Short abstract
A service evaluation of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) testing and result notification in patients attending a rapid testing service (Dean Street Express [DSE]) compared with those attending an existing ‘standard’ sexual health clinic (56 Dean Street [56DS]), and modelling the impact of the new service from 1 June 2014 to 31 May 2015. Primary outcome: time from patients’ sample collection to notification of test results at DSE compared with 56DS. Secondary outcomes estimated using a model: number of transmissions prevented and the number of new partner visits avoided and associated cost savings achieved due to rapid testing at DSE. In 2014/15, there were a total of 81,352 visits for CT/NG testing across 56DS (21,086) and DSE (60,266). Rapid testing resulted in a reduced mean time to notification of 8.68 days: 8.95 days for 56DS (95% CI 8.91–8.99) compared to 0.27 days for DSE (95% CI 0.26–0.28). Our model estimates that rapid testing at DSE would lead to 196 CT and/or NG transmissions prevented (2.5–97.5% centile range = 6–956) and lead to annual savings attributable to reduced numbers of partner attendances of £124,283 (2.5–97.5% centile range = £4260–590,331). DSE, a rapid testing service for asymptomatic infections, delivers faster time to result notification for CT and/or NG which enables faster treatment, reduces infectious periods and leads to fewer transmissions, partner attendances and clinic costs.
Keywords: Chlamydia (Chlamydia trachomatis), gonorrhoea (Neisseria gonorrhoeae), sexual behaviour
Introduction
The number of sexually transmitted infections (STIs) diagnosed in England continues to rise annually, particularly in groups such as young people and men who have sex with men (MSM).
Chlamydia trachomatis (CT) is often asymptomatic but is also associated with pelvic inflammatory disease, epididymo-orchitis and infertility. It is the most commonly diagnosed STI in genitourinary medicine (GUM) clinics in England, with over 200,000 diagnoses representing nearly 50% of all STI diagnoses in 2015.1,2 Neisseria gonorrhoeae (NG) diagnoses increased by 53% between 2012 and 2015 (26,880–41,193 cases). These increases may have resulted from changes in sexual behaviour, improved access to services, increased screening and advances in the accuracy and reliability of diagnostic technology.3
The UK Department of Health’s Framework for Sexual Health Improvement in England called for interventions and actions to improve sexual health outcomes.4 In particular, it mentioned that incorporating the latest technologies into clinical settings provided an opportunity to create sexual health services that improve access, provide prompt diagnosis and treatment, and reduce costs.
Theoretical work has shown that a rapid testing and treatment service for STIs could reduce complications, transmissions, inappropriate treatment and generate £10 million in cost savings to providers if implemented across England.5 In February 2014, Chelsea & Westminster NHS Foundation Trust opened Dean Street Express (DSE), a part of 56 Dean Street (56DS), in Soho, London, offering a walk-in, rapid STI screening service for asymptomatic individuals.6 This is the first rapid testing service of its kind in the UK.
Information on the volume and results of testing, patient service utilisation patterns and time from test to results notification is available from DSE and 56DS for all patients in Chelsea & Westminster NHS Trust. This provides an opportunity to test the theory that rapid testing and results supports improved sexual health services and yields a public health benefit. In this service evaluation, we assess the impact that DSE has had on patient care and estimate the public health benefit attained as a result of faster treatment for asymptomatic patients compared to conventional sexual health clinics. We also explore the potential impact on reducing transmissions to new partners by shortening the period between testing and treatment, and the subsequent reduction in partner treatment visits and associated cost.
Methods
As a service evaluation, ethics approval was not sought. We used SQUIRE guidelines in writing this evaluation.
56DS
Prior to the opening of DSE, both asymptomatic and symptomatic patients were seen at 56DS and processing of CT and NG samples was carried out off-site. After DSE opened, symptomatic patients continued to be seen at 56DS with the same testing model; asymptomatic patients were tested at DSE with on-site rapid NAAT testing.
56DS is a publicly-funded, confidential STI service for symptomatic patients and offers broader services such as emergency contraception and post-exposure HIV prophylaxis for sexual exposure.
