Abstract
Meiotic recombination depends upon the tightly coordinated regulation of chromosome dynamics and is essential for the production of haploid gametes. Central to this process is the formation and repair of meiotic double-stranded breaks (DSBs), which must take place within the constraints of a specialized chromatin architecture. Here, we demonstrate a role for the nucleosome remodeling and deacetylase (NuRD) complex in orchestrating meiotic chromosome dynamics in Caenorhabditis elegans. Our data reveal that the conserved Mi2 homologs Chromodomain helicase DNA-binding protein (CHD-3) and its paralog LET-418 facilitate meiotic progression by ensuring faithful repair of DSBs through homologous recombination. We discovered that loss of either CHD-3 or LET-418 results in elevated p53-dependent germ line apoptosis, which relies on the activation of the conserved checkpoint kinase CHK-1. Consistent with these findings, chd-3 and let-418 mutants produce a reduced number of offspring, indicating a role for Mi2 in forming viable gametes. When Mi2 function is compromised, persisting recombination intermediates are detected in late pachytene nuclei, indicating a failure in the timely repair of DSBs. Intriguingly, our data indicate that in Mi2 mutant germ lines, a subset of DSBs are repaired by nonhomologous end joining, which manifests as chromosomal fusions. We find that meiotic defects are exacerbated in Mi2 mutants lacking CKU-80, as evidenced by increased recombination intermediates, corpses, and defects in chromosomal integrity. Taken together, our findings support a model wherein the C. elegans Mi2 complex maintains genomic integrity through reinforcement of a chromatin landscape suitable for homology-driven repair mechanisms.
Keywords: nucleosome remodeling and deacetylase complex (NuRD), Mi2, double-strand break repair (DSBR), checkpoints, meiosis
THE ability to recognize and initiate the repair of exogenous and endogenous DNA lesions is crucial for the propagation of species, and the maintenance of genomic stability. This is of particular importance during meiosis, a specialized program of cellular division that generates haploid gametes. Paradoxically, while essential for the transmission of intact genomes, accurate meiotic chromosome segregation is reliant upon the deliberate induction of genome-wide, programmed double-stranded breaks (DSBs) (Keeney 2001). As errors affecting meiotic chromosome dynamics manifest as infertility and early embryonic death (Nagaoka et al. 2012; Webster and Schuh 2017), it is essential for all sexually reproducing organisms to be equipped with high-fidelity repair pathways to handle DSBs. To this end, processing of meiotic DSBs is accomplished predominantly via homologous recombination (HR), a tightly monitored and highly conserved repair pathway that utilizes sequence information from either sister or nonsister chromatids as a repair template (de Massy 2013; Jasin and Rothstein 2013). Given that HR is the sole means of generating the obligate crossover between homologs, it is critical that all steps of this process are carried out without incident. Crossovers enable the reciprocal exchange of genetic material between homologs as well as the resulting physical linkages that form between them, thereby ensuring the production of viable and genetically distinct gametes with the correct chromosome complement (Hunter 2015; Gray and Cohen 2016).
Though HR is the predominant repair pathway during meiosis, cells are also equipped with a number of evolutionarily conserved alternative repair routes that can act on DSBs in situations where HR is compromised (Ceccaldi et al. 2016). However, non-HR-mediated repair pathways cannot form crossovers and have a higher frequency of mutation. While there are benefits to this on an evolutionary scale, inaccurate repair of meiotic DSBs disrupts genomic stability and threatens reproductive success by causing infertility, spontaneous abortion, and congenital birth defects, and in somatic cells manifests as ataxia, immunodeficiency, and cancer (Malkova and Haber 2012; So et al. 2017). To safeguard organisms against such outcomes, there is extensive cross talk between repair machinery and checkpoint proteins, which detect DNA lesions and respond by either activating repair mechanisms or culling damaged cells by apoptosis (Giglia-Mari et al. 2011; Finn et al. 2012). Acting as quality control mechanisms, checkpoints are operative in both the soma and germ line, and play a crucial role in maintaining genomic integrity. Though the cellular contexts during which checkpoints and repair pathways are engaged differ between mitotic and meiotically dividing cells (Hartwell and Weinert 1989; Subramanian and Hochwagen 2014), there is significant conservation among many of the components involved; however, little is known about the interplay between these regulatory networks.
One requirement for both the sensing and repair of DSBs is the establishment of a specialized chromatin environment (Bakkenist and Kastan 2015; Smith and Rothstein 2017). This process necessitates alterations to existing chromatin architecture, which are carried out in part by post-translational modifications of nucleosomes and recruitment of multi-subunit remodeling complexes (Price and D’Andrea 2013; Gursoy-Yuzugullu et al. 2016; Seeber and Gasser 2017). By reshaping the local chromatin architecture surrounding DSB sites, chromatin remodeling complexes permit access to lesions, and enable the engagement of repair pathways and checkpoint signaling (Jeggo and Downs 2014). In spite of their importance, the role of chromatin remodelers in regulating meiotic DSBs remains elusive.
Here, we investigate the contributions of the Caenorhabditis elegans genes let-418 and chd-3, paralogs that encode subunits of the ATPase Mi2, and are homologous to CHD3 and CHD4 in mammals (von Zelewsky et al. 2000; Passannante et al. 2010). As in other organisms, CHD-3 and LET-418 (Mi2) associate with canonical nucleosome remodeling and deacetylase (NuRD) components in C. elegans (Passannante et al. 2010; Torrado et al. 2017). Though NuRD is important for modifying the chromatin landscape in multiple contexts (Zhang 2011; Basta and Rauchman 2015), little is known about its role during meiosis. Our data uncover a role for Mi2 in the regulation of meiotic chromosome dynamics, indicating a novel function for NuRD in facilitating HR in this context. We propose that CHD-3 and LET-418 improve gamete quality by promoting the timely repair of DSBs and inhibiting error-prone repair pathways that compromise genomic stability.
Materials and Methods
Genetics
Maintenance and genetic analysis of worms was performed using standard procedures. C. elegans var. Bristol (N2) was used as the wild-type strain. The following mutations were used in this study: LGI, rad-54(ok615); LGII, pch-2(tm1458); LGIII, cku-80(ok861) and syp-3(ok758); LGIV, zim-2(tm574); LGV, let-418(n3536) and syp-1(me17); and LGX, chd-3(eh4), chd-3(ok1651), and chd-3(vv133). All deletion strains used were outcrossed to wild-type (N2) a minimum of three times. The chd-3(vv133) strain was a gift from M. Zetka, which was generated by clustered regularly interspaced short palindromic repeats mutagenesis of the N2 wild-type strain. All other nematode strains used in this study were either provided by or generated using strains from the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources. All strains were maintained between 16 and 20°, and all experiments were performed at the permissive temperature of 20°.
Cytological analyses
Immunostaining of germ lines was performed as described with the following modifications: freeze-crack was performed after a minimum 5-min incubation of slides on an aluminum block at −80°, slides were incubated in primary antibody at 4° overnight, and slides were mounted using EMS Shield Mounting Medium with 4,6-diamidino-2-phenylindole (DAPI) and 1,4-Diazabicyclo[2.2.2]octane (DABCO) (Electron Microscopy Sciences). The following primary antibodies were purchased and used at the indicated dilutions: rabbit anti-RAD-51 (1:2000), (Novus Biologicals) and rabbit-anti-phospho-Chk1(Ser345), 1:50 (Santa Cruz Biotechnology). Rabbit anti-SYP-1 (1:200) was a generous gift from A. Villeneuve. The secondary antibody Alexa Fluor 488 goat anti-mouse IgG was used at a 1:500 dilutions and was obtained from Thermo Fisher Scientific. Postmounting, slides were sealed with nail polish and were stored at 4° prior to imaging. Images were captured using a Leica DM5500 automated upright fluorescent microscope and Leica Application Suite X (LAS X). Representative images shown were deconvolved using either LAS X software or OpenLab (Perkin-Elmer [Perkin Elmer-Cetus], Norwalk, CT) software, and were subsequently processed and analyzed using Fiji (Wayne Rasband, NIH). All images shown are projections through data stacks. Quantification of RAD-51 foci was performed using raw data from undeconvolved Z-stacks. Total data from each independently performed experiment assessing RAD-51 foci in wild-type (N2), let-418(n3536), and chd-3(eh4) germ lines were pooled, and the quantification shown in Figure 4 and Figure 6 is representative of all experiments conducted using these strains.
Figure 4.
RAD-51 disassembly is compromised in germ lines lacking Mi2. (A) Schematic of the C. elegans germ line. Nuclei divide mitotically in the proliferative zone (PZ), undergo double-strand break (DSB) formation and homolog pairing in the transition zone (TZ), and repair DSBs in early to midpachytene (EP–MP). In wild-type worms, DSB repair is complete by late pachytene (LP), and chromosomes condense in diplotene (DP) through diakinesis (DI). Gray coloring denotes regions assayed for RAD-51 foci. Total rows of nuclei in these regions were counted and divided equally into four stages (indicated by dashed lines), starting in mid-TZ, as determined by DAPI morphology. (B) Quantification of total RAD-51 foci in TZ–LP nuclei in wild-type (N2) (red), chd-3(eh4) (yellow), let-418(n3526) (dark blue), and let-418(n3536);chd-3(RNAi) (light blue). A minimum of three germ lines were scored for each genotype. Statistical comparisons between each mutant and wild-type at corresponding stages were performed using a two-tailed Mann–Whitney test. * P ≤ 0.005 and ** P ≤ 0.0001. (C–F) Representative images of RAD-51 foci in TZ (C) through LP (F) in N2, let-418(n3536), chd-3(eh4), and chd-3(RNAi);let-418(n3536). Germlines were stained with RAD-51 (yellow) and counterstained with DAPI (blue). Bar, 5 μM. Each experiment assessing RAD-51 in chd-3(RNAi);let-418(n3536) germlines was performed alongside N2 and let-418(n3536) plus L4440 empty vector controls (data not shown).
