriptional control process that leads to repression of vulval fates. | N |
| let-798 | III | wrm-1 | C. elegans β-catenin-like protein | In C. elegans, wrm-1 functions in noncanonical Wnt signaling pathways that specify cell fates in the early embryo. | N |
| let-342 | V | pmt-2 | Enzyme: phosphoethanolamine N-methyltransferase | PMT-2 lacks known mammalian orthologs, but has orthologs in the parasitic nematodes: fish, plants, and bacteria. PMT-1 only catalyzes the conversion of phosphoethanolamine to phospho-monomethylethanolamine, which is the first step in the PEAMT-pathway. | N |
| let-428 | V | K03B4.1 | Unknown | Unknown | N |
| let-455 | V | Y45G5AM.9 | Unknown | Unknown | N |
| let-463 | V | C04E6.11 | Unknown | Unknown | N |
| let-757/let-821 | III | fbn-1 | Extracellular matrix protein fibrillin | Fibrillin is a glycoprotein, which is essential for the formation of elastic fibers found in connective tissues. | N, I, M |
| let-782 | III | tag-189 | Unknown | Unknown | N, I, M |
| let-827 | III | cee-1 | Unknown | Unknown | N, I, M |
| let-327 | V | T08B1.1 | Member of solute carriers family | Predicted to have transmembrane transporter activity. | N, I, M |
| let-344 | V | sos-1 | Son of sevenless homolog | Its ortholog in mouse is a catalytic component of a trimeric complex that participates in transduction of signals from Ras to Rac by promoting the Rac-specific guanine nucleotide exchange factor activity. SOS-1 is involved in multiple Ras-dependent signaling pathways, which also interacts with LET-23 and LET-60 during vulval development. | N, I, M |
| let-348 | V | rft-1 | Member of solute carriers family, riboflavin transporter | In C. elegans, rft-1 exhibits riboflavin transporter activity and is involved in embryo development and receptor-mediated endocytosis. | N, I, M |
| let-423 | V | mig-6 | Highly similar to the extracellular matrix proteins papilin and lacunin | In C. elegans, MIG-6 activity is required for the ventral to dorsal phase of distal tip cell migration. | N, I, M |
| let-440 | V | ncx-2 | Sodium/calcium exchangers | Mediates the electrogenic exchange of Ca2+ against Na+ ions across the cell membrane, thereby contributes to the regulation of cytoplasmic Ca2+ levels and Ca2+-dependent cellular processes. | N, I, M |
| let-702 | III | hmgr-1 | Enzyme: 3-hydroxy-3-methylglutaryl-coenzyme A reductase | HMG-CoA reductase catalyzes the conversion of HMG-CoA to mevalonate, which is a rate-limiting step in sterol biosynthesis. | N, I, M, F |
| let-712 | III | prp-8 | mRNA splicing: pre-mRNA-processing-splicing factor | Its ortholog in yeast is a component of the U4/U6-U5 snRNP complex and participates in spliceosomal assembly through its interaction with the U1 snRNA. | N, I, M, F |
| let-732 | III | rpb-2 | Enzyme: RNA polymerase II (B) subunit | The second largest subunit B150 of RNA polymerase II is the enzyme that produces the primary transcript. | N, I, M, F |
| let-736 | III | cdk-12 | Enzyme: cyclin-dependent kinase | Its ortholog in yeast phosphorylates both the RNA pol II subunit to affect transcription, and the ribosomal protein to increase translational fidelity. | N, I, M, F |
| let-741 | III | rars-1 | Enzyme: arginyl(R) aminoacyl tRNA synthetase in mitochondria | The tRNA synthetase catalyzes the attachment of an amino acid to its cognate transfer RNA molecule. | N, I, M, F |
| let-743 | III | ZK686.2 | Enzyme: ATP-dependent RNA helicase | Its orthologs in yeast and human are involved in the biogenesis of ribosomal subunits. | N, I, M, F |
| let-747 | III | algn-1 | Enzyme: chitobiosyldiphosphodolichol β-mannosyltransferase | Mannosyltransferase is involved in asparagine-linked glycosylation in the endoplasmic reticulum. | N, I, M, F |
| let-753 | III | C34E10.10 | Ribosome-related protein: rRNA-processing protein | The protein is involved in ribosomal subunit biogenesis. | N, I, M, F |
| let-763 | III | T08A11.2 | mRNA splicing: splicing factor 3B subunit 1 | U2-snRNP associated splicing factor forms extensive associations with the branch site-3′ splice site-3′ exon region upon prespliceosome formation. | N, I, M, F |
| let-764 | III | byn-1 | Mammalian bystin (adhesion protein) related protein | Its ortholog in yeast is required for pre-rRNA processing and 40S ribosomal subunit synthesis. | N, I, M, F |
| let-771 | III | rfl-1 | Enzyme: ubiquitin activating enzyme | RFL-1 activity is required for proper cytokinesis and spindle orientation. | N, I, M, F |
| let-774 | III | rps-3 | Ribosome-related protein: ribosomal protein, small subunit | Protein component of the small ribosomal subunit involved in protein biosynthesis. | N, I, M, F |
| let-784 | III | gop-3 | Component of the Sorting and Assembly Machinery (SAM) complex | SAM complex binds precursors of beta-barrel proteins and facilitates their outer membrane insertion. | N, I, M, F |
| let-786 | III | unc-116 | Kinesin-related motor protein | Its ortholog in yeast is required for mitotic spindle assembly and chromosome segregation. | N, I, M, F |
| let-799 | III | ddx-23 | Enzyme: ATP-dependent RNA helicase | Its ortholog in yeast is involved in mRNA decay and rRNA processing. | N, I, M, F |
| let-826 | III | mrps-18C | Ribosome-related protein: ribosomal protein (small) in mitochondria | Ribosomal subunit biogenesis | N, I, M, F |
| let-829 | III | atp-2 | Enzyme: mitochondrial ATP synthase subunit | Evolutionarily conserved enzyme complex that is required for ATP synthesis. | N, I, M, F |
| let-832 | III | F09F7.4 | Enzyme: 3-hydroxyisobutyryl-CoA hydrolase | Biological process unknown | N, I, M, F |
| let-972 | III | hsp-110 | Heat shock protein | Its ortholog in yeast is a ATPase component of the heat shock protein Hsp90 chaperone complex and serves as nucleotide exchange factor to load ATP onto the SSA class of cytosolic Hsp70s. | N, I, M, F |
| let-326 | V | rab-1 | Enzyme: Rab family GTPase | Intracellular vesicle trafficking, such as the ER-to-Golgi step of the secretory pathway. | N, I, M, F |
| let-331 | V | prx-6 | Peroxisomal biogenesis factor | Its ortholog in yeast heterodimerizes with Pex1p and participates in the recycling of the peroxisomal signal receptor from the peroxisomal membrane to the cystosol. | N, I, M, F |
