Abstract
Background:
The use of the intra-aortic balloon pump (IABP) as a support device remains controversial due to the fact that a number of studies have shown no benefit in end mortality whilst using this device. One of the reasons for this could be the increase in vascular complications when using the pump. Therefore, the aim of the present review was to assess the current literature available with regards to IABP vascular complications during the clinical situation.
Methods:
A literature search was performed, searching for IABP complications in adult human studies between 1990 and 2016.
Results:
A total of 20 reports were identified as fitting the criteria of this study. The majority of vascular complications were limb ischemia, bleeding or mesenteric ischemia. The overall incidence of vascular complications ranged from 0.94% to 31.1%. Diabetes, peripheral vascular disease and hypertension, as well as smoking were all identified as risk factors for complications following IABP. Furthermore, studies supported the use of sheathless balloon insertion to reduce the risk of complications.
Conclusion:
Major vascular complications, including limb and mesenteric ischemia as well as bleeding and hemorrhage, have been associated with IABP. However, the incidence of these complications was generally low. Further studies are still required to truly understand the risk/benefit associated with the use of IABP.
Keywords: IABP, vascular complications, limb ischemia, mesenteric ischemia
Introduction
The intra-aortic balloon pump (IABP) is one of the most commonly used circulatory assist devices in critically ill patients with compromised cardiac function.1 This technique has been used during numerous cardiac surgical procedures, including after acute myocardial infarction and during cardiogenic shock, to both increase coronary blood flow and decrease left ventricular afterload.2
The concept of the IABP lies in the fact that the counter-pulsation caused by the pumping of the balloon causes ‘volume displacement’ of blood within the aorta at both proximal and distal locations. This action leads to a potential increase in coronary blood flow and, in addition, can lead to improvements in systemic perfusion by augmentation of the intrinsic ‘Windkessel effect’.3 IABP treatment, therefore, enhances the ventricular performance of the failing heart by facilitating an increase in myocardial oxygen supply in addition to decreasing the myocardial oxygen demand.3
Despite the evidence in favour of the IABP, recent guidelines from both sides of the Atlantic have downgraded the use of IABP for cardiogenic shock from class I to class IIa in American guidelines whereas, in Europe, IABP is now a class III treatment.4,5 Furthermore, the recent SHOCK-II trial demonstrated that IABP did not significantly reduce 12-month mortality in patients with cardiogenic shock complicating myocardial infarction undergoing early revascularization, although the self-reported quality of life was moderate to good in survivors.6 A number of recent studies, however, have challenged the outcomes of the SHOCK II trial, illustrating the fact that further work is still required to determine the benefit of this modality in the clinical situation.7,8 In addition to the SHOCK II trial data, widespread clinical use of the IABP had previously been restricted due to the fact that complications exist with regards to the insertion of the balloon pump which, despite the improvement in IABP technology, remains an important issue.9 Amongst the many vascular complications, both limb and mesenteric ischemia are the most life-threatening conditions, although recent work from our own group has shown that reducing the length of the IABP catheter may reduce ischemia without altering counter-pulsation efficacy.10
In view of this knowledge, it is, therefore, important to understand the current data set available regarding the use of IABP and the types and propensity of vascular complications that can be induced by this intervention. For these reasons, the aim of this present review was to summarize the existing literature in terms of vascular complications following IABP insertion. Ultimately, such studies should allow us to gain further insights into the risk-benefit of IABP treatment, allowing clinicians to make more informed decisions regarding use of this assist device
Materials and Methods
Study selection
Studies were compiled from carrying out a literature search of the PubMed computerized database, using the following search terms: ‘IABP complications’, ‘IABP ischemia’, ‘IABP limb ischemia’, ‘IABP vascular complications’ and ‘visceral ischemia’. All articles published between 1990 and March 2016 were selected on the basis of the title and/or abstract. Human studies with more than 10 adult patients (over 18 years old) who had an IABP inserted due to any cardiovascular condition, such as cardiogenic shock, AMI or severely reduced LVEF (<35%), were included.
Studies describing in vivo experiments, reviews, letters and case reports were excluded, Studies published in a language other than English or when no full text was available were also excluded. Furthermore, articles focusing on anticoagulation or antiplatelet safety in patients who underwent IABP or articles in which no complications where reported were not included.
