Abstract
Background
Cancer is a major cause of death in patients undergoing haemodialysis. However, information about the actual clinical practice of chemotherapy for patients with cancer undergoing haemodialysis is lacking. We conducted a nationwide survey using questionnaires on the clinical practice of chemotherapy for such patients.
Patients and methods
The nationwide survey included patients undergoing haemodialysis who were subsequently diagnosed with cancer in 20 hospitals in Japan from January 2010 to December 2012. We reviewed their clinical data, including cancer at the following primary sites: kidney, colorectum, stomach, lung, liver, bladder, pancreas and breast. The questionnaires consisted of the following subjects: (1) patient characteristics; (2) regimen, dosage and timing of chemotherapy; and (3) clinical outcome.
Results
Overall, 675 patients were registered and assessed for main primary cancer site involvement. Of 507 patients with primary site involvement, 74 patients (15%) received chemotherapy (44 as palliative chemotherapy and 30 as perioperative chemotherapy). The most commonly used cytotoxic drugs were fluoropyrimidine (15 patients), platinum (8 patients) and taxane (8 patients), and the dosage and timing of these drugs differed between institutions; however, the dosage of molecular targeted drugs (24 patients) and hormone therapy drugs (15 patients) was consistent. The median survival time of patients receiving palliative chemotherapy was 13.0 months (0.1–60.3 months). Three patients (6.8%) died from treatment-related causes and nine patients (20%) died of causes other than cancer. Of the 30 patients who received perioperative chemotherapy, 6 (20%) died of causes other than cancer within 3 years after the initiation of chemotherapy.
Conclusion
Among the haemodialysis patients with cancer who received chemotherapy, the rates of mortality from causes other than cancer might be high for both palliative and perioperative chemotherapy. Indications for the use of chemotherapy in patients undergoing haemodialysis should be considered carefully.
Keywords: anticancer drug, chemotherapy, dosage adjustment, end-stage renal disease, hemodialysis
Key questions.
What is already known about this subject?
Cancer is one of the major causes of death among haemodialysis patients.
There are no guidelines regarding chemotherapy for haemodialysis patients with cancer.
Few data are available about the actual clinical practice of chemotherapy for haemodialysis patients with cancer.
What does this study add?
Our results showed details of the treatment and clinical outcomes of haemodialysis patients who received cancer chemotherapy.
The non-cancer-related mortality is high in haemodialysis patients who receive chemotherapy.
The dosage and timing of cytotoxic drugs such as 5-fluorouracil and platinum differed between institutions.
How might this impact on clinical practice?
The indications of chemotherapy for patients with cancer undergoing haemodialysis should be carefully considered.
The optimal timing and necessary dose adjustments of anticancer drugs in the context of dialysis sessions should be investigated.
Introduction
Currently, the number of dialysis patients has increased worldwide. The dialysis population is over 2 million worldwide and 300 000 in Japan.1 2 The risk of cancers such as kidney and bladder cancer in patients undergoing haemodialysis (HD) is generally higher than in the general population,3 and cancer is one of the major causes of death among HD patients, ranking third in Japan after cardiac failure and infectious disease.2 4
Chemotherapy is a standard treatment for advanced cancers in a palliative and perioperative setting. Several randomised trials testing new treatments for various advanced cancers have shown a survival benefit. However, the subjects of these clinical trials are limited to patients with adequate organ function. There has been no clinical trial that verifies the efficacy and safety of chemotherapy in the HD patient.
As the number of HD patients increases, medical oncologists and nephrologists are more likely to treat patients with cancer undergoing HD and to confront the difficulty of managing their chemotherapy.5 6 However, there are no guidelines regarding cancer chemotherapy for HD patients due to a lack of evidence.7 Furthermore, few data are available about the actual clinical practice of managing chemotherapy in HD patients. Only one study, the CANcer and DialYsis (CANDY) study conducted in France, has reported the clinical practice of chemotherapy in patients with cancer undergoing HD.8 The CANDY study focused on the type of anticancer drugs used and dose adjustment for patients undergoing HD. However, the clinical outcomes, such as efficacy and adverse events, were not discussed; therefore, physicians still face challenges in providing cancer chemotherapy. A lack of knowledge and data concerning the use of chemotherapy may lead to improper use of chemotherapy and fatal toxic effects in patients undergoing HD.