During the observation period, 56DS used standard off-site laboratory-based nucleic acid amplification test (NAAT) testing for CT and/or NG. Test results at 56DS were manually reviewed and actioned. Patients with negative tests were sent an SMS to inform them of their result; patients with positive tests were contacted by SMS if they had received empirical treatment at their initial visit to perform partner notification. Patients with positive test results who had not been given treatment at their initial visit were called by clinic staff to arrange a follow-up appointment.
DSE
DSE is a free, confidential, walk-in STI clinic for men and women without symptoms of STIs, which opened in February 2014. On arrival at DSE, patients complete a short questionnaire on their sexual history using a touchscreen computer. The touchscreen orders the relevant swabs based on their self-reported sexual history. Patients are directed to a cubicle where they take their own swabs/samples according to the directions in a short video. Samples are then immediately delivered to the in-house laboratory via air tube and are processed by a NAAT on the GeneXpert Infinity machine (Cepheid, Sunnyvale, CA, USA). Finally, patients have a consultation with a health adviser to review their sexual history and have blood taken for syphilis, HIV and/or hepatitis B/C testing at an off-site laboratory with results notified within four hours. Empirical treatment for STIs and contacts of STIs is not given at DSE. Attenders who are symptomatic and those who reveal they are the contact of infections are directed to 56DS for management and/or treatment. However, patients receiving empirical treatment (because of being symptomatic and/or a partner) were not considered in this analysis.
Results from the GeneXpert machine and blood test results are integrated into the clinic’s electronic patient records (EPR), which allows for automatic collation and delivery of results to patients by SMS. Patients with positive test results are offered treatment at 56DS as soon as possible. More information about the service and a film of the patient pathway within clinic can be accessed online (http://www.deanstreetexpress.nhs.uk/).
Data analysis
EPR data from 56DS and DSE were extracted for the one-year period from 1 June 2014 to 31 May 2015 inclusive. This period was selected to allow a ‘bedding in’ period following the launch of DSE. The following data were available for each patient-testing attendance: anonymous patient ID, clinic site, date of clinic attendance, gender and sexual orientation (MSM, men who have sex with women [MSW] and women). The date and time of CT/NG result notification by patient ID were extracted.
From patient notes, we extracted data on the number of reported new partners in the last three months for a sample of patients attending DSE over a one-week period (27 April 2015–2 May 2015), as this was incomplete for all patient records in the EPR. In order to be representative of patients attending over the week, we selected the first 12 patients for the 15 sessions over the week (morning, afternoon, evening sessions), from MSW, MSM and women.
The total number of CT/NG testing episodes at DSE and 56DS was estimated as the total of all attendances in which English national coding data (GUMCAD1) indicated that CT/NG tests occurred: T2 (CT and NG), T3 (CT, NG and syphilis) or T4 (CT, NG, syphilis and HIV) were reported. Testing code T1 (CT only) was excluded as CT testing in isolation is not standard practice. The date and time on which patients had a testing appointment, and the date and time that their test results for CT/NG were sent via SMS were matched using patient IDs.
The time from clinic visit to sending CT/NG results was calculated for each patient, to quantify the delay to notification by clinic site.
Model description and analysis
A model was developed to estimate the potential impact of introducing a rapid testing service for asymptomatic patients at DSE. Rapid testing for asymptomatic patients was not performed at 56DS at any point during this evaluation. Therefore, the model estimated the impact of the reduction in time to result notification as if asymptomatic patients had attended 56DS rather than DSE and their CT/NG tests had been processed in that service. The model estimates the reduction in number of new sexual partners who are spared exposure to CT and/or NG for those with asymptomatic infection with rapid testing compared with slower standard off-site testing. By modelling the reduction in new sexual partners’ exposure, we estimate the reduction in partner notification and treatment (for partners exposed to CT/NG), the cost to clinics for fewer attendances for screening and treatment of partners, and potential public health impact due to transmissions averted.