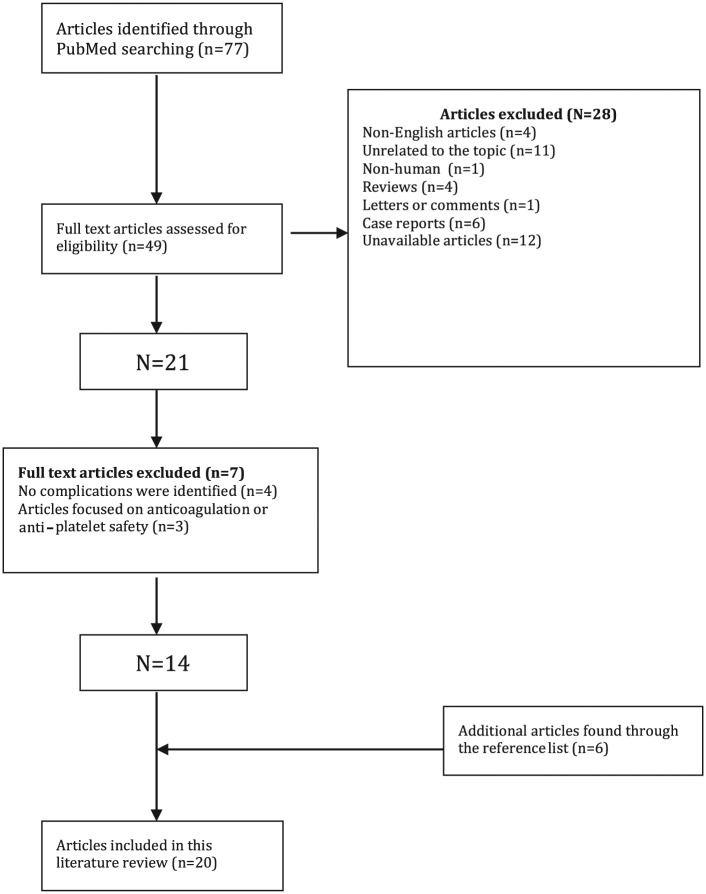
The study selection process is outlined in Figure 1. When applying the search terms, 77 articles were identified. After applying the inclusion and exclusion criteria and performing an additional manual literature search through the references lists of published articles and reviews, the final data set consisted of 20 articles.
Figure 1.
Study selection process.
Outcomes and definitions
IABP-related complications were classified as either major or minor. Major complications were defined as complications that were lethal or required surgical treatment, such as vascular injury and limb ischemia that were treated with thromboembolectomy, vascular repair or fasciotomy. Minor complications included local hematoma, infection and limb ischemia that was relieved by removal of the IABP without the need for further surgical intervention.11
Results
Twenty studies were included in this literature review, with the majority of them (n=15, 75%) being retrospective. The baseline characteristics of the 20 studies identified in this review are reported in Table 1. The majority of patients included in these trials had high risk factors for developing vascular complications, such as diabetes, hypertension, peripheral vascular disease and history of smoking. Study designs, indications, main outcomes, catheter size, insertion and IABP duration are summarized for each article (Table 1). In summary, the total number of patients was 23,731; the mean age was 63.5±4.16 years. Overall, 76.7% of the patients were male and the mean IABP support duration in these trials ranged from 8 hours to 5.4 days.
Table 1.
Baseline characteristics of patients contained within reviewed studies.
| Study Duration | Study | Diagnosis | N | Age | M/F % | DM % | Smoking % | HTN % | PVD% | Sheath | Time | Catheter Size (F) | Route | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arafa et al. (15) | 1980–1994 | R | HS | 509 | 59.8 | 66.8/33.2 | 9.60 | – | – | 19.4 | – | 78±80 h | 9.0/9.0 | FA/PC/TT |
| Arceo et al. (28) | 1989–1996 | R | AMI, CMP, UA, PCA, | 201 | 61.0 | 82.0/18.0 | – | – | – | – | Both | 48.0 h | 8.0/10.5 | FA |
| Boudoulas et al. (18) | 2008–2010 | R | HS | 36 | 60.5 | 63.0/78.0 | – | – | – | – | Yes | 2 d | 8.0 | FA |
| Christenson et al. (12) | 1998–2004 | R | CB, W CPB | 135 | 68.0 | 67.0/33.0 | 28.0 | – | – | 14.0 | No | 8–199 h | 9.5/8.0 | – |
| Cohen et al. (24) | 1993–1997 | P | AMI, UA, CS | 1119 | 65.0 | 727.0/392.0 | 27 | – | 52 | 8 | Both | – | 9.0/11.0 | FA |
| Colyer et al. (29) | 1994–1998 | R | RV | 35 | 68.0 | 54.0/46.0 | 23.0 | 77.0 | 63.0 | – | Both | – | 8.0/10.0 | PC |
| Davidson et al. (16) | 1990–1995 | – | CB+ VR | 86 | 58.0 | 62.0/38.0 | 38.40 | 21 | 64 | 8.0 | Yes | – | – | FA |
