Abstract
Innate and adaptive immune interactions within the central nervous system (CNS) and surrounding meninges contribute significantly to neural homeostasis as well as a variety of different neurological disorders. Two-photon laser scanning microscopy is a deep tissue imaging technique that provides a means to image immune cell dynamics and interactions in the living CNS with high spatial and temporal resolution. Optical access to the brain and meninges can be achieved through the creation of thinned skull windows, which can be made without inducing damage and inflammation in the underlying tissue. This protocol provides guidance on how to create a thinned skull window without causing CNS injury. We also describe a highly reproducible method to induce a mild traumatic brain injury using the thinned skull approach.
Keywords: skull thinning, two-photon, intravital imaging, neuroimmune, TBI
INTRODUCTION
The immune system contributes significantly to central nervous system (CNS) homeostasis and disease. Once thought to be ‘immune-privileged’, the CNS is home to both innate and adaptive immune cells (Carson, Doose, Melchior, Schmid, & Ploix, 2006; Galea, Bechmann, & Perry, 2007; Louveau, Harris, & Kipnis, 2015). These cells survey the CNS under steady conditions and contribute to the recruitment of immune cells from the periphery following CNS injury, infection, etc. (Fuhrmann et al., 2010; Griffin, 2003; Roth et al., 2014; Russo & McGavern, 2015; Sorbara, Misgeld, & Kerschensteiner, 2012). Recent insights into the dynamics and functions of these cells has been facilitated by the development of tools to visualize CNS immunity, which include a plethora of transgenic reporter mice, fluorescent probes, and intravital imaging technology (Cahalan, Parker, Wei, & Miller, 2002; Drobizhev, Makarov, Tillo, Hughes, & Rebane, 2011; Gossa, Nayak, Zinselmeyer, & McGavern, 2014; Herz, Zinselmeyer, & McGavern, 2012; Shaner, Steinbach, & Tsien, 2005; Sorbara et al., 2012).
Two-photon laser scanning microscopy (TPM) is a powerful tool that facilitates real-time imaging of cellular dynamics within living animals. Specifically, the use of TPM in the CNS has elucidated the role of immune (Bullen, Friedman, & Krummel, 2009; Cahalan et al., 2002; Kang & McGavern, 2009; Roth et al., 2014; Rua & McGavern, 2015), neuronal (Grutzendler, Kasthuri, & Gan, 2002; Tsai, Grutzendler, Duff, & Gan, 2004), glial (Mulligan & MacVicar, 2004; Takano et al., 2006) and vascular interactions (Chaigneau, Oheim, Audinat, & Charpak, 2003; Lecoq et al., 2011; Schaffer et al., 2006; Shih et al., 2012). Compared to single photon techniques, such as confocal microscopy, dual photon delivery of longer, lower energy wavelengths to tissue results in decreased light scatter and, therefore, increased tissue penetration. Using spectrally distributed fluorophores, multiple parameters and / or cells of interest can be imaged simultaneously in four dimensions (4D) (Drobizhev et al., 2011; Gossa et al., 2014; Shaner et al., 2005).
Utilization of thinned skull windows, allows the CNS to be imaged during homeostasis or disease without damaging underlying meningeal and parenchymal tissues. By comparison, open skull imaging preparations that require insertion of glass windows damage the meninges, disrupt flow of the cerebral spinal fluid, cause robust inflammation, and, consequently, alter physiology (Xu, Pan, Yang, & Gan, 2007; Yang, Pan, Parkhurst, Grutzendler, & Gan, 2010). Thin skull windows are created by removing upper layers of compact bone and spongy bone from the skull using a microdrill. The lower layer of compact bone is then thinned gently ~20–25 μm using a small surgical blade. At this thickness, the brain and underlying meningeal structures can be imaged by TPM without causing an inflammatory response.
Recently, our lab described a novel closed-skull model of mild traumatic brain injury (mTBI) that is an adaption of the skull thinning method (Roth et al., 2014). A focal mTBI is induced by rapidly thinning the skull to ~20–25 μm. This is followed by depressing the thinned skull bone downward using a microsurgical blade. Compared to other mTBI models, this model mirrors the meningeal damage commonly observed in human patients following mTBI and is compatible with TPM imaging (Roth et al., 2014; Xiong, Mahmood, & Chopp, 2013). This protocol provides the steps required to generate a thinned skull window for imaging without causing injury or inflammation (Basic Protocol 1) as well as a method to rapidly induce a closed skull mTBI (Basic Protocol 2).
