Abstract
A novel biopharmaceutical, consisting of the F8 monoclonal antibody (specific to a splice isoform of fibronectin) simultaneously fused to both tumor necrosis factor and interleukin-2, was found to react with the majority of solid tumors and hematological malignancies in mouse and man, but not with healthy adult tissues. The product selectively localized to neoplastic lesions in vivo, as evidenced by quantitative biodistribution studies using radioiodinated protein preparations. When the potency of the cytokine payloads was matched by a single-point mutation, the resulting fusion protein (IL2-F8-TNFmut) eradicated soft-tissue sarcomas in immunocompetent mice, which did not respond to individual antibody-cytokine fusion proteins or by standard doxorubicin treatment. Durable complete responses were also observed in mice bearing CT26, C1498 and F9 tumors. The simultaneous delivery of multiple pro-inflammatory payloads to the cancer site conferred protective immunity against subsequent tumor challenges. A fully-human homologue of IL2-F8-TNFmut, which retained a selectivity similar to its murine counterpart when tested on human material, may open new clinical applications for the immunotherapy of cancer.
Keywords: Immunotherapy, immunocytokines, interleukin-2, tumor necrosis factor, EDA domain of fibronectin
Introduction
There is a growing interest in the use of immunomodulatory approaches to boost the activity of cellular components of the patient’s immune system (e.g., T cells and NK cells) to kill tumor cells (1–3). Various strategies, based on monoclonal antibody products, have been explored for the selective activation of anti-cancer immunity at the site of disease. They include the use of intact antibodies capable of promoting antibody-dependent cell cytotoxicity (4,5), immunological check-point inhibitors (2,6) and various types of bifunctional antibody molecules (7–13).
Antibody-cytokine fusion proteins (also called “immunocytokines) represent one type of bifunctional antibody products, which is being considered for the treatment of cancer and other conditions (7,9,14,15). Immunocytokines are able to increase the therapeutic index of the corresponding cytokine payload, as a result of a selective localization at the tumor site (8,9,11,12,14,16). Various immunocytokine products, based on interleukin-2 (IL2), interleukin-12 or tumor necrosis factor (TNF), are currently being studied in clinical trials for the treatment of various cancer types (17–19).
When used as single agents, pro-inflammatory immunocytokines are rarely able to induce complete remission in mouse models of cancer and in patients (20–22). However, the combination of tumor-targeting products based on pairs of synergistic payloads (e.g., IL12/TNF, IL2/IL12, IL4/IL12) could eradicate cancer in immunocompetent mice, which did not respond to individual immunocytokines or by conventional chemotherapy (23–27).
We have recently reported that the combination of two immunocytokine products, based on IL2 and TNF, was able to induce durable complete responses in mouse models of cancer (25) and in patients with Stage IIIB/C melanoma (17). However, the clinical development of combination therapies is cumbersome, since multiple products need to be produced to industrial standards and individual dose escalation studies need to be performed. Moreover, the determination of the optimal dose for product combinations represents a complex multidimensional clinical challenge (28).
The use of products with two cytokine payloads could potentially simplify clinical development, enabling the simultaneous delivery of two immunomodulatory agents to the tumor site. The approach has previously been described by the group of Stephen Gillies (29) and by our group (30), more than a decade ago, for interleukin-12 derivatives. However, fusion proteins featuring multiple payloads have not yet been moved into clinical development programs, as a result of pharmacokinetic limitations (30) and of suboptimal matching of cytokine potency (29,30). For example, the reported maximal tolerated dose of interleukin-12 in cancer patients was less than 100 micrograms per week (18), while interleukin-2 has been dosed up to 74 milligram per week (19).
Here, we describe a novel class of dual-cytokine fusion proteins, consisting of the tumor-targeting antibody F8 (specific to the alternatively-spliced EDA domain of fibronectin, a tumor associated antigen) (31) simultaneously fused to both IL2 and TNF. The product was able to selectively localize to neoplastic lesions in vivo, as evidenced by quantitative biodistribution studies performed with radioiodinated protein preparations. When the activity of TNF was decreased by a single-point mutation in order to match the one of IL2, the resulting fusion protein (termed IL2-F8-TNFmut) was able to induce durable complete responses in mice with WEHI-164 sarcomas, which was not triggered by doxorubicin or by individual immunocytokine products (e.g., F8-IL2 or F8-TNF) used as single agents (24).
Materials and Methods
Cell lines, animals and tumor models
CHO cells, WEHI-164 fibrosarcoma cells, CTLL2 cells, F9 teratocarcinoma cells, CT26 colon carcinoma cells, L-M fibroblasts, HT1080 fibrosarcoma cells, A375 melanoma cells and C1498 acute myeloid leukemia chloroma cells were obtained from the American Type Culture Collection (ATTC) between 2015 and 2017, expanded and stored as cryopreserved aliquots in liquid nitrogen. Cells were grown according to the supplier’s protocol and kept in culture for no longer than 14 passages. Authentication of the cell lines also including check of post-freeze viability, growth properties and morphology, test for mycoplasma contamination, isoenzyme assay and sterility test were performed by the cell bank before shipment.
Eight week old female 129/SvEv mice, Balb/c mice and C57BL/6 were obtained from Janvier. Tumor cells were implanted subcutaneously in the flank using 15 x 106 cells (F9), 5 x 106 cells (WEHI-164), 2 x 106 cells (CT26) and 1 x 106 cells (C1498). Experiments were performed under a project license granted by the Veterinäramt des Kantons Zürich, Switzerland (27/2015).
Cloning, expression and protein in vitro characterization
The fusion protein IL2-F8-TNF contains the antibody F8 (31) fused to murine tumor necrosis factor alpha at the C-terminus by a 15-amino-acid linker (24) and murine interleukin 2 (gene from Eurofins Genomics) at the N-terminus by a 12-amino-acid linker. The gene encoding for the F8 antibody and the gene encoding murine TNF and murine IL2 were PCR amplified, PCR assembled and cloned into the mammalian expression vector pcDNA3.1(+) (Invitrogen) by a NheI/NotI restriction site as previously described (32).
The fusion protein IL2-F8-TNFmut contains an arginine to tryptophan mutation in the amino acid position 111 of the murine TNF gene, that was inserted by PCR and cloned into the vector pcDNA3.1(+).
The fully-human IL2-F8-TNFmut contains an arginine to alanine mutation in the amino acid position 108 of the human TNF gene, that was inserted by PCR and cloned into the vector pcDNA3.1(+).
The fusion proteins were expressed using transient gene expression in CHO cells as described previously (32,33).
The fusion proteins were purified from the cell culture medium to homogeneity by protein A chromatography and analysed by SDS-PAGE, size exclusion chromatography (Superdex200 10/300GL, GE Healthcare) and surface plasmon analysis (BIAcore) on a EDA antigen-coated sensor chip.
The biological activity of murine TNF and IL2 was determined on WEHI-164, CTLL2 cells, respectively as described before (24,34), while the biological activity of human TNF was determined on L-M fibroblasts, HT1080 andA375 cells.
Immunofluorescence studies
Antigen expression was confirmed on ice-cold acetone fixed 8-µm cryostat sections of WEHI-164, CT26, F9 and C1498 stained with IL2-F8-TNFmut (final concentration 5µg/mL) and detected with rat anti-IL2 (eBioscience 14-7022-85) and anti-rat AlexaFluor488 (Invitrogen A21208). For vascular staining goat anti-CD31 (R&D AF3628) and anti-goat AlexaFluor594 (Invitrogen A11058) antibodies were used.