| Dick et al. (23) | 1998–2003 | R/C | AMI | 187 | 69.0 | 68.0/3.2 | 31 | 45 | 58 | – | Both | 2 d | – | FA |
| Eltchaninoff et al. (25) | 1985–1990 | R | HS + CS. | 240 | 64.0 | 66.0/34.0 | 21.0 | 46.0 | 43.0 | 20.0 | Yes | 44.2 h | 8.5/10.5 | FA |
| Erdogan et al. (20) | 1985–2004 | R | – | 1211 | 67.3 | 52.4/47.6 | 26.2/24.7 | 37/ 31.7 | 12.7/12.4 | 11.1/ 3.6 | Both | 5.4 d | 9.5/11.5 | FA |
| Ferguson et al. (22) | 1996–2000 | R | CS, W CPB, UA |
16909 | 65.9 | 68.8/31.2 | 25.60 | – | – | 11.9 | Both | 53 h | 8.0/ 9.5 | FA/AA |
| Jameson et al. (26) | 1989–1990 | R | HS | 51 | 57.0 | 65.0/35.0 | – | – | – | – | Yes | – | 9.5/10.0 | FA/PC/OT |
| Meharwal and Trehan (11) | 1994–2000 | – | HS | 911 | 59.2 | 89.5/10.5 | 41.10 | 31.80 | 40.30 | 8.5 | Both | 3.8 d | BS | FA/PC/TT |
| Meisel et al. (21) | 2000– 2003 | P | ACS | 161 | 62.9 | 72.0/28.0 | 45.0 | 41.0 | 55.0 | 15.0 | No | – | 8.0 | FA |
| Rastan et al. (31) | 2007–2009 | R | HS | 621 | 67.1 | 66.7/33.3 | 49.2 | – | 71.4 | 12.7 | – | 144 h | BS | – |
| Severi et al. (13) | 1999–2011 | R | HS | 423 | 65.0 | 82.0/18.0 | 18.20 | – | 9.70 | – | No | ≥48 h | 8.0/9.0 | FA |
| Sirbe et al. (14) | 1988–1998 | R | HS | 524 | 65.2 | 66.7/27.5 | – | – | – | – | – | 1.89±1.2 d | 9.5 | FA |
| Tatar et al. (17) | 1988–1992 | R | HS | 126 | 56.1 | 80.6/19.4 82.3/17.7 |
11.6 13.3 |
– | – | 7.7/11.1 | Both | 99.5/103.9/ 117.4 h | 8.5/9.5/ 10.5 | FA/PC |
| Yildirim et al. (32) | 2008–2012 | R | HS | 107 | 69.1 | 66.4/33.6 | 16.7 | – | – | – | No | 42.4±8.7 h | 7.0/7.5/8.0 | FA |
| Yuksel et al. (19) | 2002–2011 | OS | OHS | 148 | 64.4 | 70.2/29.8 | 27.70 | 35.10 | 12.20 | 9.50 | No | – | – | FA |
Papers are displayed in alphabetical order.
R: retrospective; P: prospective; C: cohort; OS: observational study; HS: heart surgery; VR: valve replacement CB: coronary bypass; W CPB: weaning from cardiopulmonary bypass; OHS: open heart surgery; ACS: acute coronary syndromes; HTN: hypertension; DCMP: dilated cardiomyopathy; UA: unstable angina; RV: revascularization; BS: depending on body size FA: femoral artery; PC: percutaneous; OT: open technique; TT: transthoracic; AA: alternative approach.
The most frequent indications for IABP use were to provide support for high-risk coronary patients, cardiogenic shock, open-heart surgery and acute myocardial infarction. Patients also underwent IABP implantation for unstable angina, myocardial revascularization and difficulty in weaning from cardiopulmonary bypass (Table 1).
The outcomes in the included studies covered both minor and major vascular complications related to IABP insertion and are summarized in Table 2.
Table 2.
Complication Rates.