Basic Protocol 1: Preparation of an uninflamed thinned skull surgical window
Creation of a thinned skull window facilitates intravital imaging of meninges, glial limitans, and underlying brain tissue in mice. This procedure requires approximately 45 minutes.
Materials
Reagents
Induction dose of Ketamine-xylazine-acepromazine (KXA) containing ketamine 85 mg/kg, xylazine 13 mg/kg and acepromazine at 2 mg/kg
Maintenance dose of KXA containing a 1:4 dilution of KXA in sterile PBS
Ophthalmic lubricant (Lubrifresh PM Ointment, Major Pharmaceuticals, Cat No. 301909)
Cyanoacrylate glue (Loctite Super Glue Gel control, Cat No. 234790)
Artificial Cerebral Spinal Fluid (aCSF) (Harvard Apparatus, Cat No. 597316)
Equipment
Heating pad
Scalpel Blade (Fine Science Tools, Cat No. 10050-00)
-
Skull thinning platform
To stabilize for intravital imaging experiments, our lab uses a custom-built metal imaging platform stage (15.5 cm × 7.5 cm) with adjustable thumb screws and a metal cranial brace (43mm × 21 mm with a 5.5 mm diameter center hole) with O-ring as shown in Figure 1. Surgical forceps and scissors
Micro-Drill Kit (Braintree Scientific, Cat No. MD-1200) with 0.7m Burrs for micro drill (Fine Science Tools, Cat No. 19007-07)
Miniature Blade Round Tip, Sharp Full Radius and Sides Blade, Double Bevel, Straight (Surgistar, Cat No. 6961)
Dissecting scope with illumination unit (Olympus)
-
Two-photon microscope
Our lab uses an SP8 two-photon microscope (Leica) equipped with a Mai Tai HP DeepSee laser (Spectra-Physics) and an Insight DS laser (Spectra-Physics), an 12,000-Hz resonant scanner, a 25×/1.0 NA color corrected objective, a quad HyD external detector array, and a custom-environment chamber
Figure 1. Skull thinning platform used for two-photon imaging.
A custom metal imaging platform consisting of a base, two support posts, a brace, and a rubber O-ring to contain aCSF can be quickly constructed for two-photon imaging studies of mice with thinned skull windows. The support posts should be threaded and have nuts to fasten the metal brace that is glued directly to the skull bone. The thinned skull window should be created and imaged within the hole cut in the center of the metal brace.
-
Anesthetize mouse with intraperitoneal (i.p.) injection of KXA at a dose of 5 μl/gram of body weight. Detailed information on mouse anesthesia can be found in CPI Unit 1.4.
Animal should become non-reflexive between 10–15 minutes. If animal begins to show reflexive behavior during the experiment, animal should be provided with a subcutaneous dose of maintenance anesthesia at 10μl/gram of body weight. Other anesthetics instead of KXA (e.g. isoflurane) can be used if necessary. -
Once the animal is non-reflexive, remove hair on the scalp with a scalpel blade or electronic shaver. While removing the scalp hair, place animal on a heating pad for thermal support. It is also important to place the metal skull thinning platform on the heating pad so that it can prewarm.
Monitor body temperature closely, as animals can overheat easily. To remove the scalp fur, we have found that a sharp scalpel blade often works better than an electronic shaver. Place ophthalmic lubricant on both eyes to prevent drying.
Using forceps, lift the shaved scalp and create a midline excision caudal to rostral, from between ears to immediately superior to the eyes, then retract the scalp skin laterally toward the ears to expose the skull.
-
Using dull forceps, gently remove the periosteum atop the skull bone by brushing forceps tips along the top of the intact skull.
The periosteum is a translucent tissue that wraps the skull. Remove the periosteum gently. -
Place a layer of Loctite Super Glue Gel around the circular opening of the metal brace, on the opposite side of the O-ring, that attaches to skull thinning platform (Figure 1). Flip the metal brace and place onto a flat portion of the skull bone. The brace can be glued to different regions of the skull bone so long as the surface is relatively flat and the brace makes a tight seal with the glue. Wait 10–15 minutes for the glue to dry.
