Abstract
Background
The relationship between folate status and asthma-related outcomes has not been carefully examined in low- and middle-income countries where folate deficiency is common.
Methods
Ancillary analysis of an unmatched case-control study in which we analyzed serum folate concentrations in 412 children with asthma and 342 controls living in peri-urban communities in Lima, Peru. We examined baseline associations between folate and asthma, atopy, total serum IgE, pulmonary function, and fractional exhaled nitric oxide. We then followed children with asthma longitudinally for 6–9 months and assessed associations between folate and odds of uncontrolled asthma (defined as Asthma Control Test score ≤ 19) and of ≥1 emergency visits during follow-up.
Results
A 10 ng/mL decrease in serum folate was associated with 45% higher adjusted odds of asthma (OR=1.45, 95% CI 1.05–2.02). The folate-asthma relationship differed by atopic status: a 10 ng/mL decrease in serum folate was associated with a 2.4-fold higher odds of asthma among children without atopy (2.38, 1.20–4.72) and 23% higher odds of asthma in children with atopy (1.23, 0.85–1.80). Among children with asthma, a 10 ng/mL decrease in serum folate was associated with 62% higher odds of uncontrolled asthma (1.62, 1.02–2.56) and 73% higher odds of ≥1 emergency visits during follow-up (1.73, 1.05–2.85).
Conclusions
Serum folate concentrations were inversely associated with asthma, but this effect was stronger in children without atopy. Among children with asthma, lower serum folate concentrations were associated with higher risk of uncontrolled asthma.
Keywords: Asthma, Folate, Asthma Control, Atopy, Pediatric Asthma, Peruvians
INTRODUCTION
Although the prevalence of asthma in high-income countries has reached a relative plateau, asthma prevalence has continued to increase in many low- and middle-income (LMIC) countries1,2 with asthma emerging as one of the most prevalent non-communicable diseases3. Asthma is the most common chronic disease in childhood, with an estimated 14% of children worldwide experiencing asthma symptoms in the previous year4. The underlying mechanisms that help to explain the global asthma epidemic are probably multifactorial and likely affected by multiple genetic and environmental or lifestyle factors, including dietary intake5.
Folate, and other nutrients acting as methyl donors, could contribute to asthma risk by affecting DNA methylation and, ultimately, gene expression6,7. DNA methylation, a type of epigenetic regulation, is a mechanism underlying some gene-environment interactions of complex diseases such as asthma8. In particular, changes in DNA methylation can affect the pathogenesis of asthma by increasing or decreasing the expression of disease-susceptibility genes. Although initial findings from mouse models have generated substantial interest regarding the potential role of folate in the pathogenesis of asthma, their relevance to human beings remains unclear. Neither ecological evidence9 nor evidence from two recent independent reviews10,11 support a strong effect of periconceptional folate supplementation on increased risk of asthma in humans.
Much of the focus on folate’s role in asthma has centered on studies examining the relationship between prenatal use of folate and new-onset asthma. However, few studies have examined whether folate status is associated with disease morbidity or disease severity in subjects with established asthma; whether folate status affects asthma control in individuals who already have asthma is also unclear10,12. Low folate levels have been shown to be associated with increased risks of wheeze, atopy, severe asthma exacerbations, or an elevated total IgE in cross-sectional studies of children and adults13,14,15.
Given evidence from previous studies, we hypothesized that lower serum folate levels are significantly associated with increased odds of asthma, atopy and with decreased asthma control. Given the relative lack of large-scale studies of asthma and folate in the context of LMICs, we sought to examine the relationship between serum folate and asthma outcomes in a cohort of children and adolescents living in two peri-urban communities of Lima, Peru. To test these hypotheses, we examined the relationships among serum folate levels and asthma status, atopy, and asthma control; in addition, we explored the associations between serum folate and pulmonary function measures (spirometry), markers of allergy (IgE, atopy), and markers of inflammation (FeNO).
METHODS
Study population and setting
The study population was composed of children and adolescents 9 to 19 years of age living in the two communities, Pampas de San Juan de Miraflores and Villa El Salvador, located approximately 25 km south of central Lima, Peru. Pampas de San Juan and Villa El Salvador have grown rapidly over the last two decades both economically and in population size; a higher proportion of inhabitants in Pampas was born in the highlands as compared to Villa, and a lower proportion is native to Lima. The two communities also differ in age structure and socioeconomic status (SES); Pampas is less urbanized, has a slightly younger population, and has an overall lower SES. We chose to carry out this study in two communities to increase our recruitment pool of asthma cases. A study conducted in 2010 by our research group in 725 adolescents 13–15 years of age living in Pampas determined that 22% of participants had lifetime wheeze, 12% had current asthma symptoms, and 13% had a physician diagnosis of asthma16. This current study was approved by the Institutional Review Boards at the Johns Hopkins University School of Medicine, Baltimore, USA, and A.B. PRISMA in Lima, Peru.