Frozen tumor and normal tissue specimens in microarray format were obtained from Amsbio and stained with a biotinylated preparation of the fully human IL2-F8-TNFmut fusion protein and detected with Streptavidin-AlexaFluor488 (Invitrogen S11223). Cell nuclei were counterstained with DAPI (Invitrogen D1306).
For ex-vivo immunofluorescence analysis, mice were injected according to the therapy schedule and sacrificed 24h after injection. Tumors were excised and embedded in cryoembedding medium (Thermo Scientific) and cryostat sections (8µm) were stained using the following antibodies: rat anti-IL2 (eBioscience 14-7022-85), rat anti-CD4 (Biolegend 100423), rat anti-CD8 (Biolegend 100702), rat anti-FoxP3 (eBioscience 14-5773-82), rabbit anti-Asialo GM1 (Wako 986-10001), rabbit anti-Caspase3 (Sigma C8487), rat anti-CD31 (BD 553370), goat anti-CD31 (R&D AF3628), rat anti-NKp46 (Biolegend 137601); and detected with anti-rat AlexaFluor488 (Invitrogen A21208), anti-rabbit AlexaFluor488 (Invitrogen A11008), anti-goat AlexaFluor594 (Invitrogen A11058), anti-rat AlexaFluor594 (Invitrogen A21209). Slides were mounted with fluorescent mounting medium and analysed with Axioskop2 mot plus microscope (Zeiss).
Biodistribution studies
The capability of targeting EDA in vivo was assessed by quantitative biodistribution analysis, according to previously published experimental procedures (31). 5-10µg of radioiodinated fusion protein was injected into the lateral tail vein of F9 tumor-bearing mice (32). Mice were sacrificed 24h after injection, organs were excised, weighed and the radioactivity of organs and tumors was measured using a Cobra γ counter and expressed as percentage of injected dose per gram of tissue (%ID/g ± SEM), (n = 3-4 mice per group).
Therapy studies and in vivo depletion of CD4+ T cells, CD8+ T cells and NK cells
Mice were monitored daily and tumor volume was measured with a calliper (volume = length x width2 x 0.5). When tumors reached a suitable volume (approx. 70-100 mm3), mice were injected three times into the lateral tail vein with the pharmacological agents. Fusion proteins were dissolved in phosphate buffered saline (PBS), also used as negative control, and administered every 48h or 72h. The commercial anti-PD-1 antibody (clone J43, BioXCell) was administered i.v. once at a dose of 200 µg. For the tumor re-challenge study, mice with complete responses were injected subcutaneously with 5 x 106 WEHI-164 cells in the flank.
For the in vivo depletion of CD4+ T cells, CD8+ T cells and NK cells, WEHI-164 tumor bearing mice were injected intra-peritoneally with 30 µL anti-Asialo GM1 (Wako 986-10001), 250 µg anti-CD4 (clone GK1.5 BioXCell) or 250 µg anti-CD8 (clone 2.43 BioXCell) antibodies on day 2, 5, 8 and 11 after tumor implantation. A saline group and a treatment group without depletion were included as controls.
Results are expressed as tumor volume in mm3 ± SEM. For WEHI-164 studies, 5 mice per group (6 mice per group for the depletion experiment) were used. For therapy studies with C1498, CT26 and F9 tumors, 4 mice per group were used.
Toxicity assessment
12 week-old Balb/c mice were injected three times into the lateral tail vein with immunocytokine (50µg). The fusion protein was dissolved in phosphate buffered saline (PBS), also used as negative control, and administered every 48h. Two days after the last injections mice were sacrificed and a necropsy was performed by a veterinary pathologist. An initial macroscopic examination of the external surface of the body, all orifices, the cranial, thoracic, and abdominal cavities and their contents and organs and tissues from every animal was performed.
Selected tissues were fixed in 10% neutral buffered formalin, dehydrated, embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined microscopically.
Results
Production and characterization of dual cytokine-antibody fusions
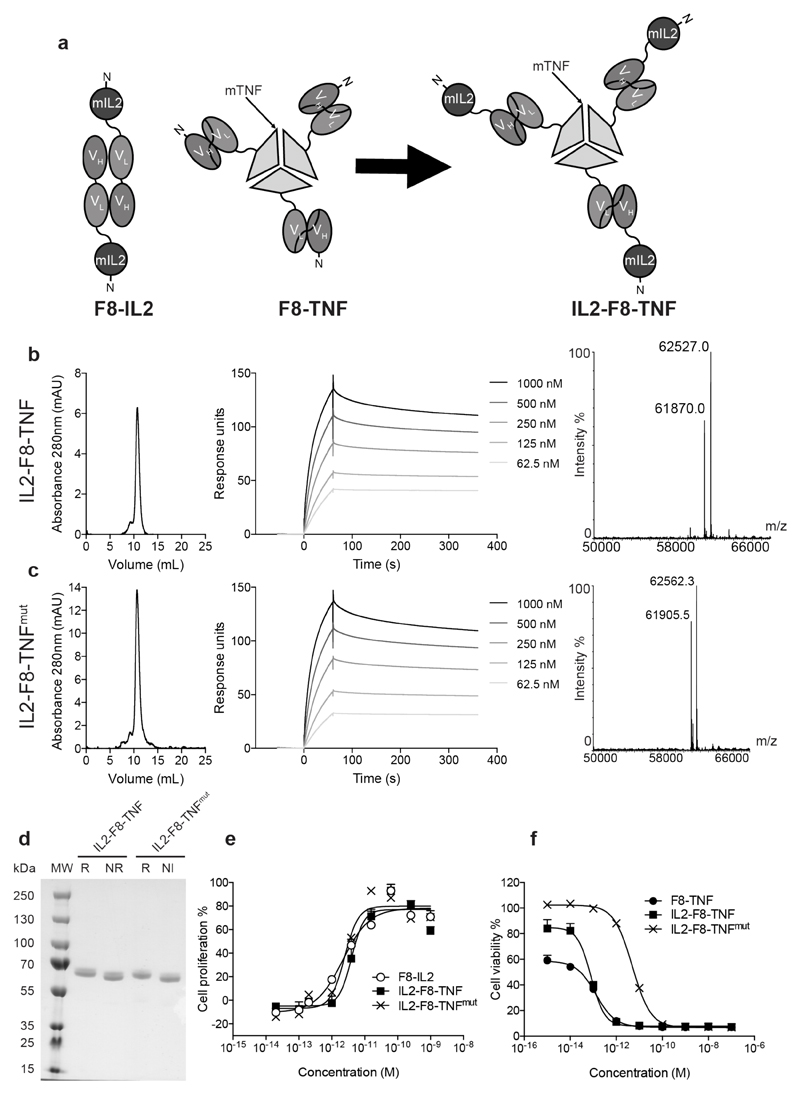
Antibody fragments specific to splice isoforms of fibronectin, fused to IL2 or to TNF, display promising therapeutic activity in preclinical models of cancer and in clinical trials (17,25,35). Because of their different potency, IL2 fusions are typically administered at 10-fold higher doses compared to TNF fusions, both in mouse and in man (17,19,24,25,36,37). In order to combine the IL2 and TNF moieties into a single molecular entity, a fusion protein was generated and expressed in mammalian cells, which sequentially incorporated (starting from the N-terminus) murine IL2, the F8 antibody in scFv format and murine TNF. These functional moieties were connected using short peptide linkers of 12 and 15 amino acids, respectively [Figure 1A and Supplementary Figure S1]. In addition, in order to match the IL2 and TNF potency, several mutations of TNF (38) were screened during an initial scouting study [Supplementary Figure S2] and the mutant with the best performance based on in vitro cell killing was further characterized. A fusion protein was generated, which featured the R467W mutation (38) in the murine TNF moiety. The two fusion proteins, termed IL2-F8-TNF and IL2-F8-TNFmut, were expressed in CHO cells and purified to homogeneity exploiting the binding properties of the VH domain of the F8 antibody to Protein A resin (31). Figure 1B-D shows the analytical characterization of IL2-F8-TNF and IL2-F8-TNFmut by SDS-PAGE, gel-filtration, ESI-MS and BIAcore analysis on antigen-coated microsensor chips. Analysis of the in vitro activity of the two fusion proteins indicated that IL2-F8-TNF and IL2-F8-TNFmut displayed a comparable IL2 activity (based on a cell line proliferation assay), while TNF activity was decreased in the R467W mutant (based on a cell killing assay) [Figure 1E,F].