It is very important that the metal brace is glued tightly to the skull bone. A loose brace can result in unstable imaging and movement artifacts. Even a small hole or crack in the glue seal can result in leakage of aCSF applied in a later step. -
Once the glue has dried, secure animal to the metal posts on the skull thinning platform (Figure 1) and place the animal under the dissecting scope. Use the dissecting scope and microsurgical drill fitted with a 0.7m drill bit, carefully drill a circle into the skull bone (~1 mm diameter) above the brain region you intend to image. The murine skull bone is ~300 μm thick and consists of three layers of bone: cortical (compact upper bone layer), cancellous (spongy middle layer of bone), and cortical (compact lower bone layer). Drill through the upper cortical and cancellous bone layers with careful circular motions until the white puncta in the bone begin to disappear. The goal is to drill completely through the upper cortical and cancellous bone layers until only the lower cortical bone remains.
Drilling slowly is imperative to prevent damage to the CNS. To prevent injury while drilling, it is also important not press the drill bit downward. Drill the bone by applying lateral circular motions. Heat buildup from rapid and excessive periods of drilling on dry bone can also damage underlying structures and cause robust inflammation in the meninges. Thinning two layers of murine skull bone with a microdrill should take ~10–15 minutes.Optional: The skull can be drilled with aCSF on the surface to reduce heat damage. -
After removing the upper cortical and cancellous bone layers, use a small Round Tip Miniature Microsurgical Blade to thin the lower cortical bone manually until about ~20–25 μm remains. As discussed in step 9, skull thickness can be assessed on the two-photon microscope. Bone thicknesses greater than 25 μm will result in chromatic aberrations and optical distortions when imaging.
It is very important not to press downward with the microsurgical blade while manually thinning the skull bone. Thin the bone by scraping laterally and applying minimal downward pressure. The skull bone will start to become pliable at this stage and downward pressure will cause extensive damage and inflammation in the underlying tissue. Thinning this third layer of bone should take ~10–15 minutes. -
Once thinning is complete, pipette 200–300 μl of aCSF into the rubber ring on the metal brace that is glued to the skull bone (Figure 1).
Before proceeding with your intravital imaging experiment, the skull thickness can be quickly assessed by measuring second harmonic signal using a two-photon microscope. The skull bone generates second harmonic signal when exposed to two-photon light at a variety of wavelengths. We typically tune our two-photon laser to 905nm to generate second harmonic signal, but other wavelengths can be used as well. If the skull thickness is greater than 25 μm, animals can be returned to the dissecting scope and thinned further using the microsurgical blade. Place the animal and imaging platform on the stage of a two-photon microscope. To maintain the animal’s body temperature while under anesthesia, it is ideal if the two-photon microscope is enclosed in an environmental chamber that supports a constant temperature.
-
Dip the lens of the two-photon microscope into the aCSF solution atop the skull bone and focus on the underlying brain and meninges.
If it is difficult to see through the thinned bone into the underlying tissue, then the skull bone is likely not thinned enough. After focusing on an area of interest, tune the laser to a wavelength that will optimally excite your fluorophore(s) / fluorescent protein(s) of interest and begin imaging. For more details about two-photon imaging and analysis, there are number of excellent reviews available (Bullen et al., 2009; Cahalan & Parker, 2008; Drobizhev et al., 2011; Herz et al., 2012; Zinselmeyer et al., 2009).
-
After the imaging experiment, the animal can be euthanized or maintained for consecutive imaging experiments scheduled for different days.
The maintaining a mouse for consecutive imaging experiments requires establishment of a survival surgery approved by an institutional animal care and use committee. A thinned skull window can typically be imaged 2–3 times. However, as the thinned bone begins to regrow, the viewing area can become cloudy and will need to be gently thinned again with a microsurgical blade. Usually, only a few scrapes with the microsurgical blade are required to remove the excess bone. It should be noted, however, that it is very easy to injure the underlying meninges and brain when re-thinning previously thinned areas. Re-thinning should only be performed if it is no longer possible to focus through the skull bone.
Basic Protocol 2: Generation of a mild traumatic brain injury (mTBI) beneath the thinned skull window
It is possible to create a highly reproducible mTBI model by rapidly thinning the skull and compressing the bone into the underlying meninges (Roth et al., 2014). This injury only requires 1–2 min to generate and the resultant immune response can be imaged shortly thereafter by two-photon microscopy.