Study design
This is an ancillary analysis of an unmatched case-control study conducted to determine the role of ambient air pollution on asthma control. For the parent study, Genetics of Asthma Susceptibility to Pollution (GASP), we enrolled children 9 to 19 years of age living in either of the two study communities. We excluded children with a recorded history of any of the following: ocular, abdominal, or thoracic surgery in the past 3 months, history of hospitalization for cardiac reasons in the past 3 months, a diagnosis of tuberculosis or currently receiving treatment for tuberculosis, a chronic respiratory condition other than asthma, or were pregnant at enrollment. We recruited participants using household census surveys conducted in the study communities. We identified and visited all potential asthma cases aged 9 to 19 years in the two study communities using birthdate and a positive response to a census question identifying individuals with wheeze or use of asthma medications in the past 12 months, or a lifetime physician diagnosis of asthma. We identified children without asthma using a simple random sample of children aged 9–19 years in our census that responded negatively to all asthma-related census questions. We defined children with asthma as having self- or parental-report of any occurrence of wheezing in the chest or any use of asthma medications in the past year. We confirmed asthma status at enrollment and evaluated asthma severity in accordance with NAEPP-3 guidelines17. We defined children without asthma as having no occurrence of self- or parentally-reported wheeze symptoms consistent with asthma in the past year and no use of asthma medications in the past year. In total, we had a final enrollment of 258 and 248 children with asthma, and 374 and 297 without asthma, in Pampas and Villa, respectively. The publication of the parent study, GASP, is currently under review. However, two separate ancillary studies from the parent study have already been published18,19.
Questionnaires
We administered a baseline questionnaire, which included questions regarding demographic information, socioeconomic status, asthma medication use, history of allergic rhinitis and eczema, and smoking history.
Clinical Measurements
At enrollment, we conducted spirometry in all participants; we used a flow-based portable spirometer (SpiroPro, Jaeger/ERT, Hoechberg, Germany), obtaining at least three acceptable and reproducible spirometry maneuvers for a maximum of eight in accordance ATS/ERS guidelines20. We determined predicted values and Z-scores using multi-ethnic reference values derived by the Global Lung Health Initiative21. We measured fractional exhaled nitric oxide (FeNO) using the handheld NIOXMINO (Aerocrine, Solna, Sweden). We measured both total serum IgE and specific IgE antibody to mixes of three common allergens (animal, mold, and dust mite) using the ImmunoCAP 250 (ThermoFisher Scientific, Kalamazoo, MI). An IgE level of > 0.1 kU/L indicated a positive IgE antibody response, and a positive response to any of the three mixes indicated atopy.
To assess asthma control, we used a validated questionnaire to measure an Asthma Control Test (ACT) score22,23,24; for children under 12, the childhood ACT was scored from 0 to 27, and for children 12 years and older, the ACT was scored from 0 to 25. An ACT score ≤ 19 is indicative of uncontrolled asthma. We continued to measure ACT scores and health care utilization visits in children with asthma for up to 9 months. We defined a health care utilization visit during 9-month follow-up as an ED visit or hospitalization as a result of the child’s asthma.
Measurement of serum folate levels
We collected one blood sample per participant using standard phlebotomy techniques at baseline. Blood samples were drawn in the morning and done in a fasting state. We then separated and centrifuged samples within two hours of extraction and stored samples at −80°C; we safely stored samples by taking precautions to prevent light exposure before processing. We quantified serum folate levels (ng/mL) at the Analytics Laboratory at Nemours Children Health System, Jacksonville, FL using a Folate Accubind ELISA kit (Monobind). We measured serum folate in duplicate and mean serum folate values for each participant were used to increase reliability and to account for repeated measures. Seasonality was defined based on season of blood draw with the two seasons specified as January–April and September–December.
Biostatistical methods
We used multivariable logistic regression to model the association between folate and asthma status, atopy status, and the odds of one or more emergency visits for asthma during 9-month follow-up. Asthma models were also stratified by atopy. We used multivariable linear regressions to model associations between serum folate levels and the following outcomes: pre-bronchodilator FEV1 Z-scores, pre-bronchodilator FVC Z-scores, pre-bronchodilator FEV1/FVC Z-scores, FeNO (log scale), and total serum IgE (log scale). The regression models for asthma and atopy were adjusted for the following confounders: age, sex, BMI, SES, season, and (in the asthma model) atopy. The models for pulmonary function, FeNO, and total serum IgE: age, sex, BMI, and season. Pulmonary function, FeNO, and total serum IgE models were also stratified by asthma status. The regression models for uncontrolled asthma were adjusted for the following confounders: age, sex, BMI, SES, atopy, season. We graphed the unadjusted odds of asthma across increasing vigintiles of serum folate concentrations (ng/mL); we stratified serum folate concentrations into 20 equal quantiles with each stratum considered a vigintile (similar to how stratifying a distribution into quartiles results in 4 equal strata; quintiles, 5, etc.). The stratification of serum folate concentrations into vigintiles was determined a priori.