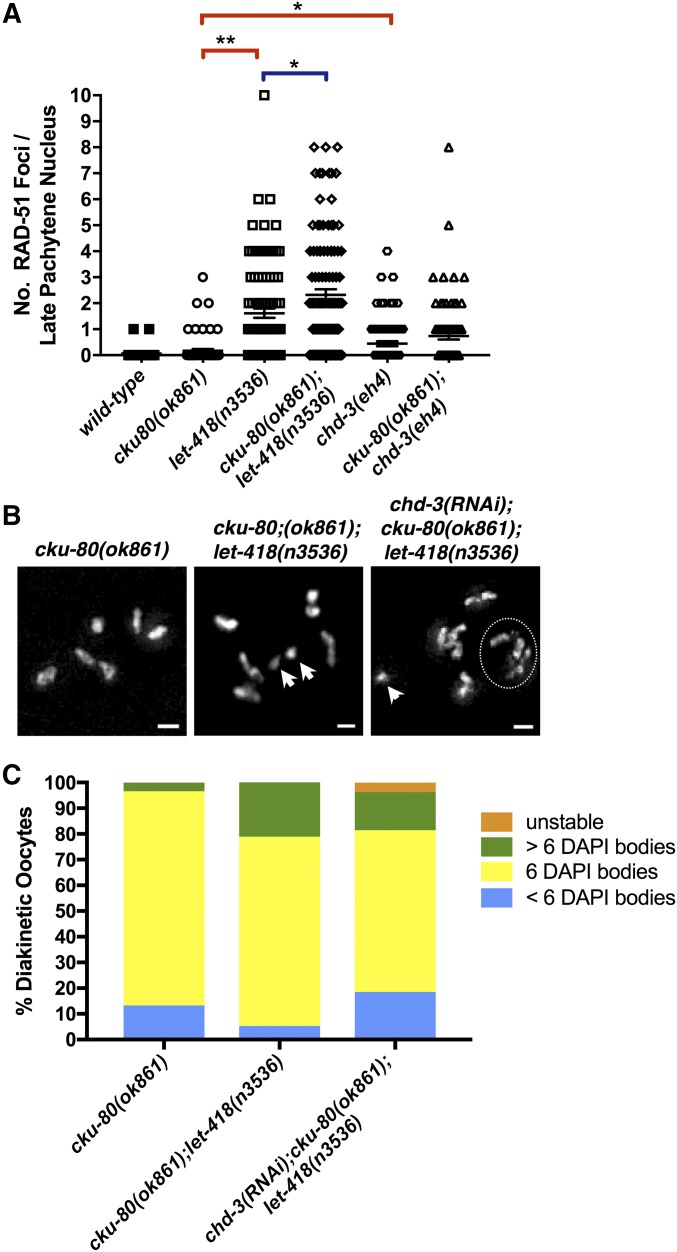
Figure 6.
Absence of nonhomologous end joining (NHEJ) exacerbates repair defects in let-418 mutants. (A) Quantification of RAD-51 foci per late pachytene nucleus in cku-80(ok861), let-418(n3536), cku-80(ok861);let-418(n3536);chd-3(eh4), and cku-80(ok861);chd-3(eh4) mutants. Statistical comparisons between single and double mutants were performed using a two-tailed Mann–Whitney test. * P ≤ 0.02 and ** P ≤ 0.0001. (B) Representative images of DAPI staining bodies in diakinetic (DI) nuclei in each mutant, which were quantified in (C): cku-80(ok861), (n = 30), cku-80(ok861);let-418(n3536), (n = 19), and chd-3(RNAi);cku-80(ok861);let-418(n3536), (n = 26). Number of visibly stained bodies is indicated in upper left corner of image. Arrowheads indicate extra DAPI-stained bodies, and circle indicates region with multiple fragmented chromosomes. Bar, 2 μM. Fragmentation (more than six DAPI bodies) was assessed using a Fisher’s exact test and was significant for the following comparisons: let-418 vs. cku-80;let-418: P = 0.0002; let-418 vs. chd-3(RNAi)cku-80;let-418: P = 0.0010; and cku-80 vs. chd-3(RNAi);cku-80;let-418: P = 0.0425. Chromosome fusions (less than six DAPI bodies) were identical between all three mutants: cku-80 vs. cku-80;let-418: P = 0.3478 and cku-80 vs. chd-3(RNAi);cku-80;let-418: P = 0.4304.
RNA-mediated interference (RNAi) analysis
RNAi experiments were performed at 20° using the feeding method (Timmons et al. 2001). L4 larvae were fed RNAi-inducing HT115(DE3) bacteria strains or the same bacteria transformed with the empty feeding vector, L4440. All constructs used were generated from N2 genomic DNA using primers corresponding to exon-containing regions, and were cloned into the Topo2.1 vector (Thermo Fisher Scientific) and subcloned into L4440. Cultures were plated onto NGM plates containing 25 µg/ml Carbenicillin (Sigma [Sigma Chemical], St. Louis, MO) and 1 mM IPTG (Zymo), induced at 37° for ∼4 hr, and were used within 2 weeks of plating.
Analysis of fragmented chromosomes
Germ lines were dissected as described above and fixed in 5% paraformaldehyde, followed by 1 min incubation in −20° ethanol following freeze-crack. Slides were washed a minimum of 4 × 10 min in 0.1% PBS Tween prior to mounting in EMS Shield Mounting Medium with DAPI and DABCO (Electron Microscopy Sciences). The number of DAPI-stained bodies within diakinesis (DI) nuclei were scored from Z-stacks captured using LAS X software. Significance was analyzed using a two-tailed Fisher’s exact test to compare the prevalence of chromosomal abnormalities (identified as all nuclei with more than six or less than six DAPI bodies) and a one-tailed Fisher’s exact test to identify elevated fragmentation (more than six DAPI bodies) or fusions (less than six DAPI bodies).
Brood size analysis
L4 hermaphrodite worms were plated and transferred to new plates approximately every 24 hr over the course of 3 days. Total hatched progeny on each plate were counted 2–3 days after being laid. All strains were assessed in parallel and data were pooled from multiple rounds of analyses. Significance for all experiments was assessed using a Student’s t-test with Welch’s correction.
Quantification of germ line apoptosis
Apoptotic germ line nuclei were scored in indicated worms by acridine orange and ced-1::GFP expression as described (Jaramillo-Lambert and Engebrecht 2010). Significance for all data sets was analyzed using a two-tailed Mann–Whitney test. Data represented in Figure 1D, Supplemental Material, Figure S2D, Figure S8A, and Table 1 were from independently conducted experiments.
Figure 1.
Loss of Mi2 activates the recombination checkpoint. (A) Schematic of C. elegans recombination checkpoint pathway. (B and C) mRNA of proapoptotic BH3-only family member egl-1 is upregulated in Mi2 mutants, as determined by quantitative RT-PCR. zim-2(tm574) mutants were used as a positive control as a known activator of the recombination checkpoint without activating the synapsis checkpoint. (B) Data are expressed as 2−ΔCq in which egl-1 levels were normalized to the reference gene cdc-42. Error bars indicate SD of four biological replicates. Statistical comparisons between data sets were conducted using a two-tailed Student’s t-test: wild-type vs. zim-2, P = 0.0068; wild-type vs. let-418, P = 0.0157; and wild-type vs. chd-3, P = 0.0113. (C) Data are expressed as fold change in egl-1 expression normalized to wild-type (N2). Error bars represent range of expression: let-418 = 2.5–3.1-fold, chd-3 = 2.1– 2.9-fold, and zim-2 = 4.3–6.1-fold. (D) Apoptosis in let-418(n3536) and chd-3(eh4) germ lines is suppressed by cep-1(RNAi) and chk-1(RNAi). Assessment of apoptotic bodies per gonad arm was measured using acridine orange staining. Total number of gonads examined: N2 L4440, n = 52; cep-1(RNAi) L4440, n = 25; chk-1(RNAi) L4440, n = 16; zim-2(tm574) L4440, n = 118; zim-2(tm574);cep-1(RNAi) n = 83; zim-2(tm574);chk-1(RNAi), n = 35; let-418(n3536) L4440, n = 100; let-418(n3536);cep-1(RNAi), n = 28; let-418(n3536);chk-1(RNAi), n = 49; chd-3(eh4) L4440, n = 111; chd-3(eh4);cep-1(RNAi), n = 88; and chd-3(eh4);chk(RNAi), n = 19. Error bars correspond to SEM. Statistical comparisons between data sets in (D) were conducted using a two-tailed Mann–Whitney test. For comparisons in (B and D), * P ≤ 0.05, ** P ≤ 0.005, and *** P ≤ 0.0001. Unst., unstable.
Table 1. Germline apoptosis in NuRD complex mutants.
| Genotype | Number of apoptotic bodies/gonad arm | n |
|---|---|---|
| wild-type (N2) | 0.55 ± 0.1 | 77 |
| zim-2(tm574) | 2.30 ± 0.8** | 20 |
| hda-1(RNAi) | 2.66 ± 0.3** | 56 |
| let-418(n3536) | 2.36 ± 0.3** | 77 |
| chd-3(eh4) | 1.62 ± 0.3** | 47 |
| chd-3(vv133) | 1.22 ± 0.3* | 37 |
| chd-3(ok1641) | 0.91 ± 0.2* | 38 |
| chd-3(RNAi); let-418(n3536) | 3.11 ± 0.3** | 57 |
Apoptotic bodies quantified by scoring acridine orange stained bodies per gonad arm. zim-2 was used as a control as it is a viable mutant defective in chromosome V pairing and synapsis (Phillips and Dernburg 2006). All animals were staged and scored as adults at ∼48 hr after the L4 larval stage. All experiments were performed at 20°. n = total gonad arms scored per genotype. Data are mean ± SEM. Statistical comparisons between wild-type and each strain were conducted using a two-tailed Mann–Whitney test.
P ≤ 0.05 and ** P ≤ 0.0001. Statistical comparison between let-418 and chd-3(RNAi);let-418: P = 0.0639.
Quantitative RT-PCR
For RNA extraction, worms were washed 4× with M9 buffer. Per 15 µl of worm pellet, 250 µl of a phenol/chloroform/guanidine isothiocyanate solution was added (IBI Isolate, IBI Scientific). Worms were solubilized via three rounds of freeze/thaw at −80° with extensive vortexing. RNA was extracted from 250 µl of IBI Isolate using chloroform, and contaminating DNA in the aqueous fraction was removed (Spin Away Filter, Zymo Research). RNA was further purified using a column-based method with DNase I treatment (Quick RNA Miniprep, Zymo Research). The integrity of purified RNA was analyzed by gel electrophoresis and A260/A280 ratios were ≥ 2.0 (NanoDrop Lite, Thermo Fisher Scientific). cDNA was synthesized using 125 ng input RNA and oligo d(T)23VN primers (OneTaq RT-PCR, New England Biolabs, Beverly, MA). Quantitative RT-PCR (qRT-PCR) was performed using 2.0 µl cDNA per well (in triplicate) in 20 µl final reaction volume containing 0.3 µM primers and 1× iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA). PCR conditions (CFX96 Real-Time PCR Detection System, Bio-Rad) were as follows: initial denaturation at 94° for 30 sec, followed by 40 cycles of denaturation at 94° for 5 sec, and annealing/extension at 60° for 30 sec. Primers were designed to span at least one exon–exon junction to eliminate genomic DNA amplification. The exception was the egl-1 primer set, although these primers were separated by an intron. cdc-42 was chosen as a normalizer gene as its expression in C. elegans was shown to be highly stable, as determined by meta-analysis of microarray expression data and qRT-PCR (Hoogewijs et al. 2008). Standard curves using the various primer sets and fivefold serial dilutions of zim-2 cDNA were performed to assess the suitability of the comparative quantification cycle (Cq) method of analysis. Slopes of the dilution curves were analyzed mathematically and differences were shown to be insignificant. qPCR data were expressed as mean 2−ΔCq ± SD (Schmittgen and Livak 2008). For statistical analysis of 2−ΔCq data, differences between mean egl-1 expression were analyzed using a two-tailed Student’s t-test using an α level of 0.05. These data were also expressed as fold change in expression compared to wild-type (N2) using the comparative Cq method (Livak and Schmittgen 2001). Error bars indicate the range of egl-1 expression and were calculated as follows: the SD of each ΔΔCq was calculated by propagating the error of the target gene and normalizer gene Cq values. Range of egl-1 expression was calculated as mean ΔΔCq ± SD. These values were then transformed (2−ΔΔCq) and graphed. Data were obtained from four independent experiments.