Reagents
Induction dose of Ketamine-xylazine-acepromazine (KXA) containing ketamine 85 mg/kg, xylazine 13 mg/kg and acepromazine at 2 mg/kg
Maintenance dose of KXA containing a 1:4 dilution of KXA in sterile PBS
Ophthalmic lubricant (Lubrifresh PM Ointment, Major Pharmaceuticals, Cat No. 301909)
Cyanoacrylate glue (Loctite Super Glue Gel control, Cat No. 234790)
Artificial Cerebral Spinal Fluid (aCSF) (Harvard Apparatus, Cat No. 597316)
Equipment
Heating pad
Scalpel Blade (Fine Science Tools, Cat No. 10050-00)
-
Skull thinning platform
To stabilize for intravital imaging experiments, our lab uses a custom-built metal imaging platform stage (15.5 cm × 7.5 cm) with adjustable thumb screws and a metal cranial brace (43mm × 21 mm with a 5.5 mm diameter center hole) with O-ring as shown in Figure 1. Surgical forceps and scissors
Micro-Drill Kit (Braintree Scientific, Cat No. MD-1200) with 0.7m Burrs for micro drill (Fine Science Tools, Cat No. 19007-07)
Miniature Blade Round Tip, Sharp Full Radius and Sides Blade, Double Bevel, Straight (Surgistar, Cat No. 6961)
Dissecting scope with illumination unit (Olympus)
-
Two-photon microscope
Our lab uses an SP8 two-photon microscope (Leica) equipped with a Mai Tai HP DeepSee laser (Spectra-Physics) and an Insight DS laser (Spectra-Physics), an 12,000-Hz resonant scanner, a 25×/1.0 NA color corrected objective, a quad HyD external detector array, and a custom-environment chamber
-
Anesthetize mouse with intraperitoneal (i.p.) injection of KXA at a dose of 5 μl/gram of body weight.
Animal should become non-reflexive between 10–15 minutes. If animal begins to show reflexive behavior during the experiment, animal should be provided with a subcutaneous dose of maintenance anesthesia at 10μl/gram of body weight. -
Once the animal is non-reflexive, remove hair on the scalp with a scalpel blade or electronic shaver. While removing the scalp hair, place animal on a heating pad for thermal support. It is also important to place the metal skull thinning platform on the heating pad so that it can prewarm.
Monitor body temperature closely, as animals can overheat easily. To remove the scalp fur, we have found that a sharp scalpel blade often works better than an electronic shaver. Place ophthalmic lubricant on both eyes to prevent drying.
Using forceps, lift the shaved scalp and create a midline excision caudal to rostral, from between ears to immediately superior to the eyes, then retract the scalp skin laterally toward the ears to expose the skull.
-
Using dull forceps, gently remove the periosteum atop the skull bone by brushing forceps tips along the top of the intact skull.
The periosteum is a translucent tissue that wraps the skull. Remove the periosteum gently. -
Place a layer of Loctite Super Glue Gel around the circular opening of the metal brace, on the opposite side of the O-ring, that attaches to skull thinning platform (Figure 1). Flip the brace and place onto a flat portion of the skull bone. The brace can be glued to different regions of the skull bone so long as the surface is relatively flat and the brace makes a tight seal with the glue. Wait 10–15 minutes for the glue to dry.
It is very important that the metal brace is glued tightly to the skull bone. A loose brace can result in unstable imaging and movement artifacts. Even a small hole or crack in the glue seal can result in leakage of aCSF applied in a later step. Once the glue has dried, secure animal to the metal posts on the skull thinning platform (Figure 1) and place the animal under the dissecting scope. Use the dissecting scope and microsurgical drill fitted with a 0.7m drill bit, rapidly drill a circle into the skull bone (~1–2 mm diameter) above the brain region you intend to injure and image. Drill through all three layers of skull bone (upper cortical, cancellous, and lower cortical) until ~20–25 μm of lower cortical bone remains. It is not necessary to drill slowly for this procedure, as the goal here is to induce an injury. The drilling should only take ~45–60 seconds to complete.
-
Utilizing the blunt end of the microsurgical blade, gently press the skull bone downward into the CNS parenchyma. Press the thinned skull bone downward 5–10 times to achieve a consistent injury.
This pressure may cause the thinned skull window to become concave, but it should not crack. Once the injury is complete, pipette 200–300 μl of aCSF into the rubber ring on the metal brace that is glued to the skull bone and proceed immediately to the two-photon microscope.