We conducted a nationwide survey of patients with cancer undergoing HD and receiving chemotherapy. We reviewed the clinical outcome in addition to the regimen and dosage of chemotherapy.
Patients and methods
This retrospective case series study was conducted by the Onconephrology Consortium in Japan with clinical investigators, both medical oncologists and nephrologists, from 20 institutions. We enrolled patients undergoing HD who were subsequently diagnosed with cancer in the participating institutes from January 2010 to December 2012. We reviewed the clinical courses of those patients who met the following selection criteria: (1) primary sites of the cancer were in the kidney, colorectum, stomach, lung, bladder, liver, breast or pancreas; and (2) the initial treatments were palliative chemotherapy or surgery followed by perioperative chemotherapy. We selected the eight primary sites because they were represented at a high frequency in our preliminary survey. We excluded patients with a history of renal transplantation.
Data collection
In July 2014, the same questionnaires were sent by email to the members of the Onconephrology Consortium of 20 institutions in Japan. Twelve of these were general hospitals and eight were university hospitals. The questionnaires consisted of the following sections: (1) patient characteristics (age, sex, primary disease of renal failure, duration of HD, symptoms due to cancer, disease status and Eastern Cooperative Oncology Group performance status); (2) regimen, dosing and timing of chemotherapy; and (3) clinical outcome (response rate, adverse events, survival time and cause of death). The data were collected from medical records until the point of the most recent follow-up. The deadline for submission was November 2015.
Response and toxicity evaluation
Objective response was assessed according to the Response Evaluation Criteria in Solid Tumors (V.1.1). The toxicity was evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (V.4.0). Treatment-related death was defined as any cause of death that occurred within 30 days after the initiation of chemotherapy.
Statistical analysis
Overall survival was calculated from the initiation of treatment to the date of death from any cause. Survival curves were calculated by the Kaplan-Meier method using IBM statistics software (V.21.0; IBM, Armonk, New York, USA).
Results
Subjects
Overall, 675 patients with cancer undergoing HD were registered in this study. The primary cancer sites were kidney (161 patients), colorectum (84 patients), stomach (73 patients), lung (64 patients), bladder (37 patients), liver (35 patients), breast (27 patients) and pancreas (26 patients). Among these patients, 396 cancers were assessed to be surgically resectable, 107 were assessed to be unresectable and the disease status of the remaining 4 patients was unknown. Of the 107 patients with unresectable cancer, 44 underwent chemotherapy, 36 received best supportive care and 27 patients underwent other therapies. The reasons for best supportive care were older age (14 patients), poor performance status (11 patients), no indication for chemotherapy (9 patients), critical comorbidities such as severe heart disease and cerebral infarction (8 patients), patient/family decision (7 patients) and unknown (5 patients) (13 patients overlapped). Finally, 74 patients met the selection criteria and received chemotherapy as the initial treatment (44 as palliative chemotherapy and 30 as perioperative chemotherapy). The consort diagram is shown in online supplementary figure 1. The baseline characteristics of the 74 patients are shown in table 1. The median age of patients and duration of HD were 64 (range, 44–81) and 8.8 (range, 0.3–37.2) years, respectively, in the palliative group, and 68 (range, 43–85) and 9.2 (range, 0.1–27.7) years, respectively, in the perioperative group. The primary causes of renal failure were chronic glomerulonephritis and diabetic nephropathy in both groups. The primary cancer sites were kidney (18 patients), lung (9 patients) and colorectum (7 patients) in the palliative group, and breast (17 patients) and colorectum (6 patients) in the perioperative group.