Primer sequences were as follows:
SDS-PAGE and western blotting
For preparation of whole-worm lysates for western blotting, worms were washed 3× with cold M9. Worm pellets were resuspended in TNN buffer (50 nM Tris pH 8, 120 nM NaC1, 0.5% NP-40, and 1 mM dithiothreitol) plus 1× protease/phosphatase inhibitor cocktail (Pierce Protease and Phosphatase Inhibitor Mini Tablets, Thermo Fisher Scientific) at a ratio of 4 v/v of worm pellet. Samples were snap frozen at −80°, partially thawed, and sonicated for three rounds of 7 sec each. Worm lysates were incubated for 15 min at 4° with constant inversion, cellular debris was removed via centrifugation, protein was quantified via Bradford assay (Bradford Reagent, Bio-Rad), and samples were stored at −80°. For SDS-PAGE, 12 µg samples were resuspended in reduced sample buffer, boiled for 5 min, and fractionated on a 12% polyacrylamide gel. Proteins were electroblotted via wet transfer to PVDF membrane (Millipore, Bedford, MA) at 70 V for 2 hr. Membranes were blocked with TBST/5% bovine serum albumin (BSA) for 1 hr and probed overnight at 4° with rabbit anti-pChk1 (Ser345) (#2341; Cell Signaling Technologies) or mouse anti-Actin (clone C4; MP Biomedical). Primary antibodies were diluted in TBST/5% BSA at a ratio of 1:1000 or 1:10,000, respectively. Blots were probed for 1 hr at room temperature with goat anti-rabbit-HRP or goat anti-mouse-HRP diluted 1:10,000 (Millipore) in TBST/5% BSA. Secondary antibodies were detected via enhanced chemiluminescence (Clarity Max ECL Substrate; Bio-Rad) and exposure to film. To quantify western blotting results, films were scanned at 600 dpi (dots per inch) and densitometry was performed using ImageJ software. pChk-1 signal in Mi2 mutants was normalized to actin and then normalized to wild-type (N2) to obtain fold expression.
Data availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Results and Discussion
NuRD machinery attenuates germ line apoptosis
In C. elegans, the germ line is the only tissue to undergo apoptosis as a response to stress, making it an ideal system for the identification of genes involved in maintaining cellular homeostasis and promoting genetic integrity (Gartner et al. 2008; Kirienko et al. 2010). Our prior work revealed increased germ line apoptosis in response to RNAi knockdown of several classes of chromatin remodelers, including the HDAC1 homolog hda-1 (Checchi and Engebrecht 2011), which encodes a histone deacetylase that functions as part of a conserved NuRD complex (Shi and Mello 1998; Passannante et al. 2010). In mammals, NuRD is essential for maintaining genomic stability and dysregulation of its core catalytic components, the Mi2 subunits CHD4 and CHD3, has been implicated in several human cancers as well as in heritable developmental disabilities (Weiss et al. 2016; Mills 2017). The C. elegans genome encodes two Mi2 paralogs, chd-3 and let-418. Although the intersubunit connectivities among mammalian NuRD subunits are controversial (Torrado et al. 2017), biochemical data in worms support a model wherein CHD-3 and LET-418 bind several conserved core NuRD components, including HDA-1 (HDAC1), LIN-53 (RbAP), and LIN-40 (MTA1), and can function as members of separate NuRD complexes (Passannante et al. 2010). To investigate the role of both Mi2 subunits in maintaining genetic integrity, we assessed the consequences of CHD-3 and LET-418 (CHD4) loss both individually and in combination in the C. elegans germ line.
With the exception of chd-3, null mutations in NuRD complex-encoding genes result in sterility and/or embryonic lethality in C. elegans (Johnsen and Baillie 1991; Lu and Horvitz 1998; von Zelewsky et al. 2000; Dufourcq et al. 2002). To assess the role of both Mi2 paralogs during meiosis, we took advantage of let-418(n3536), a hypomorphic allele that is larval lethal at 25° but viable at lower temperatures (Andersen 2006). We quantified germ cell corpses in let-418(n3536) hypomorphs at 20° as well as three putative null alleles of chd-3, and discovered significantly elevated apoptosis in all mutants examined as compared to wild-type (Table 1).
Interestingly, combined knockdown of both Mi2 subunits did not increase the number of apoptotic bodies as compared to let-418(n3536) alone (Table 1, P = 0.064). This result was somewhat surprising given that let-418(n3536);chd-3(eh4) double mutants are inviable, even at lower temperatures (Käser-Pébernard et al. 2016; C. A. Turcotte and P. M. Checchi, unpublished observations). The lack of either an additive or synergistic phenotype with regard to germ line apoptosis in chd-3(RNAi);let-418(n3536) germ lines hints that, in certain contexts, Mi2 subunits can function nonredundantly. These data are consistent with several other reports in worms and mammals proposing nonredundant functions for Mi2 (Passannante et al. 2010; Nitarska et al. 2016).
These results are also intriguing given that no prior phenotypes have been observed in chd-3 single mutants with regard to fecundity (von Zelewsky et al. 2000; Passannante et al. 2010). We validated these results by assessing apoptosis in worms expressing CED-1::GFP, a reporter that marks engulfed apoptotic nuclei in the adult gonad (Zhou et al. 2001; Gartner et al. 2008). In chd-3(RNAi);CED-1::GFP germ lines, we found significantly higher numbers of corpses as compared to CED-1::GFP (empty vector) controls (P < 0.0001, Figure S1). These data support our inference that maintenance of germ line integrity in C. elegans requires both Mi2 subunits, and suggest that loss of either subunit triggers removal of faulty germ cells.
Activation of the recombination/DNA damage response checkpoint (DDR) in germ lines lacking Mi2
Germ line apoptosis in C. elegans is initiated by several types of stressors, which can be genotoxic, physiological, or due to environmental insults or pathogens (Gartner et al. 2008; Lant and Derry 2014). Although all of these stressors result in cell death via engagement of the core apoptotic machinery, the preceding steps differ. For example, checkpoint activation is only involved in a subset of these scenarios, such as in response to defects in chromosome dynamics or threats to genomic integrity. As Mi2 regulates the response to DNA damage in somatic tissues in mammals (O’Shaughnessy and Hendrich 2013) and suppresses transgene activation (a known source of genotoxic stress) in worms (O’Neil and Rose 2006; McMurchy et al. 2017), we speculated that the increase in corpses in Mi2 mutant germ lines (Table 1) was likely a downstream consequence of checkpoint activation.
To test whether loss of Mi2 activates checkpoints, we first used quantitative RT-PCR to measure levels of egl-1 expression. egl-1 encodes one of two proapoptotic BH3 family members activated in response to insults stemming from DNA-damaging agents or from defective chromosome dynamics; as a direct consequence, the core apoptotic machinery is engaged (Hofmann et al. 2002; Ye et al. 2014; Wang and Yang 2016; Figure 1A). We found significantly elevated egl-1 in both let-418(n3536) and chd-3(eh4) mutants as compared to wild-type controls (Figure 1, B and C). These data indicate that apoptosis in Mi2 mutants is a downstream consequence of EGL-1 activity and is likely to be checkpoint-dependent.
As egl-1 is a known target of several checkpoint pathways operating in meiosis (Garcia-Muse and Boulton 2005; Ye et al. 2014), we next asked which of these checkpoints were activated in Mi2 mutants. As a chromatin remodeler, we suspected that loss of Mi2 disrupts one or more aspects of genomic integrity and thus would activate checkpoints that recognize genomic instability. Therefore, we assessed apoptosis in Mi2 mutants depleted for components of the DDR and a mechanistically related version of the DDR known as the recombination checkpoint. Whereas the DDR is engaged in the response to genomic damage, the recombination checkpoint monitors the first meiotic prophase and is triggered in response to defects in meiotic chromosome dynamics, such as unrepaired recombination intermediates (Longhese et al. 2009; Figure 1A). Although the source of their activation differs, most of the machinery involved, as well as the net outcome (additional time for repair or culling of damaged germ cells), are preserved.
The DDR/recombination checkpoints converge at activation of the C. elegans p53 homolog CEP-1, which in turn activates egl-1 transcription (Derry 2001; Schumacher et al. 2001; Hofmann et al. 2002; Garcia-Muse and Boulton 2005; Figure 1A). Importantly, loss of CEP-1 does not disrupt other meiotic checkpoint pathways, and the synapsis checkpoint remains intact in cep-1 germ lines (Xu et al. 2012). We therefore tested whether cep-1 inactivation suppressed apoptosis in let-418 and chd-3 germ lines. In both cep-1(RNAi);let-418(n3536) and cep-1(RNAi);chd-3(eh4) worms, we observed a significant reduction in corpses, suggesting that the presence of both Mi2 paralogs is necessary to prevent the activation of the DDR/recombination checkpoint (Figure 1D). These findings also provide a plausible explanation for the increased brood size recently reported in cep-1;let-418 double mutants (McMurchy et al. 2017), which our data indicate is at least in part due to a reduction in germ line apoptosis. As a complementary approach, we also tested whether apoptosis is suppressed in Mi2 mutants lacking an upstream component of the recombination/DDR pathways, checkpoint kinase CHK-1. chk-1 encodes an essential protein that is required for the transduction of checkpoint signaling in response to DNA lesions in multiple species, and its absence eliminates the DDR (Kalogeropoulos et al. 2004; Olsen et al. 2006; Zhang and Hunter 2014; Figure 1A). During C . elegans meiosis, CHK-1 is activated in response to impaired chromosome pairing or defective DSB repair (Jaramillo-Lambert et al. 2010; Kim and Colaiácovo 2014). We found that like cep-1, RNAi depletion of chk-1 resulted in a significant reduction in corpses in let-418 and chd-3 germ lines (Figure 1D).