Results
Clinical data
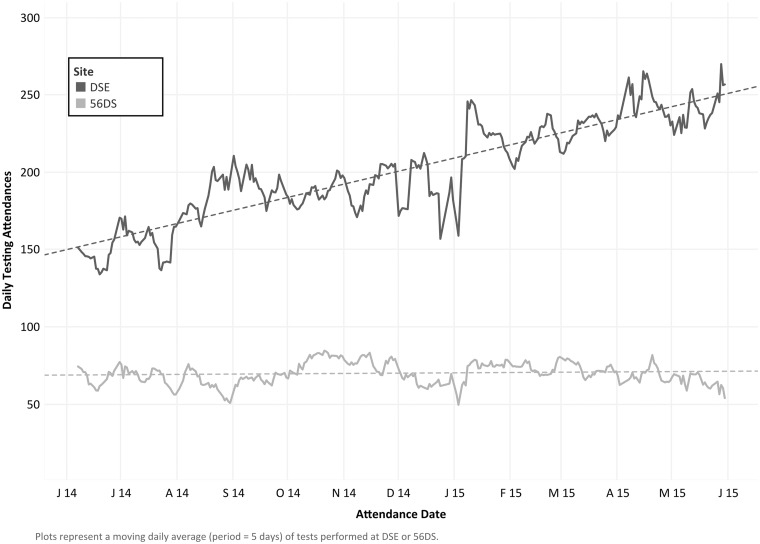
In 2014/15, there were a total of 81,352 visits for CT/NG testing across 56DS (21,086) and DSE (60,266). The volume of testing attendances increased from an initial five-day moving average of 152.7 per day to 241.0 per day at DSE over the observation period (p for trend = < 0.001), a relative increase of 58% over the year. The volume of testing attendances at 56DS remained relatively static (p for trend = 0.088) over the observation period (Figure 1). The time between patients’ sample collection and notification of CT and NG test results at DSE and 56DS over the observation period are shown in Figure 2.
Figure 1.
Moving average of daily attendances for testing at Dean Street Express (DSE) and 56 Dean Street (56DS) over the observation period. Plots represent a moving daily average (period = 5 days) of tests performed at DSE or 56DS. Dashed lines represent the output of unadjusted linear regression of the moving five-day average of daily testing attendances versus attendance date and are provided for illustrative purposes only (p for trend = <0.001 and 0.088 for DSE and 56DS, respectively).
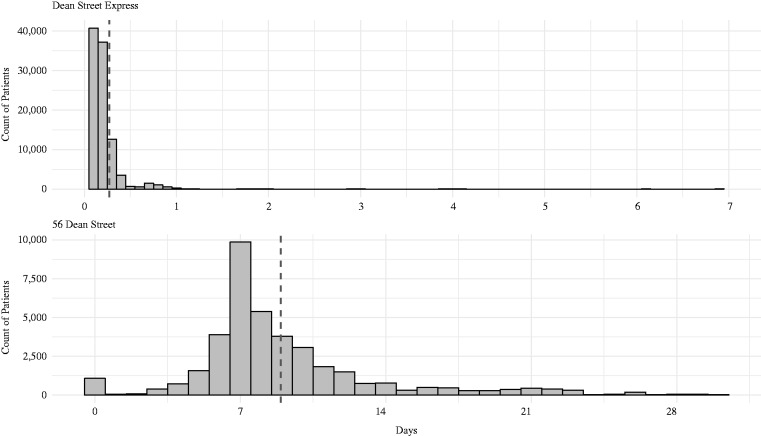
Figure 2.
Time from testing visit to results being reported to patients by text message; for Dean Street Express and 56 Dean Street. Broken vertical lines denote the mean.
Visits for CT/NG testing generated a total of 40,982 CT/NG test notifications from 56DS and 102,060 from DSE. Ninety-seven per cent of these results (138,936) were matched to data describing dates and times for testing and result notification. Of these, 138,261 (99.5%) were eligible for inclusion (delay between time of appointment and test notification is positive and ≤ 30 days).