Place the animal and imaging platform on the stage of a two-photon microscope. To maintain the animal’s body temperature while under anesthesia, it is ideal if the two-photon microscope is enclosed in an environmental chamber that supports a constant temperature.
-
Dip the lens of the two-photon microscope into the aCSF solution atop the skull bone and focus on the underlying brain and meninges.
If it is difficult to see through the thinned bone into the underlying tissue, then the skull bone is likely not thinned enough. After focusing on an area of interest, tune the laser to a wavelength that will optimally excite your fluorophore(s) / fluorescent protein(s) of interest and begin imaging. For more details about two-photon imaging and analysis, there are number of excellent reviews available (Bullen et al., 2009; Cahalan & Parker, 2008; Drobizhev et al., 2011; Herz et al., 2012; Zinselmeyer et al., 2009).
-
After the imaging experiment, the animal can be euthanized or maintained for consecutive imaging experiments scheduled for different days.
The maintaining a mouse for consecutive imaging experiments requires establishment of a survival surgery approved by an institutional animal care and use committee. A thinned skull window can typically be imaged 2–3 times. However, as the thinned bone begins to regrow, the viewing area can become cloudy and will need to be gently thinned again with a microsurgical blade. Usually, only a few scrapes with the microsurgical blade are required to remove the excess bone. It should be noted, however, that it is very easy to injure the underlying meninges and brain when re-thinning previously thinned areas. Re-thinning should only be performed if it is no longer possible to focus through the skull bone.
COMMENTARY
Background Information
The ability to image cellular interactions and dynamics in vivo has reached unprecedented clarity with advances in two-photon imaging technology. Compared to conventional imaging techniques, two-photon microscopy provides superior depth penetration and temporal resolution (Cahalan et al., 2002). By using higher wavelengths to excite tissue, light scatter is eliminated and greater tissue penetration can be achieved without photobleaching or damaging tissue. Improved temporal resolution is achieved through the utilization of resonant scanners with frequencies up to 16 kHz. This permits imaging of events that require video-rate acquisition, such as calcium dynamics, in 4 dimensions (Bullen et al., 2009; Mank et al., 2008; Stosiek, Garaschuk, Holthoff, & Konnerth, 2003; Wang, Wong, Flores, Vosshall, & Axel, 2003).
Cellular dynamics and interactions can be studied in different cell types simultaneously using multiple fluorescent probes. These probes are separated spectrally using optical filters. The spectral properties of fluorescent molecules are important to consider. Single wavelength excitation and emission spectra of fluorescent proteins do not often translate to their two photon absorption spectra (Drobizhev et al., 2011). However, the two photon absorption spectra of many fluorescent proteins are published and readily accessible (Drobizhev et al., 2011; Shaner et al., 2005). For unpublished fluorophores, testing spectral properties prior to experimentation is necessary (Gossa et al., 2014; Herz et al., 2012). The quality of fluorophore imaging is dictated by many factors include brightness, photo stability, excitation / emission spectra, toxicity, rejectability, etc. It is important to carefully plan imaging experiments in advance and think about how fluorescent probe properties / combinations, surgical preparation, imaging equipment, and heat loads will influence your experimental outcome. These experimental variables can potentially give rise to artefactual findings and lead investigators astray.
Critical Parameters & Troubleshooting
Critical Parameters
The CNS and surrounding meninges are closely monitored by dynamic myeloid cells that constantly survey their surroundings and respond rapidly to damage (Davalos et al., 2005; Nayak, Zinselmeyer, Corps, & McGavern, 2012; Nimmerjahn, Kirchhoff, & Helmchen, 2005; Roth et al., 2014). Because CNS injury can trigger inflammation and alter normal physiology, it is important that surgical preparations to access living tissues be minimally invasive, lest they alter the biology under investigation. A previous study demonstrated that use of open skull windows to image the brain caused pathophysiological inflammatory responses that altered the underlying biology (Xu et al., 2007). As an alternative, this study recommended use of thinned skull preparations, which are far less injurious to the meninges and brain. However, it is important to note that poorly prepared thin skull windows can induce inflammation like the damage observed beneath open skull windows. In fact, we describe in Protocol 2 a rapid and highly reproducible model of mTBI that relies on an injurious thinned skull preparation (Roth et al., 2014). If the goal is not to induce an injury, then preparation of thinned skull windows should be slow and deliberate. Prior to conducting experiments, individuals should become competent in creating undamaged thin skull windows – a process that usually requires 2–3 months of weekly practice. Becoming consistent at generating reproducible mTBI lesions also requires a few months of practice.