| let-332 | V | C05C8.7 | Enzyme: Mannose phosphate isomerase | Its ortholog in yeast catalyzes the interconversion of fructose-6-P and mannose-6-P, which is required for early steps in protein mannosylation. | N, I, M, F |
| let-334 | V | slc-17.8 | Member of solute carriers family | Predicted to have transmembrane transporter activity. | N, I, M, F |
| let-335 | V | C37C3.2 | Translation initiation factor | Based on the homology to yeast, the products of C37C3.2 are predicted to function during translation initiation as GTPase activators to stimulate GTP hydrolysis by eIF2-GTP-Met-tRNAi. | N, I, M, F |
| let-338 | V | rpac-40 | Enzyme: RNA polymerase I/III (A/C) shared subunit | Common component of RNA polymerases I and III, which synthesize ribosomal RNA precursors and small RNAs, such as 5S rRNA and tRNAs. | N, I, M, F |
| let-343 | V | cpsf-2 | Cleavage and polyadenylation specificity factor | Required for 3′ processing, splicing, and transcriptional termination of mRNAs and snoRNAs. | N, I, M, F |
| let-346 | V | soap-1 | HEAT repeat-containing protein | SOAP is involved in a pathway that controls the apical delivery of E-cad and morphogenesis. | N, I, M, F |
| let-350 | V | C37H5.5 | Nucleolar complex protein-like DNA replication regulator | Its ortholog in yeast binds to chromatin at active replication origins and is required for pre-RC formation as well as maintenance during DNA replication licensing. | N, I, M, F |
| let-402 | V | erfa-1 | Translation termination factor | Subunit of the heterodimeric translation release factor complex involved in the release of nascent polypeptides from ribosomes. | N, I, M, F |
| let-408 | V | snap-1 | α-soluble NSF attachment protein | Its ortholog in mouse is required for vesicular transport between the endoplasmic reticulum and the Golgi apparatus. | N, I, M, F |
| let-409 | V | asns-1 | Enzyme: asparagine synthase (glutamine-hydrolyzing) | Catalyzes the synthesis of L-asparagine from L-aspartate in the asparagine biosynthetic pathway. | N, I, M, F |
| let-410 | V | dlst-1 | Enzyme: dihydrolipoamide S-succinyltransferase | Its ortholog in yeast is a component of the mitochondrial alpha-ketoglutarate dehydrogenase complex, which catalyzes the oxidative decarboxylation of alpha-ketoglutarate to succinyl-CoA in the TCA cycle. | N, I, M, F |
| let-411 | V | xpo-1 | Exportin-1, an importin-β-like protein orthologous to Drosophila, vertebrates, and yeast exportin-1/CRM1 | XPO-1 is predicted to function as a nuclear export receptor for proteins containing leucine-rich nuclear export signals. | N, I, M, F |
| let-415 | V | hsp-6 | Nuclear-encoded mitochondrion-specific chaperone that is a member of the DnaK/Hsp70 superfamily of molecular chaperones | In C. elegans, hsp-6 is involved in the mitochondrial unfolded protein response. | N, I, M, F |
| let-417 | V | ceh-34 | Homeobox protein | In C. elegans, ceh-34 activity is required for regulation of the programmed cell death of a pharyngeal neuron, the sister of the M4 motor neuron. | N, I, M, F |
| let-419 | V | pqn-51 | Transcription initiation factor IIA | Its ortholog in yeast is involved in transcriptional activation and acts as antirepressor or coactivator. | N, I, M, F |
| let-420 | V | adss-1 | Enzyme: adenylosuccinate synthetase | Catalyzes the first step in the synthesis of adenosine monophosphate from inosine 5′monophosphate during purine nucleotide biosynthesis. | N, I, M, F |
| let-422 | V | hmgs-1 | Enzyme: hydroxymethylglutaryl-CoA synthase | Its ortholog in yeast catalyzes the formation of HMG-CoA from acetyl-CoA and acetoacetyl-CoA. | N, I, M, F |
| let-424 | V | vars-1 | Enzyme: valyl-aminoacyl tRNA synthetase in mitochondria | The tRNA synthetase catalyzes the attachment of an amino acid to its cognate transfer RNA molecule. | N, I, M, F |
| let-439 | V | M03F8.3 | mRNA splicing: crooked neck pre-mRNA splicing factor | The crooked neck gene of Drosophila is essential for embryogenesis and is thought to be involved in cell cycle progression and pre-mRNA splicing. | N, I, M, F |
| let-442 | V | C05C8.2 | Ribosome-related protein: small subunit processome component | A nucleolar protein required for rRNA synthesis and ribosomal assembly. | N, I, M, F |
| let-447 | V | egl-8 | Enzyme: phospholipase C β | In yeast, the ortholog (Plc1p) and inositol polyphosphates are required for acetyl-CoA homeostasis, which regulates global histone acetylation. | N, I, M, F |
| let-459 | V | hpo-18 | Enzyme: ATP synthase | Unknown | N, I, M, F |
| let-470 | V | F11A3.2 | Translation initiation factor | These proteins help stabilize the formation of the functional ribosome around the start codon and also provide regulatory mechanisms in translation initiation. | N, I, M, F |
The table is sorted by evolutionary conservation, chromosome (Chr), and lethal name (let-Name). N, nematodes; I, invertebrates (Drosophila); M, mammals (mouse, human); F, fungi (Saccharomycetaceae).
Because of the functional importance of essential genes, one would expect that they are conserved and found in most organisms. Consistent with this, we found that 44 out of 60 (73.3%) of our essential genes have orthologs in all the examined organisms but eight of the 60 (13.3%) are nematode-specific. This is consistent with the results found in a previous study based on a transcriptome analysis of the phylum Nematoda (Parkinson et al. 2004). Eight genes were found in nematodes, invertebrates, and mammals, but not in single-cell fungi, suggesting that there are essential genes that are specific to multicellular organisms. For instance, let-757/fbn-1 encodes the extracellular matrix protein fibrillin, which is highly conserved in multicellular organisms and is a key component of elastic fiber in connective tissues (Ritty et al. 2003).
Four new protein domains revealed by examining missense lethal mutations
Protein functions can be affected by missense mutations that reside in conserved regions, such as functional domains. Thus, we want to explore whether missense lethal mutations are more likely to be located within annotated Pfam domains.