Table 1.
Patients’ characteristics
| Palliative group, n=44 | Perioperative group, n=30 | |
| Median age, year (range) | 64 (44–81) | 68 (43–85) |
| Sex | ||
| Male | 36 (82%) | 10 (33%) |
| Female | 8 (18%) | 20 (67%) |
| Primary causes of renal failure | ||
| Chronic glomerulonephritis | 15 (34%) | 8 (27%) |
| Diabetic nephropathy | 11 (25%) | 6 (20%) |
| Nephrosclerosis | 6 (14%) | 1 (3%) |
| Others | 12 (27%) | 15 (50%) |
| Median duration of haemodialysis, year (range) | 8.8 (0.3–37.2) | 9.2 (0.1–27.7) |
| Primary site | ||
| Kidney | 18 | 0 |
| Lung | 9 | 0 |
| Colorectum | 7 | 6 |
| Stomach | 4 | 1 |
| Pancreas | 3 | 3 |
| Breast | 2 | 17 |
| Bladder | 1 | 3 |
| Symptoms due to cancer | ||
| Yes | 21 (48%) | 18 (60%) |
| No | 23 (52%) | 12 (40%) |
| Disease status | ||
| Resectable disease | 30 (100%) | |
| Locally advanced disease | 6 (14%) | |
| Metastatic disease | 38 (86%) | |
| ECOG performance status | ||
| 0–1 | 27 (61%) | 18 (60%) |
| ≥2 | 8 (18%) | 1 (3%) |
| Unknown | 9 (21%) | 11 (37%) |
ECOG, Eastern Cooperative Oncology Group.
esmoopen-2017-000301supp001.jpg (1.3MB, jpg)
Anticancer drugs prescribed in this study
The anticancer drugs prescribed in this study were cytotoxic drugs in 34 patients, molecular targeted drugs in 24 patients, hormone therapy drugs in 15 patients and other drugs in 4 patients (table 2). The cytotoxic drugs used most commonly were fluoropyrimidine (15 patients), platinum (8 patients) and taxane (8 patients). Most of the molecular targeted drugs were used for renal cell cancer (17 patients), and all hormone therapy drugs were used for breast cancer. Regarding the dosage and timing of chemotherapy, the 5-fluorouracil (5-FU) dose was reduced by 20%–30% in three patients in consideration of renal dysfunction. Most of the taxanes, gemcitabine and monoclonal antibodies were administered on non-dialysis days. Notably, the dosage and timing of platinum differed among institutions. In eight patients who received platinum-containing chemotherapy at eight different institutions, the timing of platinum administration was just before the HD session on a dialysis day in four patients and on a non-dialysis day in four patients. The dosage of oxaliplatin was reduced by 30% in two patients and the dosage of cisplatin was reduced by 50% in one patient.
Table 2.
Anticancer drugs prescribed in this study
| n | Dosage adjustment | ||
| No (%) | Yes (%) | ||
| (Cytotoxic drugs) | |||
| Fluoropyrimidine | |||
| 5-Fluorouracil | 9 | 67 | 33 |
| Tegafur/uracil | 6 | 17 | 67 |
| Platinum | |||
| Oxaliplatin | 4 | 50 | 50 |
| Carboplatin | 2 | 50 | 50 |
| Cisplatin | 2 | 50 | 50 |
| Taxane | |||
| Paclitaxel | 4 | 25 | 75 |
| Docetaxel | 4 | – | 100 |
| Others | |||
| Gemcitabine | 7 | 57 | 43 |
| Irinotecan | 3 | 33 | 67 |
| Other drugs | 4 | – | – |
| Molecular targeted drugs | |||
| Sorafenib | 6 | – | 100 |
| Sunitinib | 4 | – | 100 |
| Temsirolimus | 4 | 100 | – |
| Everolimus | 2 | 50 | 50 |
| Erlotinib | 2 | 100 | – |
| Trastuzumab | 2 | 100 | – |
| Cetuximab | 1 | 100 | – |
| Panitumumab | 1 | 100 | – |
| Imatinib | 1 | 100 | – |
| Axitinib | 1 | 100 | – |
| Hormonal therapy drugs | |||
| Aromatase inhibitor | 11 | 100 | – |
| LH-RH agonist | 2 | 100 | – |
| Tamoxifen | 2 | 100 | – |
The status of tegafur/uracil dosage adjustment is unknown in one patient.