Although neither cep-1 RNAi nor chk-1 RNAi reduced apoptosis to basal levels in either mutant examined, we attribute this to incomplete knockdown, as suppression was comparable with what we observed in response to cep-1 knockdown in zim-2 controls (Figure 1D), which activates only the recombination checkpoint (Phillips and Dernburg 2006). Further, inactivation of an alternate checkpoint pathway known as the synapsis checkpoint does not suppress apoptosis in germ lines lacking other NuRD components such as HDA-1 (M. Romer and P. M. Checchi, unpublished observations), a binding partner of both Mi2 subunits in worms and mammals (Denslow and Wade 2007; Passannante et al. 2010). Knowing this, we predicted that apoptosis was unlikely to be affected in Mi2 mutants lacking the synapsis checkpoint. To rule out involvement of the synapsis checkpoint, we constructed double mutants lacking LET-418 as well as PCH-2, a highly conserved ATPase that culls damaged cells in response to synapsis failure (Bhalla et al. 2005). In pch-2(tm1458) mutants, the synapsis checkpoint is nonfunctional, yet the DNA damage/recombination checkpoints remain intact, which provides a means of distinguishing whether one or both checkpoints are activated in different mutant contexts. In syp-1(me17) mutants, for example, the central element of the synaptonemal complex (SC) fails to load, and both the recombination and synapsis checkpoints are activated; consequently, partial suppression of apoptosis is observed in pch-2;syp-1 double mutants (Figure S2; MacQueen 2002; Bhalla et al. 2005). In contrast, we observed no difference in the number of apoptotic bodies in let-418 vs. pch-2;let-418 double mutants, and apoptosis was significantly elevated in the double mutants as compared to wild-type controls (P < 0.0001; Figure S2). Combined with our aforementioned analyses using cep-1(RNAi) and chk-1(RNAi), these data indicate that loss of Mi2 triggers the recombination checkpoint but not the synapsis checkpoint.
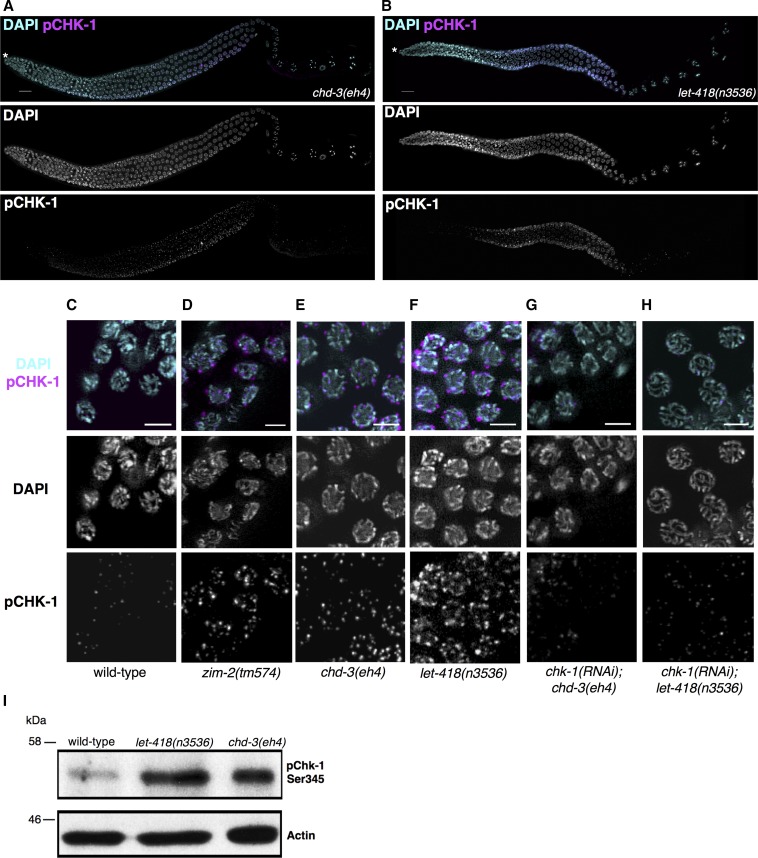
As our genetic data reveal a relationship between Mi2 and activation of the DDR/recombination checkpoint, we next sought to explore the cellular and molecular involvement of this pathway in let-418 and chd-3 mutant germ lines. We probed chd-3(eh4) and let-418(n3536) germ lines using a phospho-specific CHK-1 (pCHK-1) antibody (Figure 2, A–G). In both mutants, we detected increasing levels of pCHK-1 in meiotic germ cells that peaked in late pachytene, thus reinforcing our hypothesis that let-418 and chd-3 mutants incur meiotic defects (Figure 2, A and B). In support of this, pCHK-1 was not detected in wild-type germ lines and was suppressed by chk-1 RNAi in all animals examined (Figure 2, C–H). As confirmation, we quantified pCHK-1 protein in these animals and found that levels were elevated 3.7-fold and 2.2-fold in let-418 and chd-3, respectively (Figure 2I and Figure S3A). The specificity of this phospho-specific antibody was confirmed using chk-1 RNAi (Figure S3B). Total levels of CHK-1 protein were not assessed due to the lack of a specific antibody for use in western blotting that cross-reacts with C. elegans. However, we observed no difference in chk-1 mRNA levels in N2, zim-2, let-418, and chd-3 (Figure S3C), suggesting that CHK-1 protein levels were equivalent in these strains. These results indicate that the loss of either Mi2 paralog alone is sufficient to activate CHK-1, even in the absence of exogenous damage.
Figure 2.
Checkpoint kinase CHK-1 is activated in germ lines lacking Mi2. Phosphorylated CHK-1 (pChk1) accumulates during prophase I and peaks in late pachytene in chd-3(eh4) (A) and let-418(n3536) (B) germ lines. Germ lines were stained with anti-pChk1 (Ser345) (magenta) and counterstained with DAPI (cyan). Germ lines are oriented from left to right with white asterisk denoting the distal tip (regions of prophase I are described in Figure 4A). Bar, 15 μM. (C–F) Representative images of late pachytene nuclei in N2, zim-2(tm574), chd-3(eh4), and let-418(n3536). pChk1 specificity was determined using chk-1 RNAi for all strains, and results are shown for chd-3(eh4) (G) and let-418(n3536) (H). Exposure parameters were identical for all data collected. Bar, 5 μM. (I) Wild-type (N2), let-418, and chd-3 whole-worm lysates were fractionated by SDS-PAGE and levels of pCHK-1 were analyzed by western blot. Actin served as a loading control. A representative blot of three independent experiments is shown. Compared to wild-type (N2) controls, levels of pChk1 protein are elevated 3.8-fold in let-418(n3536) and 2.3- in chd-3(eh4). Specificity of the pChk1 antibody used for western blotting is shown in Figure S3B. No., number; RNAi, RNA-interference.
Reduced Mi2 function compromises fertility
During meiosis, an advantage of checkpoint-mediated apoptosis is that it provides a protective mechanism in response to altered chromatin dynamics, thereby preventing the formation of gametes with abnormal chromosomes (Subramanian and Hochwagen 2014). In some instances, meiotic quality control mechanisms have been reported to mask nearly all errors, thereby drastically improving gamete quality (Mei et al. 2015). In spite of this, activation of the recombination/DDR checkpoints alone is not sufficient to eliminate all defective nuclei. The remaining fraction of damaged nuclei that are neither repaired nor removed can manifest as damaged gametes, which compromise fertility and can result in developmental defects in offspring (Jaramillo-Lambert et al. 2010; Bailly and Gartner 2013). Similar defects are observed in in mammals, wherein aberrant survival of defective germ cells is associated with the formation of germ cell tumors (De Felici and Klinger 2015). Therefore, we asked whether defects in Mi2 activity affect subsequent generations. Our findings that chd-3 mutants display elevated checkpoint-dependent apoptosis (Figure 1 and Figure 2) led us to speculate that the loss of CHD-3 alone is enough to compromise fertility. To test this, we performed a brood size analysis of all three predicted null alleles of chd-3 as well as let-418(n3536) hypomorphs raised at 20°. Indeed, brood sizes of all chd-3 alleles was significantly lower than in wild-type, and were consistent with the extent to which apoptosis was elevated in these strains (Figure 3 and Table 1).
Figure 3.
Mi2 mutants have reduced fertility. Total hatched offspring were scored in the F1 generation of the following strains, wherein n = total number of animals examined for brood size: wild-type (N2), n = 17; chd-3(eh4) n = 19; chd-3(vv133), n = 29; let-418(n3536), n = 10; chd-3(ok1651), n = 17; and chd-3(RNAi);let-418(n3536), n = 8. All animals scored were F1 generation, with the exception of chd-3(RNAi);let-418(n3536), which were the F2 offspring of let-418(n3536); chd-3(RNAi) F1 progeny hatched on chd-3(RNAi) plates. Each data point is representative of the total number of viable offspring corresponding to a single hermaphrodite parent. Horizontal lines correspond to mean for each data set, which was compared to N2 or let-418 and assessed using a two-tailed Student’s t-test with Welch’s correction. * P < 0.05 compared to N2. ** P ≤ 0.0001. RNAi, RNA-interference.
Corroborating recent findings revealing fertility defects in let-418(n3536) grown at restrictive temperatures (McMurchy et al. 2017), we also observed a reduction in brood size of let-418(n3536) hypomorphs that was exacerbated by chd-3 RNAi (Figure 3). Unlike let-418(RNAi);chd-3(eh4) animals, wherein all of the F1 progeny reportedly died as embryos (von Zelewsky et al. 2000), we saw no obvious lethality in chd-3(RNAi);let-418 offspring, as unhatched embryos were not observed in any of our brood size analyses (data not shown). However, the majority of F1 survivors were sterile and possessed ectopic vulva, indicating a synergistic interaction consistent with the previously reported role for LET-418 and CHD-3 in the development of the somatic gonad (data not shown; von Zelewsky et al. 2000). As neither embryonic lethality nor a significant increase in apoptosis in chd-3(RNAi);let-418 germ lines as compared to other Mi2 mutants was observed (Table 1 and data not shown), it is likely that somatic defects at least partially account for the severe reduction in brood size observed when both Mi2 mutants are depleted.
Mi2 facilitates repair of meiotic DSBs
As several studies have reported a role for NuRD in response to DNA damage in somatic tissue (Larsen et al. 2010; Polo et al. 2010; Smeenk et al. 2010; Gong et al. 2015), we questioned whether a similar scenario could occur in adult germ cells. To date, no studies have investigated the contributions of NuRD during meiosis, and we therefore asked whether Mi2 plays a role in the regulation of meiotic DSBs. To test this, we took advantage of the unique spatiotemporal organization of the C. elegans gonad, which allows for cytological analysis of the formation and repair of DSBs (Lui and Colaiácovo 2013; Hillers 2017; Figure 4A). To determine whether loss of either Mi2 subunit manifests in defective DSB repair, we monitored the kinetics of the recombinase RAD-51 in wild-type worms, let-418(n3536) hypomorphs, and chd-3(eh4) null mutants, both singly and in combination (Figure 4, B–F).