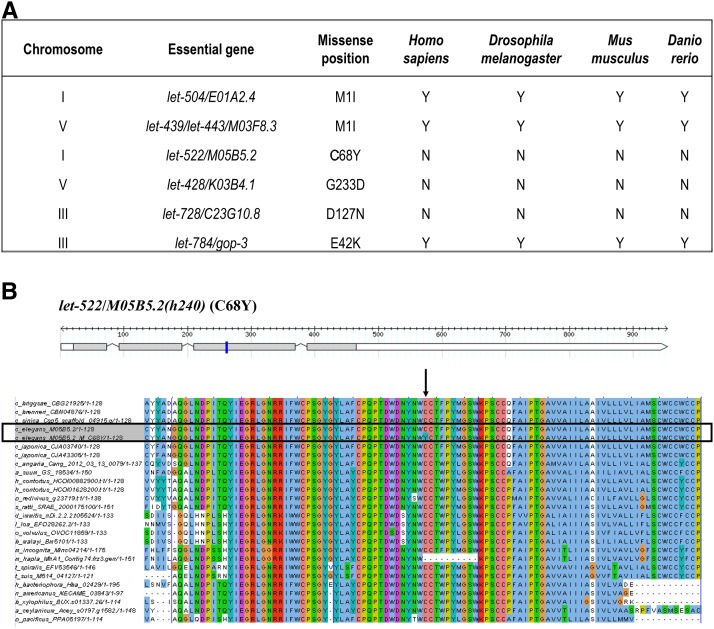
To gain a better understanding of lethal mutations in C. elegans, we wanted a large dataset and so incorporated 62 essential genes identified in previous studies that examined lethal mutations balanced by sDp2 on chromosome I (Chu et al. 2014) and eT1 on chromosome V (Jones et al. 2007), and the essential genes identified in this study. We found 70 missense mutations in 59 essential genes of which 64 are within Pfam domains of 53 genes, suggesting the functions of the essential proteins are disrupted. We looked at the remaining six missense mutations in six essential genes (Figure 3A).
Figure 3.
(A) Six missense mutations in six essential genes that are not in annotated functional domains. (B) One gene model (let-522/M05B5.2) that has a missense mutation h240 (C68Y) that does not reside in an annotated domain (Chu et al. 2014). The gene model was created using the Perl module FeatureStack. Exons are drawn to scale relative to each other in gray boxes; black lines with triangle shapes represent introns, which are not drawn to scale; white boxes represent UTRs. Blue bars indicate the missense mutation. Multiple alignments were performed between the essential genes and their orthologs in 25 other nematodes and four model organisms (Homo sapiens, Drosophila melanogaster, Mus musculus, and Danio rerio). However, no orthologs were found in the four model organisms. Left columns show the names of nematode species. The alignment figure shows a fragment of 100 amino acids. The mutated amino acid (C68Y) as indicated by the black arrow is in the center with 50 amino acids upstream and 49 amino acids downstream. The alignment color scheme is based on “ClustalX,” in which the color of a symbol depends on the residue type and the occurrence frequency in one column. Black boxes highlight the input genes with both wild type and mutant copy.
Two missense mutations were nucleotide transitions (G->A) that changed the ATG start codon to ATA. It is possible that the next in-frame ATG would be recognized as an initiation codon, but the truncated product could have a hypomorphic defect (Maser et al. 2001). In both cases in our study, the next ATG was not in-frame, which probably resulted in a lack of wild-type products causing loss of function (Schnabel et al. 1992). The observed lethal phenotypes also show that start codon mutations can be deleterious.
For the other four missense mutations, even though they are not located within annotated domains (by searching InterPro database), it turns out that the altered amino acids are still highly conserved in orthologs from different species. In a window size of 100 amino acids, which is the average size of protein domains based on the statistical analysis on the size of Pfam domains from all protein isoforms (data not shown), the sequence regions containing the target amino acids are also highly conserved (one example is shown in Figure 3B). It suggests new protein domains that had not been identified as functional domains. It is also possible that the effected amino acids are important for maintaining protein structures and the mutations disrupt these structures, resulting in malfunctioning proteins.
GO analysis of essential genes
To conduct GO analysis, four groups of genes were used for comparison. Group one (G1): essential genes that were isolated through genetic screens in the Baillie laboratory or collaborations with the Baillie laboratory and have been fully sequenced or rescued by fosmids (Maciejowski et al. 2005; Jones et al. 2007; Chu et al. 2014; S. Ono, personal communication) (143 in total, including 60 genes from the current study). Group two (G2): essential genes that have published lethal alleles (1208 in total). Group three (G3): genetically identified nonessential genes (796 in total). Group four (G4): genes with no observed phenotype by either RNAi or alleles (12,811 in total). This putatively nonessential group contains the majority of protein-coding genes in C. elegans.
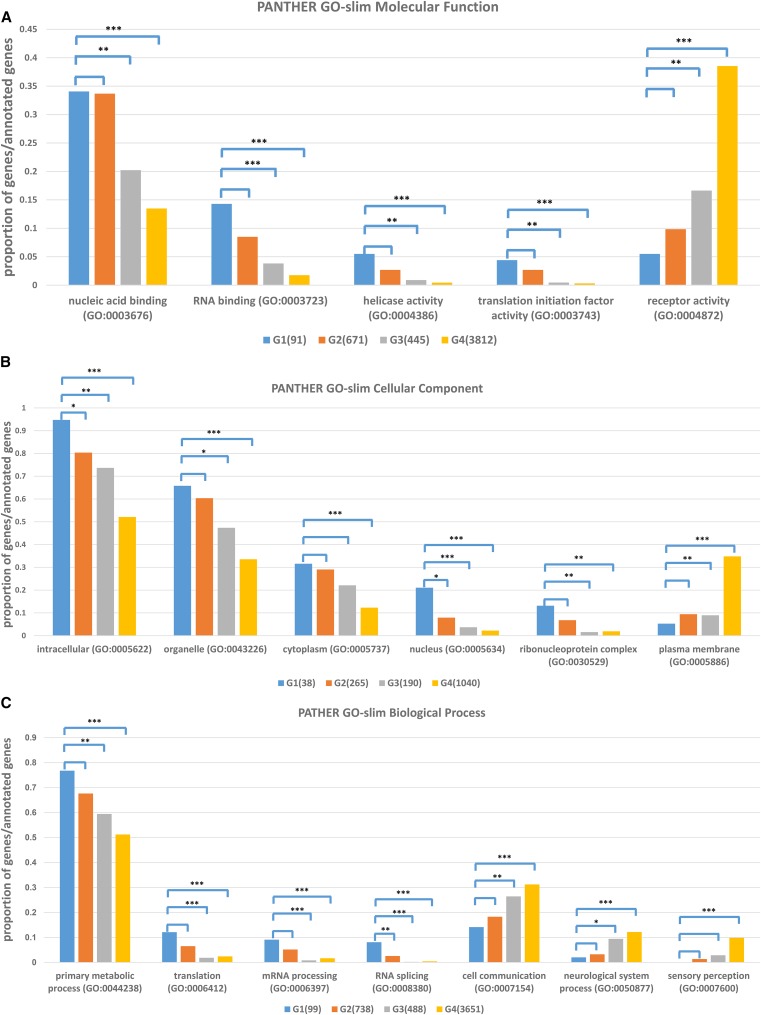
We compared the G1 essential genes to genes in the other three groups based on three GO categories: cellular component, molecular function, and biological process (Figure 4).