The mean delay between eligible pairs of clinic appointments and test result notifications was 8.95 days (95% CI 8.91–8.99 days) for 56DS and 0.27 days (95% CI 0.26–0.28 days) at DSE (Figure 2). This resulted in a reduction in time to result notification of 8.68 days.
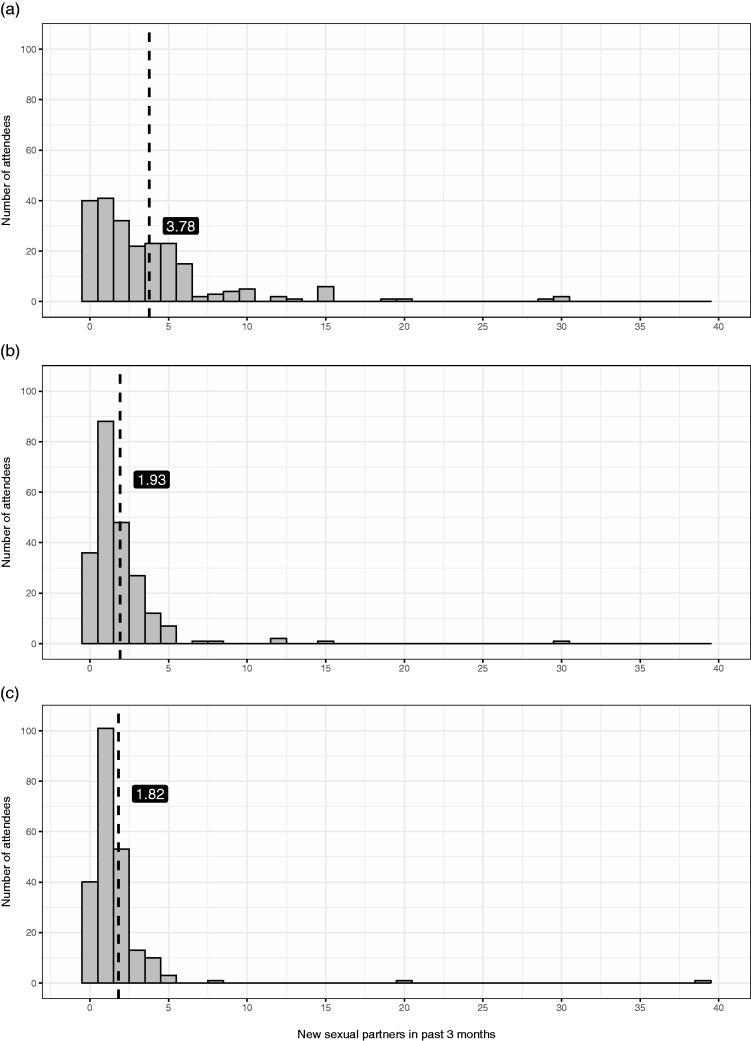
Data on the mean (and median) number of new partners in the past three months for women, MSW and MSM were 1.82 (1), 1.93 (1) and 3.78 (2), respectively (distribution show in Figure 3).
Figure 3.
Total number of new partners reported in the last three months for (a) MSM, (b) MSW and (c) women; broken vertical line denote the mean.MSM: men who have sex with men; MSW: men who have sex with women.
The distributions used for our Monte Carlo simulations were based on our observed results and data from existing literature; the distributions and their coefficients are described in the supplementary table (Table 2 in Appendix 1).
Our model estimated that implementing a rapid CT/NG testing service at DSE led to 854 partner attendances averted (2.5–97.5% centile range = 31–4040), prevented 196 CT and/or NG transmissions (2.5–97.5% centile range = 6–956) and led to annual savings attributable to reduced numbers of partner attendances of £124,283 (IQR = £4260–590,331).