Loading of RAD-51 occurred normally in all strains examined, with foci first appearing in midtransition zone (leptotene/zygotene) nuclei, consistent with nuclear morphology indicative of chromosome pairing (Figure 4, B and C and data not shown). In wild-type germ lines, RAD-51 foci decrease in number throughout pachytene, concomitant with the repair of DSBs via HR (Alpi et al. 2003; Figure 4, A, B, and D–F). In contrast, let-418(n3536) and chd-3(eh4) mutants displayed persisting RAD-51 foci that were significantly elevated throughout early and midpachytene, and were still visible in late pachytene (Figure 4, B and D–F). Quantification of these data revealed a significant increase in RAD-51 foci in let-418 and chd-3 mutants as compared to wild-type throughout pachytene (Figure 4B), indicating a role for both paralogs in mediating the repair of DSBs through HR. While partial loss of both paralogs resulted in a high frequency of animals with either partial or complete sterility and/or impaired gonad development, chd-3(RNAi);let-418(n3536) mutants possessing intact germ lines contained no difference in the number of RAD-51 foci as compared to let-418 mutants (data not shown and Figure 4B). This result indicates that although both LET-418 and CHD-3 are each required for ameliorating HR, their roles are likely nonredundant in this context. Notably, RAD-51 foci were not observed in mitotically dividing germ cells of either mutant, singly or in combination (Figure S4). This result, along with our observations that pCHK-1 foci are limited to meiotic nuclei (Figure 2, A and B), indicates that loss of Mi2 is not linked to endogenous replicative damage, as is the case for other HR repair mutants that incur spontaneous DSBs (Bailly et al. 2010).
Our findings thus far support a model whereby checkpoint activation in let-418(n3536) and chd-3(eh4) germ lines is at least in part due to aberrant handling of meiosis-specific DSBs, which could result from either excessive DSB production, defective processing, or both. To discern between these possibilities, we quantified RAD-51 foci in the absence of RAD-54, a protein required for RAD-51 dissociation whose loss in C. elegans “traps” recombination intermediates, thereby enabling an estimation of total DSBs formed per nucleus (Solinger et al. 2002; Mets and Meyer 2009). We found an equivalent number of RAD-51 foci in rad-54(ok615);let-418(n3536) mutants vs. rad-54(ok615) alone, indicating that loss of LET-418 does not affect the number of DSBs produced (Figure S5). As rad-54 germ lines retain all SPO-11-induced DSBs (Mets and Meyer 2009), the equivalent number of RAD-51 foci observed in rad-54;let-418 germ lines indicates that persisting DSBs in let-418 germ lines are most likely SPO-11-dependent. This interpretation is supported by the absence of RAD-51 or pCHK-1 foci in mitotic germ cells of either let-418 or chd-3, as would be expected in mutants that accrue SPO-11-independent damage (Figure 2, A and B and Figure S4 and data not shown). Nonetheless, as these data do not formally exclude the alternative explanation that the increase in late-stage recombination intermediates is due to spontaneous damage, future experiments will be conducted to address this possibility.
As RAD-51 removal is delayed in both let-418(n3536) and chd-3(eh4) germ lines, we next asked whether compromised DSB repair in either mutant could manifest in visible defects in chromosome integrity. In C. elegans, these types of structural defects can be detected in late prophase nuclei as either additional DAPI-staining bodies, chromosomal fusions, or fragmented DNA (Hillers 2017). As in wild-type worms, nearly all chd-3 and let-418 mutants possessed six intact DAPI-staining bodies in DI-stage oocytes, corresponding to six paired homologs (Figure 5, A and B). In contrast, structural defects were seen in 33% of chd-3(RNAi);let-418 DI nuclei, the majority of which contained four or five DAPI-staining structures, indicative of chromosome fusions (Figure 5, A and B). Fusions are elicited in response to defective DSB repair, and can result from either spurious activation of error-prone repair or telomerase deficiency (Wong et al. 2000; Lowden et al. 2011; Hillers 2017). As either scenario would be consistent with elevated checkpoints and increased genomic instability, we conclude that the presence of Mi2 is required for promoting HR and the prevention of gross chromosomal abnormalities that would compromise fertility, as evidenced by the significant reduction in viable gametes in chd-3(RNAi);let-418 mutants (Figure 3).
Figure 5.
Mi2 deficiency results in defects in diakinetic chromosome structure. Representative images (A and C) and percent diakinetic (DI) nuclei (B and D) of DAPI-stained chromosomes from a DI −1 oocyte (stage immediately prior to spermatheca entry) in (B) wild-type (N2) L4440 (n = 77), chd-3(eh4) (n = 167), let-418(n3536) L4440 (n = 78), and chd-3(RNAi);let-418(n3536) (n = 24), and (D) syp-3(ok758) (n = 27), syp-3(ok758);let-418(n3536) L4440 (n = 16), and chd-3(RNAi); syp-3(ok758);let-418(n3536) (n = 13). Number of visibly stained bodies is indicated in upper left corner of image. Abnormal chromosome structures are indicated by white arrowheads. Nuclei for which individual DAPI bodies could not be identified were scored as unstable (Unst.). Bar, 2 μM. Chromosomal abnormalities in DI −1 nuclei in (B and D) were assessed using a Fisher’s exact test, and were significant following comparisons: N2 L4440 vs. chd-3(RNAi);let-418: P < 0.0001; chd-3 vs. chd-3(RNAi);let-418: P < 0.0001; let-418 L4440 vs. chd-3(RNAi);let-418: P = 0.0003; and syp-3 vs. syp-3;let-418: P = 0.0012; syp-3 vs. chd-3(RNAi);syp-3;let-418: P = 0.0378.
The fact that visible defects in chromosome integrity are uncovered only when both Mi2 subunits are disrupted (Figure 5, A and B) is supported by biochemical data suggesting that both LET-418 and CHD-3 operate independently in two distinct NuRD complexes in worms (Passannante et al. 2010). Corroborating these data, recent findings by Hoffmeister et al. (2017) provide strong biochemical evidence demonstrating that mammalian CHD3 and CHD4 also form isoform-specific NuRD complexes. Perhaps most compelling is the discovery that these distinct CHD3 and CHD4-NuRD-containing complexes are also independently recruited to sites of induced DNA damage, suggesting overlapping functionality in this context (Hoffmeister et al. 2017). These data provide a particularly fitting explanation for the phenotypes we observed in Mi2-deficient worms with regard to DSB repair (Figure 4 and Figure 5).
We next asked how LET-418 and CHD-3 contribute toward HR repair. Defects in meiotic DSB repair can involve HR by either disrupting interhomolog interactions, intersister interactions, or both. Disruption of interhomolog repair inhibits crossovers, and in all sexually reproducing organisms, an outcome of defective crossovers is aneuploidy. In C. elegans, aneuploidy is detected in offspring as a Him phenotype (high incidence of males) as a result of X chromosome nondisjunction, and is typically accompanied by embryonic lethality (Hodgkin et al. 1979; Schedl 2013; MacLennan et al. 2015). To test whether aneuploidy contributes to low brood size in animals lacking Mi2, which would be indicative of interhomolog repair defects, we determined the percentage of male offspring in chd-3(eh4) and let-418(n3536) hypomorphs. Consistent with prior experiments, total brood size (as measured by average number of offspring produced by eight mutants per strain examined) was significantly reduced in both Mi2 mutants as compared to wild-type controls (Figure 3 and Figure S6A). However, no males were observed in the offspring of let-418 hermaphrodites (n = 703 total animals examined), and only one male was observed in the progeny of chd-3(eh4) mutants (n = 1227; Figure S6A). Furthermore, no males were found in the F1 offspring of chd-3(RNAi);let-418 mutants (n = 433), indicating that animals are still competent to form crossovers even in the absence of Mi2 (Figure S6A).
Given that an absence of viable, aneuploid offspring can be masked by high levels of apoptosis (Mei et al. 2015), we also performed cytological analyses to probe Mi2-deficient germ lines for defects that could impact HR. We monitored SC loading in let-418(n3536) and chd-3(eh4) mutants, both singly and in combination. SC assembly links homologous chromosomes and is often disrupted in mutants with compromised HR (Cahoon and Hawley 2016). In C. elegans, several mutants that disrupt chromosome pairing fail to load SC components without activating the synapsis checkpoint (Bhalla 2005; Phillips and Dernburg 2006). Our data indicate that pairing and synapsis are normal in mutants lacking one or both Mi2 subunits, as the SC central element protein SYP-1 was detected on all pachytene chromosomes (Figure S6B). These data support our assertion that decreased brood size in Mi2 mutants is not due to aneuploidy, and are consistent with the lack of male offspring or inviable embryos in these mutants (Figure S6 and data not shown).
An absence of aneuploid embryos indicates that chromosomes are competent for crossover formation, an outcome of one type of HR known as interhomolog repair. As interactions between homologs appeared normal in Mi2 mutants (Figure S6), we next considered the possibility that Mi2 could instead mediate HR by promoting recombination between sister chromatids. To address this, we monitored recombination in mutants lacking a functional SC. In syp mutants, interhomolog interactions do not occur, although germ cells remain competent for intersister repair (Smolikov et al. 2007; Adamo et al. 2008; Bickel et al. 2010). This results in 12 distinct, univalent chromosomes in oocytes. However, when syp mutants are combined with mutants that disrupt intersister repair, the resulting oocytes display chromosome fragmentation as both interhomolog and intersister repair pathways are disrupted (Colaiácovo et al. 2003; Smolikov et al. 2007; Adamo et al. 2008; Bickel et al. 2010). As a consequence, syp mutants provide a sensitized background that can reveal gene products that mediate HR but are not implicitly required for the formation of crossovers. These mutants allow us to interrogate the role of Mi2 in HR between sister chromatids, and to address this we generated syp-3(ok758);let-418(n3536) mutants.
As expected, univalents appeared structurally normal in syp-3 single mutants, yet several syp-3(ok758);let-418(n3536) oocytes contained frequent chromosomal aberrations, including decondensed chromatin and more or fewer than expected DAPI bodies, indicative of either fusions or fragmentation, respectively (Figure 5, C and D). From this, we conclude that although HR is not fully abolished when Mi2 function is compromised, LET-418 could play a role in processing recombination intermediates or repairing DSBs once constraints for interhomolog bias are lifted (Figure 7). Notably, when combined with RNAi knockdown of chd-3, DAPI morphology in several chd-3(RNAi);syp-3;let-418 germ lines was disrupted to the extent that chromosome number was unable to be counted, indicating severe instability (Figure 5, C and D). In addition to a possible role in mediating intersister repair, the presence of increased fusions in both chd-3(RNAi);let-418 as well as the sensitized syp-3;let-418 background hints at a possible role for Mi2 involvement in noncrossover pathways, which, when defective, can activate alternative repair routes such as nonhomologous end joining (NHEJ) and manifest in this phenotype (Shrivastav et al. 2008; Hillers 2017).