Troubleshooting
Preparative Damage
Damage to the underlying brain and meninges can occur during preparation of a thinned skull window. Damage can be assessed using several methods including administration of vascular dyes and / or imaging fluorescent protein reporter mice as shown in Figure 2 and 3. We routinely use CX3CR1gfp/+ mice (Jung et al., 2000) to assess the degree of injury in thinned skull preparations. Damage caused by the thinned skull preparation results in loss of meningeal macrophages and morphological transformation of microglia at the glial limitans (Figure 2 & 3). Skull thinning can also cause damage to meningeal blood vessels. It is easy to identify this damage by injecting mice intravenously with a fluorescent vascular tracer. Damaged vessels will either be occluded or leaking. Potential causes of meningeal or parenchymal damage include cracking the skull, thinning the skull too quickly, or applying too much downward pressure when thinning the bone with the microsurgical blade or microdrill.
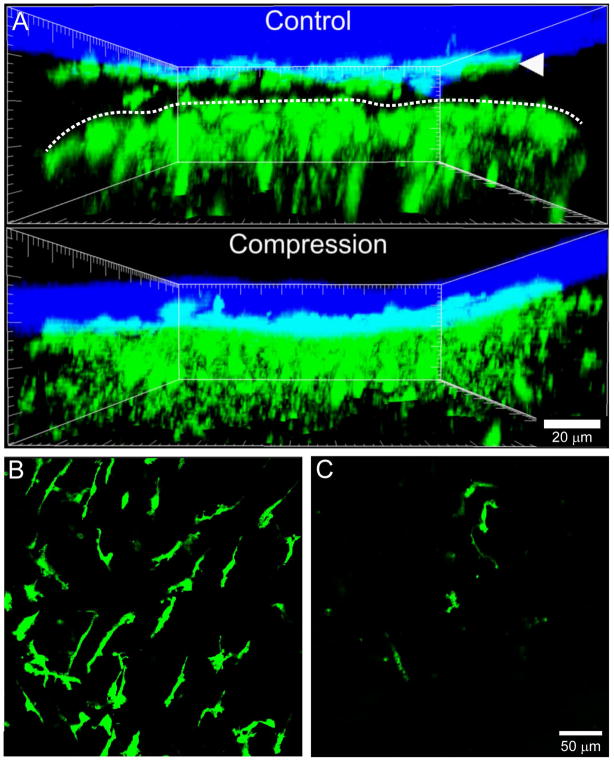
Figure 2. Compression of the thinned skull window to induce a mTBI.
A. Representative xz projections captured by two-photon microscopy through a thinned skull window are shown for a naïve vs. compressed CX3CR1gfp/+ mice. Meningeal macrophages (green, white arrowhead) reside in the dura mater just beneath the skull bone (blue) and are readily visible in an uninjured thinned skull preparation. In addition, there should be a visible space between these macrophages and the glial limitans (denoted with a dotted white line). Beneath the glial limitans are microglia (green), which should have a ramified, non-activated appearance. The application of downward pressure to the skull bone compresses the meningeal space, kills meningeal macrophages, and activates microglia beneath the glial limitans. Note the absence of a meningeal space in the compressed CX3CR1gfp/+ mouse 5 min following the injury. B–C. Following compression of the thinned skull window, meningeal macrophages in the dura mater are rapidly killed. Representative xy projections captured in the dura mater of naïve (B) and compressed (C) CX3CR1gfp/+ mice show the clear loss of meningeal macrophages (green) in the injured animal relative to the uninjured control.