Discussion
The rapid STI testing service at DSE has reduced the time from clinic attendance for asymptomatic CT and NG testing and notification of results by over a week to a few hours – a relative reduction of 97%. Adoption of rapid CT/NG testing has the potential to realise a number of ‘knock-on’ public health benefits, some of which are incorporated into our model: fewer transmissions and partners to contact trace, and fewer tests and empirical treatments for contacts. Our findings complement analyses demonstrating the savings in baseline costs associated with deployment of rapid testing in open-access GUM clinics.5,7
The ability of DSE to consistently deliver rapid testing and notification of CT/NG samples is, in part, due to the lean service delivery model that has been adopted. The service was designed to minimise the number of steps involved in the patient journey and the management of test results. The direct linkage between the results data generated by the GeneXpert platform and DSE’s EPR system enables the clinic to notify patients as they become available, compared to relying on manual result collection and notification. Although not captured in our analysis, it will also mean less staff time (and costs) to notify patients of results.
Concerns about the potentially deleterious effect that the immediacy of result notification may have on patient willingness to attend for testing have been raised by other studies examining the role of rapid tests for CT and NG.8,9 Conversely, daily demand for the rapid testing service offered at DSE has increased by 59% over the observation period. This may indicate that the DSE services have answered an unmet need for rapid STI testing and treatment services.
Our study is based on a large sample of patients across two clinic sites over the same year-long observation period, minimising any transient seasonal differences in sexual behaviours, patterns of social mixing, prevalence or health-seeking behaviours. Whilst our simulations show a wide range in the distribution of savings, rapid test notification consistently leads to monetary benefits across the various combinations of input parameters in our simulations.
There are several limitations to this analysis. First, our results may not be wholly generalizable to other settings across the UK and internationally due to differences in which patients access services or how these services are delivered. The majority of NG/CT tests in DSE and 56DS are in MSM, whereas nationally MSM account for 12% of STI testing episodes tests.1 This suggests that there may not be as large a public health benefit, as MSM have higher rates of new partners than MSW and women. However, clinics outside central London could still benefit from the same model of rapid testing and faster treatment, in terms of reductions in transmissions and partner treatment visits averted.
Second, we did not have access to the paired GeneXpert-EPR data from the clinic for notification of results. This meant that we could not directly categorise CT and/or NG positivity rates by gender and sexual orientation. Instead, we relied on proxy measures to identify samples originating from MSM and classify results as being from ‘MSM’ and ‘non-MSM’. The anatomical site for each sample result was unavailable. Third, whilst we know the exact date and time attendees were notified of their results via SMS, we do not know when they have read or actioned the message. Fourth, we have not analysed the impact of infections for bisexual or transgender patients attending our service.
In this analysis, we assumed that the delay between result notification and attendance for treatment is the same; earlier analysis has shown this is similar for DSE and 56DS.10,11 In the absence of other data, we have used a low daily transmission probability for CT and NG in our model, which may underestimate the magnitude of public health benefits as a result of this service reconfiguration. We have assumed that sexual behaviour with regards to new sexual partners remains the same in the period between testing and receiving results as before testing; if this is lower, it may overestimate the impact of rapid testing. Because we took partner data from a small sample of patients, we have missed some of the patients who report very high numbers of partners; this will reduce the mean and underestimate the impact of DSE leading to more conservative results. Also, those with CT and/or NG may have higher partner numbers than those without infection and our model has not modelled the impact of this, perhaps underestimating the effects of rapid results.
Our model ignored any public health benefits of rapidly notifying for other infections tested at DSE such as syphilis, HIV and hepatitis B and C.
Further research is warranted to assess the reproducibility of the services offered at DSE in other clinics in a range of rural and urban settings. It would also be useful to estimate the initial set-up costs for the infrastructure required for rapid testing and notification, the effect this service may have on the systematic collection of data describing the prevalence and patterns of antimicrobial resistance and what impact expanding rapid testing to other organisms such as syphilis and HIV may have.
Although we have not modelled the effect that rapid testing may have on the prevalence of CT and NG beyond the potential for shortening infectious periods and subsequently decreasing the number of transmission opportunities, existing evidence indicates that rapid CT/NG testing has the potential to considerably reduce the prevalence of CT and NG.
Our evaluation provides real-world evidence to enable decision-makers and commissioners to rationalise expenditure and maximise outcomes when considering the funding and structure of their STI services. It shows the unmet need for a rapid testing service based on the large increases in patient attendances, and the potential public health benefits and cost savings of implementing such a service.