Figure 1. Cloning expression and characterisation of IL2-F8-TNF and IL2-F8-TNFmut.
(A) Schematic representation of the domain assembly of F8-IL2 in diabody format, as well as of F8-TNF and IL2-F8-TNF in non-covalent homotrimeric format. (B and C) Starting from left: size exclusion chromatography profile, BIAcore analysis on EDA-coated sensor chip and ESI-MS profile of IL2-F8-TNF and of IL2-F8-TNFmut, respectively. (D) SDS-Page analysis, MW: molecular weight, R: reducing conditions, NR: non-reducing conditions. (E) IL2 bioactivity assay, based on the proliferation of CTLL2 cells. (F) TNF bioactivity assay, based on the killing of WEHI-164 cells.
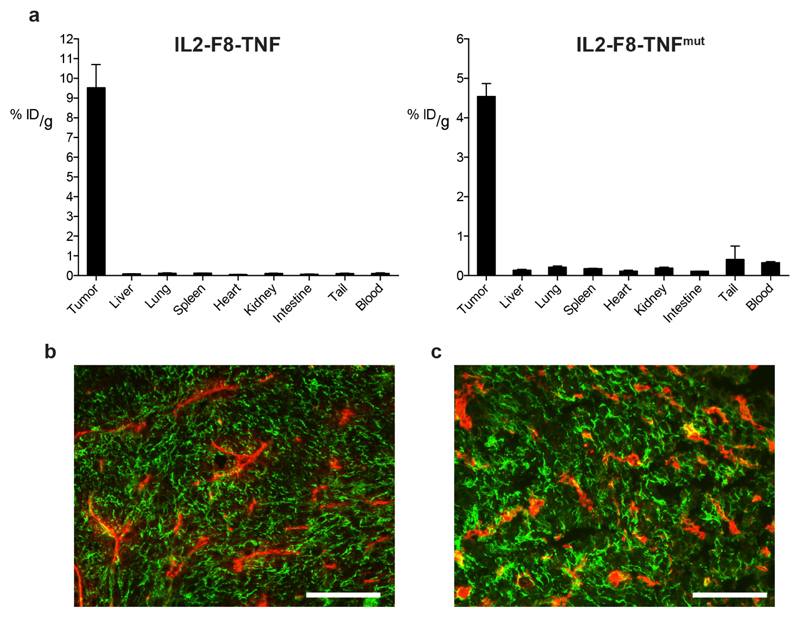
The tumor-targeting properties of radioiodinated preparations of IL2-F8-TNF and IL2-F8-TNFmut were assessed by quantitative biodistribution analysis in immunocompetent mice, bearing subcutaneously grafted murine F9 teratocarcinomas (23,24,31). Both fusion proteins selectively localized to solid tumors, with tumor:organ and tumor:blood ratios > 10:1, twenty-four hours after intravenous administration [Figure 2A]. The tumor targeting performance was also assessed in mice with WEHI-164 sarcomas (24). A microscopic fluorescence analysis of tumor sections, obtained from non-injected animals and from mice 24 hours after administration of IL2-F8-TNFmut, confirmed that the fusion protein could uniformly localize to its cognate EDA(+)-fibronectin antigen within the tumor mass in vivo [Figure 2B,C].
Figure 2. Tumor-targeting properties of IL2-F8-TNF and IL2-F8-TNFmut.
(A) Quantitative biodistrubution analysis of radioiodinated IL2-F8-TNF (left) and IL2-F8-TNFmut (right) in immunocompetent mice bearing F9 teratocarcinoma tumors. 5-10µg of radioiodinated fusion protein was injected i.v. into the lateral tail vein and mice were sacrificed 24h after injection, organs were excised, weighed and the radioactivity of organs and tumors was measured. Results are expressed as percentage of injected dose per gram of tissue (%ID/g ± SEM), (n = 3-4 mice per group). (B) Microscopic fluorescence analysis of EDA expression on WEHI-164 tumor section detected with IL2-F8-TNFmut (green for anti-murine IL2, Alexa Fluor 488) and anti-CD31 (red, Alexa Fluor 594), 20x magnification, scale bars = 100µm. (C) Confirmation by immunofluorescence analysis of tumor targeting properties 24 hours after IL2-F8-TNFmut injection in mice bearing WEHI-164 sarcomas, cryosections were stained with anti-murine IL2 (green, Alexa Fluor 488) and anti-CD31 (red, Alexa Fluor 594), 20x magnification, scale bars = 100µm.