Figure 7.
Model: Mi2 maintains gamete quality by promoting the timely and error-free repair of programmed meiotic double-strand breaks (DSBs). (A) Programmed meiotic DSBs occur in the transition zone (TZ) and are progressively repaired throughout pachytene [early pachytene (EP to late pachytene (LP); midpachytene (MP)] in wild-type germ lines. Chromosomes condense through diplotene (DP) and diakinesis (DI), prior to fertilization and formation of viable gametes. In wild-type germ lines (A), LET-418 and CHD-3 encourage homologous recombination (HR)-mediated repair of programmed DSBs and ensure production of fertile offspring. Absence of Mi2 (B) allows accrual of unrepaired recombination intermediates and generates genotoxic stress, as evidenced by checkpoint-mediated apoptosis. Loss of Mi2 additionally releases constraints on mutagenic repair, permitting the engagement of pathways such as nonhomologous end joining (NHEJ). Elevated apoptosis in mutants lacking Mi2 contributes to infertility and reduced gamete quality.
Evidence for Mi2 involvement in the suppression of NHEJ during meiosis
Although checkpoint activation and defects in the timely removal of RAD-51 in let-418(n3536) and chd-3(eh4) germ lines strongly support a role for Mi2 in promoting the repair of meiotic DSBs (Figure 1, Figure 2, and Figure 4), these findings alone do not address an underlying mechanism. Several classes of ATP-dependent chromatin remodelers have been directly implicated in establishing a chromatin landscape conducive to DNA repair (Lans et al. 2012). This led us to ask whether LET-418 and CHD-3 could play a similar role in generating a chromatin environment suitable for the recruitment and engagement of HR machinery in the germ line. In human somatic cells, CHD4 specifies a nucleosomal arrangement that is suitable for the repair of DSBs, and mutations in either Mi2 subunit cause structural defects in chromatin that compromise this process (Goodarzi et al. 2011; Sims and Wade 2011). Intriguingly, although CHD4 knockdown significantly impairs HR (Pan et al. 2012), other forms of repair appear to remain intact, as NHEJ can effectively repair DSBs in CHD4-depleted cells (Qi et al. 2016). Given our observations that recombination intermediates disappear by diplotene combined with the frequent occurrence of chromosome fusions in DI-stage chd-3(RNAi);let-418(n3536) nuclei (Figure 4 and Figure 5, A and B), we asked whether NHEJ contributes to the repair of meiotic DSBs in let-418(n3536) and chd-3(eh4) germ lines. As it cannot generate crossovers, NHEJ is normally blocked in C. elegans by the CtIP homolog COM-1 (Lemmens et al. 2013). However, there are several reports of spurious NHEJ activation in scenarios wherein HR is compromised due to defects in either SC assembly or RAD-51 processing (Martin et al. 2005; Smolikov et al. 2007; Yin and Smolikove 2013; Lawrence et al. 2016).
C. elegans possess homologs of both Ku70 and Ku80 (cku-70 and cku-80, respectively), and an absence of either subunit disrupts heterodimerization and blocks NHEJ (Clejan 2006). We examined RAD-51 kinetics throughout prophase I in cku-80(ok861);let-418(n3536) and cku-80(ok861);chd-3(eh4) germ lines. Although the number of RAD-51 foci was comparable to let-418 and chd-3 single mutants in earlier stages (transition zone through midpachytene), we found consistently elevated numbers of RAD-51 foci in cku-80;let-418 germ lines in late pachytene (Figure 6A and Figure S7, and data not shown), indicating that at least a subset of meiotic DSBs are repaired by NHEJ in scenarios where LET-418 activity is compromised. As an additive effect on the number of persisting DSBs is only observed at the end of pachytene, our data indicate that additional mechanisms are still intact to block NHEJ in earlier stages. Additionally, the fact that increases in RAD-51 were only observed in cku-80;let-418, but not cku-80;chd-3, germ lines is consistent with the more pronounced phenotypes we observed in let-418 compared to chd-3 (Figure 4, B–F and Figure 6A).
If an absence of LET-418 enables the engagement of NHEJ as a secondary mode of repair, we speculated that an increase in RAD-51 foci in cku-80;let-418 double mutants would be accompanied by chromosomal fragmentation. To address this possibility, we assessed chromosome structure in DI-stage cku-80;let-418 germ lines and found that 26% of the double mutants possessed chromosomal abnormalities, nearly all of which were extra DAPI-staining bodies, indicating fragmentation (Figure 6, B and C). We next asked whether the combined loss of CHD-3 and LET-418 exacerbates repair defects in NHEJ-deficient germ lines. Though pronounced sterility and gonad deformities in chd-3(RNAi);cku-80;let-418 precluded extensive analysis, we discovered defects in 39% of DI oocytes examined (Figure 6C). These data are consistent with our findings that Mi2 deficiency results in persisting recombination intermediates and chromosomal fusions, a hallmark of spurious repair of meiotic DSBs via NHEJ (Figure 4 and Figure 5; Hillers 2017). Further, these findings indicate that defective let-418 nuclei that bypass the recombination checkpoint directly contribute to fertility defects, which we speculate is at least in part due to aberrant engagement of NHEJ (Figure 7). An intriguing possibility is that perhaps there is a relationship between Mi2 and the Fanconi anemia pathway, which regulates chromatin loading of HR proteins and, when defective, manifests in genetic instability that is in part due to spurious NHEJ (Cohn and D’Andrea 2008; Adamo et al. 2010; Michl et al. 2016).
In accordance with the pleiotropic nature of let-418 hypomorphs, we also discovered that a subset of cku-80(ok861);let-418(n3536) germ lines possessed disorganized nuclear morphology, which was not seen in either single mutant (Figure S7F). Both cku-80(ok861);let-418(n3536) and cku-80(ok861);chd-3(eh4) germ lines contained multiple highly condensed nuclei with numerous RAD-51 foci, reminiscent of corpses (Figure S7, E and F). Although the extent of germ line disorganization varied among these mutants, consistent with their pleiotropic nature, we also observed more apoptotic nuclei and fewer offspring in cku-80;let-418 as compared to let-418 single mutants (Figure S8). As severe morphological defects were only occasionally observed in cku-80;let-418 animals and were not found in cku-80;chd-3 mutants (Figure S7, E and F), these data corroborate our prior findings that loss of CHD-3 alone disrupts meiotic progression, but not to the same extent as its paralog (Figure 4). These observations are in agreement with previously reported interpretations of the phylogenetic relationship between the Mi2 paralogs, proposing that gene duplication gave rise to an essential copy (let-418) as well as a nonessential copy (chd-3), which is speculated to have acquired new developmental roles (Passannante et al. 2010).
Interestingly, CKU-80 also plays an auxiliary role in maintaining telomere stability that is predominant in situations where telomere length is compromised, which is independent from its role in promoting NHEJ (Boulton and Jackson 1996; Li et al. 2014). The excess of late pachytene DSBs that we observed in cku-80;let-418 mutants is consistent with a defect in NHEJ (Figure 6 and Figure S7). Further, as aberrant telomere regulation is coincident with chromosomal fusions and not fragmentation (Ahmed and Hodgkin 2000; Cheung 2006; Yanowitz 2008), our observations that a loss of CKU-80 significantly increased fragmentation in Mi2 mutants is indicative of errors stemming from CKU-80’s canonical role in NHEJ (Figure 6C). In support of this, there was no statistical difference in the number of chromosomal fusions observed in chd-3(RNAi);cku-80;let-418 mutants vs. cku-80 alone (Figure 6C). However, these data do not entirely exclude the prospect of telomere involvement, and future studies will address such possibilities. Notably, either scenario is consistent with our findings and the recent work of other laboratories (McMurchy et al. 2017), pinpointing LET-418 as a critical regulator of genome maintenance.
Conclusions
Faithful genome transmission necessitates extensive cross talk between checkpoint pathways and repair machinery, as well as a specialized chromatin environment in which such activities are carried out (Papamichos-Chronakis and Peterson 2012; Gospodinov and Herceg 2013). The latter is accomplished in part by ATP-dependent chromatin remodelers, whose activity directly affects access to DSBs and influences repair pathway selection (Price and D’Andrea 2013; Jeggo and Downs 2014). Given that defects in DSB repair are a direct cause of cancers, immune disorders, and age-related diseases in humans, the role of chromatin remodelers in these contexts has been the subject of numerous recent investigations (Alt et al. 2013; White and Vijg 2016; Raschellà et al. 2017; So et al. 2017; Tubbs and Nussenzweig 2017). However, in spite of their importance, the mechanisms by which chromatin modifiers coordinate DSB repair in meiotically dividing cells are poorly understood. Defining their contributions in this context is of considerable importance, as (1) germ cells are portals for all of our genetic information, which must remain intact to be passed on to subsequent generations, yet (2) all germ cells are subjected to self-inflicted DSBs, whose repair must be carried out with precision to form viable gametes. In this study, we have uncovered a previously undescribed role for the NuRD chromatin remodeling complex during meiosis, wherein Mi2 facilitates meiotic chromosome dynamics. Our findings that ATPase Mi2 promotes timely and accurate repair of DSBs in meiotic nuclei underscores its importance in protecting the germ line and ensuring gamete quality (Figure 7). These data are particularly intriguing in light of recent findings identifying LET-418 as one of several classes of heterochromatin factors required to suppress repetitive elements in both worms and mammals (Montoya-Durango et al. 2016; McMurchy et al. 2017). As CHD4 interacts directly with both checkpoint proteins and the HR machinery in human cell lines (Urquhart et al. 2011; Pan et al. 2012), an attractive possibility is that similar functional interactions may take place during meiosis. Although a role for Mi2 in the repression of repetitive elements is consistent with our observations that let-418 and chd-3 mutants have elevated checkpoint-dependent apoptosis, the finding that persisting recombination intermediates and pCHK-1 recruitment are limited to meiotic nuclei (Figure 2, Figure 4, and Figure S4) indicates that transgene suppression is not the sole means by which Mi2 maintains genetic stability. Instead, we favor an updated model wherein Mi2 functions in multiple capacities to maintain genetic integrity throughout development (Figure 7). Although future experiments will be necessary to explore these exciting possibilities, our findings emphasize the extensive cross talk between repair pathways and checkpoint signaling that occurs in the germ line, and uncover several novel roles for Mi2 in the coordination of such events.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.118.300686/-/DC1.
Acknowledgments
We thank M. Zetka and the Caenorhabditis Genetics Center for providing strains; A. Villeneuve for antibodies; J. Engebrecht, M. Dennis, and A. Scott for helpful discussions and critical reading of the manuscript; and A. Peruffo, J. Laibach, A. Nowakowski, M. Romer, and E. Leonard for technical assistance with experiments. This work was supported by the National Institutes of Health (grant 1R15 GM-117479 to P.M.C.) and the Barry Goldwater Scholarship and Excellence in Education Foundation (C.A.T.).