| Author | N | Total Complications N (%) |
Vascular Complications N (%) |
Limb ischemia N (%) |
Hemorrhage/ Bleeding N (%) |
Hematoma N (%) |
Pseudoaneurysm N (%) |
Amputation N (%) |
Mesenteric Ischemia N (%) |
|---|---|---|---|---|---|---|---|---|---|
| Arafa et al. (15) | 509 | All- 57 (9.7) Major- 41 (8) |
57 (9.7) | 41 (8) | 4 (0.8) | 7 (1.4) | 3 (0.6) | – | – |
| Arceo et al. (28) | 201 | 22 (10.4) | 18 (9.0) | 12 (5.7) | 5 (2) | – | 1 (0.4) | – | – |
| Boudoulas et al. (18) | 36 | ≤2 days:5 (18) >2 days: 6 (66) |
– | ≤2 days:1 (3.7) | ≤2 days:4 (14.8) >2 days: 6 (66) |
– | – | – | ≤2 days:1 (3.7) |
| Christenson et al. (12) | 135 | 20 (14.8) | 20 (14.8) | Major – 12 (8.9) Minor– 6 (4.4) |
2 (1.5) | – | – | – | – |
| Cohen et al. (24) | 1119 | All– 166 (15) Major– 126 (11) |
– | 37 (3.3) | 52 (4.6) | – | – | 1 (0.1) | – |
| Colyer et al. (29) | 35 | – | – | 2 (5) | 4 (11.4) | ||||
| Davidson et al. (16) | 86 | – | 3 (3.5) | 3 (3.5) | – | – | – | 3 (3.5) | – |
| Dick et al. (23) | 187 | – | – | 19 (10) | – | – | – | 4 (2.1) | – |
| Eltchaninoff et al. (25) | 240 | 31 (13) | 29 (12.1) | 12 (8.8) | 1 (0.4) | 7 (2.9) | – | – | – |
| Erdogan et al. (20) | 1211 | 146 (12.1) | 146 (12.1) | 129 (10.7) | 2 (0.2) | 6 (0.5) | – | 1 (0.1) | – |
| Ferguson et al. (22) | 16909 | All-1183 (7) Major- 473 (2.8) |
– | All – 490 (2.9) Major- 152 (0.9) |
All- 406 (2.4) Major-135 (0.9) |
– | – | 17 (0.1) | – |
| Jameson et al. (26) | 51 | – | 20 (39.2) | 9 (17.6) | – | 2 (3.9) | – | – | – |
| Meharwal and Trehan (11) | 911 | 107 (11.7) | Major- 54 (5.9) Minor- 53 (5.8) |
25 (2.7) | – | – | – | – | – |
| Meisel et al. (21) | 161 | 11 (6.8) | 11 (6.8) | Major-2 (1.2) Minor-2 (1.2) |
Major- 1 (0.6) Minor- 4 (2.4) |
– | 2 (1.2) | – | – |
| Rastan et al. (31) | 621 | – | – | – | – | – | – | – | 61/63 (87.3)* |
| Severi et al. (13) | 423 | 8 (1.9) | 4 (0.94) | 4 (0.94) | – | – | – | – | – |
| Sirbe et al. (14) | 524 | – | 163 (31.1) | 149 (26.7) | – | 16 (3.1) | – | 5 (3.5) | – |
| Tatar et al. (17) | 126 | – | 25 (19.6) | 20 (16) | – | 4 (3.2) | – | – | – |
| Yildirim et al. (32) | 107 | 5 (4.7) | 5 (4.7) | 3 (2.8) | 1 (0.7) | 1 (0.7) | |||
| Yuksel et al. (19) | 148 | 18 (12.1) | 18 (12.1) | 13 (8.7) | 4 (2.7) | – | – | 1 (0.7) | – |
Mesenteric ischemia diagnosed with computed tomography scan, which occurred in 63 of the total 621 patients.
Papers are displayed in alphabetical order.
The incidence of IABP-related vascular complications varied widely, from 0.94% to 31.1% (mean 15.1 ± 12.5).12–18 Only one study distinguished between early and late complications, with early complications being observed in 56 patients (11%).15 In addition, only a small number of studies (n=5, 20%) divided vascular complications into major and minor. Two of them indicated that major vascular complications occurred in 6.7% and 8.1% of patients, while minor complications took place in 5.7% and 2.9% of cases.11,15
The most frequent vascular complication associated with IABP insertion was limb ischemia which had an incidence ranging from 0.9% to 26.7% (mean 8.03 ± 7.4).12–16,18–29 Arceo et al. concluded that limb ischemia occurred early after balloon placement and observed ischemia in 12 (5.7%) patients. In half of these patients, the ischemia was transient and resolved itself after removal of the IABP.28 Colyer et al. reported that only two patients among 37 (5.4%) experienced this complication.29
Meharwel and Thehan included 911 patients in their study in which 25 (2.7%) of the patients developed limb ischemia which needed thrombolectomy, while 3.6% of limb ischemia was relieved by IABP removal.11 Christenson et al. distinguished major and minor limb ischemia that was observed in 6 and 12 patients, respectively (overall incidence, 8.9 and 6.6%).12 Severi et al., Yildirim et al. and Meisel et al. showed the lowest rate of limb ischemia, occurring in 4 (0.94%), 3 (2.8%) and 2 (1.2%) patients, respectively.13,21,30 In the latter study, the low rate of such a complication could be due to the use of low-profile IABP 8Fr catheters and sheathless technique IABP.21
Treatment time is considered to be one of the major factors driving IABP vascular complications, with an increased duration of treatment leading to a higher risk of complications. Time duration for IABP insertion in the studies found ranged from 8 hours to 5.4 days. One study directly supports the fact that prolonged IABP treatment time leads to more vascular complications (vascular complications were seen in 20 patients (14.8%) in total).12 Boudoulas et al. also demonstrated that the duration of IABP assistance has a great impact on limb ischemia since the incidence of this complication increased dramatically when the balloon had been used for more than two days.18 In five studies, amputation for limb ischemia was reported at rates varying from 0.1 to 3.5%.14,16,19,24,28