LH-RH, luteinizing hormone-releasing hormone.
However, the dosage of molecular targeted drugs (24 patients) and hormone therapy drugs (15 patients) was consistent among participating institutions; most of the hormone therapy drugs and molecular targeted drugs were used without dose adjustment (table 2). The dosage of sorafenib was reduced by 50% (400 mg/day) in all patients and the dosage of sunitinib was reduced by 25%–50% (37.5 mg/day or 25 mg/day) in four patients.
Response, clinical course and adverse events
Of the 22 patients with measurable lesions in the palliative group, 5 patients achieved partial response and 8 patients were stable. The adverse events, grade 3 or higher, of cytotoxic and molecular drugs are listed in table 3. Among the 10 patients who received perioperative cytotoxic chemotherapy, 5 patients completed the planned regimen. Three patients who received tegafur/uracil at an adjusted dosage completed the planned regimen without experiencing grade 3 or higher adverse events. Three patients, who received 5-FU or gemcitabine at the standard dose, discontinued adjuvant chemotherapy due to severe adverse events, including grade 4 sepsis, grade 3 pneumonitis and grade 3 small intestinal mucositis. Among 10 patients who received molecular targeted drug monotherapy at the standard dose in the palliative group, 8 patients could continue chemotherapy at the initial dosage without severe adverse events. One patient who received erlotinib required a dose adjustment due to diarrhoea, and one patient who received everolimus discontinued chemotherapy due to grade 3 pneumonitis. As for pneumonitis, three patients—receiving gemcitabine at an 80% dosage, sorafenib at a 50% dosage or everolimus at the standard dosage—experienced grade 3 or higher pneumonitis within 2 months of the initiation of chemotherapy. All three patients improved with the discontinuation of chemotherapy and steroid therapy, yet they were unable to receive subsequent chemotherapy.
Table 3.
Adverse events of grade 3 or higher
| Cytotoxic drugs only (n=31) | Molecular targeted drugs only (n=21) | Cytotoxic and molecular targeted drugs (n=3) | Total (N=55) | |
| Neutropaenia | 5 (16%) | 1 (5%) | 1 (33%) | 7 (13%) |
| Leucocytopaenia | 3 (10%) | 1 (5%) | 1 (33%) | 5 (9%) |
| Anaemia | 5 (16%) | 3 (14%) | 1 (33%) | 9 (16%) |
| Thrombocytopaenia | 3 (10%) | 3 (14%) | 6 (11%) | |
| Febrile neutropaenia | 1 (3%) | 1 (2%) | ||
| Nausea | 1 (3%) | 1 (2%) | ||
| Anorexia | 1 (3%) | 1 (33%) | 2 (4%) | |
| Diarrhoea | 1 (3%) | 1 (2%) | ||
| Small intestinal mucositis | 1 (3%) | 1 (2%) | ||
| Enterocolitis infection | 1 (3%) | 1 (2%) | ||
| Colonic haemorrhage | 1 (3%) | 1 (2%) | ||
| Rectal ulcer | 1 (3%) | 1 (2%) | ||
| Skin-related toxicities | 1 (33%) | 1 (2%) | ||
| Peripheral sensory neuropathy | 1 (33%) | 1 (2%) | ||
| Stroke | 1 (5%) | 1 (2%) | ||
| Pneumonitis | 1 (3%) | 2 (10%) | 3 (5%) | |
| Sepsis | 1 (3%) | 1 (2%) |
Severe adverse events leading to hospitalisation or death were reported in 15 patients, including three treatment-related deaths: sudden death in two patients and sepsis in one patient. However, there were no severe adverse events in the hormone therapy group.