Therapy experiments
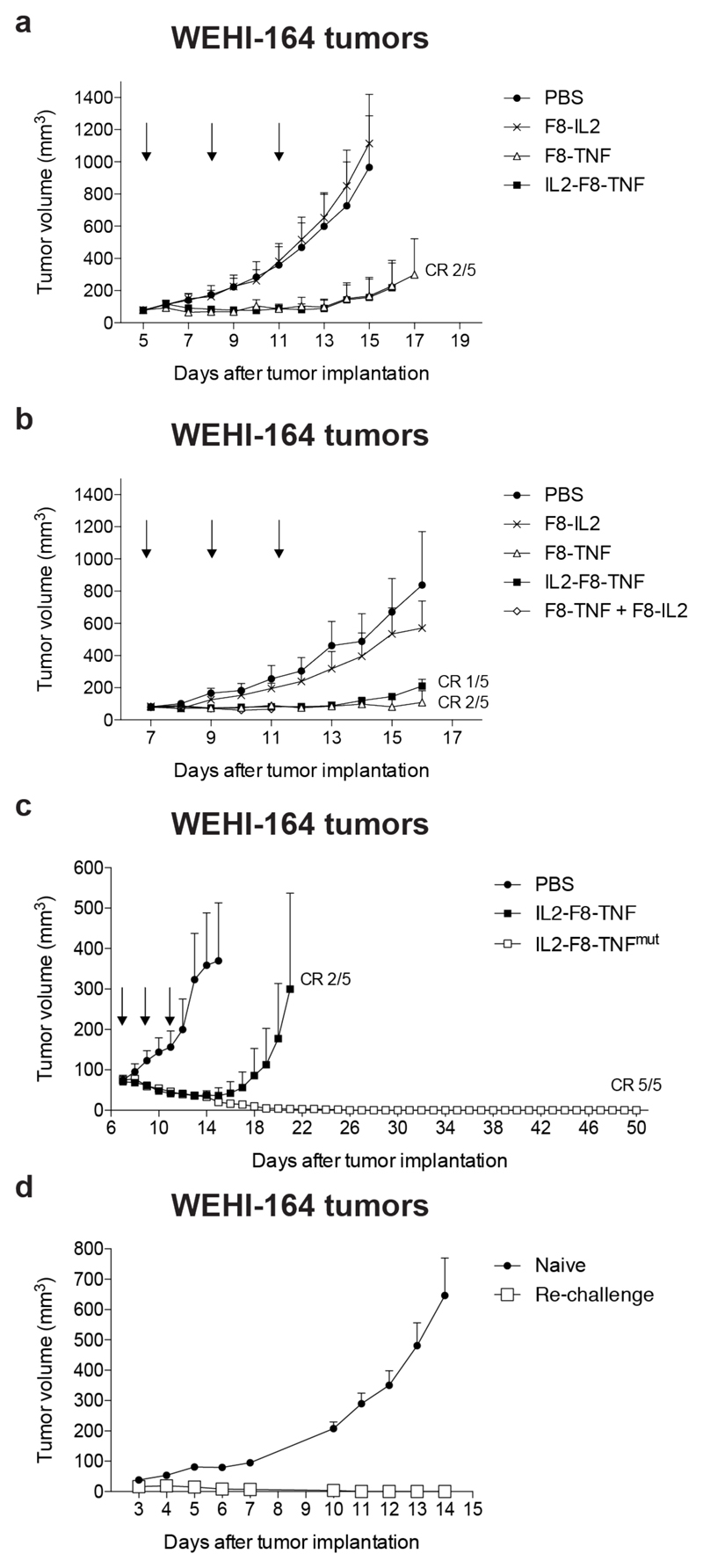
First, we compared the therapeutic activity of F8-IL2, F8-TNF and IL2-F8-TNF in immunocompetent Balb/c mice, bearing WEHI-164 sarcomas. Mice received equimolar doses of the fusion proteins (2µg F8-TNF, 2µg F8-IL2 and 2.8 µg IL2-F8-TNF) on day 5, 8 and 11 after tumor cell implantation. F8-TNF and IL2-F8-TNF displayed a comparable therapeutic activity, but the dual cytokine-antibody fusion protein was better tolerated, as evidenced by the comparison of body weight profiles [Figure 3A and Supplementary Figure S3]. As expected, treatment with F8-IL2 resulted in a tumor growth profile similar to the one obtained with saline, as the agent was used at a 10-fold lower dose, compared to regimens which had previously been shown to be active (25,35).
Figure 3. Therapeutic performance of IL2-F8-TNF and IL2-F8-TNFmut in balb/c mice bearing WEHI-164 fibrosarcoma tumors.
Data represent mean tumor volume ± SEM, n = 5 mice per group, CR = complete response. (A) Comparison of single agents F8-IL2 and F8-TNF versus IL2-F8-TNF, treatment started when tumors reached a volume of 80 mm3 and mice were injected three times intravenously every 72 hours with either PBS, 2µg F8-TNF, 2µg F8-IL2 or 2.8µg IL2-F8-TNF. (B) Comparison of single agents F8-IL2 and F8-TNF, combination of F8-IL2 and F8-TNF versus IL2-F8-TNF, treatment started when tumors reached a volume of 70 mm3 and mice were injected three times intravenously every 48 hours with either PBS, 4µg F8-TNF, 4µg F8-IL2, the combination of F8-IL2 and F8-TNF or 5.6µg IL2-F8-TNF. (C) Therapeutic activity of the potency-matched IL2-F8-TNFmut compared to IL2-F8-TNF, treatment started when tumors reached a volume of 70 mm3 and mice were injected three times intravenously every 48 hours with either PBS, 10µg IL2-F8-TNF or 50µg IL2-F8-TNFmut. (D) Tumor re-challenge study. After 50 days, mice with complete responses were injected subcutaneously with 5 x 106 WEHI-164 cells in the flank.
In a second study, we doubled the injected quantities of the therapeutic agents (4µg F8-TNF, 4µg F8-IL2 and 5.6 µg IL2-F8-TNF), in order to reach the maximal tolerated dose of IL2-F8-TNF. A potent inhibition of tumor cell growth was observed with F8-TNF, IL2-F8-TNF and the combination of F8-IL2 + F8-TNF. This last combination regimen, however, was not well tolerated and mice in the group had to be sacrificed at day 11 [Figure 3B and Supplementary Figure S3].
The therapeutic activity of the potency-matched IL2-F8-TNFmut (administered at a dose of 50µg) was compared to the one of IL2-F8-TNF (used at 10µg). The dual cytokine-antibody fusion protein mutant induced durable complete responses in 100% of mice in the study group, while only a transient tumor regression was observed in the IL2-F8-TNF group. The body weight profiles for the two fusion proteins were comparable [Figure 3C and Supplementary Figure S3]. A necroscopic analysis of mice after IL2-F8-TNFmut treatment at the maximal tolerated dose revealed certain macroscopic and microscopic findings. Macroscopically, a 2.3-fold increase in spleen size (weight) was observed. Microscopically, a number of abnormalities could be observed two days after the administration of the last dose of immunocytokine, including areas of necrosis and fibrosis in the bone marrow, increased extramedullary hematopoiesis (spleen, liver, lung) and cortical lymphoid depletion in the thymus. [Supplementary Figure S4].
Mice with complete responses obtained with IL2-F8-TNFmut treatment were re-challenged with WEHI-164 cells 50 days after the primary tumor implantation and were found to have acquired a protective immunity against the tumor [Figure 3D].
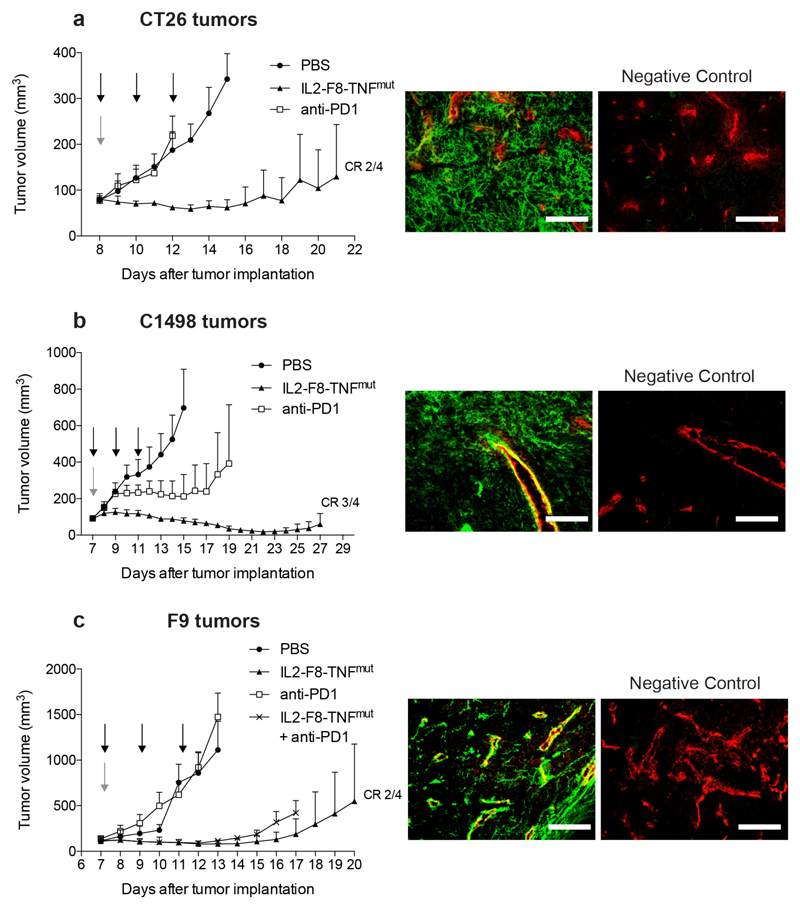
In order to characterize product activity in additional tumor models, the potency-matched IL2-F8-TNFmut product was also tested in immunocompetent mice, bearing subcutaneous C1498 acute myeloid leukemia chloroma lesions, CT26 colorectal carcinomas and F9 teratocarcinomas. Also in these models, the product displayed a potent therapeutic activity, which was superior to the one of an anti-mouse PD-1 treatment [Figure 4]. The patterns of EDA expression were different in the various cancer models: a diffuse staining of the interstitium was observed in C1498 and CT26 tumors, while the antigen was mainly found around tumor blood vessels in F9 teratocarcinomas [Figure 4].