Appendix 1
The model calculated results separately for MSM, MSW and women, and then results were added together. Input parameters came from observed data (Table 1) and published sources (Table 2).12–17 We also determined the positivity rates for CT and NG in patients attending DSE by taking CT/NG test results from DSE’s GeneXpert platform, as this was unavailable directly from the EPR. The test reference numbers incorporated an embedded unique patient identifier and therefore multiple samples could be correctly attributed to a single patient.
Table 1.
Descriptive statistics of patient attendances, number of sexual partners, CT and/or NG positivity and days between attendance and notification of test results for 56 Dean Street and Dean Street Express.
|
56 Dean Street |
Dean Street Express |
Overall |
|||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Attendancesa | MSW | 4856 | 19% | 13,100 | 13% | 17,956 | 17% |
| MSM | 24,726 | 58% | 39,373 | 65% | 64,099 | 60% | |
| Women | 8336 | 23% | 15,856 | 22% | 24,192 | 23% | |
| Total | 37,918 | 100% | 68,329 | 100% | 106,247 | 100% | |
| Attendances for CT/NG testing | MSW | 3020 | 14% | 12,053 | 20% | 15,073 | 19% |
| MSM | 13,293 | 63% | 33,348 | 55% | 46,641 | 57% | |
| Women | 4773 | 23% | 14,865 | 25% | 19,638 | 24% | |
| Total | 21,086 | 100% | 60,266 | 100% | 81,352 | 100% | |
|
n with data |
Mean |
||||||
| New sexual partners in past three months | MSW | 224 | 1.93 | ||||
| Women | 224 | 1.82 | |||||
| MSM | 224 | 3.78 | |||||
|
Mean |
SD |
||||||
| CT positivity | MSW/females | 3.65% | 0.42% | ||||
| MSM | 6.39% | 0.52% | |||||
| NG positivity | MSW/females | 1.70% | 0.27% | ||||
| MSM | 11.21% | 0.88% | |||||
| Co-positivity | MSW/females | 0.02% | 0.06% | ||||
| MSM | 1.27% | 0.27% | |||||
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
||
| Days between attendance and result notification | 8.95 | 4.43 | 0.27 | 0.82 | |||
CT: Chlamydia trachomatis; MSM: men who have sex with men; MSW: men who have sex with women; NG: Neisseria gonorrhoeae.
Three attendances were excluded from analysis on the basis of missing gender data.
Model coding, input parameterisation, input sampling and tabulation of results were performed in R V3.3.0 for Windows; data visualisations were constructed in Tableau V10.0 for Windows. Statistical significance was assumed at α = 0.05.
Direct linkage of GeneXpert data to clinic EPRs was precluded by the absence of a global patient identifier, making disaggregation of test results by gender and sexual orientation impossible. Data were unavailable to determine the anatomical site of each sample. Therefore, we assumed that any patients who had three samples (urine, rectal and throat swabs) taken on the same date were MSM.
A patient was considered positive for CT and/or NG on a specific date if any of the samples from their batch of tests was reported as positive. Co-positivity on a given date was defined as a patient testing positive for both infections across any of their results from a given batch (i.e. sample 1 tested positive for both CT and NG or sample 1 tested positive for CT and sample 2 tested positive for NG). Estimated patient positivity was used to estimate point prevalence of CT, NG and co-infection with CT and NG.
The primary outputs from this model were the number of transmissions avoided, the partner attendances averted as a direct result of reductions in time to notification and treatment and the cost savings from reduced attendances. We assumed that there would be no difference in the time from notification of a positive result to attending for treatment by clinic site; hence, the potential benefits are approximated by the reduced time from sample collection to notification of results with a rapid test. We also assumed that the rate of sexual partner change remains the same after testing at DSE.
The total number of infections was estimated by multiplying the annual number of attendances for CT/NG testing using DSE data by the proportion of samples with positive results for CT, NG or both. This was thought to better approximate the number of infections than the diagnostic codes from the clinic, as this was inconsistently assigned to either the testing visit retrospectively or the follow-up treatment visit.