Mesenteric ischemia is thought to be an important vascular complication during IABP treatment, however, there are actually very few studies that have directly assessed mesenteric ischemia complications in patients. The incidence appears to be quite low, with the exception of the study of Rastan et al. where the flow in the superior mesenteric artery was compromised in 61 out of a subset of 63 (87%) patients.31 In another study, it was reported that only one patient had mesenteric ischemia (0.9%); however, this did ultimately lead to patient death.32 For these reasons, additional studies should be carried out to directly assess the occurrence of mesenteric ischemia to determine the risk that this complication may confer in IABP patients. Other vascular complications are rarely mentioned in the literature; however, a number of studies do report on the incidence of pseudoaneurysm, hematoma and amputation. Limb ischemia was reported in two studies, with a low occurrence rate (1.2, 0.5%).15,21 Hematoma was described in six studies, with an incidence rate varying from 0.4 to 3.9%.14,15,17,20,25,26
Bleeding or hemorrhage was mentioned in a number of studies, with the percentage of the incidence ranging from 0.4 to 27.7% (mean 5.27±8.5).18–21,25,28,29,32
In addition to analyzing the vascular complications themselves, the majority of studies also took into account the known and suspected risk factors associated with complications and IABP. These included peripheral vascular disease, hypertension, diabetes mellitus, smoking, gender and sheathed insertion.
Diabetes mellitus was the most common risk factor which occurred in 16 studies (80%) followed by peripheral vascular disease in 12 (60%) and hypertension in a total of 11 studies (55%). The least common risk factor was smoking which occurred in eight studies (40%).
Five studies used sheathless insertion while four used sheathed insertion. The rest of the studies used a combination of the two whilst three studies did not mention which type of balloon insertion was used.
Many studies supported the fact that sheathless insertion and smaller size catheters can minimize the risk of vascular complications. There were two retrospective studies that compared modality of insertion and identified that the sheathed insertion technique is a major risk factor for vascular complications during the use of IABP.17,20 The first study showed that the incidence of ischemic complications was lower in the sheathless group (5.2%) compared with the sheathed group (12.4%, p = 0.001).20 However, there were no differences between other complications, such as bleeding and hematoma, in the two groups. The other study showed that vascular complication rate in the sheathless group was 8.8% versus 25.9% in the sheathed group (p<0.01) while hematoma occurred in 0.7% and 2.3%, respectively (p<0.05).17 Together, these studies indicate that the sheathless approach leads to a reduced risk of complications when compared to the sheathed approach, although the data set remains limited.
Arafa et al. pointed out that there is a significant decrease in the major vascular complication rate with IABP insertion in the last five years due to an increase in the use of catheters with smaller diameters.15 Furthermore, Eltchaninoff et al. showed that there was a reduction in the complication profile when smaller size catheters were utilised.25 The catheter size in the included studies ranged from 7 Fr to 11.5 Fr. Some studies reported that the significant decrease in major vascular complications that has occurred over previous years is due to the increased use of catheters with smaller diameters.15,22 The Benchmark IABP Registry revealed that the smaller 8 Fr IABP catheter might reduce the incidence of complications such as limb ischemia.22
The IABP catheter was inserted percutaneously through the femoral artery in all studies except in a few reports that used alternative routes, such as the trans-thoracic approach.11,15,22
Some studies also utilised the subclavian artery approach which is associated with limited morbidity and no vascular complications were reported using this approach. However, additional studies are needed to evaluate long-term outcomes.
Complications tended to be more common in female patients and many studies noted that female gender should be considered as a risk factor. Nonetheless, only one study actually examined the role of gender on unwanted vascular events, demonstrating that female gender should be taken into account as a risk factor during IABP insertion.24
Discussion
This review explores vascular complications during intra-aortic balloon pump assistance in the existing literature and it highlights the most common risk factors following IABP insertion. Identifying the occurrence rates of vascular complications and the major risk factors associated with deleterious effects of IABP may prevent or delay the onset of complications along with reducing the morbidity and mortality rate.