Figure 4. Therapeutic performance of IL2-F8-TNFmut compared to anti-mouse PD1 treatment.
Data represent mean tumor volume ± SEM, n = 4 mice per group, CR = complete response. (Left panels) (A) Therapy in balb/c mice bearing CT26 colon carcinoma lesions. Treatment started when tumors reached a volume of 80 mm3, mice were injected three times intravenously every 48 hours with either PBS, 50µg IL2-F8-TNFmut (black arrows) or once with 200µg anti-mouse PD1 (grey arrow). (B) Therapy in C57BL/6 mice bearing C1498 acute myeloid leukemia chloroma tumors. Treatment started when tumors reached a volume of 90 mm3, mice were injected three times intravenously every 48 hours with either PBS, 50µg IL2-F8-TNFmut (black arrows) or once with 200µg anti-mouse PD1 (grey arrow). (C) Therapy in 129SvEv mice bearing F9 teratocarcinoma tumors. Treatment started when tumors reached a volume of 100 mm3, mice were injected three times intravenously every 48 hours with either PBS, 40µg IL2-F8-TNFmut (black arrows), once with 200µg anti-mouse PD1 (grey arrow) or the combination IL2-F8-TNFmut + anti-mouse PD1. (Right panels) Microscopic fluorescence analysis of EDA expression on CT26 (A), C1498 (B) or F9 (C) tumor section detected with IL2-F8-TNFmut (green for anti-murine IL2, Alexa Fluor 488) and anti-CD31 (red, Alexa Fluor 594), as negative control staining without the fusion protein was performed, 20x magnification, scale bars = 100µm (right).
Mechanistic studies
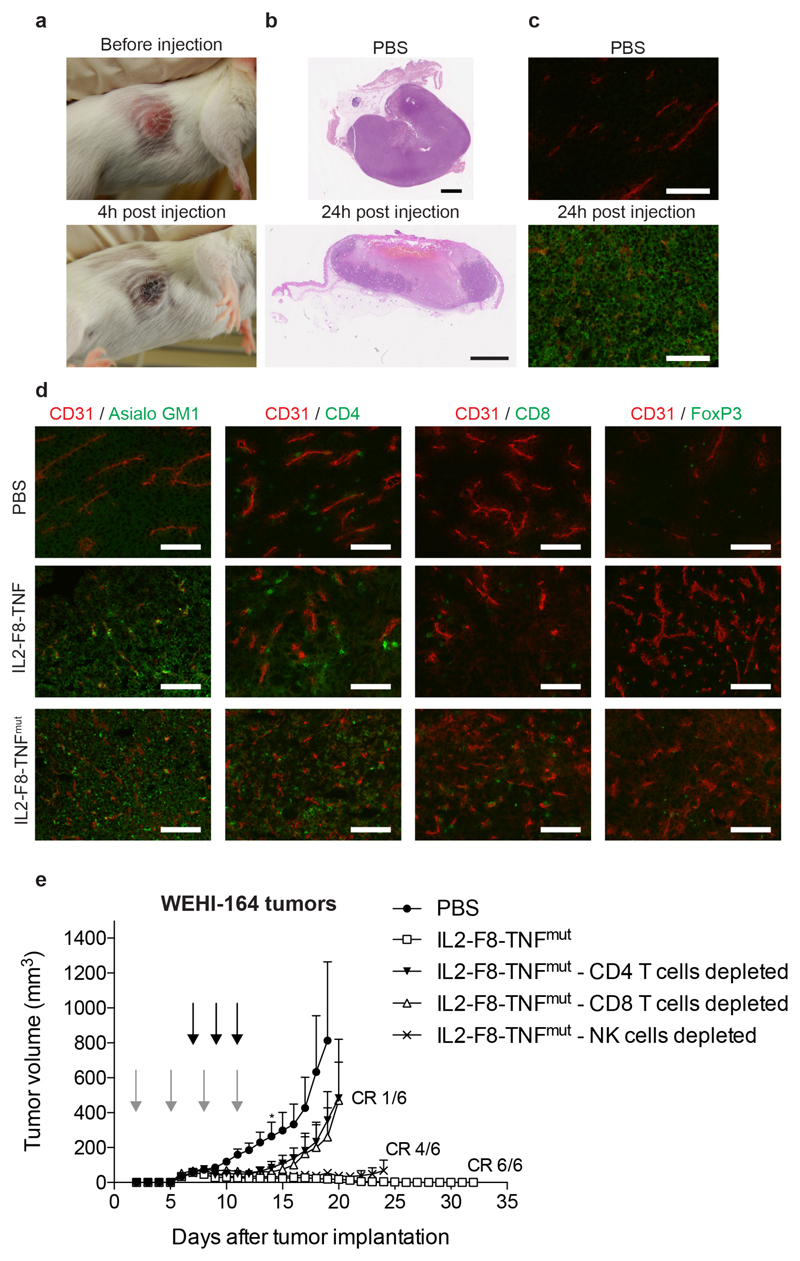
At a macroscopic level, the induction of necrosis within the tumor lesion was already detectable a few hours after the first immunocytokine treatment [Figure 5A]. A microscopic H&E analysis of tumor sections confirmed a rapid onset of tumor cell death within the neoplastic mass. Within 24 hours, the tumor had been converted into necrotic mass following IL2-F8-TNF treatment, except for small areas in close proximity with the abdomen [Figure 5B]. Pharmacological treatment with IL2-F8-TNFmut led to a homogenous cell death within the neoplastic lesion, as evidenced by H&E staining (not shown) and by caspase-3 staining [Figure 5C]. A microscopic analysis of tumor sections, obtained 24h after the first injection of IL2-F8-TNF and IL2-F8-TNFmut, revealed a substantial increase in the density of leukocytes stained by antibodies specific to the asialogylcoprotein receptor (mainly NK cells), compared to mice treated with saline, moreover the infiltration of NK cells was confirmed by staining with a different monoclonal antibody (anti-NKp46) [Figure 5D and Supplementary figure S5]. By contrast, the influx of CD4+ and CD8+ T cells was less prominent, suggesting that these lymphocytes may be more relevant at a later stage, contributing to the killing of residual tumor cells after initial debulking and to the development of protective immunity (23).