In the absence of robust observed data, the daily transmission probabilities for CT and NG were assumed to be 5% (SD 1%) and 10% (SD 2%), respectively, based on the estimations of Turner et al.5 and accounts for variations in condom use, type of sex acts and number of sex acts per partner per day. The transmission probability of a dual-positive individual passing on either CT, NG or both was estimated under the assumption that the probabilities of transmitting either infection were independent (i.e. .
Table 2.
Characteristics of parameter distributions used in Monte Carlo simulations.
| Variable | Sampling distribution | Distribution characteristics | Source/note | |
|---|---|---|---|---|
| CT positivity | MSM | Log-normalb | = −2.75, σ = 0.02 | |
| MSW/womena | Log-normalb | = −3.31, σ = 0.03 | ||
| NG positivity | MSM | Log-normalb | = −2.18, σ = 0.02 | DSE GeneXpert platform |
| MSW/Womena | Log-normalb | = −4.06, σ = 0.04 | ||
| Co-positivity | MSM | Log-normalb | = −4.36, σ = 0.05 | |
| MSW/Womena | Log-normalb | = −6.62, σ = 0.14 | ||
| New partners in past three months | Sampled directly from raw data, with replacement | |||
| Proportion of partners attending | Log-normalb | = −0.87, σ = 0.07 | Estcourt et al.18 | |
| Time (days) to notification | DSE | Log-normal | = −1.70, σ = 0.63 | Clinic EPRs |
| 56DS | Weibull | Shape = 2.01, Scale = 10.00 | ||
| Daily transmission probability (per partner) | CT | Log-normalb | = −3.00, σ = 0.20 | Turner et al.5 |
| NG | Log-normalb | = −2.30, σ = 0.20 | ||
CT: Chlamydia trachomatis; DSE: Dean Street Express; EPR: electronic patient record; MSM: men who have sex with men; MSW: men who have sex with women; NG: Neisseria gonorrhoeae.
The positivity in MSW/women was estimated from samples in which only one sample was done within 24 h per patient ID. No data on patient characteristics were available to differentiate MSW and women.
Indicates distribution fitted and parameterised a priori according to the characteristics of the underlying data for this variable. Parameters for lognormal distributions were derived from observed means and standard deviations of source data.
The cost of a GUM attendance for partners was assumed to be the mean of the single and multi-professional first attendance (£135) from the draft non-mandatory UK National Tariff for 2016/17.10
Patients that test positive are advised at the treatment visit that their partners also need to attend for treatment. According to Estcourt et al.,18 approximately 41.9% (95% CI 39.1–44.6%) of a positive patient’s partners will attend a GUM clinic for treatment. We assumed that asymptomatic patients who had a test will not alter their sexual behaviour whilst awaiting test results. Therefore, we estimated the mean number of partner clinic appointments that could be averted by using a rapid test by multiplying the daily number of partners and the reduction in turnaround time by the estimated partner attendance. The number of averted clinic visits was multiplied by the tariff reimbursement per visit.
A Monte Carlo simulation was performed to assess the robustness of the estimated outcomes. We ran the model over 100,000 combinations of input parameters, each independently sampled from appropriate distributions that were fitted according to the characteristics of the data or selected as the optimal fit (based on the Akaike Information Criterion) to the observed data (Table 1), or by using the raw data (Table 2).
Authors’ note
The work presented is entirely the authors’ own. All authors have completed the International Committee of Medical Journal Editors disclosure at http://www.icmje.org/conflicts-of-interest/.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: NL received personal fees from Aquarius Population Health during the conduct of the study. EJA, MJH and DCG work for Aquarius Population Health that has received grants for other sexual health and/or point of care modelling projects from St Georges University of London, Enigma Diagnostics, Cepheid, Atlas Genetics, and AstraZeneca outside the submitted work. GGW and AM have received speaker fees and travel expenses from Cepheid to speak at Symposia about the initial outcomes from the introduction of their GeneXpert technology at DSE.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Cepheid and Chelsea & Westminster NHS Foundation Trust part-funded Aquarius Population Health for the authors’ contribution to the manuscript.