Figure 4.
Genes from each group annotated with three ontology terms: (A) molecular function, (B) cellular component, and (C) biological process. The x-axis lists several GO terms in each GO category and the y-axis is the proportion of genes for each GO term over the total number of annotated genes in each group. Four groups are shown in separate colors: G1 in blue, G2 in orange, G3 in gray, and G4 in gold. Statistical difference was calculated for G1 vs. G2, G3, and G4 individually by using Fisher’s exact test (* P-value < 0.05, ** P-value < 0.01, *** P-value < 0.001).
Essential proteins from G1 and G2 have no significant difference in any molecular function related annotation, including nucleic acid binding and especially RNA binding (GO:0003723). These annotations are significantly lower for the proteins of nonessential genes for both G3 and G4 (Figure 4A). This is consistent with our observation in the cellular component analysis, in which annotations of the ribonucleoprotein complex (GO:0030529) are high in essential proteins (Figure 4B). In contrast, receptor activity (GO:0004872) is lower in essential proteins (Figure 4A). This might also explain the observation that the proportion of nonessential proteins located in plasma membranes (GO:0005886) is significantly higher than that of essential proteins (Figure 4B).
With regard to biological processes, essential proteins in G1 and G2 are significantly enriched for primary metabolic processes, as well as translation and mRNA processing, suggesting that essential genes tend to be involved in protein synthesis. In contrast, nonessential proteins are significantly enriched for regulation of cellular functions, such as cell communication and sensory perception (Figure 4C). If there is a disruption in these processes, the worms might show visible phenotypes; however, these are generally not lethal.
GO term analysis indicated that essential genes tend to execute enzyme and nucleic acid binding activities during fundamental processes, such as DNA replication, translation, and transcription intracellularly.
Gene essentiality analysis
Gene essentiality, gene duplicability, protein interaction networks, and gene expression are biological factors that can influence the evolutionary rate of proteins (Liao et al. 2006). Thus, to develop an understanding of how essential proteins function in cells, we assessed the properties of essential genes from three perspectives: gene duplicability, protein interactions, and gene expression, applying the same dataset used in GO term analysis.
Gene essentiality vs. gene duplicability:
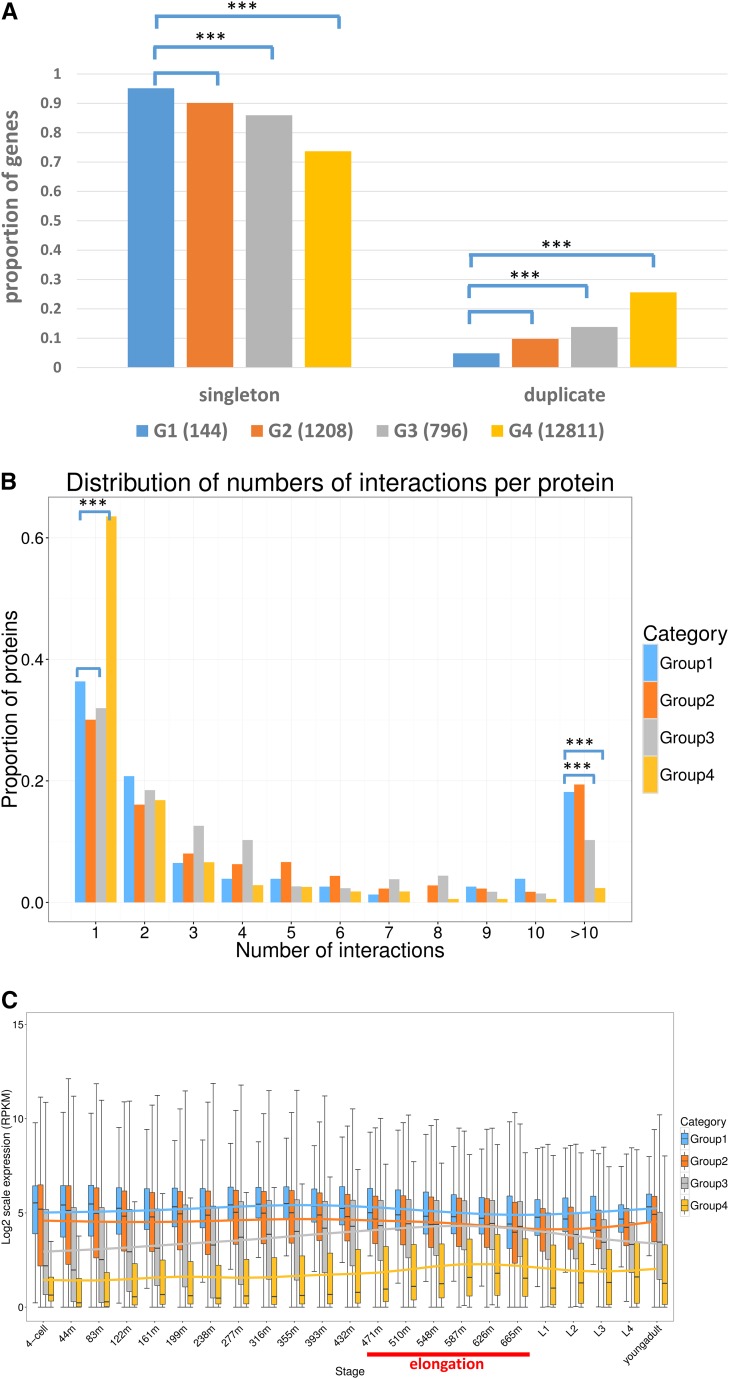
In C. elegans, researchers showed that closely related gene duplicates are responsible for mitigating the effects of mutations (Conant and Wagner 2004), which is also known as mutational robustness (Mendonca et al. 2011). Studies performed in yeast showed that duplicate genes have a higher probability of functional compensation than singletons (Gu et al. 2003). However, there are analyses in both yeast and mouse suggesting that duplicate genes are as essential as singletons (Wagner 2000; Liang and Li 2007; Liao and Zhang 2007). Thus, we want to revisit this question using our collected datasets. Using the most stringent criteria of e-value with 1E−20 for quantitative comparison, we see that most collected genes are singletons, no matter which group they belong to (Figure 5A). This is consistent with the theory that redundant duplicates are not evolutionarily stable and tend to be removed over long periods of time (Nowak et al. 1997), although this theory is still debated (Yona et al. 2015). Despite that, the proportions of singletons to duplicates in each group show differences. The proportions of both singletons and duplicates in G1 are similar to those in G2, which is as we expected, considering both groups contain only essential genes. On the other hand, the proportions of singletons are significantly less in non-essential genes (both G3 and G4). Based on this analysis, essential genes are more likely to be unique, with over 90% of essential genes present as single copies (in both G1 and G2) compared with nonessential genes.