The most common vascular complication related to IABP insertion is limb ischemia. The occurrence rate of limb ischemia increases in patients who have risk factors such as peripheral vascular disease and diabetes mellitus. For these reasons, careful assessment of the aorto-femoral vascular tree should be carried out during early diagnosis and intervention to prevent limb loss. It should also be noted that minor limb ischemia can normally be relieved by IABP removal, whilst this is not the case when major limb ischemia occurs, indicating that the ischemia is initially most likely caused by intermittent obstruction.
In fact, some patients will suffer from limb amputation as a consequences of irreversible limb ischemia due to IABP insertion. This is a very rare and serious complication, so careful physical examination is vital to identify early vascular changes and treated immediately before they become established. Emergency revascularization via a thrombectomy can stabilize the patient to avoid further complications.
Bleeding and hematoma have also been reported to occur in several studies, with varying rates. In most cases, bleeding is related to the insertion site and can vary from mild to severe. It can lead to thrombotic complications which may require transfusion or surgery. Many studies have stated that IABP duration time increases the risk of moderate and major bleeding if it used for a long period of time, even if it is more than two days. Furthermore, sheathless insertion is found not to be associated with high bleeding rate.
Mesenteric ischemia is a rare event, but is considered a potentially life-threating condition which can lead to serious conditions, such as gangrene of the bowel wall. However, only a small proportion of patients had mesenteric ischemia during IABP insertion in the included studies, due to the fact the majority of the research in this field has been carried out in animal studies or are reported in case reports, both of which were excluded in this review. Early diagnosis is important in any patient suspected of having mesenteric ischemia to prevent further complications.
Studies from our own group have investigated the association between intra-aortic balloon catheter size and visceral flow. In a number of studies, we have compared the use of a shorter balloon and compared this with the standard size catheters. Final results from these studies have concluded that the short balloon decreased mesenteric blood flow to a lesser extent when compared with the standard-size balloon, without losing IABP beneficial effects. In fact, it even improves visceral flows in comparison with the conventional IABP catheter.10,33 Furthermore, in an additional study, Gelsomino et al. investigated the effect of IABP weaning strategy on visceral flow and found that mesenteric blood flows decreased in both forms of weaning. These data indicate that the weaning strategy should not affect the occurrence of this complication, although further clinical research would be required to validate this.34
There is a lack of published information regarding the risk of pseudoaneurysm after IABP. The literature tends to only mention the number of patients without exploring the related risk factors. Once again, this should be a focus of future work
In the studies reviewed, most procedures were carried out using the femoral artery for insertion of the IABP catheter. However, a number of studies used alternative approaches. The subclavian artery approach could be associated with a lower morbidity rate. However, there are some things that should be taken into considerations. It is more time-consuming than the femoral approach and, therefore, is not recommended in the emergency setting. Additionally, there is still a chance of developing vascular complications, such as stroke and limb ischemia, due to the fact that the balloon catheter traverses the arch of the aorta. Studies, therefore, are needed to investigate whether this approach may reduce the risk of complications.
In assessing the vascular complications of IABP, several risk factors must be taken into consideration, such as female gender, smaller diameter catheters, peripheral vascular disease and diabetes mellitus. Complications, in general, due to IABP tend to be more common in females due to the smaller size of the femoral artery, so whether this increased risk could be overcome by utilizing alternative access points remains to be seen.
Several studies have linked vascular complications with diabetes mellitus and peripheral vascular disease. Therefore, careful vascular assessment is required when inserting IABP catheters into patients with these diseases to reduce the risk of vascular complication, as has been reported in many studies.
Sheathless insertion and smaller-size catheters can minimize the risk of vascular complications. It has been previously shown that there is a significant decrease in the rate of major vascular complications with IABP insertion in the last five years and this has been due to the increased use of catheters with smaller diameters. Moreover, the use of 6 Fr catheters has shown a great benefit in minimizing the risk of vascular complications, but further studies are required to cover the complications risk of using a smaller-sized catheter. Finally, long treatment time with an IABP catheter is considered an independent risk factor for the development of vascular complications. The longer duration of IABP support seemed to increase the risk for the of occurrence vascular complications.
Limitations
There are a number of limitations that should be noted in this review. A meta-analysis could not be performed due to heterogeneity of the studies as they investigated different outcomes, had different designs, as well as variable patient populations. In addition, data for a number of parameters, such as IABP duration, were not available in some studies. Finally, in a number of studies, it is likely that not all of the observed complications are reported, therefore, it is likely we under report on the rates of complication during IABP.
Conclusions
In conclusion, we have carried out a literature review assessing the current knowledge with regards to IABP complications. The major vascular complications include limb and mesenteric ischemia as well as hemorrhage. However, the incidence of these complications was generally low, although there is some study-to-study variability. Furthermore, there are a number of confounding factors that contribute to complication rates, including disease status, especially with regards to diabetes mellitus and peripheral vascular disease. Additionally, the method of catheter placement and catheter size as well as time on pump have been shown to be detrimental in the incidence rate of complications.