Footnotes
Communicating editor: M. Colaiácovo
Literature Cited
- Adamo A., Montemauri P., Silva N., Ward J. D., Boulton S. J., et al. , 2008. BRC-1 acts in the inter-sister pathway of meiotic double-strand break repair. EMBO Rep. 9: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo A., Collis S. J., Adelman C. A., Silva N., Horejsi Z., Ward J. D., Martinez-Perez E., Boulton S. J., La Volpe A., 2010. Preventing nonhomologous end joining suppresses DNA repair defects of Fanconi Anemia. Mol. Cell 39: 25–35. [DOI] [PubMed] [Google Scholar]
- Ahmed S., Hodgkin J., 2000. MRT-2 checkpoint protein is required for germline immortality and telomere replication in C. elegans. Nature 403: 159–164. [DOI] [PubMed] [Google Scholar]
- Alpi A., Pasierbek P., Gartner A., Loidl J., 2003. Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 112: 6–16. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Zhang Y., Meng F.-L., Guo C., Schwer B., 2013. Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell 152: 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen E. C., 2006. C. elegans ISWI and NURF301 antagonize an Rb-like pathway in the determination of multiple cell fates. Development 133: 2695–2704. [DOI] [PubMed] [Google Scholar]
- Bailly A. P., Freeman A., Hall J., Déclais A. -C., Alpi A., et al. , 2010. The Caenorhabditis elegans homolog of Gen1/Yen1 resolvases links DNA damage signaling to DNA double-strand break repair. PLoS Genet. 6: e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly A., Gartner A., 2013. Germ cell apoptosis and DNA damage responses, pp. 249–276. in Germ Cell Development, edited by Elegans C., Schedl T. Springer, New York, NY. [DOI] [PubMed] [Google Scholar]
- Bakkenist C. J., Kastan M. B., 2015. Chromatin perturbations during the DNA damage response in higher eukaryotes. DNA Repair (Amst.) 36: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta J., Rauchman M., 2015. The nucleosome remodeling and deacetylase complex in development and disease. Transl. Res. 165: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla N., 2005. A conserved checkpoint monitors meiotic chromosome synapsis in Caenorhabditis elegans. Science 310: 1683–1686. [DOI] [PubMed] [Google Scholar]
- Bickel J. S., Chen L., Hayward J., Yeap S. L., Alkers A. E., et al. , 2010. Structural maintenance of chromosomes (SMC) proteins promote homolog-independent recombination repair in meiosis crucial for germ cell genomic stability. PLoS Genet. 6: e1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton S. J., Jackson S. P., 1996. Identification of a Saccharomyces cerevisiae Ku80 homologue: roles in DNA double strand break rejoining and in telomeric maintenance. Nucleic Acids Res. 24: 4639–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon C. K., Hawley R. S., 2016. Regulating the construction and demolition of the synaptonemal complex. Nat. Struct. Mol. Biol. 23: 369–377. [DOI] [PubMed] [Google Scholar]
- Ceccaldi R., Rondinelli B., D’Andrea A. D., 2016. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 26: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checchi P. M., Engebrecht J., 2011. Caenorhabditis elegans histone methyltransferase MET-2 shields the male X chromosome from checkpoint machinery and mediates meiotic sex chromosome inactivation. PLoS Genet. 7: e1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung I., 2006. High incidence of rapid telomere loss in telomerase-deficient Caenorhabditis elegans. Nucleic Acids Res. 34: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clejan I., 2006. Developmental modulation of nonhomologous end joining in Caenorhabditis elegans. Genetics 173: 1301–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. A., D’Andrea A. D., 2008. Chromatin recruitment of DNA repair proteins: lessons from the fanconi anemia and double-strand break repair pathways. Mol. Cell 32: 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaiácovo M. P., MacQueen A. J., Martinez-Perez E., McDonald K., Adamo A., et al. , 2003. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev. Cell 5: 463–474. [DOI] [PubMed] [Google Scholar]
- De Felici M., Klinger F. G., 2015. Programmed cell death in mouse primordial germ cells. Int. J. Dev. Biol. 59: 41–49. [DOI] [PubMed] [Google Scholar]
- de Massy B., 2013. Initiation of meiotic recombination: how and where? Conservation and specificities among eukaryotes. Annu. Rev. Genet. 47: 563–599. [DOI] [PubMed] [Google Scholar]
- Denslow S. A., Wade P. A., 2007. The human Mi-2/NuRD complex and gene regulation. Oncogene 26: 5433–5438. [DOI] [PubMed] [Google Scholar]
- Derry W. B., 2001. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294: 591–595. [DOI] [PubMed] [Google Scholar]
- Dufourcq P., Victor M., Gay F., Calvo D., Hodgkin J., et al. , 2002. Functional requirement for histone deacetylase 1 in Caenorhabditis elegans gonadogenesis. Mol. Cell. Biol. 22: 3024–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn K., Lowndes N. F., Grenon M., 2012. Eukaryotic DNA damage checkpoint activation in response to double-strand breaks. Cell. Mol. Life Sci. CMLS 69: 1447–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Muse T., Boulton S. J., 2005. Distinct modes of ATR activation after replication stress and DNA double-strand breaks in Caenorhabditis elegans. EMBO J. 24: 4345–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner, A., P. R. Boag, and T. K. Blackwell, 2008 Germline survival and apoptosis (September 4, 2008), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.145.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Giglia-Mari G., Zotter A., Vermeulen W., 2011. DNA damage response. Cold Spring Harb. Perspect. Biol. 3: a000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong F., Chiu L. -Y., Cox B., Aymard F., Clouaire T., et al. , 2015. Screen identifies bromodomain protein ZMYND8 in chromatin recognition of transcription-associated DNA damage that promotes homologous recombination. Genes Dev. 29: 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi A. A., Kurka T., Jeggo P. A., 2011. KAP-1 phosphorylation regulates CHD3 nucleosome remodeling during the DNA double-strand break response. Nat. Struct. Mol. Biol. 18: 831–839. [DOI] [PubMed] [Google Scholar]
- Gospodinov A., Herceg Z., 2013. Chromatin structure in double strand break repair. DNA Repair (Amst.) 12: 800–810. [DOI] [PubMed] [Google Scholar]
- Gray S., Cohen P. E., 2016. Control of meiotic crossovers: from double-strand break formation to designation. Annu. Rev. Genet. 50: 175–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursoy-Yuzugullu O., House N., Price B. D., 2016. Patching broken DNA: nucleosome dynamics and the repair of DNA breaks. J. Mol. Biol. 428: 1846–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L., Weinert T., 1989. Checkpoints: controls that ensure the order of cell cycle events. Science 246: 629–634. [DOI] [PubMed] [Google Scholar]
- Hillers, K. J., V. Jantsch, E. Martinez-Perez, and J. L. Yanowitz, 2017 Meiosis (May 4, 2017), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.178.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Hodgkin J., Horvitz H. R., Brenner S., 1979. Nondisjunction mutants of the nematode CAENORHABDITIS ELEGANS. Genetics 91: 67–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister H., Fuchs A., Erdel F., Pinz S., Gröbner-Ferreira R., et al. , 2017. CHD3 and CHD4 form distinct NuRD complexes with different yet overlapping functionality. Nucleic Acids Res. 45: 10534–10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann E. R., Milstein S., Boulton S. J., Ye M., Hofmann J. J., et al. , 2002. Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr. Biol. CB 12: 1908–1918. [DOI] [PubMed] [Google Scholar]
- Hoogewijs D., Houthoofd K., Matthijssens F., Vandesompele J., Vanfleteren J. R., 2008. Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in C. elegans. BMC Mol. Biol. 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N., 2015. Meiotic recombination: the essence of heredity. Cold Spring Harb. Perspect. Biol. 7: a016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo-Lambert A., Engebrecht J., 2010. A single unpaired and transcriptionally silenced X chromosome locally precludes checkpoint signaling in the Caenorhabditis elegans germ line. Genetics 184: 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo-Lambert A., Harigaya Y., Vitt J., Villeneuve A., Engebrecht J., 2010. Meiotic errors activate checkpoints that improve gamete quality without triggering apoptosis in male germ cells. Curr. Biol. CB 20: 2078–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M., Rothstein R., 2013. Repair of strand breaks by homologous recombination. Cold Spring Harb. Perspect. Biol. 5: a012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A., Downs J. A., 2014. Roles of chromatin remodellers in DNA double strand break repair. Exp. Cell Res. 329: 69–77. [DOI] [PubMed] [Google Scholar]
- Johnsen R. C., Baillie D. L., 1991. Genetic analysis of a major segment [LGV(left)] of the genome of Caenorhabditis elegans. Genetics 129: 735–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeropoulos N., Christoforou C., Green A. J., Gill S., Ashcroft N. R., 2004. chk-1 is an essential gene and is required for an S-M checkpoint during early embryogenesis. Cell Cycle Georget. Tex 3: 1196–1200. [PubMed] [Google Scholar]
- Käser-Pébernard S., Pfefferli C., Aschinger C., Wicky C., 2016. Fine-tuning of chromatin composition and polycomb recruitment by two Mi2 homologues during C. elegans early embryonic development. Epigenetics Chromatin 9: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S., 2001. Mechanism and control of meiotic recombination initiation. Curr. Top. Dev. Biol. 52: 1–53. [DOI] [PubMed] [Google Scholar]
- Kim H.-M., Colaiácovo M. P., 2014. ZTF-8 interacts with the 9–1-1 complex and is required for DNA damage response and double-strand break repair in the C. elegans germline. PLoS Genet. 10: e1004723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirienko N. V., Mani K., Fay D. S., 2010. Cancer models in Caenorhabditis elegans. Dev. Dyn. 239: 1413–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lans H., Marteijn J. A., Vermeulen W., 2012. ATP-dependent chromatin remodeling in the DNA-damage response. Epigenetics Chromatin 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lant, B., and W. B. Derry, 2014 Analysis of apoptosis in Caenorhabditis elegans. Cold Spring Harb. Protoc. 2014: pdb.top070458.10.1101/pdb.top070458. [DOI] [PubMed]
- Larsen D. H., Poinsignon C., Gudjonsson T., Dinant C., Payne M. R., et al. , 2010. The chromatin-remodeling factor CHD4 coordinates signaling and repair after DNA damage. J. Cell Biol. 190: 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence K. S., Tapley E. C., Cruz V. E., Li Q., Aung K., et al. , 2016. LINC complexes promote homologous recombination in part through inhibition of nonhomologous end joining. J. Cell Biol. 215: 801–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens B. B. L. G., Johnson N. M., Tijsterman M., 2013. COM-1 promotes homologous recombination during Caenorhabditis elegans meiosis by antagonizing ku-mediated non-homologous end joining. PLoS Genet. 9: e1003276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., O’Neil N. J., Moshgabadi N., Hieter P., 2014. Synthetic cytotoxicity: digenic interactions with TEL1/ATM mutations reveal sensitivity to low doses of camptothecin. Genetics 197: 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Longhese M. P., Bonetti D., Guerini I., Manfrini N., Clerici M., 2009. DNA double-strand breaks in meiosis: checking their formation, processing and repair. DNA Repair (Amst.) 8: 1127–1138. [DOI] [PubMed] [Google Scholar]