Survival and cause of death
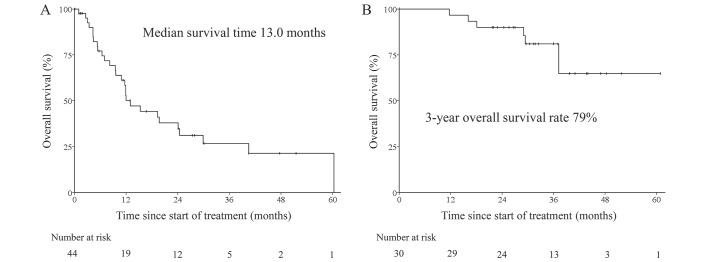
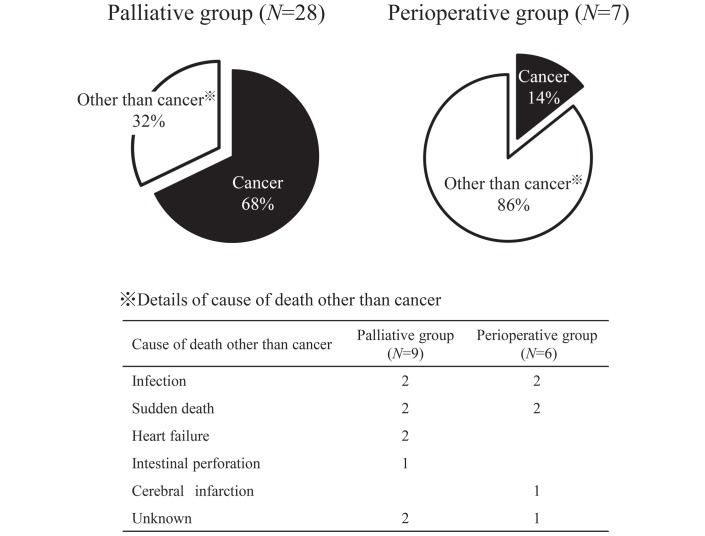
After a median follow-up time of 14.1 months (0.1–52.2 months), the median survival time of 44 patients who received palliative chemotherapy was 13.0 months (figure 1). Regarding the cause of death, 19 patients (68%) died of cancer and 9 patients (32%) died of causes other than cancer (figure 2). Among five patients whose primary cause of renal failure was diabetic nephropathy, three patients (60%) died of causes other than cancer, including two treatment-related deaths (infection, sudden death). Of 30 patients who received perioperative chemotherapy, the 3-year survival rate was 79% after a median follow-up time of 31.5 months (11.7–60.9 months). Regarding the cause of deaths, one patient (14%) died of cancer and six patients (86%) died of causes other than cancer within 3 years after chemotherapy was initiated. All non-cancer-related causes of death occurred after chemotherapy had ended; therefore, they were considered unrelated to chemotherapy.
Figure 1.
Kaplan-Meier curves for overall survival: (A) palliative group and (B) perioperative group.
Figure 2.
Cause of death.
Discussion
This is the largest case series study of chemotherapy in patients with cancer undergoing HD. It will enable us to recognise what we should consider when managing chemotherapy for these patients in clinical practice.
The prognosis of dialysed patients is poor compared with non-dialysed patients because dialysed patients are compromised and have several complications. According to the results of a Japanese nationwide survey, the annual death rate of dialysed patients has remained in the range of 9.2%–10.2% since 1992, whereas since 1995 that of the general Japanese population aged 60–64 years, 70–74 years and 80–84 years has remained in the range of 0.6%–0.9%, 1.5%–2.2% and 4.4%–6.9%, respectively.2 9 This poor prognosis of dialysed patients mainly due to cardiac failure and infectious disease may have influenced the high non-cancer-related death rate in this study.