We used the chi-square test to compare proportions between children with asthma and controls, and analysis of variance to compare means between these groups. We used a smoothing spline to visualize the relationship between serum folate levels and unadjusted odds of asthma. We generated a composite score for SES using Principal Component Analysis (PCA) techniques with a higher score indicating a higher SES. Variables used in the PCA analysis included number of individuals living in the household, 24-hour availability of running water, salary, and parental education, among others. We used random forest methods to impute missing observations (<10% of data)25. We conducted analyses in STATA 14 (Stata Corp., College Station, Texas) and in R (www.r-project.org).
RESULTS
Characteristics of the study population
Of 1177 children enrolled in our study, 884 (413 with asthma, 471 healthy controls) had serum available for analysis of folate. Mean serum folate concentrations (range: 7.6–42.8 ng/mL) were found to be 20.1 ng/mL (SD 4.98) in children with asthma and 21.1 ng/mL (SD 4.77) in children without asthma.
There were no differences in age (13.4 vs. 13.5 years, p=0.48), sex (50.9% vs. 53.4% male, p=0.45), atopy (69.7 % vs. 79.0% with atopy, p=0.39), pre-bronchodilator FEV1 Z-score (1.3 vs. 1.4, p=0.57), or body mass index (BMI) (21.9 vs. 21.5, p=0.13) between individuals with and without a blood sample, respectively. There was a significant difference in the proportion with asthma (54.6% vs. 35.7%, p < 0.001) in children with and without a blood sample. Among the 884 children with analyzed blood samples, mean age at enrollment was 13.9 years (SD = 2.72), 53.4% were boys, and 52.6% lived in Villa. Despite differences in SES between study communities, but the proportion of children with asthma in each study community was similar.
We summarized baseline characteristics of children with asthma as compared to controls (Table 1). Compared to controls, children with asthma were more likely to have lower total serum folate levels, higher BMIs, higher prevalence of atopy, higher prevalence of allergic rhinitis, higher FeNO, lower pre-FEV1 Z-scores, lower pre-FEV1/FVC ratio Z-scores, and higher SES. They also differed from controls by season of blood draw. Children with asthma did not differ from controls in age at enrollment, gender distribution, or current smoking status.
Table 1.
Participant characteristics among children with and without asthma
| Children with asthma | Children without asthma | p-value | |
|---|---|---|---|
| Sample size (n) | 412 | 342 | |
| Folate Measures | |||
| Total serum folate (ng/ml), mean (SD) | 20.1 (4.98) | 21.1 (4.93) | 0.004 |
| Demographics | |||
| Gender | |||
| n, (%) boys | 234 (56.8) | 238 (50.4) | 0.16 |
| Age in years | |||
| mean (SD) | 13.9 (2.65) | 13.8 (2.74) | 0.70 |
| Anthropometry | |||
| Height in cm, mean (SD) | 153.7 (11.2) | 152.2 (10.6) | 0.08 |
| BMI in kg/m2, mean (SD) | 22.2 (4.23) | 21.4 (3.88) | 0.01 |
| BMI-for-age z-score, mean (SD) | 1.03 (1.19) | 0.82 (1.14) | 0.01 |
| Socioeconomics, n (%) | |||
| Maternal education ≥ 6 years | 273 (70.9) | 180 (55.6) | <0.001 |
| 6 or more household members | 154 (37.4) | 140 (40.9) | 0.54 |
| SES Score | −0.29 (1.67) | 0.17 (1.68) | <0.001 |
| Smoking, n (%) | |||
| Current smoker | 6 (20) | 9 (36) | 0.51 |
| Pulmonary function and allergy | |||
| Pre-BD FEV1 z-score, mean (SD) | 1.08 (1.40) | 1.60 (1.20) | <0.001 |
| Pre-BD FVC z-score, mean (SD) | 1.38 (1.41) | 1.35 (1.25) | 0.73 |
| Pre-BD FEV1/FVC z-score, mean (SD) | −0.35 (1.17) | 0.47 (0.81) | <0.001 |
| Either parent with history of physician-diagnosed asthma, n (%) | 95 (25.2) | 29 (9.3) | 0.07 |
| Atopy (≥ 1 positive), n (%) | 315 (78.2) | 199 (59.1) | <0.001 |
| Pollen allergy (self-report), n (%) | 38 (9.82) | 8 (2.52) | 0.50 |
| Animal dander allergy (self-report), n (%) | 107 (27.6) | 13 (3.99) | 0.06 |
| Ever having allergic rhinitis (self-report), n (%) | 290 (75.1) | 102 (31.1) | <0.001 |
| Either parent with history of allergic rhinitis (self-report), n (%) | 124 (32.6) | 63 (19.8) | 0.07 |
| Ever having eczema (self-report), n (%) | 52 (13.5) | 20 (6.12) | 0.38 |
| Either parent with history of eczema (self-report), n (%) | 12 (3.16) | 12 (3.79) | 0.93 |
| Exhaled nitric oxide in ppb, mean(SD) | 34.4 (32.9) | 16.3 (15.7) | <0.001 |
| Season of blood draw, n, (%) | |||
| January–April | 115 (27.8) | 63 (18.5) | 0.003 |
| September–December | 298 (72.2) | 278 (81.5) |
Relationship between total serum folate concentrations and asthma, atopy
We plotted the unadjusted odds of asthma for each vigintile of serum folate concentrations (Figure 1). We observed a consistent inverse relationship between unadjusted odds of asthma across increasing vigintiles of serum folate concentrations. The unadjusted odds of asthma crossed 1.0 at approximately vigintile 5, which corresponds to a serum folate level of 16.3 ng/ml. There was also a significant difference in the proportion of children who were considered folate deficient (≤ 20 ng/mL) in each subgroup (children with asthma 52%, controls 42%, p=0.01) (Figure 2).