- Lowden M. R., Flibotte S., Moerman D. G., Ahmed S., 2011. DNA synthesis generates terminal duplications that seal end-to-end chromosome fusions. Science 332: 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Horvitz H. R., 1998. lin-35 and lin-53, two genes that antagonize a C. elegans ras pathway, encode proteins similar to Rb and its binding protein RbAp48. Cell 95: 981–991. [DOI] [PubMed] [Google Scholar]
- Lui D. Y., Colaiácovo M. P., 2013. Meiotic development in Caenorhabditis elegans, pp. 133–170. in Germ Cell Development, edited by Elegans C., Schedl T. Springer, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan M., Crichton J. H., Playfoot C. J., Adams I. R., 2015. Oocyte development, meiosis and aneuploidy. Semin. Cell Dev. Biol. 45: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen A. J., 2002. Synapsis-dependent and -independent mechanisms stabilize homolog pairing during meiotic prophase in C. elegans. Genes Dev. 16: 2428–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova A., Haber J. E., 2012. Mutations arising during repair of chromosome breaks. Annu. Rev. Genet. 46: 455–473. [DOI] [PubMed] [Google Scholar]
- Martin J. S., Winkelmann N., Petalcorin M. I. R., McIlwraith M. J., Boulton S. J., 2005. RAD-51-dependent and -independent roles of a Caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol. Cell. Biol. 25: 3127–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurchy A. N., Stempor P., Gaarenstroom T., Wysolmerski B., Dong Y., et al. , 2017. A team of heterochromatin factors collaborates with small RNA pathways to combat repetitive elements and germline stress. Elife 6: e21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F., Chen P. F., Dombecki C. R., Aljabban I., Nabeshima K., 2015. A defective meiotic outcome of a failure in homologous pairing and synapsis is masked by meiotic quality control. PLoS One 10: e0134871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mets D. G., Meyer B. J., 2009. Condensins regulate meiotic DNA break distribution, thus crossover frequency, by controlling chromosome structure. Cell 139: 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Zimmer J., Tarsounas M., 2016. Interplay between Fanconi anemia and homologous recombination pathways in genome integrity. EMBO J. 35: 909–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A. A., 2017. The Chromodomain helicase DNA-binding chromatin remodelers: family traits that protect from and promote cancer. Cold Spring Harb. Perspect. Med. 7: a026450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya-Durango D. E., Ramos K. A., Bojang P., Ruiz L., Ramos I. N., et al. , 2016. LINE-1 silencing by retinoblastoma proteins is effected through the nucleosomal and remodeling deacetylase multiprotein complex. BMC Cancer 16: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka S. I., Hassold T. J., Hunt P. A., 2012. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitarska J., Smith J. G., Sherlock W. T., Hillege M. M. G., Nott A., et al. , 2016. A functional switch of NuRD chromatin remodeling complex subunits regulates mouse cortical development. Cell Rep. 17: 1683–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A., Vantipalli M. C., Lithgow G. J., 2006. Checkpoint Proteins control survival of the postmitotic cells in Caenorhabditis elegans. Science 312: 1381–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil, N., and A. Rose, 2006 DNA repair (January 13, 2006), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.54.1, http://www.wormbook.org.
- O’Shaughnessy A., Hendrich B., 2013. CHD4 in the DNA-damage response and cell cycle progression: not so NuRDy now. Biochem. Soc. Trans. 41: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M.-R., Hsieh H.-J., Dai H., Hung W.-C., Li K., et al. , 2012. Chromodomain helicase DNA-binding protein 4 (CHD4) regulates homologous recombination DNA repair, and its deficiency sensitizes cells to poly(ADP-ribose) polymerase (PARP) inhibitor treatment. J. Biol. Chem. 287: 6764–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichos-Chronakis M., Peterson C. L., 2012. Chromatin and the genome integrity network. Nat. Rev. Genet. 14: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passannante M., Marti C.-O., Pfefferli C., Moroni P. S., Kaeser-Pebernard S., et al. , 2010. Different Mi-2 complexes for various developmental functions in Caenorhabditis elegans. PLoS One 5: e13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C. M., Dernburg A. F., 2006. A family of zinc-finger proteins is required for chromosome-specific pairing and synapsis during meiosis in C. elegans. Dev. Cell 11: 817–829. [DOI] [PubMed] [Google Scholar]
- Polo S. E., Kaidi A., Baskcomb L., Galanty Y., Jackson S. P., 2010. Regulation of DNA-damage responses and cell-cycle progression by the chromatin remodelling factor CHD4. EMBO J. 29: 3130–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price B. D., D’Andrea A. D., 2013. Chromatin remodeling at DNA double-strand breaks. Cell 152: 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W., Chen H., Xiao T., Wang R., Li T., et al. , 2016. Acetyltransferase p300 collaborates with chromodomain helicase DNA-binding protein 4 (CHD4) to facilitate DNA double-strand break repair. Mutagenesis 31: 193–203. [DOI] [PubMed] [Google Scholar]
- Raschellà G., Melino G., Malewicz M., 2017. New factors in mammalian DNA repair-the chromatin connection. Oncogene 36: 4673–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl T., 2013. Germ Cell Development in C. elegans. Springer, New York, NY. [Google Scholar]
- Schmittgen T. D., Livak K. J., 2008. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- Schumacher B., Hofmann K., Boulton S., Gartner A., 2001. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 11: 1722–1727. [DOI] [PubMed] [Google Scholar]
- Seeber A., Gasser S. M., 2017. Chromatin organization and dynamics in double-strand break repair. Curr. Opin. Genet. Dev. 43: 9–16. [DOI] [PubMed] [Google Scholar]
- Shi Y., Mello C., 1998. A CBP/p300 homolog specifies multiple differentiation pathways in Caenorhabditis elegans. Genes Dev. 12: 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastav M., De Haro L. P., Nickoloff J. A., 2008. Regulation of DNA double-strand break repair pathway choice. Cell Res. 18: 134–147. [DOI] [PubMed] [Google Scholar]
- Sims J. K., Wade P. A., 2011. Mi-2/NuRD complex function is required for normal S phase progression and assembly of pericentric heterochromatin. Mol. Biol. Cell 22: 3094–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeenk G., Wiegant W. W., Vrolijk H., Solari A. P., Pastink A., et al. , 2010. The NuRD chromatin-remodeling complex regulates signaling and repair of DNA damage. J. Cell Biol. 190: 741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Rothstein R., 2017. Poetry in motion: increased chromosomal mobility after DNA damage. DNA Repair (Amst.) 56: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolikov S., Eizinger A., Hurlburt A., Rogers E., Villeneuve A. M., et al. , 2007. Synapsis-defective mutants reveal a correlation between chromosome conformation and the mode of double-strand break repair during Caenorhabditis elegans meiosis. Genetics 176: 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So A., Le Guen T., Lopez B. S., Guirouilh-Barbat J., 2017. Genomic rearrangements induced by unscheduled DNA double strand breaks in somatic mammalian cells. FEBS J. 284: 2324–2344. [DOI] [PubMed] [Google Scholar]
- Solinger J. A., Kiianitsa K., Heyer W.-D., 2002. Rad54, a Swi2/Snf2-like recombinational repair protein, disassembles Rad51:dsDNA filaments. Mol. Cell 10: 1175–1188. [DOI] [PubMed] [Google Scholar]
- Subramanian V. V., Hochwagen A., 2014. The meiotic checkpoint network: step-by-step through meiotic prophase. Cold Spring Harb. Perspect. Biol. 6: a016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L., Court D. L., Fire A., 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263: 103–112. [DOI] [PubMed] [Google Scholar]
- Torrado M., Low J. K. K., Silva A. P. G., Schmidberger J. W., Sana M., et al. , 2017. Refinement of the subunit interaction network within the nucleosome remodelling and deacetylase (NuRD) complex. FEBS J. 284: 4216–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs A., Nussenzweig A., 2017. Endogenous DNA damage as a source of genomic instability in cancer. Cell 168: 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart A. J., Gatei M., Richard D. J., Khanna K., 2011. ATM mediated phosphorylation of CHD4 contributes to genome maintenance. Genome Integr. 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zelewsky T., Palladino F., Brunschwig K., Tobler H., Hajnal A., et al. , 2000. The C. elegans Mi-2 chromatin-remodelling proteins function in vulval cell fate determination. Development 127: 5277–5284. [DOI] [PubMed] [Google Scholar]
- Wang X., Yang C., 2016. Programmed cell death and clearance of cell corpses in Caenorhabditis elegans. Cell. Mol. Life Sci. CMLS 73: 2221–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A., Schuh M., 2017. Mechanisms of aneuploidy in human eggs. Trends Cell Biol. 27: 55–68. [DOI] [PubMed] [Google Scholar]
- Weiss K., Terhal P. A., Cohen L., Bruccoleri M., Irving M., et al. , 2016. De Novo mutations in CHD4, an ATP-dependent chromatin remodeler gene, cause an intellectual disability syndrome with distinctive dysmorphisms. Am. J. Hum. Genet. 99: 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. R., Vijg J., 2016. Do DNA double-strand breaks drive aging? Mol. Cell 63: 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. K., Chang S., Weiler S. R., Ganesan S., Chaudhuri J., et al. , 2000. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nat. Genet. 26: 85–88. [DOI] [PubMed] [Google Scholar]
- Xu J., Sun X., Jing Y., Wang M., Liu K., et al. , 2012. MRG-1 is required for genomic integrity in Caenorhabditis elegans germ cells. Cell Res. 22: 886–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanowitz J. L., 2008. Genome integrity is regulated by the Caenorhabditis elegans Rad51D homolog rfs-1. Genetics 179: 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye A. L., Ragle J. M., Conradt B., Bhalla N., 2014. Differential regulation of germline apoptosis in response to meiotic checkpoint activation. Genetics 198: 995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Smolikove S., 2013. Impaired resection of meiotic double-strand breaks channels repair to nonhomologous end joining in Caenorhabditis elegans. Mol. Cell. Biol. 33: 2732–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., 2011. Biology of the Mi-2/NuRD complex in SLAC (stemness, longevity/ageing, and cancer). Gene Regul. Syst. Bio. 5: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Hunter T., 2014. Roles of Chk1 in cell biology and cancer therapy: Chk1 review. Int. J. Cancer 134: 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Hartwieg E., Horvitz H. R., 2001. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 104: 43–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.