References
- 1.Public Health England. Infection report – sexually transmitted infections and chlamydia screening in England, 2015. Health Prot Rep 2016; 10: 8–34.
- 2.Public Health England. Sexually transmitted infections and chlamydia screening in England, 2014. Health Prot Rep 2015; 11: 1–20.
- 3.Hughes G and, Field N. The epidemiology of sexually transmitted infections in the UK: impact of behavior, services and interventions. Future Microbiol 2015; 10: 35–51. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health. A framework for sexual health improvement in England. London. Report No.: 18420, https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/142592/9287-2900714-TSO-SexualHealthPolicyNW_ACCESSIBLE.pdf (2013 March, accessed 21 June 2016).
- 5.Turner KME, Round J, Horner P, et al. An early evaluation of clinical and economic costs and benefits of implementing point of care NAAT tests for Chlamydia trachomatis and Neisseria gonorrhoea in genitourinary medicine clinics in England. Sex Transm Infect 2014; 90: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chelsea and Westminster NHS Foundation Trust. Dean Street Express, https://deanstreetexpress.chelwest.nhs.uk/fm_personal_info.php (accessed 8 April 2016).
- 7.Adams EJ, Ehrlich A, Turner KME, et al. Mapping patient pathways and estimating resource use for point of care versus standard testing and treatment of chlamydia and gonorrhoea in genitourinary medicine clinics in the UK. BMJ Open 2014; 4: e005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Natoli L, Guy RJ, Shephard M, et al. Public health implications of molecular point-of-care testing for chlamydia and gonorrhoea in remote primary care services in Australia: a qualitative study. BMJ Open 2015; 5: e006922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui BB, Wilson DP, Ward JS, et al. The potential impact of new generation molecular point-of-care tests on gonorrhoea and chlamydia in a setting of high endemic prevalence. Sex Health 2013; 10: 348–356. [DOI] [PubMed] [Google Scholar]
- 10.Cooper F, Appleby T, Chislett L, et al. Innovative, rapid and effective: asymptomatic screening in 2014. 23rd IUSTI Europe conference, September, Valetta, Malta, 2014. Oral Abstract O4.
- 11.Byrne R, Cooper F, Appleby T, et al. Can express treatment reduce onward transmission? BASHH conference, Glasgow, 1–3 June 2015. Oral Abstract O19.
- 12.Monitor. 2016/17 National Tariff Payment System: draft prices, https://www.gov.uk/government/publications/201617-national-tariff-payment-system-draft-prices (2016, accessed 21 June 2016).
- 13.Althaus CL, Turner KME, Schmid BV, et al. Transmission of Chlamydia trachomatis through sexual partnerships: a comparison between three individual-based models and empirical data. J R Soc Interface 2012; 9: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garnett GP, Mertz KJ, Finelli L, et al. The transmission dynamics of gonorrhoea: modelling the reported behaviour of infected patients from Newark, New Jersey. Philos Trans R Soc B Biol Sci 1999; 354: 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooper RR, Reynolds GH, Jones OG, et al. Cohort study of venereal disease. I: the risk of gonorrhea transmission from infected women to men. Am J Epidemiol 1978; 108: 136–144. [DOI] [PubMed] [Google Scholar]
- 16.Platt R Rice PA andMcCormack WM.. Risk of acquiring gonorrhea and prevalence of abnormal adnexal findings among women recently exposed to gonorrhea. JAMA 1983; 250: 3205–3209. [PubMed] [Google Scholar]
- 17.Public Health England. Sexually transmitted infections (STIs): annual data tables, https://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables (2016, accessed 21 June 2016).
- 18.Estcourt CS, Sutcliffe LJ, Copas A, et al. Developing and testing accelerated partner therapy for partner notification for people with genital Chlamydia trachomatis diagnosed in primary care: a pilot randomised controlled trial. Sex Transm Infect 2015; 91: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]