Figure 5.
Gene essentiality analysis. Group one (G1): essential genes that were isolated through genetic screens in the Baillie laboratory or collaborations and are fully sequenced or rescued by fosmids (Maciejowski et al. 2005; Jones et al. 2007; Chu et al. 2014; S. Ono, personal communication) (143 in total, including 60 genes from the current study). Group two (G2): essential genes that have published alleles supporting lethal phenotypes (1208 in total). Group three (G3): nonessential genes that have known alleles supporting nonlethal phenotypes (796 in total). Group four (G4): no-phenotype supporting genes that have no observable phenotypes caused by either RNAi or known alleles (12,811 in total). Given that this group contains the majority of protein-coding genes in C. elegans, most genes in this group are expected to be nonessential. The four groups are labeled in blue, orange, gray, and gold, respectively. (A) The proportion of singletons and duplicates in the four groups. The x-axis shows singletons and duplicates from the groups. Significant differences (*** P-value < 0.001) were observed between G1 and G3, and between G1 and G4. (B) The distribution of number of interactions per protein (Stark et al. 2006). The x-axis shows the number of interactions per protein and the y-axis is the proportion of proteins. (C) The developmental stage-specific expression pattern and expression level of genes in four groups. The x-axis shows 23 developmental stages, embryos to young adults, from four cells. m in the x-axis is short for minutes of embryonic development. Each bar represents the distribution of the normalized expression level (RPKM in log2 scale) of each gene in each of four groups for each developmental stage. The linear regression trend line is drawn for each group in their respective color. The stages of embryonic elongation are underlined in red. The boxplot was computed using R and the function ggplot2, which uses a 95% C.I.
Gene essentiality vs. gene connectivity:
Proteins do not function alone; they function as parts of pathways, macromolecular structures, and regulatory networks (Dolinski and Botstein 2007). By characterizing the yeast proteome, Jeong et al. (2001) found that the most highly connected proteins in a cell are the most important ones for survival (Jeong et al. 2001). Based on this theory, we hypothesize that essential proteins tend to engage in protein interaction hubs, with more interactors connecting to them than to nonessential proteins.
Using the whole genome protein interactions in C. elegans from BioGRID (Stark et al. 2006), Figure 5B shows the distribution of the number of interactions per protein, essential proteins from G1 and G2 tend to have more interaction partners, i.e., >10 proteins compared with G3 and G4 (P-value < 0.001). This suggests that essential proteins tend to be in the interaction hubs. It is interesting to note that proteins that have no phenotypic consequence are much more likely to have only one interactor.
Gene essentiality vs. gene expression:
Expression levels are frequently used to infer the activities and functions of gene products. Expression levels have also been used as a parameter to analyze the nonessential gene datasets in the human genome (Hart et al. 2014). Therefore, we want to examine the expression level of all C. elegans genes in our collected data by using the gene expression dataset from the GExplore (version 1.4) expression search interface (Hutter et al. 2009) and the NHGRI modENCODE project (Hillier et al. 2009; Gerstein et al. 2010).
Figure 5C shows that essential genes from G1 and G2 have significantly higher expression level in all developmental stages than nonessential genes from G3 and G4. With respect to the expression pattern over the developmental stages, essential genes are more consistently expressed across all developmental stages than nonessential genes. However, the expression levels of nonessential genes show a twofold increase in the embryonic elongation stage (470–640 min). During this period, morphogenesis is happening, which in more complex multicellular organisms could be considered as the assembly of cells into functioning tissues and organs. Morphogenesis likely proceeds via conserved signaling pathways, regulatory mechanisms, and effector genes (Edgar 1999) (Figure 5C).
Discussion
A total of 62 out of 89 essential genes were successfully identified and 60 out of 62 were matched to their molecular counterpart. This large number demonstrates the value of our method. However, we were not able to identify any appropriate mutations in the sequences of the other 27 genes. Complementation tests and PCR sequencing were conducted to validate possible candidates for seven of those 27; however, we found that they were not essential genes. We do not know the reason(s) for this, but we hypothesize several possible reasons. First is strain mix-ups; however, this is unlikely because during the construction of the strains containing let-500 the terminal phenotypes of all the lethals were the same as noted when the lethals were first analyzed. Second is sequencing errors, as it is possible that there were not enough sequencing reads to support some of the lethal mutations. For example, only one out of 32 sequencing reads (3%) supports C to T change in unc-46(e177) in the let-417(s204) strain, but in the remaining 78 sequenced mutants with unc-46(e177), the variation frequencies of e177 range from 23 to 78%. This reason is not adequate because most of the time there was sufficient read support to get good results. Third, mutations may have been mismapped. An example is let-443 and let-439, which were mapped genetically into two different zones. Sequence analysis showed they were actually the same gene. This reason is weak because this result shows that we can find mutations in different zones but careful scanning did not find mutations in any zone for the unsolved genes. Fourth, mutations may reside in noncoding regions. An example is let-470 where no mutation was found in its genetic zone for s1629 but a splicing acceptor mutation in F11A3.2 was found for s1581 by a zone boundary, and fosmid rescue assays showed that F11A3.2 rescues let-470(s1629). This reason does not explain why we found a mutation in noncoding DNA for one allele but not for the other. As stated above, we do not have a good reason to explain why 27 genes were not identified, but we are pleased that 62 were identified, with 60 of the 62 mapped to their molecular counterparts.
Of the 60 identified essential genes, five are without RNAi evidence supporting their lethal phenotype (Table S1 in File S1). This finding is meaningful because it indicates that although RNAi is an efficient method for essential gene identification, it does miss roughly 8.3% (five out of 60) of the genes that can be isolated in mutagenesis screens.
Among the 143 essential genes collected in this study, we found 15 genes related to cell division and chromosome partitioning; surprisingly, 11 of those are on chromosome I. However, there was no other functional clustering of genes observed on chromosome III or V. Nevertheless, chemoreceptor gene families are unusually enriched on chromosome V, especially on the chromosomal arms, which might be responsible for signal transduction in C. elegans (Robertson and Thomas 2006). Members of this large gene family are not essential genes but inhabit a lot of chromosome V(left), which is consistent with the fact that chromosome V(left) has few essential genes relative to the other autosomes and has a high degree of gene duplications (Kamath et al. 2003). The concentration of functionally related genes on chromosomes in a nonrandom pattern is important for understanding how genomes function and evolved. Besides, it may have clinical significance in understanding or predicting disease-causing. clustered genes (Andrews et al. 2015).