Figure 1.
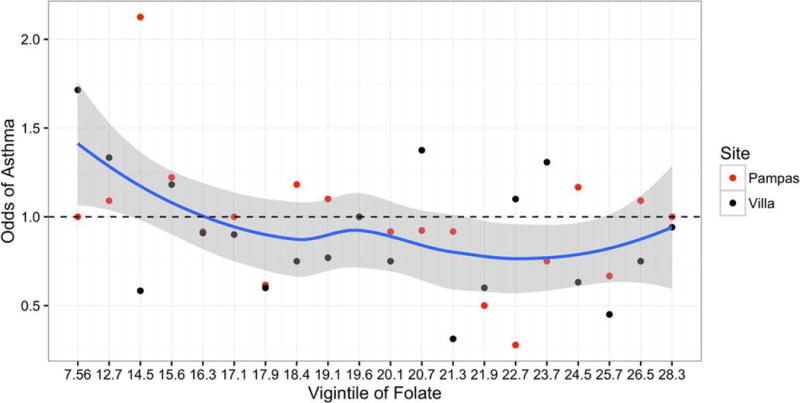
Unadjusted odds of asthma across increasing vigintiles of serum folate concentrations (ng/mL). The solid blue line represents the lowess smoothed curve and the gray shaded region represent confidence intervals for this curve. The black dots indicate the odds of current asthma in Pampas de San Juan, and the red dots indicate the odds of current asthma in Villa El Salvador. The size of the dot is proportional to the number of individuals included in calculation of the odds. The numbers along the x-axis correspond to the lower thresholds of each subsequent vigintile.
Figure 2.
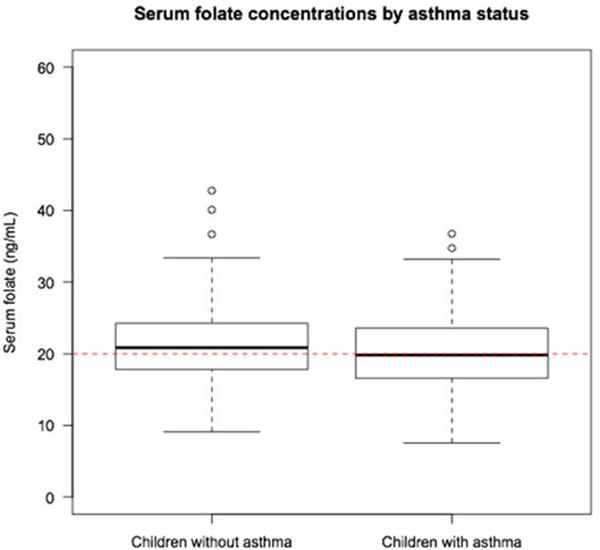
Boxplot of serum folate concentrations (ng/mL) by asthma status. The red dotted line represents a serum folate concentration of 20 ng/mL; this has been deemed as a possible cutoff for folate deficiency associated with asthma-related outcomes. Percentage of children who were considered folate deficient (serum folate ≤ 20 ng/mL) in each subgroup: children with asthma 52%, controls 42%.
We tabulated the results of the multivariable logistic regressions of the associations between serum folate levels and the following outcomes: asthma and atopy (Table 2). After adjusting for confounders, a 10 ng/mL decrease in serum folate was associated with a 45% higher odds of asthma (OR=1.45, 95% CI 1.05 to 2.02; p=0.03). After stratifying by atopy, we found a difference nearing significance in the association between folate and odds of asthma after adjusting for confounders. Children without atopy had a 2.38 times the odds of having asthma with each 10 ng/mL decrease in serum folate concentrations (95% CI 1.20 to 4.72; p=0.01) whereas among children with atopy, a 10 ng/mL decrease in folate was not associated with having asthma (OR = 1.23, 95% CI 0.85 to 1.80; p=0.28). In both the single variable and multivariable analyses, a 10 ng/mL decrease in serum folate was not associated with having atopy (OR=1.14, 95% CI 0.81 to 1.61; p=0.45).
Table 2.