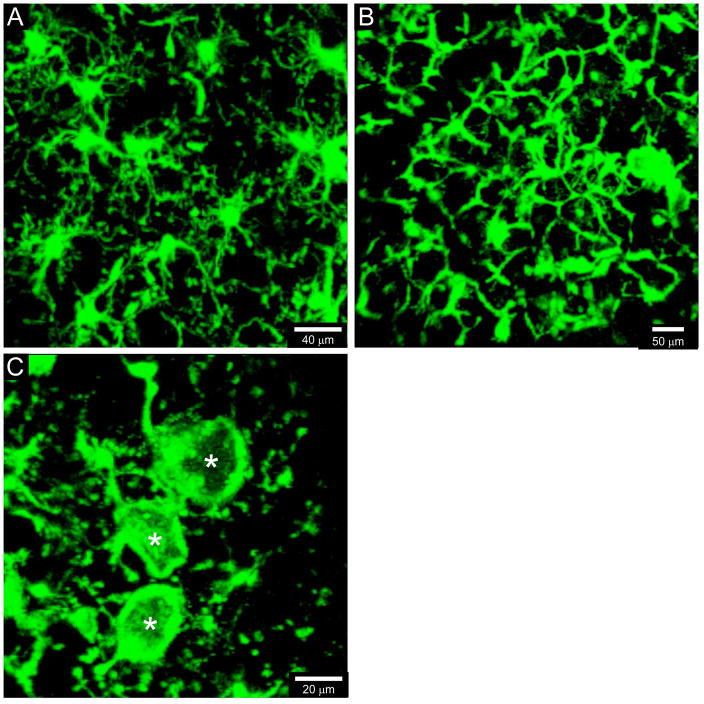
Figure 3. Comparison of microglia morphologies visualized through uninjured versus injured thinned skull windows.
When visualized by two-photon microscopy through an uninjured thinned skull window, naïve CX3CR1gfp/+ microglia (green) should have small cell bodies and appear highly ramified, with a complex network of branched processes extending outward in all directions (A). By contrast, accidental or intentional damage to thinned skull windows will cause microglia to rapidly transform, especially at the glial limitans. Microglia will adopt honeycomb (B) and phagocytic “jellyfish-like” (C, white asterisks) morphologies within 20–30 min of injury. Observance of these morphological transformations is a clear sign that the thinned skull preparation has induced damage in the underlying tissue.
Photobleaching and Laser Damage
The relative brightness of fluorescent probes is an important consideration when performing intravital imaging through a thinned skull window. Utilization of high laser powers to image dim fluorophores or fluorescent proteins can cause photobleaching and significant heat damage to the CNS / meninges. Cells residing in the upper layers of the meninges (i.e., the dura and arachnoid mater) are particularly susceptible to laser injury due to their proximity to the objective. Laser damage can cause injury to blood vessels and cell death, resulting in inflammation. It is important to minimize laser power when imaging through a thinned skull bone, especially when imaging over long periods of time.
Tissue drift and movement artifacts
Time lapses collected by two-photon microscopy through a thinned skull window can become unstable, drift, or move at a periodic rate. This can result from thermal expansion, unstable attachment of the metal brace to the skull bone, or issues relating to the depth of anesthesia. Steady z drift can occur when the metal brace and/or glue attached to the skull bone expands when placed in an environmental chamber surrounding the two-photon microscope. Environmental chamber temperatures are usually kept around 33–35°C to keep animals warm while imaging. However, the metal brace and glue can expand when placed into the environmental chamber if they are not prewarmed to this temperature. This type of z drift will usually subside once the metal / glue equilibrates with the temperature of the imaging chamber. Movement artifacts can also be created when the metal brace attached to the skull bone becomes loose or unstable. This can be easily fixed by removing the brace and reapplying the glue. Lastly, imaging can also become unstable if the animal is not at the appropriate level of anesthesia. A rapid rate of respiration can create movements appearing at regular intervals. This can be resolved by inducing a deeper state of anesthesia by administering a maintenance dose of anesthesia.
ANTICIPATED RESULTS
Basic Protocol 1 facilitates imaging of immune and neural cell dynamics in the CNS under steady state and pathophysiological conditions. By contrast, Basic Protocol 2 allows one to study the dynamics of a sterile injury response to CNS damage. The results of your experiment will depend on the model system and fluorescent reporters under investigation. Figures 2 and 3 show some of the expected results from Basic Protocol 1 and 2 when imaging CX3CR1gfp/+ mice. As described in these figures, the two skull thinning preparations have vastly different effects on myeloid cells that reside in the meninges and brain parenchyma.