Generally, the main purpose of perioperative chemotherapy is to reduce cancer recurrence and to prolong survival. Therefore, perioperative chemotherapy is indicated for patients who are expected to survive for extended periods after their cancer is cured. For example, the non-cancer-related 5-year mortality rate of patients with breast cancer who were treated with surgery followed by adjuvant tamoxifen was 3.7%.10 Similarly, the non-cancer-related 6-year mortality rate of patients with colorectal cancer who were treated with surgery followed by adjuvant oxaliplatin, folinic acid and 5-fluorouracil (FOLFOX4) therapy was 5.8%.11 However, in this study, six patients (20%) died of causes other than cancer within 3 years after the initiation of perioperative chemotherapy. Compared with the results for non-dialysed patients, the non-cancer-related mortality rate in HD patients was clearly higher. This suggests that the prognosis should be taken into account when considering the indications for perioperative chemotherapy in patients undergoing HD.
In the palliative chemotherapy group (n=44), the rate of treatment-related death was 6.8%. One cause of treatment-related death was infection. A 76-year-old man with a wild-type UGT1A1 allele received irinotecan at a dosage of 150 mg/m2 as part of irinotecan, folinic acid and 5-fluorouracil (FOLFIRI) plus cetuximab chemotherapy. This patient died of sepsis with grade 4 neutropaenia within 1 month of treatment. Fujita et al12 previously reported that in patients with severe renal failure, the area under the concentration–time curve for SN-38, the active metabolite of irinotecan, was much greater than that of patients with normal kidney function, although neither irinotecan nor SN-38 is excreted by the kidneys. This might be the cause of the severe neutropaenia and infection in this patient. Because patients undergoing HD are potentially compromised, we should carefully decide the regimen and dosage of chemotherapy to avoid faecal infection.
The other problem is that the dosage and timing of cytotoxic drugs such as fluoropyrimidine and platinum differed between institutions in this study. This different administration of chemotherapy is due to the paucity of pharmacokinetic data. For example, there is a lack of data available about whether platinum can be dialysed. Several case reports have recommended that a dialysis session is initiated immediately after the administration of oxaliplatin to remove circulating platinum molecules derived from oxaliplatin, which have biological activity.13–15 However, most circulating platinum molecules are undialysable because they are immediately bound to plasma proteins or distributed to the tissue. Therefore, the adequate dosage and timing of platinum administration are unclear. However, by referring to data from the literature with sufficient pharmacokinetic data, the dosage of sorafenib in this study was consistently reduced.16 Pharmacokinetic study is needed to establish the optimal chemotherapy for patients with cancer undergoing HD. Therefore, we conducted a pharmacokinetic study of 5-FU and oxaliplatin, both of which were commonly used in this study, in patients with cancer undergoing HD.17
This study has some limitations. First, this study is retrospective; therefore, the possibility of bias exists in the selection of patients. In this study, we collected the data only from the patients undergoing HD with cancer who were treated in a cancer hospital because we could not collect the data of those who could not be treated for reasons such as patient refusal or their medical condition. Therefore, this might affect the evaluation of efficacy and safety of chemotherapy for patients with cancer undergoing HD. Second, the subjects in this study included various cancers and chemotherapy regimens. Although the incidence of grade 3 or higher adverse events was relatively low, it might be due to reduced dosage of anticancer drugs and underestimated by retrospective analysis. Therefore, it is rather difficult to evaluate the efficacy and adverse events of a given chemotherapy regimen. Third, the follow-up period to evaluate the survival data is short. However, the problem of poor outcome and the method of anticancer drugs administration can be discussed. Furthermore, 12 (27%) of 44 patients with unresectable cancer lived more than 2 years in this study; effective chemotherapy may provide a chance of long survival even for HD patients with unresectable cancer. In the current situation where there is a lack of information, our present data may facilitate clinical decision making and future advancements in cancer chemotherapy for patients undergoing HD.