Multivariable logistic regression analysis of the association between serum folate concentrations and odds of current asthma and atopy among children and adolescents in Lima, Peru.
| n | OR (95% CI) | p-value | |
|---|---|---|---|
|
|
|||
| Asthma | |||
| Overall* | 739 | 1.45 [1.05, 2.02] | 0.03 |
| Non-Atopy | 513 | 2.38 [1.20, 4.72] | 0.01 |
| Atopy | 226 | 1.23 [0.85, 1.80] | 0.28 |
| Atopy | |||
| Unadjusted | 866 | 1.21 [0.90, 1.63] | 0.21 |
| Adjusted† | 865 | 1.15 [0.81, 1.63] | 0.43 |
Values in boldface are statistically significant at p<0.05 level Folate expressed as per 10 ng/mL decrease in serum folate
Overall model adjusted for age, sex, BMI, SES, atopy, season
Adjusted for age, sex, BMI, SES, season, case/control status
Total serum folate concentrations and pulmonary function
In the unadjusted model for the overall population, pre-FEV1 was 0.24 Z-scores lower with every 10 ng/mL decrease in serum folate (95% CI −0.42 to −0.07) (Table 3). We found a similar result in unadjusted, bivariate models when limiting analyses to children with asthma (−0.29; 95% CI −0.57 to −0.02). However, after adjusting for confounders, decreases in pre-FEV1 were not significant for either the overall model or models stratified by asthma status.
Table 3.
Multivariable linear regression analysis of the associations between serum folate levels (expressed as per 10 ng/ml decrease) and markers of inflammation, allergy, and measures of pulmonary function.
| Overall | Children with Asthma | Children without Asthma | ||||
|---|---|---|---|---|---|---|
| Unadjusted Beta (95% CI) |
Adjusted Beta* (95% CI) |
Unadjusted Beta (95% CI) |
Adjusted Beta* (95% CI) |
Unadjusted Beta (95% CI) |
Adjusted Beta* (95% CI) |
|
| Pulmonary function | ||||||
| Pre-bronchodilator FEV1 Z-score |
−0.24 [−0.42,−0.07] |
−0.18 [−0.38, 0.02] |
−0.29 [−0.57, −0.02] |
−0.22 [−0.50, 0.05] |
−0.15 [−0.41, 1.06] |
−0.14 [−0.41, 0.14] |
| Pre-bronchodilator FVC Z-score |
−0.09 [−0.27, 0.09] |
−0.08 [−0.28, 0.12] |
−0.09 [−0.37, 0.18] |
−0.04 [−0.31, 0.24] |
−0.17 [−0.44, 0.10] |
−0.16 [−0.44, 0.12] |
| Pre-bronchodilator FEV1/FVC Z-score |
−0.23 [−0.37, −0.08] |
−0.15 [−0.30, 0.00] |
−0.29 [−0.52, −0.06] |
−0.28 [−0.52, −0.05] |
0.01 [−0.16, 0.19] |
0.03 [−0.15, 0.21] |
| Markers of inflammation | ||||||
| FeNO (log scale) |
0.22 [0.10, 0.34] |
0.07 [−0.06, 0.19] |
0.21 [0.02, 0.40] |
0.12 [−0.07, 0.32] |
0.07 [−0.07, 0.22] |
0.01 [−0.15, 0.16] |
| Markers of allergy | ||||||
| Total serum IgE (log scale) | 0.04 [−0.17, 0.24] |
0.01 [−0.21, 0.23] |
−0.05 [−0.32, 0.23] |
0.02 [−0.27, 0.31] |
−0.09 [−0.40, 0.23] |
0.00 [−0.34, 0.34] |
Values in boldface are statistically significant at p<0.05 level
Adjusted for age, sex, BMI, SES. Overall also adjusted for asthma status
In the unadjusted model, pre-FEV1/FVC was 0.23 Z-scores lower (95% CI −0.08 to −0.37) with each 10 ng/mL decrease in serum folate. For children with asthma, a 10 ng/mL decrease in serum folate was associated with a 0.28 lower pre-bronchodilator FEV1/FVC ratio Z-score (95% CI −0.52 to −0.05). However, in children without asthma, we found no statistically significant change in pre-FEV1/FVC ratio Z-scores. In all multivariable models, we did not find an association between pre-FVC Z-scores and serum folate.
Total serum folate concentrations and markers of inflammation (FeNO) and allergy (IgE)
In the overall, single model of FeNO and serum folate, a 10 ng/mL decrease in folate resulted in a 0.22 higher FeNO level (95% CI 0.10 to 0.34, p<0.01) (Table 3). However, after adjusting for confounders, this result was not significant (0.07, 95% CI −0.06 to 0.19; p = 0.31). We found a similar result in unadjusted, bivariate models for children with asthma (0.21, 95% CI 0.02 to 0.40, p = 0.03). However, in multivariate models, we found no significant association between serum folate concentrations and FeNO levels (log scale).