Figure 5. Macro- and microscopic analysis of therapeutic performance of dual cytokine-fusion proteins and in vivo depletion of CD4+ T cells, CD8+ T cells and NK cells.
(A) Macroscopic indication of necrotic changes 4 hours after IL2-F8-TNF treatment. (B) H&E analysis of tumor sections 24 hours after administration of IL2-F8-TNF, scale bars: 2mm. (C) Detection of Caspase3 by immunofluorescence in WEHI-164 tumor sections 24 hours after treatment with IL2-F8-TNFmut stained with anti-Caspase3 (green, Alexa Fluor 488) and anti-CD31 (red, Alexa Fluor 594), 20x magnification, scale bars = 100µm. (D) Immunofluorescence analysis of tumor infiltrating cells on WEHI-164 tumor sections 24 hours after treatment with PBS, IL2-F8-TNF or IL2-F8-TNFmut, marker specific for NK cells (Asialo GM1), CD4+ T cells (CD4), CD8+ T cells (CD8) and T regs (FoxP3) were stained in green (Alexa Fluor 488), anti-CD31 (red, Alexa Fluor 594), 20x magnification, scale bars = 100µm. (E) In vivo depletion experiment. When tumors (WEHI-164) reached a volume of approximately 60 mm3, mice were injected three times intravenously every 48 hours with 50µg IL2-F8-TNFmut (black arrows). Depletion antibodies were administered at day 2, 5, 8 and 11 (grey arrows) after tumor implantation. A PBS treated negative control and an undepleted IL2-F8-TNFmut treated positive group were included. Data represent mean tumor volume ± SEM, n = 6 mice per group (for PBS group n = 5 from day 14*), CR = complete response.
In order to gain more insights about the leukocyte contribution to the therapy experiments, we evaluated the therapeutic activity of IL2-F8-TNFmut in mice bearing WEHI-164 sarcomas after antibody-based depletion of CD4+ T cells, CD8+ T cells or NK cells [Figure 5E]. Both CD4+ and CD8+ T cells contribute to the onset of an anti-cancer immunity, while the contribution of NK cells was dispensable in this setting.
A fully-human IL2-F8-TNFmut fusion protein for clinical applications
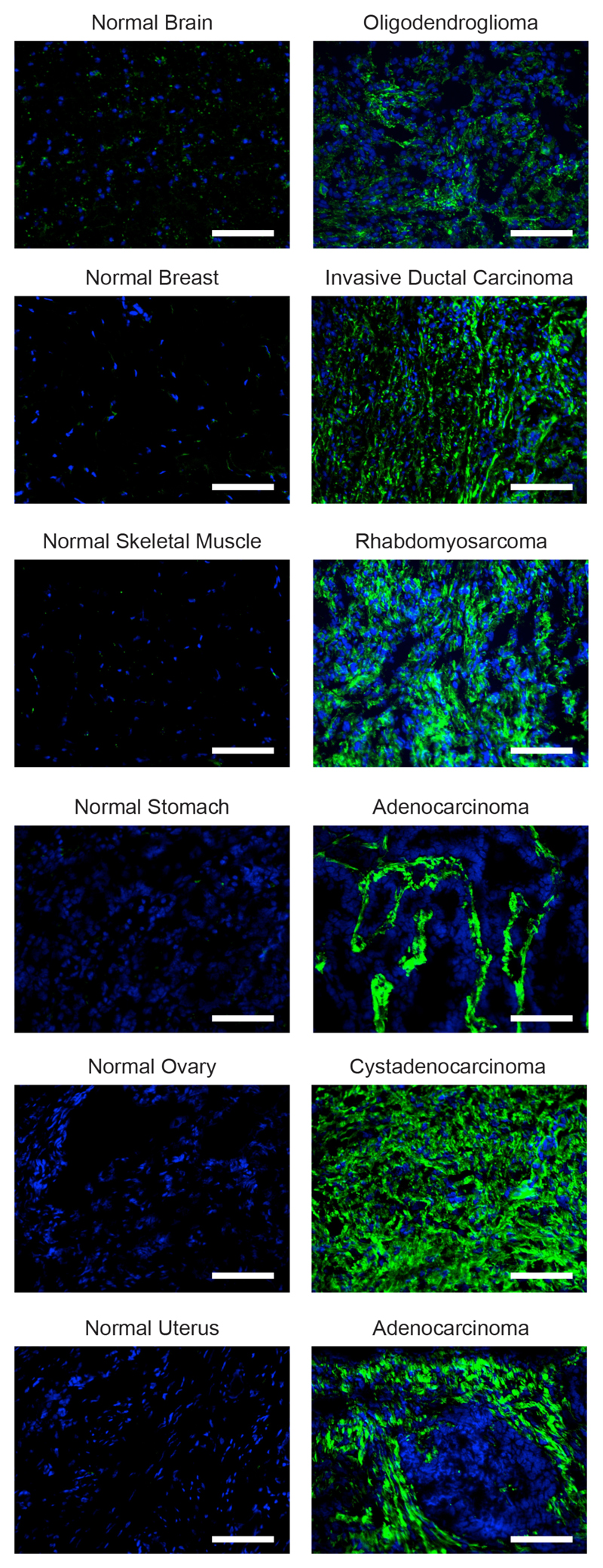
In order to prepare for clinical applications different amino acid substitutions at position R108 of human TNF were investigated using an in vitro cell killing assay [Supplementary Figure S6]. Finally, a fusion protein was cloned, expressed and purified, which consisted of human IL2 and of the human TNF R108A mutant fused to the F8 antibody [Supplementary Figure S7A]. The product was well-behaved in biochemical assays [Supplementary Figure S7B], selectively localized to solid tumors in vivo [Supplementary Figure S7C] and displayed a matched in vitro activity of the IL2 and TNF moieties, using cellular assays based on the proliferation of murine CTLL-2 lymphocytes [Supplementary Figure S7D] and on the killing of human HT-1080 and A375 tumor cell lines [Supplementary Figure S7D]. A labeled preparation of the IL2-F8-TNFmut fusion protein exhibited an intense staining in most human solid tumors [Figure 6], but not of healthy adult organs. For many tumors, the staining was confined to the sub-endothelial extracellular matrix of tumor-blood vessels, as previously reported by our group for the parental F8 antibody (20,39,40).
Figure 6. Microscopic fluorescence analysis of EDA expression of frozen tumor and normal tissues.
A microarray containing normal tissue specimens (left) and their tumoral counterpart (right) was stained with a biotinylated preparation of the fully human IL2-F8-TNFmut (green, Alexa Fluor 488), cell nuclei were counterstained with DAPI (blue), 20x magnification, scale bars = 100µm.
Discussion
The integration of IL2 and TNF moieties into a single antibody-based fusion protein has led to the generation of a new class of immunostimulatory products, with highly selective tumor homing properties. The potency-matched IL2-F8-TNFmut product was able to eradicate syngeneic sarcomas, which do not respond to conventional chemotherapy (24,41). Mice with durable complete responses acquired a protective immunity against subsequent tumor challenges. Similarly, complete tumor eradications were observed in mouse models of leukemia and of colorectal cancer, which did not respond well to PD-1 blockade.