In conclusion, among patients with cancer who were undergoing HD and received chemotherapy, the rates of mortality from causes other than cancer might be high for both palliative and perioperative chemotherapy. The prognosis of dialysed patients is poor compared with that of non-dialysed patients. Therefore, the indications of chemotherapy for patients undergoing HD should be carefully considered.
Acknowledgments
We especially thank the patients and their family members. We also thank all investigators and clinical research coordinators who contributed to this study at the 20 institutions, members of the Japan Onconephrology Consortium (Sapporo City General Hospital, University of Tsukuba Hospital, Toranomon Hospital, St Marianna University School of Medicine, Kyorin University Hospital, Shiga Medical Center for Adults, Japanese Red Cross Otsu Hospital, National Hospital Organization Kyoto Medical Center, Mitsubishi Kyoto Hospital, Kyoto City Hospital, Kyoto Min-iren Chuo Hospital, Kyoto University Hospital, Kitano Hospital, Takatsuki General Hospital, Kobe University Hospital, Kobe City Medical Center General Hospital, Okayama University Hospital, Kyushu University Hospital, Fukuoka Red Cross Hospital and Kumamoto University Hospital).
Footnotes
Contributors: All authors contributed to conception and design of the study and acquisition of data. TF contributed to the analysis of data and drafting of the manuscript. All authors edited and approved the manuscript.
Funding: This work was supported by the Japanese Association of Dialysis Physicians (JADP Grant 2014-11).
Competing interests: MN has received honoraria from Daiichi Sankyo and Taiho Pharmaceutical. MasM has received research grants from Chugai, Kyowa Hakko Kirin, Takeda, Daiichi Sankyo, Astellas, Otsuka, Baxter, Teijin Pharma and Shionogi. TS has received research grants from Baxter, Astellas, Kyowa Hakko Kirin, Teijin Pharma and Takeda. EB has received research grants from Takeda, Chugai, Eli Lilly, Merck Serono, Shionogi and Taiho. He has also received honorarium from Eli Lilly. KT has received research grants from Kyowa Hakko Kirin, Chugai, Takeda, Kissei, Otsuka, Daiichi Sankyo and Torii. He has also received honoraria from Kyowa Hakko Kirin, Chugai and Sanofi. His institution has received funding from Baxter. AN has received research grant from Daiichi Sankyo. HY has received honoraria from Medicon, Taiho, Chugai, Yakult Honsha, Bristol-Myers Squibb, Takeda and Kyowa Hakko Kirin. MY has been on the advisory board of Astellas and received research grants from Astellas, Chugai, Daiichi Sankyo, Fuji Yakuhin, Kyowa Hakko Kirin, Mitsubishi Tanabe, MSD, Nippon Boehringer Ingelheim, Baxter, Takeda Pharmaceutical Company, Fuso Pharmaceutical Industries and Terumo Corporation. ManM has received research grant from Chugai, Yakult Honsha, Ono Pharmaceutical, Ayumi Pharmaceutical, Showa Yakuhin Kako, Shionogi, Taiho, Terumo and Nippon Zoki Pharmaceutical Corporations.
Patient consent: Detail has been removed from this case description/these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval: This study was approved by the institutional review board or ethics committee at each participating institution.
Provenance and peer review: Not commissioned; externally peer reviewed.
Presented at: Part of this study was presented at the annual meeting of ESMO 2016 Congress.