We found no statistically significant change in total serum IgE levels (log scale). In the adjusted model, a decrease in serum folate was not associated with total serum IgE levels (log scale). Moreover, we found no significant differences in this effect by asthma status.
Relationship between total serum folate concentrations and measures of longitudinal asthma control
In the adjusted model, a 10 ng/mL decrease in serum folate was associated with a 62 percent higher odds of uncontrolled asthma (ACT score ≤ 19) (OR=1.62; 95% CI 1.02 to 2.56; p = 0.03) (Table 4). A 10 ng/mL decrease in serum folate was also associated with 1.73 times the odds of having 1 or more health care utilization visits during follow-up (95% CI 1.05, 2.85; p = 0.03), but was not associated with having 1 or more follow-up visit with an ACT score ≤ 19 (OR 1.07; 95% CI 0.71 to 1.63; p = 0.74).
Table 4.
Multivariable logistic regression analysis of the associations between serum folate levels and asthma control measures
| n | OR (95% CI) | p-value | |
|---|---|---|---|
|
|
|||
|
Uncontrolled asthma (ACT score ≤ 19) |
|||
| Unadjusted | 412 | 1.50 [0.98,2.30] | 0.06 |
| Adjusted* | 403 | 1.62 [1.02, 2.56] | 0.03 |
| 1+health care visit during follow-up | |||
| Unadjusted | 412 | 1.18 [0.76, 1.84] | 0.47 |
| Adjusted* | 403 | 1.73 [1.05, 2.85] | 0.03 |
| Longitudinal ACT | |||
| Unadjusted | 412 | 0.97 [0.66, 1.43] | 0.87 |
| Adjusted* | 403 | 1.07 [0.71, 1.63] | 0.74 |
Values in boldface are statistically significant at p<0.05 level Folate expressed as per 10 ng/mL decrease
Models adjusted for age, sex, BMI, SES, atopy, season
DISCUSSION
We demonstrate that lower serum folate concentration is associated with a higher odds of asthma in Peruvian children and adolescents with a greater effect observed in children without atopy. Among Peruvian children with asthma, lower serum folate concentrations were also associated with worse asthma control.
The findings that support an inverse association between folate concentrations and odds of asthma are consistent with some26,27, but not all, previous studies15,28. Two cross-sectional studies reported no association between folate and physician-diagnosed asthma16 or current asthma symptoms28, one reported an inverse association between dietary folate intake and physician-diagnosed asthma27, and another reported an inverse association between serum folate and physician-diagnosed asthma but no association with airflow obstruction26. To our knowledge, this is the first report of an association between serum folate and asthma in a population of children in a LMIC setting. Moreover, we demonstrate that higher serum folate levels offer greater control of one’s asthma in Peruvian children and adolescents.
Some human studies have reported an association between high maternal folate status and early childhood wheezing29; however, others have not found an association between folate status during pregnancy or at birth and asthma 10,11,30. Moreover, two systematic reviews and/or meta-analyses of birth cohort studies concluded that there is no evidence of a major effect of folic acid supplementation during pregnancy on asthma10,11.
In contrast to the extensive literature on folate status in early life and asthma, little is known about folate status (beyond the perinatal period) and either asthma or asthma control in children with established asthma. One study has recently shown that folate deficiency (defined as serum folate ≤ 20 ng/mL) is associated with increased degree of atopy and severe asthma exacerbations in school-aged Puerto Ricans13. Our data showed a similar finding to Blatter et al13 and NHANES findings15 of increased asthma risk when folate concentrations were ≤ 16.3 ng/mL. It should be noted, however, that clinical folate deficiency is not considered to exist until circulating serum folate falls below 3 ng/mL (based on development of neural tube defects in newborns), suggesting that the threshold at which the association of circulating folate with asthma exists is considerably higher than the threshold conventionally associated with folate deficiency. To our knowledge, we do not have a normal range for serum folate concentrations in our population. The occurrence of folate deficiency in this population suggests poor dietary intake especially of foods rich in folate. A balanced diet rich in sources of folate and antioxidants can potentially correct this deficiency.
Though we show an association between low serum folate levels and asthma and worse asthma control, we did not observe a significant relationship between serum folate levels and atopy or total serum IgE. Our findings are in general agreement with Thuesen et. al (2010) in which two objective markers of folate deficiency were associated with self-reported doctor-diagnosed asthma and attacks of shortness of breath, but not with lung function or atopy26. On the other hand, previous studies have also demonstrated that increases in folate may afford a protective effect in certain populations against allergic inflammatory outcomes14,15 such as high total IgE, atopy, and wheeze. A cross-sectional study of 8,083 children and adults (aged 2–85 yr) showed that serum folate was inversely associated with wheeze, total IgE, and atopy (defined as a positive skin test to at least one allergen)15. Similarly, serum folate was inversely associated with total IgE in a study of 120 adults with asthma14. The discrepancy between our findings and results from other studies 14,15 may be explained by differences in sample size, age, race/ethnicity of participants, timing of folate measurement, and degree of adjustment for potential confounders.