This review provides a comprehensive overview of the current knowledge regarding IABP complications and indicates that particular care should be taken in IABP implantation in patients with diabetes and peripheral vascular disease. Furthermore, smaller and sheathless insertion of balloons reduce the risk of complications. It is hoped that these data will be used to be able to assess the risks that the use of IABP may confer on patients from various pathophysiological backgrounds.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Parissis H, Graham V, Lampridis S, Lau M, Hooks G, Mhandu PC. IABP: history-evolution-pathophysiology-indications: what we need to know. J Cardiothorac Surg 2016; 11: 122 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4972967/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Nunen LX, Noc M, Kapur NK, Patel MR, Perera D, Pijls NHJ. Usefulness of intra-aortic balloon pump counterpulsation. Am J Cardiol 2016; 117: 469–476. [DOI] [PubMed] [Google Scholar]
- 3. Krishna M, Zacharowski K. Principles of intra-aortic balloon pump counterpulsation. Contin Educ Anaesth Crit Care Pain 2009; 9: 24–28. [Google Scholar]
- 4. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61: e78–140. [DOI] [PubMed] [Google Scholar]
- 5. The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33: 2569–2619. [DOI] [PubMed] [Google Scholar]
- 6. Thiele H, Zeymer U, Neumann F-J, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet 2013; 382: 1638–1645. [DOI] [PubMed] [Google Scholar]
- 7. Deppe A-C, Weber C, Liakopoulos OJ, et al. Preoperative intra-aortic balloon pump use in high-risk patients prior to coronary artery bypass graft surgery decreases the risk for morbidity and mortality—a meta-analysis of 9,212 patients. J Card Surg 2017; 32: 177–185. [DOI] [PubMed] [Google Scholar]
- 8. Iqbal MB, Al-Hussaini A, Rosser G, et al. Intra-aortic balloon pump counterpulsation in the post-resuscitation period is associated with improved functional outcomes in patients surviving an out-of-hospital cardiac arrest: insights from a dedicated heart attack centre. Heart Lung Circ 2016; 25: 1210–1217. [DOI] [PubMed] [Google Scholar]
- 9. Elahi MM, Chetty GK, Kirke R, Azeem T, Hartshorne R, Spyt TJ. Complications related to intra-aortic balloon pump in cardiac surgery: a decade later. Eur J Vasc Endovasc Surg 2005; 29: 591–594. [DOI] [PubMed] [Google Scholar]
- 10. Gelsomino S, Lozekoot PWJ, Lorusso R, et al. Comparing short versus standard-length balloon for intra-aortic counterpulsation: results from a porcine model of myocardial ischaemia–reperfusion. Eur J Cardiothorac Surg 2016; 49: 1361–1369. [DOI] [PubMed] [Google Scholar]
- 11. Meharwal ZS, Trehan N. Vascular complications of intra-aortic balloon insertion in patients undergoing coronary reavscularization: analysis of 911 cases. Eur J Cardiothorac Surg 2002; 21: 741–747. [DOI] [PubMed] [Google Scholar]
- 12. Christenson JT, Sierra J, Romand J-A, Licker M, Kalangos A. Long intraaortic balloon treatment time leads to more vascular complications. Asian Cardiovasc Thorac Ann 2007; 15: 408–412. [DOI] [PubMed] [Google Scholar]
- 13. Severi L, Vaccaro P, Covotta M, Landoni G, Lembo R, Menichetti A. Severe intra-aortic balloon pump complications: a single-center 12-year experience. J Cardiothorac Vasc Anesth 2012; 26: 604–607. [DOI] [PubMed] [Google Scholar]
- 14. Sirbu H, Busch T, Aleksic I, Friedrich M, Dalichau H. Ischaemic complications with intra-aortic balloon counter-pulsation: incidence and management. Cardiovasc Surg 2000; 8: 66–71. [DOI] [PubMed] [Google Scholar]
- 15. Arafa OE, Pedersen TH, Svennevig JL, Fosse E, Geiran OR. Vascular complications of the intraaortic balloon pump in patients undergoing open heart operations: 15-year experience. Ann Thorac Surg 1999; 67: 645–651. [DOI] [PubMed] [Google Scholar]
- 16. Davidson J, Baumgariner F, Omari B, Milliken J. Intra-aortic balloon pump: indications and complications. J Natl Med Assoc 1998; 90: 137–140. [PMC free article] [PubMed] [Google Scholar]