References
- 1.Liyanage T, Ninomiya T, Jha V, et al. . Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015;385:1975–82. 10.1016/S0140-6736(14)61601-9 [DOI] [PubMed] [Google Scholar]
- 2.Masakane I, Nakai S, Ogata S, et al. . An overview of regular dialysis treatment in Japan (As of 31 December 2013). Ther Apher Dial 2015;19:540–74. 10.1111/1744-9987.12378 [DOI] [PubMed] [Google Scholar]
- 3.Maisonneuve P, Agodoa L, Gellert R, et al. . Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet 1999;354:93–9. 10.1016/S0140-6736(99)06154-1 [DOI] [PubMed] [Google Scholar]
- 4.de Jager DJ, Grootendorst DC, Jager KJ, et al. . Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009;302:1782–9. 10.1001/jama.2009.1488 [DOI] [PubMed] [Google Scholar]
- 5.Kitai Y, Matsubara T, Yanagita M. Onco-nephrology: current concepts and future perspectives. Jpn J Clin Oncol 2015;45:617–28. 10.1093/jjco/hyv035 [DOI] [PubMed] [Google Scholar]
- 6.Kitai Y, Matsubara T, Funakoshi T, et al. . Cancer screening and treatment in patients with end-stage renal disease: remaining issues in the field of onco-nephrology. Renal Replacement Therapy 2016;2:1–9. 10.1186/s41100-016-0046-y [DOI] [Google Scholar]
- 7.Janus N, Thariat J, Boulanger H, et al. . Proposal for dosage adjustment and timing of chemotherapy in hemodialyzed patients. Ann Oncol 2010;21:1395–403. 10.1093/annonc/mdp598 [DOI] [PubMed] [Google Scholar]
- 8.Janus N, Launay-Vacher V, Thyss A, et al. . Management of anticancer treatment in patients under chronic dialysis: results of the multicentric CANDY (CANcer and DialYsis) study. Ann Oncol 2013;24:501–7. 10.1093/annonc/mds344 [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health and Welfare. Vital Statistics of Japan 1995-2015. Tokyo, Japan: Statistics and Information Department, Minister’s Secretariat, Ministry of Health and Welfare. [Google Scholar]
- 10.Davies C, Godwin J, Gray R, et al. . Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84. 10.1016/S0140-6736(11)60993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.André T, Boni C, Navarro M, et al. . Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16. 10.1200/JCO.2008.20.6771 [DOI] [PubMed] [Google Scholar]
- 12.Fujita K, Masuo Y, Okumura H, et al. . Increased Plasma Concentrations of Unbound SN-38, the Active Metabolite of Irinotecan, in Cancer Patients with Severe Renal Failure. Pharm Res 2016;33:269–82. 10.1007/s11095-015-1785-0 [DOI] [PubMed] [Google Scholar]
- 13.Watayo Y, Kuramochi H, Hayashi K, et al. . Drug monitoring during FOLFOX6 therapy in a rectal cancer patient on chronic hemodialysis. Jpn J Clin Oncol 2010;40:360–4. 10.1093/jjco/hyp176 [DOI] [PubMed] [Google Scholar]
- 14.Horimatsu T, Miyamoto S, Morita S, et al. . Pharmacokinetics of oxaliplatin in a hemodialytic patient treated with modified FOLFOX-6 plus bevacizumab therapy. Cancer Chemother Pharmacol 2011;68:263–6. 10.1007/s00280-011-1633-9 [DOI] [PubMed] [Google Scholar]
- 15.Gori S, Lunardi G, Inno A, et al. . Pharmacokinetics of oxaliplatin in a hemodialyzed patient: chemotherapy dose adjustment and timing of dialysis. Clin Colorectal Cancer 2014;13:260–3. 10.1016/j.clcc.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 16.Kennoki T, Kondo T, Kimata N, et al. . Clinical results and pharmacokinetics of sorafenib in chronic hemodialysis patients with metastatic renal cell carcinoma in a single center. Jpn J Clin Oncol 2011;41:647–55. 10.1093/jjco/hyr015 [DOI] [PubMed] [Google Scholar]
- 17.Funakoshi T, Horimatsu T, Yamada A, et al. . 1606PPharmacokinetics and safety of FOLFOX therapy in patients undergoing hemodialysis. Ann Oncol 2017;28(suppl_5):v543–67. 10.1093/annonc/mdx388.063 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2017-000301supp001.jpg (1.3MB, jpg)