In general agreement with our results, a multivariable analysis of data from a 1-year prospective study of 144 children with persistent asthma (aged 5–17 years) showed that serum folate was not significantly associated with lung function, fractional exhaled nitric oxide, number of positive skin tests to allergens, or asthma-related hospitalizations12. The findings from NHANES15 were not observed among Hispanic subjects, indicating that ethnic differences may affect the relationship between folate status and atopy. Moreover, our study population was comprised of children and adolescents furthering the hypothesis that folate may have differing effects across different life stages; specifically, the effects of folate may differ by the timing of exposure as in utero exposure appears to confer risk while exposure in later childhood may be protective.
There are several proposed mechanisms underlying our observations. Although folate has a myriad of biologic effects, the prevailing hypothesis posits that folate (acting as a methyl donor) may promote DNA methylation thereby suppressing the expression of key immune regulatory genes31. Although these findings point to the potential for folate to act via epigenetic mechanisms during early childhood, folate has many roles in cellular function so that it may act on the pathogenesis of allergic sensitization by other mechanisms.
We recognize several limitations to this study. Of note, the study population had a very low reported use (<5%) of inhaled corticosteroids, which virtually eliminates corticosteroid use as a confounding factor. An important limitation of this study is the inability to assess temporality or causality. Finally, our findings may not be generalizable to non-Peruvian children. However, our results may be broadly relevant to underserved populations at high risk for asthma morbidity. Although we do not have dietary intake information reported here, this study makes use of an objective laboratory measure to assess folate levels. This study enrolled a large sample of children in comparison to the majority of case-control studies examining the relationship between serum folate and asthma. Furthermore, this study contributes important information regarding the link between folate and asthma in LMIC settings where folate deficiency is common. Future studies are needed to define the temporal relationships among serum folate levels and allergy and asthma and to also determine potential mechanisms of action. Moreover, future studies should investigate a possible cutoff for serum folate in individuals with established disease.
In summary, the results of our study suggest that there is an inverse relationship between serum folate levels and asthma with a stronger relationship among individuals within the non-allergic phenotype in a population of Peruvian children. Moreover, we report a direct relationship between serum folate and asthma control. Our study does not support associations of total serum folate concentrations with pulmonary function measures or markers of lung inflammation (FeNO) or allergy (IgE). There is a need for well-designed and comprehensive studies that address the effects of folate on asthma severity and possible mechanisms of the existing association between folate and asthma.
Lower serum folate concentration is associated with a higher odds of asthma.
We observed a stronger effect for this relationship in children without atopy.
Lower serum folate concentrations were associated with worse asthma control.
A threshold for the association of circulating folate with asthma is proposed.
Acknowledgments
The authors are indebted to all participants who kindly agreed to participate in the study. We also gratefully acknowledge the team in Lima, Peru for their commitment and hard work throughout the study as well all other GASP Study Investigators for their contributions (listed below)
Other members of the GASP Study Investigators: Katherine Tomaino MSPH, Patrick Breysse PhD, D’Ann Williams DrPH, Caroline Johnson BS, Sonali Bose MD MPH, Lindsay Underhill MS, Rocío Galvez RD, Chen Chen MSPH, Kirsten Koehler PhD.
The authors are indebted to all participants who kindly agreed to participate in the study. Special thanks to the field team in Lima, Peru for their commitment and hard work throughout the study.
Funding: This project was funded by a grant from the National Institute of Environmental Health Sciences, National Institutes of Health (R01ES018845, R01ES018845-S1) and Biomedical Research, Nemours Children’s Health System. Suzanne Pollard was supported by a grant from the Fogarty International Center, Office of AIDS Research. Suzanne Pollard was further supported by a grant from the Fogarty International Center, Office of AIDS Research, National Cancer Center, National Heart, Blood, and Lung Institute, and the NIH Office of Research for Women’s Health through the Fogarty Global Health Fellows Program Consortium comprised of the University of North Carolina, Johns Hopkins University, Morehouse School of Medicine, and Tulane University (5R25TW009340). William Checkley was supported by a Pathway to Independence Award (R00HL096955) from the National Heart, Lung and Blood Institute, National Institutes of Health. Andrew Nicholson was supported by the Johns Hopkins Center for Global Health. Sponsors had no role in the design of the study, the collection of the data, the data analysis, or in the preparation of the manuscript.
Abbreviations
- ACT
Asthma Control Test
- BMI
Body Mass Index
- FeNO
Fractional exhaled nitric oxide
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- IgE
Immunoglobulin E
- SES
Socioeconomic status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: None declared.
Author Contributions: SP, KR, CTM, GMG, JL, EM, NH, and WC conceived, designed and supervised the overall study. KR, CTM, GMG, and SP coordinated and supervised fieldwork activities in Lima; in addition, they contributed to data collection and data management. J.L. and E.M. were responsible for serum folate analysis and laboratory quality control, and also participated in writing of the manuscript. AN, SP, and WC contributed to data analysis, interpretation of results, and manuscript writing; AN wrote the first draft and SP, NH, and WC participated in writing the manuscript. WC had primary responsibility for final content. All authors read and approved the final manuscript.