- 17. Tatar H, Ciçek S, Demirkilic U, et al. Vascular complications of intraaortic balloon pumping: unsheathed versus sheathed insertion. Ann Thorac Surg 1993; 55: 1518–1521. [DOI] [PubMed] [Google Scholar]
- 18. Boudoulas KD, Bowen T, Pederzolli A, Pfahl K, Pompili VJ, Jr ELM. Duration of intra-aortic balloon pump use and related complications. Acute Card Care 2014; 16: 74–77. [DOI] [PubMed] [Google Scholar]
- 19. Yuksel V, Huseyın S, Ozdemır AC, Ege T. Vascular complications of the intra-aortic balloon pump in patients undergoing open heart surgery: 10 years’ experience. Thorac Cardiovasc Surg 2013; 61: 453–455. [DOI] [PubMed] [Google Scholar]
- 20. Erdogan HB, Goksedef D, Erentug V, et al. In which patients should sheathless IABP be used? An analysis of vascular complications in 1211 cases. J Card Surg 2006; 21: 342–346. [DOI] [PubMed] [Google Scholar]
- 21. Meisel S, Shochat M, Sheikha SA, et al. Utilization of low-profile intra-aortic balloon catheters inserted by the sheathless technique in acute cardiac patients: clinical efficacy with a very low complication rate. Clin Cardiol 2004; 27: 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferguson JJ, III, Cohen M, Freedman RJ, Jr, et al. The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. J Am Coll Cardiol 2001; 38: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 23. Dick P, Mlekusch W, Delle-Karth G, Nikfardjam M, Schillinger M, Heinz G. Decreasing incidence of critical limb ischemia after intra-aortic balloon pump counterpulsation. Angiology 2009; 60: 235–241. [DOI] [PubMed] [Google Scholar]
- 24. Cohen M, Dawson MS, Kopistansky C, McBride R. Sex and other predictors of intra-aortic balloon counterpulsation-related complications: prospective study of 1119 consecutive patients. Am Heart J 2000; 139: 282–287. [DOI] [PubMed] [Google Scholar]
- 25. Eltchaninoff H, Dimas AP, Whitlow PL. Complications associated with percutaneous placement and use of intraaortic balloon counterpulsation. Am J Cardiol 1993; 71: 328–332. [DOI] [PubMed] [Google Scholar]
- 26. Jameson JS, Sayers RD, Macpherson DS, Spyt TJ. Management of lower limb ischaemia associated with the use of intra-aortic balloon pumps during cardiac surgery. Eur J Vasc Endovasc Surg 1995; 10: 327–329. [DOI] [PubMed] [Google Scholar]
- 27. Yamada T, Mizuguchi Y, Sakamoto S, Taniguchi N, Nakajima S, Takahashi A. Use of novel 6 French intra-aortic balloon pump catheter for patients undergoing percutaneous coronary intervention. ASAIO J 2013; 59: 493–496. [DOI] [PubMed] [Google Scholar]
- 28. Arceo A, Urban P, Dorsaz P-A, et al. In-hospital complications of percutaneous intraaortic balloon counterpulsation. Angiology 2003; 54: 577–585. [DOI] [PubMed] [Google Scholar]
- 29. Colyer WR, Burket MW, Ansel GM, et al. Intra-aortic balloon pump placement following aorto-iliac angioplasty and stent placement. Catheter Cardiovasc Interv 2002; 55: 163–168. [DOI] [PubMed] [Google Scholar]
- 30. Sommer S-P, Lange V, Yildirim C, et al. Cardiac surgery and hematologic malignancies: a retrospective single-center analysis of 56 consecutive patients. Eur J Cardiothorac Surg 2011; 40: 173–178. [DOI] [PubMed] [Google Scholar]
- 31. Rastan AJ, Tillmann E, Subramanian S, et al. Visceral arterial compromise during intra-aortic balloon counterpulsation therapy. Circulation 2010; 122: S92–99. [DOI] [PubMed] [Google Scholar]
- 32. Yildirim Y, Pecha S, Kubik M, et al. Efficacy of prophylactic intra-aortic balloon pump therapy in chronic heart failure patients undergoing cardiac surgery. Artif Organs 2014; 38: 967–972. [DOI] [PubMed] [Google Scholar]
- 33. Gelsomino S, Lozekoot PW, Lorusso R, et al. A new 35-mm short intra-aortic balloon catheter: a suitable option also for non-small-sized patients? Innovations (Phila) 2016; 11: 46–53; discussion 53. [DOI] [PubMed] [Google Scholar]
- 34. Gelsomino S, Lozekoot PWJ, Lorusso R, et al. The optimal weaning strategy for intraaortic balloon counterpulsation: volume-based versus rate-based approach in an animal model. Ann Thorac Surg 2016; 101: 1485–1493. [DOI] [PubMed] [Google Scholar]