We noticed that nonsense mutations are significantly enriched relative to missense mutations over duplication and translocation balancers (data not shown). Considering the heterozygosity background for each mutation, we propose that it might be due to the different functional effects of missense mutations and nonsense mutations in heterozygotes. Missense alleles might confer negative interaction with the wild-type allele in heterozygotes by being located in protein functional domains or the subunit interfaces, causing selection against these heterozygotes during genetic screens (Phillips et al. 1995; Thomas et al. 1997). On the other hand, the nonsense-mediated mRNA decay (NMD) surveillance system degrades nonsense allele products, which suggests that almost no interfering nonsense mutant products are produced, therefore there is no such negative interaction using either duplication or translocation balancers. Consistent with this theory, we found that nonsense mutations are located toward N-terminal protein sequences. To test our hypothesis, one could utilize NMD defective mutants that harbor a genetically balanced strain to isolate lethal mutations. One would expect that the selective enrichment of nonsense alleles would be reduced in a NMD mutant background, because the amount of truncated protein would be greater in the defective NMD background.
This allele-dependent property of essential genes is related to functional effects causing human diseases. Human orthologs of mouse essential genes are linked to numerous human diseases that affect a variety of physiological systems. It has been shown that mutations in human orthologs of embryonic lethal phenotypes, caused by loss-of-function essential mouse genes, can cause lethality in human pregnancies and may result in spontaneous abortions (Goh et al. 2007). However, phenotypic symptoms induced by defective essential genes are not restricted to developmental abnormalities, but can also contribute to adult diseases. Unlike null alleles that cause loss of function, some point mutations in these genes do not necessarily lead to a complete loss of protein function (Dickerson et al. 2011). There are orthologs in human of mouse essential genes with missense mutations that may lead to abnormal phenotypes in the heterozygous state (International PPH Consortium et al. 2000; Ragge et al. 2005). Therefore, not only miscarriage and birth defects, but also other human diseases can be induced by defective essential genes, including different types of cancer (Dickerson et al. 2011).
How many human orthologs of the essential genes in C. elegans that we identified are related to human disorders? Of the 143 essential genes that were gathered for the functional analysis, 108 were identified as having a putative ortholog in human. Among those 108 genes, 97 (90%) are associated with 1218 different diseases. For instance, SF3B1, which encodes an RNA-splicing factor and is orthologous to let-763/sftb-1, has mutations related to myeloid cancers and refractory anemias (Papaemmanuil et al. 2011; Broseus et al. 2013). In another case, the ortholog of F11A3.2 in human is a translation initiation factor EIF2B4, which is associated with the inheritable disease Vanishing White Matter (Kanbayashi et al. 2015). Moreover, 60 of the 97 genes have existing variations that were found to be responsible for 163 different diseases. Consistent with previous findings (Dickerson et al. 2011), mutations in these genes can lead to a broad spectrum of human disorders. The most abundant diseases tend to be related to cancer or carcinogenesis, including breast carcinoma and colorectal cancer, suggesting links between adult cellular abnormalities and developmental functions.
A large number of essential genes were molecularly identified in this study, which significantly enlarges the database of essential genes in C. elegans. The identified lethal alleles provide a rich resource for future studies on essential genes, considering the difficulty of isolating and maintaining lethal mutations. Moreover, conserved essential genes could be good gene models for developing our understanding of the functions of homologous genes in humans, especially disease-related genes. On the other hand, according to a recent study, the essential genes that are only conserved in nematodes can play critical roles in core eukaryotic processes, especially in chromosome segregation (Verster et al. 2017). Meanwhile, species-specific essential genes could be good candidates for targeting pathogenic nematodes. Considering the functional importance of essential genes, studies can be conducted on the ones that currently have no annotated functions.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.300338/-/DC1.
Acknowledgments
We thank the National Bioresource Project for the Experimental Animal “Nematode C. elegans,” the Caenorhabditis Genetics Center for providing strains, and the Michael Smith Genome Sciences Centre for Illumina sequencing. Special thanks go to Ann Rose for helping with the sequencing. We also thank Jiarui Li for advice and comments. This research was sponsored by grants from the Natural Sciences and Engineering Research Council of Canada Discovery Grants Program and the Canada Research Chairs Program to D.B. The authors declare that they have no competing interests.
Author contributions: J.S.-C.C. and Z.Q. prepared genomic DNA for WGS. Z.Q. analyzed the WGS data of chromosome V mutants and S.Y. analyzed the WGS data of chromosome III mutants. S.Y. and Z.Q. conducted essential gene identification analyses on both chromosome III and chromosome V mutants. R.J. performed genetic crosses. Z.Q. performed all the functional studies. D.L.B. and N.C. led the study. Z.Q., S.Y., J.S.-C.C., and N.C. wrote the article. All authors read and approved the final article.