We had previously observed that the combination of immunocytokines, based on antibodies against splice isoforms of fibronectin fused to IL2 or TNF, could eradicate tumors in mice, which did not respond to the individual products used as single agents (19,25,36,42). Furthermore, we have recently described that the combination of two immunocytokine products, based on IL2 and TNF as immunostimulatory payloads, was potently active for the intralesional treatment of patients with stage IIIB,C melanoma (17). The combination of the two agents led not only to a disappearance of the injected lesion, but also to an objective response in the majority of non-injected lesions (17), providing a rationale for a combination Phase III clinical trial, which has recently started [EudraCT number 2015-002549-72].
The possibility to incorporate potency-matched IL2 and TNF moieties into the same biopharmaceutical product offers several biotechnological advantages. A single product needs to be developed, thus decreasing costs and simplifying dose-finding clinical trials, compared to the combination of two immunocytokines. The simultaneous delivery of two immunomodulatory agents to the tumor site leads to a rapid tumor cell killing and creates a pro-inflammatory environment, which facilitates the influx and activation of various types of leukocytes into the neoplastic mass. The favorable biodistribution profiles of IL2-F8-TNF and IL2-F8-TNFmut, with tumor:blood and tumor:organ ratios greater than 10:1 in tumor-bearing mice at 24h after injection, suggest that the vasoactive properties of TNF (43,44) and of IL2 (43,45) may contribute to an efficient extravasation and tumor homing of the corresponding homotrimeric fusion protein.
We have previously observed that some tumors respond better to IL2-based immunocytokines, while other tumor types respond better to TNF-based products (20,24). This situation is likely to also occur in cancer patients (36,37). By incorporating both immunomodulatory moieties into a single product, we may be able to increase the proportion of patients who benefit from pharmacological intervention. TNF can kill tumor cells by direct interaction with cognate receptors on the cell surface (46). In addition, it promotes hemorrhagic necrosis and apoptosis of the tumor endothelium, thus enhancing vascular permeability (47). The targeted delivery of IL2 leads to an influx and activation of T cells and NK cells into the neoplastic mass (21,48,49). Interestingly, the depletion studies performed in therapy experiments with IL2-F8-TNFmut have revealed a dominant role of CD4+ and CD8+ T cells for the tumor rejection process [Figure 5E]. By contrast, the therapeutic activity of the single fusion protein F8-TNF depended on both CD8+ T cells and NK cells, while depletion of CD4+ T cells had an impact on therapy only if performed prior to tumor implantation (50).
Potency-matched immunocytokines may find clinical applications for the same indications in which individual antibody-cytokine fusions are currently being considered. Future research efforts will elucidate which therapeutic modalities (e.g., radiation, cytotoxic agents or immunotherapeutics) may be suitable for combination therapy. The products described in this article, equipped with the human homologues of IL2 and TNF, could in principle be used for the treatment of various types of cancer, as the alternatively-spliced EDA domain of fibronectin is strongly expressed in the majority of solid tumors (24,45), lymphomas (23) and acute leukemias (20), while being undetectable in the majority of normal adult tissues (25).
Supplementary Material
Acknowledgements
We would like to thank Baptiste Gouyou (Philochem AG, Otelfingen) for his help with experimental procedures.
Financial support: D. Neri gratefully acknowledges ETH Zürich, the Swiss National Science Foundation, the European Research Council (ERC Advanced Grant “Zauberkugel”), the Swiss Federal Commission for Technology and Innovation (CTI Project “DUAL CYTOKINE-ANTIBODY FUSIONS”) and the “Stiftung zur Krebsbekämpfung”.
References
- 1.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 2.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 3.Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med. 2016 doi: 10.1038/nm.4200. advance online publication http://www.nature.com/nm/journal/vaop/ncurrent/abs/nm.4200.html-supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6(5):343–57. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 5.Carter PJ. Introduction to current and future protein therapeutics: a protein engineering perspective. Exp Cell Res. 2011;317(9):1261–9. doi: 10.1016/j.yexcr.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Sledzinska A, Menger L, Bergerhoff K, Peggs KS, Quezada SA. Negative immune checkpoints on T lymphocytes and their relevance to cancer immunotherapy. Mol Oncol. 2015;9(10):1936–65. doi: 10.1016/j.molonc.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neri D, Sondel PM. Immunocytokines for cancer treatment: past, present and future. Curr Opin Immunol. 2016;40:96–102. doi: 10.1016/j.coi.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess C, Venetz D, Neri D. Emerging classes of armed antibody therapeutics against cancer. MedChemComm. 2014;5(4):408. doi: 10.1039/c3md00360d. [DOI] [Google Scholar]
- 9.Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today. 2012;17(11–12):583–90. doi: 10.1016/j.drudis.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Muller D. Antibody fusions with immunomodulatory proteins for cancer therapy. Pharmacol Ther. 2015;154:57–66. doi: 10.1016/j.pharmthera.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Kontermann RE. Antibody–cytokine fusion proteins. Archives of Biochemistry and Biophysics. 2012;526(2):194–205. doi: 10.1016/j.abb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Lode HN, Xiang R, Becker JC, Gillies SD, Reisfeld RA. Immunocytokines: A Promising Approach to Cancer Immunotherapy. Pharmacology & Therapeutics. 1998;80(3):277–92. doi: 10.1016/S0163-7258(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 13.Baeuerle PA, Reinhardt C. Bispecific T-Cell Engaging Antibodies for Cancer Therapy. Cancer Research. 2009;69(12):4941. doi: 10.1158/0008-5472.CAN-09-0547. [DOI] [PubMed] [Google Scholar]
- 14.Bootz F, Neri D. Immunocytokines: a novel class of products for the treatment of chronic inflammation and autoimmune conditions. Drug Discov Today. 2016;21(1):180–9. doi: 10.1016/j.drudis.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwager K, Bootz F, Imesch P, Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-10 inhibits endometriosis in a syngeneic mouse model. Hum Reprod. 2011;26(9):2344–52. doi: 10.1093/humrep/der195. [DOI] [PubMed] [Google Scholar]
- 16.Penichet ML, Morrison SL. Antibody–cytokine fusion proteins for the therapy of cancer. Journal of Immunological Methods. 2001;248(1–2):91–101. doi: 10.1016/S0022-1759(00)00345-8. [DOI] [PubMed] [Google Scholar]
- 17.Danielli R, Patuzzo R, Di Giacomo AM, Gallino G, Maurichi A, Di Florio A, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunology, Immunotherapy. 2015;64(8):999–1009. doi: 10.1007/s00262-015-1704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gollob JA, Mier JW, Veenstra K, McDermott DF, Clancy D, Clancy M, et al. Phase I Trial of Twice-Weekly Intravenous Interleukin 12 in Patients with Metastatic Renal Cell Cancer or Malignant Melanoma: Ability to Maintain IFN-γ Induction Is Associated with Clinical Response. Clinical Cancer Research. 2000;6(5):1678. [PubMed] [Google Scholar]