TIME CONSIDERATIONS
Preparation of a mouse for two-photon imaging can take 40–60 minutes, which includes all steps. Generating thinned skull window (Basic Protocol 1) requires more time than the mTBI preparation (Basic Protocol 2) because the skull must be carefully and gently thinned without applying downward pressure. The thinning alone for Basic Protocol 1 should take about 20–25 minutes. By contrast, the thinned for Basic Protocol 2 requires 1–2 minutes. After thinning the bone, a mouse can be imaged varying lengths of time, depending on the experimental goals. We have imaged mice for up to 10 hours, although routine two-photon imaging sessions typically last for 30 to 90 minutes.
Acknowledgments
This research was supported by the intramural research program at the National Institute of Neurological Disease (NINDS), National Institute of Health (NIH).
LITERATURE CITED
- Bullen A, Friedman RS, Krummel MF. Two-photon imaging of the immune system: a custom technology platform for high-speed, multicolor tissue imaging of immune responses. Curr Top Microbiol Immunol. 2009;334:1–29. doi: 10.1007/978-3-540-93864-4_1. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol. 2002;2(11):872–880. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc Natl Acad Sci U S A. 2003;100(22):13081–13086. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, … Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Drobizhev M, Makarov NS, Tillo SE, Hughes TE, Rebane A. Two-photon absorption properties of fluorescent proteins. Nat Methods. 2011;8(5):393–399. doi: 10.1038/nmeth.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, … Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci. 2010;13(4):411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28(1):12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Gossa S, Nayak D, Zinselmeyer BH, McGavern DB. Development of an immunologically tolerated combination of fluorescent proteins for in vivo two-photon imaging. Sci Rep. 2014;4:6664. doi: 10.1038/srep06664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE. Immune responses to RNA-virus infections of the CNS. Nat Rev Immunol. 2003;3(6):493–502. doi: 10.1038/nri1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420(6917):812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Herz J, Zinselmeyer BH, McGavern DB. Two-photon imaging of microbial immunity in living tissues. Microsc Microanal. 2012;18(4):730–741. doi: 10.1017/S1431927612000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, McGavern DB. Inflammation on the mind: visualizing immunity in the central nervous system. Curr Top Microbiol Immunol. 2009;334:227–263. doi: 10.1007/978-3-540-93864-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoq J, Parpaleix A, Roussakis E, Ducros M, Goulam Houssen Y, Vinogradov SA, Charpak S. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nat Med. 2011;17(7):893–898. doi: 10.1038/nm.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Harris TH, Kipnis J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015;36(10):569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mank M, Santos AF, Direnberger S, Mrsic-Flogel TD, Hofer SB, Stein V, … Griesbeck O. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat Methods. 2008;5(9):805–811. doi: 10.1038/nmeth.1243. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431(7005):195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nayak D, Zinselmeyer BH, Corps KN, McGavern DB. In vivo dynamics of innate immune sentinels in the CNS. Intravital. 2012;1(2):95–106. doi: 10.4161/intv.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505(7482):223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rua R, McGavern DB. Elucidation of monocyte/macrophage dynamics and function by intravital imaging. J Leukoc Biol. 2015;98(3):319–332. doi: 10.1189/jlb.4RI0115-006RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MV, McGavern DB. Immune Surveillance of the CNS following Infection and Injury. Trends Immunol. 2015;36(10):637–650. doi: 10.1016/j.it.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer CB, Friedman B, Nishimura N, Schroeder LF, Tsai PS, Ebner FF, … Kleinfeld D. Two-photon imaging of cortical surface microvessels reveals a robust redistribution in blood flow after vascular occlusion. PLoS Biol. 2006;4(2):e22. doi: 10.1371/journal.pbio.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2(12):905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- Shih AY, Driscoll JD, Drew PJ, Nishimura N, Schaffer CB, Kleinfeld D. Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J Cereb Blood Flow Metab. 2012;32(7):1277–1309. doi: 10.1038/jcbfm.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbara C, Misgeld T, Kerschensteiner M. In vivo imaging of the diseased nervous system: an update. Curr Pharm Des. 2012;18(29):4465–4470. doi: 10.2174/138161212802502279. [DOI] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100(12):7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7(11):1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112(2):271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007;10(5):549–551. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5(2):201–208. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinselmeyer BH, Dempster J, Wokosin DL, Cannon JJ, Pless R, Parker I, Miller MJ. Chapter 16. Two-photon microscopy and multidimensional analysis of cell dynamics. Methods Enzymol. 2009;461:349–378. doi: 10.1016/S0076-6879(09)05416-0. [DOI] [PubMed] [Google Scholar]