Footnotes
Communicating editor: S. Lee
Literature Cited
- Amsterdam A., Nissen R. M., Sun Z., Swindell E. C., Farrington S., et al. , 2004. Identification of 315 genes essential for early zebrafish development. Proc. Natl. Acad. Sci. USA 101: 12792–12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews T., Honti F., Pfundt R., de Leeuw N., Hehir-Kwa J., et al. , 2015. The clustering of functionally related genes contributes to CNV-mediated disease. Genome Res. 25: 802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broséus J., Alpermann T., Wulfert M., Florensa Brichs L., Jeromin S., et al. , 2013. Age, JAK2(V617F) and SF3B1 mutations are the main predicting factors for survival in refractory anaemia with ring sideroblasts and marked thrombocytosis. Leukemia 27: 1826–1831. [DOI] [PubMed] [Google Scholar]
- Chu J. S., Johnsen R. C., Chua S. Y., Tu D., Dennison M., et al. , 2012. Allelic ratios and the mutational landscape reveal biologically significant heterozygous SNVs. Genetics 190: 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. S., Chua S. Y., Wong K., Davison A. M., Johnsen R., et al. , 2014. High-throughput capturing and characterization of mutations in essential genes of Caenorhabditis elegans. BMC Genomics 15: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. V., Johnsen R. C., McKim K. S., Baillie D. L., 1990. Analysis of lethal mutations induced in a mutator strain that activates transposable elements in Caenorhabditis elegans. Genome 33: 109–114. [DOI] [PubMed] [Google Scholar]
- Conant G. C., Wagner A., 2004. Duplicate genes and robustness to transient gene knock-downs in Caenorhabditis elegans. Proc. Biol. Sci. 271: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. W., Hammarlund M., Harrach T., Hullett P., Olsen S., et al. , 2005. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics 6: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb N. J., Maruyama I. N., Krause M., Karn J., 1989. Sequence analysis of the complete Caenorhabditis elegans myosin heavy chain gene family. J. Mol. Biol. 205: 603–613. [DOI] [PubMed] [Google Scholar]
- Dickerson J. E., Zhu A., Robertson D. L., Hentges K. E., 2011. Defining the role of essential genes in human disease. PLoS One 6: e27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinski K., Botstein D., 2007. Orthology and functional conservation in eukaryotes. Annu. Rev. Genet. 41: 465–507. [DOI] [PubMed] [Google Scholar]
- Edgar L. G., 1999. Morphogenesis and organogenesis in Caenorhabditis elegans, pp. 269–297 in Development, edited by V. E. A. Russo, D. J. Cove, L. G. Edgar, R. Jaenisch, F. Salamini. Springer, Berlin, Heidelberg. [Google Scholar]
- Edgley M. L., Riddle D. L., 1990. The nematode Caenorhabditis elegans. Genetic Maps 5: 111–133. [Google Scholar]
- Finn R. D., Bateman A., Clements J., Coggill P., Eberhardt R. Y., et al. , 2014. Pfam: the protein families database. Nucleic Acids Res. 42: D222–D230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flibotte S., Edgley M. L., Chaudhry I., Taylor J., Neil S. E., et al. , 2010. Whole-genome profiling of mutagenesis in Caenorhabditis elegans. Genetics 185: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech C., Choo C., Chen N., 2012. FeatureStack: Perl module for comparative visualization of gene features. Bioinformatics 28: 3137–3138. [DOI] [PubMed] [Google Scholar]
- Gerdes S., Edwards R., Kubal M., Fonstein M., Stevens R., et al. , 2006. Essential genes on metabolic maps. Curr. Opin. Biotechnol. 17: 448–456. [DOI] [PubMed] [Google Scholar]
- Gerstein M. B., Lu Z. J., Van Nostrand E. L., Cheng C., Arshinoff B. I., et al. , 2010. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science 330: 1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh K. I., Cusick M. E., Valle D., Childs B., Vidal M., et al. , 2007. The human disease network. Proc. Natl. Acad. Sci. USA 104: 8685–8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Steinmetz L. M., Gu X., Scharfe C., Davis R. W., et al. , 2003. Role of duplicate genes in genetic robustness against null mutations. Nature 421: 63–66. [DOI] [PubMed] [Google Scholar]
- Hart T., Brown K. R., Sircoulomb F., Rottapel R., Moffat J., 2014. Measuring error rates in genomic perturbation screens: gold standards for human functional genomics. Mol. Syst. Biol. 10: 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier L. W., Reinke V., Green P., Hirst M., Marra M. A., et al. , 2009. Massively parallel sequencing of the polyadenylated transcriptome of C. elegans. Genome Res. 19: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter H., Ng M. P., Chen N., 2009. GExplore: a web server for integrated queries of protein domains, gene expression and mutant phenotypes. BMC Genomics 10: 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International PPH Consortium. Lane K. B., Machado R. D., Pauciulo M. W., Thomson J. R., Phillips J. A., III, et al. , 2000. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat. Genet. 26: 81–84. [DOI] [PubMed] [Google Scholar]
- Janke D. L., Schein J. E., Ha T., Franz N. W., O’Neil N. J., et al. , 1997. Interpreting a sequenced genome: toward a cosmid transgenic library of Caenorhabditis elegans. Genome Res. 7: 974–985. [DOI] [PubMed] [Google Scholar]
- Jeong H., Mason S. P., Barabasi A. L., Oltvai Z. N., 2001. Lethality and centrality in protein networks. Nature 411: 41–42. [DOI] [PubMed] [Google Scholar]
- Johnsen R. C., Baillie D. L., 1988. Formaldehyde mutagenesis of the eT1 balanced region in Caenorhabditis elegans: dose-response curve and the analysis of mutational events. Mutat. Res. 201: 137–147. [DOI] [PubMed] [Google Scholar]
- Johnsen R. C., Baillie D. L., 1991. Genetic analysis of a major segment [LGV(left)] of the genome of Caenorhabditis elegans. Genetics 129: 735–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. R., Maydan J. S., Flibotte S., Moerman D. G., Baillie D. L., 2007. Oligonucleotide array comparative genomic hybridization (oaCGH) based characterization of genetic deficiencies as an aid to gene mapping in Caenorhabditis elegans. BMC Genomics 8: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. R., Chua S. Y., O’Neil N. J., Johnsen R. C., Rose A. M., et al. , 2009. High-resolution array comparative genomic hybridization analysis reveals unanticipated complexity of genetic deficiencies on chromosome V in Caenorhabditis elegans. Mol. Genet. Genomics 282: 37–46. [DOI] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., et al. , 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. [DOI] [PubMed] [Google Scholar]
- Kanbayashi T., Saito F., Matsukawa T., Oba H., Hokkoku K., et al. , 2015. Adult-onset vanishing white matter disease with novel missense mutations in a subunit of translational regulator, EIF2B4. Clin. Genet. 88: 401–403. [DOI] [PubMed] [Google Scholar]
- Koboldt D. C., Zhang Q., Larson D. E., Shen D., McLellan M. D., et al. , 2012. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 22: 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., 2000. How many genes can make a cell: the minimal-gene-set concept. Annu. Rev. Genomics Hum. Genet. 1: 99–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koressaar T., Remm M., 2007. Enhancements and modifications of primer design program Primer3. Bioinformatics 23: 1289–1291. [DOI] [PubMed] [Google Scholar]
- Kramer J. M., French R. P., Park E. C., Johnson J. J., 1990. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol. Cell. Biol. 10: 2081–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., et al. , 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R., 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., et al. , 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Li W. H., 2007. Gene essentiality, gene duplicability and protein connectivity in human and mouse. Trends Genet. 23: 375–378. [DOI] [PubMed] [Google Scholar]