Ethics approval: Ethical approval was obtained from the relevant Institutional Review Board committees at the Johns Hopkins University School of Medicine, Baltimore, USA, and A.B. PRISMA in Lima, Peru.
References
- 1.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;32:1–14. [PubMed] [Google Scholar]
- 2.Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 3.Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 4.Network, G.A. The Global Asthma Report 2014. Auckland, New Zealand: 2014. [Google Scholar]
- 5.Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994;49:171–174. doi: 10.1136/thx.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovinsky-Desir S, Miller RL. Epigenetics, asthma, and allergic diseases: a review of the latest advancements. Curr Allergy Asthma Rep. 2012;12:211–220. doi: 10.1007/s11882-012-0257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132:2333S–2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- 8.Miller RL, Ho SM. Environmental epigenetics and asthma: current concepts and call for studies. Am J Respir Crit Care Med. 2008;177:567–573. doi: 10.1164/rccm.200710-1511PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ownby DR. Has mandatory folic acid supplementation of foods increased the risk of asthma and allergic disease? J Allergy Clin Immunol. 2009;123:1260–1261. doi: 10.1016/j.jaci.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Blatter J, Han YY, Forno E, et al. Folate and asthma. Am J Respir Crit Care Med. 2013;188:12–17. doi: 10.1164/rccm.201302-0317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crider KS, Cordero AM, Qi YP, et al. Prenatal folic acid and risk of asthma in children: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:1272–1281. doi: 10.3945/ajcn.113.065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JH, Matsui W, Aloe C, et al. Relationships between folate and inflammatory features of asthma. J Allergy Clin Immunol. 2013;131:918–920. doi: 10.1016/j.jaci.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blatter J, Brehm J, Sordillo J, et al. Folate Deficiency, Atopy, and Severe Asthma Exacerbations in Puerto Rican Children. Annals of the American Thoracic Society. 2016;13:223–230. doi: 10.1513/AnnalsATS.201508-549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farres MN, Shahin RY, Melek NA, et al. Study of folate status among Egyptian asthmatics. Internal medicine. 2011;50:205–11. doi: 10.2169/internalmedicine.50.4424. [DOI] [PubMed] [Google Scholar]
- 15.Matsui EC, Matsui W. Higher serum folate levels are associated with a lower risk of atopy and wheeze. J Allergy Clin Immunol. 2009;123:1253–1259.e2. doi: 10.1016/j.jaci.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson CL, Baumann LM, Romero K, et al. Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax. 2011 doi: 10.1136/thx.2011.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Program, N.A.E.a.P. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma Full Report 2007. 2007 [Google Scholar]
- 18.Pollard S, Lima J, Romero K, et al. Associations between serum 25(OH)D concentrations and prevalent asthma among children living in communities with differing levels of urbanization: a cross-sectional study. Asthma Research and Practice. 2017;3(1) doi: 10.1186/s40733-017-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollard S, Lima J, Mougey E, et al. Free 25(OH)D concentrations are associated with atopy and lung function in children with asthma. Annals of Allergy, Asthma & Immunology. 2017;119(1):37–41. doi: 10.1016/j.anai.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 21.Quanjer PH, Stanojevic S, Cole T, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan R, Sorkness C, Kosinski M, et al. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Schatz M, Kosinski M, Yarlas A, et al. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4):719–723.e1. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 24.Schatz M, Sorkness C, Li J, et al. Asthma Control Test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 26.Thuesen BH, Husemoen LL, Ovesen L, et al. Atopy, asthma, and lung function in relation to folate and vitamin B(12) in adults. Allergy. 2010;65:1446–1454. doi: 10.1111/j.1398-9995.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 27.Patel BD, Welch AA, Bingham SA, et al. Dietary antioxidants and asthma in adults. Thorax. 2006;61:388–93. doi: 10.1136/thx.2004.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods RK, Walters EH, Raven JM, et al. Food and nutrient intakes and asthma risk in young adults. Am J Clin Nutr. 2003;78:414–421. doi: 10.1093/ajcn/78.3.414. [DOI] [PubMed] [Google Scholar]
- 29.Haberg SE, London SJ, Nafstad P, et al. Maternal folate levels in pregnancy and asthma in children at age 3 years. J Allergy Clin Immunol. 2011;127:262–4. 264.e1. doi: 10.1016/j.jaci.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Valk RJ, Kiefte-de Jong JC, Sonnenschein-van der Voort AM, et al. Neonatal folate, homocysteine, vitamin B12 levels and methylenetetrahydrofolate reductase variants in childhood asthma and eczema. Allergy. 2013;68:788–795. doi: 10.1111/all.12146. [DOI] [PubMed] [Google Scholar]
- 31.Nadeau K, McDonald-Hyman C, Noth EM, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126:845–852.e10. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]