- Liao B. Y., Zhang J., 2007. Mouse duplicate genes are as essential as singletons. Trends Genet. 23: 378–381. [DOI] [PubMed] [Google Scholar]
- Liao B. Y., Scott N. M., Zhang J., 2006. Impacts of gene essentiality, expression pattern, and gene compactness on the evolutionary rate of mammalian proteins. Mol. Biol. Evol. 23: 2072–2080. [DOI] [PubMed] [Google Scholar]
- Maciejowski J., Ahn J. H., Cipriani P. G., Killian D. J., Chaudhary A. L., et al. , 2005. Autosomal genes of autosomal/X-linked duplicated gene pairs and germ-line proliferation in Caenorhabditis elegans. Genetics 169: 1997–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser R. S., Zinkel R., Petrini J. H., 2001. An alternative mode of translation permits production of a variant NBS1 protein from the common Nijmegen breakage syndrome allele. Nat. Genet. 27: 417–421. [DOI] [PubMed] [Google Scholar]
- McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., et al. , 2010. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça A. G., Alves R. J., Pereira-Leal J. B., 2011. Loss of genetic redundancy in reductive genome evolution. PLOS Comput. Biol. 7: e1001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H., Muruganujan A., Casagrande J. T., Thomas P. D., 2013. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 8: 1551–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok C. A., Au V., Thompson O. A., Edgley M. L., Gevirtzman L., et al. , 2017. MIP-MAP: high-throughput mapping of Caenorhabditis elegans temperature-sensitive mutants via molecular inversion probes. Genetics 207: 447–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder N., Apweiler R., 2007. InterPro and InterProScan: tools for protein sequence classification and comparison. Methods Mol. Biol. 396: 59–70. [DOI] [PubMed] [Google Scholar]
- Nowak M. A., Boerlijst M. C., Cooke J., Smith J. M., 1997. Evolution of genetic redundancy. Nature 388: 167–171. [DOI] [PubMed] [Google Scholar]
- Papaemmanuil E., Cazzola M., Boultwood J., Malcovati L., Vyas P., et al. , 2011. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N. Engl. J. Med. 365: 1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J., Mitreva M., Whitton C., Thomson M., Daub J., et al. , 2004. A transcriptomic analysis of the phylum Nematoda. Nat. Genet. 36: 1259–1267. [DOI] [PubMed] [Google Scholar]
- Phillips J. P., Tainer J. A., Getzoff E. D., Boulianne G. L., Kirby K., et al. , 1995. Subunit-destabilizing mutations in Drosophila copper/zinc superoxide dismutase: neuropathology and a model of dimer dysequilibrium. Proc. Natl. Acad. Sci. USA 92: 8574–8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragge N. K., Brown A. G., Poloschek C. M., Lorenz B., Henderson R. A., et al. , 2005. Heterozygous mutations of OTX2 cause severe ocular malformations. Am. J. Hum. Genet. 76: 1008–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remm M., Storm C. E., Sonnhammer E. L., 2001. Automatic clustering of orthologs and in-paralogs from pairwise species comparisons. J. Mol. Biol. 314: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Ritty T. M., Broekelmann T. J., Werneck C. C., Mecham R. P., 2003. Fibrillin-1 and -2 contain heparin-binding sites important for matrix deposition and that support cell attachment. Biochem. J. 375: 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. M., Thomas J. H., 2006. The putative chemoreceptor families of C. elegans. WormBook 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. T., Thorvaldsdottir H., Winckler W., Guttman M., Lander E. S., et al. , 2011. Integrative genomics viewer. Nat. Biotechnol. 29: 24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A. M., O’Neil N. J., Bilenky M., Butterfield Y. S., Malhis N., et al. , 2010. Genomic sequence of a mutant strain of Caenorhabditis elegans with an altered recombination pattern. BMC Genomics 11: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth R. E., Baillie D. L., 1981. The genetic analysis of a reciprocal translocation, eT1(III; V), in Caenorhabditis elegans. Genet. Res. 99: 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth R. E., Cuddeford C., Baillie D. L., 1983. Mutagenesis in Caenorhabditis elegans. I. A rapid eukaryotic mutagen test system using the reciprocal translocation eTI(III;V). Mutat. Res. Fundam. Mol. Mech. Mutagen. 110: 39–48. [Google Scholar]
- Rosenbluth R. E., Cuddeford C., Baillie D. L., 1985. Mutagenesis in Caenorhabditis elegans. II. A spectrum of mutational events induced with 1500 r of γ-radiation. Genetics 109: 493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth R. E., Rogalski T. M., Johnsen R. C., Addison L. M., Baillie D. L., 1988. Genomic organization in Caenorhabditis elegans: deficiency mapping on linkage group V(left). Genet. Res. 52: 105–118. [Google Scholar]
- Sarin S., Prabhu S., O’Meara M. M., Pe’er I., Hobert O., 2008. Caenorhabditis elegans mutant allele identification by whole-genome sequencing. Nat. Methods 5: 865–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel D., Schroder M., Furst W., Klein A., Hurwitz R., et al. , 1992. Simultaneous deficiency of sphingolipid activator proteins 1 and 2 is caused by a mutation in the initiation codon of their common gene. J. Biol. Chem. 267: 3312–3315. [PubMed] [Google Scholar]
- Stark C., Breitkreutz B. J., Reguly T., Boucher L., Breitkreutz A., et al. , 2006. BioGRID: a general repository for interaction datasets. Nucleic Acids Res. 34: D535–D539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart H. I., Rosenbluth R. E., Baillie D. L., 1991. Most ultraviolet irradiation induced mutations in the nematode Caenorhabditis elegans are chromosomal rearrangements. Mutat. Res. 249: 37–54. [DOI] [PubMed] [Google Scholar]
- Stewart H. I., O’Neil N. J., Janke D. L., Franz N. W., Chamberlin H. M., et al. , 1998. Lethal mutations defining 112 complementation groups in a 4.5 Mb sequenced region of Caenorhabditis elegans chromosome III. Mol. Gen. Genet. 260: 280–288. [DOI] [PubMed] [Google Scholar]
- Thomas J. T., Kilpatrick M. W., Lin K., Erlacher L., Lembessis P., et al. , 1997. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat. Genet. 17: 58–64. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G., 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson O., Edgley M., Strasbourger P., Flibotte S., Ewing B., et al. , 2013. The million mutation project: a new approach to genetics in Caenorhabditis elegans. Genome Res. 23: 1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdottir H., Robinson J. T., Mesirov J. P., 2013. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14: 178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B. C., et al. , 2012. Primer3–new capabilities and interfaces. Nucleic Acids Res. 40: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara I. A., Frech C., Chen N., 2012. CooVar: co-occurring variant analyzer. BMC Res. Notes 5: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster A. J., Styles E. B., Mateo A., Derry W. B., Andrews B. J., et al. , 2017. Taxonomically restricted genes with essential functions frequently play roles in chromosome segregation in Caenorhabditis elegans and Saccharomyces cerevisiae. G3 (Bethesda) 7: 3337–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A., 2000. Robustness against mutations in genetic networks of yeast. Nat. Genet. 24: 355–361. [DOI] [PubMed] [Google Scholar]
- Yona A. H., Frumkin I., Pilpel Y., 2015. A relay race on the evolutionary adaptation spectrum. Cell 163: 549–559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. Table S1 in File S1 contains detailed information of all identified essential genes and lethal mutations. Table S2 in File S1 includes a total of 86 strains with eT1(III;V) that were sequenced. Table S3 in File S1 contains a total of 49 strains with sDp3 sequenced. Table S4 in File S1 includes all the strains used in the complementation tests. The WGS data of all 135 sequenced strains are publicly available on the NCBI with the BioProject accession number PRJNA416306.