- 19.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutbrodt KL, Schliemann C, Giovannoni L, Frey K, Pabst T, Klapper W, et al. Antibody-Based Delivery of Interleukin-2 to Neovasculature Has Potent Activity Against Acute Myeloid Leukemia. Science Translational Medicine. 2013;5(201):201ra118. doi: 10.1126/scitranslmed.3006221. [DOI] [PubMed] [Google Scholar]
- 21.Yang RK, Kalogriopoulos NA, Rakhmilevich AL, Ranheim EA, Seo S, Kim K, et al. Intratumoral hu14.18-IL-2 (IC) induces local and systemic antitumor effects that involve both activated T and NK cells as well as enhanced IC retention. J Immunol. 2012;189(5):2656–64. doi: 10.4049/jimmunol.1200934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogue SL, Taura T, Bi M, Yun Y, Sho A, Mikesell G, et al. Targeting Attenuated Interferon-alpha to Myeloma Cells with a CD38 Antibody Induces Potent Tumor Regression with Reduced Off-Target Activity. PLoS One. 2016;11(9):e0162472. doi: 10.1371/journal.pone.0162472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemmerle T, Neri D. The antibody-based targeted delivery of interleukin-4 and 12 to the tumor neovasculature eradicates tumors in three mouse models of cancer. Int J Cancer. 2014;134(2):467–77. doi: 10.1002/ijc.28359. [DOI] [PubMed] [Google Scholar]
- 24.Hemmerle T, Probst P, Giovannoni L, Green AJ, Meyer T, Neri D. The antibody-based targeted delivery of TNF in combination with doxorubicin eradicates sarcomas in mice and confers protective immunity. Br J Cancer. 2013;109(5):1206–13. doi: 10.1038/bjc.2013.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwager K, Hemmerle T, Aebischer D, Neri D. The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF. J Invest Dermatol. 2013;133(3):751–8. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- 26.Wigginton JM, Komschlies KL, Back TC, Franco JL, Brunda MJ, Wiltrout RH. Administration of Interleukin 12 With Pulse Interleukin 2 and the Rapid and Complete Eradication of Murine Renal Carcinoma. Journal of the National Cancer Institute. 1996;88(1):38–43. doi: 10.1093/jnci/88.1.38. [DOI] [PubMed] [Google Scholar]
- 27.Wigginton JM, Wiltrout RH. IL-12/IL-2 combination cytokine therapy for solid tumours: translation from bench to bedside. Expert Opin Biol Ther. 2002;2(5):513–24. doi: 10.1517/14712598.2.5.513. [DOI] [PubMed] [Google Scholar]
- 28.List T, Neri D. Immunocytokines: a review of molecules in clinical development for cancer therapy. Clin Pharmacol. 2013;5:29–45. doi: 10.2147/CPAA.S49231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillies SD, Lan Y, Brunkhorst B, Wong WK, Li Y, Lo KM. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol Immunother. 2002;51(8):449–60. doi: 10.1007/s00262-002-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halin C, Gafner V, Villani ME, Borsi L, Berndt A, Kosmehl H, et al. Synergistic Therapeutic Effects of a Tumor Targeting Antibody Fragment, Fused to Interleukin 12 and to Tumor Necrosis Factor α. Cancer Research. 2003;63(12):3202. [PubMed] [Google Scholar]
- 31.Villa A, Trachsel E, Kaspar M, Schliemann C, Sommavilla R, Rybak JN, et al. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int J Cancer. 2008;122(11):2405–13. doi: 10.1002/ijc.23408. [DOI] [PubMed] [Google Scholar]
- 32.Pasche N, Woytschak J, Wulhfard S, Villa A, Frey K, Neri D. Cloning and characterization of novel tumor-targeting immunocytokines based on murine IL7. Journal of Biotechnology. 2011;154(1):84–92. doi: 10.1016/j.jbiotec.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Rajendra Y, Kiseljak D, Baldi L, Hacker DL, Wurm FM. A simple high-yielding process for transient gene expression in CHO cells. Journal of Biotechnology. 2011;153(1–2):22–6. doi: 10.1016/j.jbiotec.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Frey K, Schliemann C, Schwager K, Giavazzi R, Johannsen M, Neri D. The Immunocytokine F8-IL2 Improves the Therapeutic Performance of Sunitinib in a Mouse Model of Renal Cell Carcinoma. The Journal of Urology. 2010;184(6):2540–8. doi: 10.1016/j.juro.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 35.Pretto F, Elia G, Castioni N, Neri D. Preclinical evaluation of IL2-based immunocytokines supports their use in combination with dacarbazine, paclitaxel and TNF-based immunotherapy. Cancer Immunology, Immunotherapy. 2014;63(9):901–10. doi: 10.1007/s00262-014-1562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eigentler TK, Weide B, de Braud F, Spitaleri G, Romanini A, Pflugfelder A, et al. A Dose-Escalation and Signal-Generating Study of the Immunocytokine L19-IL2 in Combination with Dacarbazine for the Therapy of Patients with Metastatic Melanoma. Clinical Cancer Research. 2011;17(24):7732. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- 37.Spitaleri G, Berardi R, Pierantoni C, De Pas T, Noberasco C, Libbra C, et al. Phase I/II study of the tumour-targeting human monoclonal antibody–cytokine fusion protein L19-TNF in patients with advanced solid tumours. Journal of Cancer Research and Clinical Oncology. 2013;139(3):447–55. doi: 10.1007/s00432-012-1327-7. [DOI] [PubMed] [Google Scholar]
- 38.Van Ostade X, Tavernier J, Prangé T, Fiers W. Localization of the active site of human tumour necrosis factor (hTNF) by mutational analysis. The EMBO Journal. 1991;10(4):827–36. doi: 10.1002/j.1460-2075.1991.tb08015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwager K, Kaspar M, Bootz F, Marcolongo R, Paresce E, Neri D, et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Research & Therapy. 2009;11(5):R142. doi: 10.1186/ar2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rybak JN, Roesli C, Kaspar M, Villa A, Neri D. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res. 2007;67(22):10948–57. doi: 10.1158/0008-5472.CAN-07-1436. [DOI] [PubMed] [Google Scholar]
- 41.Borsi L, Balza E, Carnemolla B, Sassi F, Castellani P, Berndt A, et al. Selective targeted delivery of TNFα to tumor blood vessels. Blood. 2003;102(13):4384. doi: 10.1182/blood-2003-04-1039. [DOI] [PubMed] [Google Scholar]
- 42.McDermott DF, Cheng SC, Signoretti S, Margolin KA, Clark JI, Sosman JA, et al. The high-dose aldesleukin “select” trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2015;21(3):561–8. doi: 10.1158/1078-0432.CCR-14-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khawli LA, Miller GK, Epstein AL. Effect of seven new vasoactive immunoconjugates on the enhancement of monoclonal antibody uptake in tumors. Cancer. 1994;73(S3):824–31. doi: 10.1002/1097-0142(19940201)73:3+<824::AID-CNCR2820731312>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 44.Folli S, Épèlegrin A, Chalandon Y, Yao X, Buchegger F, Lienard D, et al. Tumornecrosis factor can enhance radio-antibody uptake in human colon carcinoma xenografts by increasing vascular permeability. International Journal of Cancer. 1993;53(5):829–36. doi: 10.1002/ijc.2910530521. [DOI] [PubMed] [Google Scholar]
- 45.Moschetta M, Pretto F, Berndt A, Galler K, Richter P, Bassi A, et al. Paclitaxel enhances therapeutic efficacy of the F8-IL2 immunocytokine to EDA-fibronectin-positive metastatic human melanoma xenografts. Cancer Res. 2012;72(7):1814–24. doi: 10.1158/0008-5472.CAN-11-1919. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes[quest] Acta Pharmacol Sin. 2008;29(11):1275–88. doi: 10.1111/j.1745-7254.2008.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Horssen R, ten Hagen TLM, Eggermont AMM. TNF-α in Cancer Treatment: Molecular Insights, Antitumor Effects, and Clinical Utility. The Oncologist. 2006;11(4):397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 48.Carnemolla B, Borsi L, Balza E, Castellani P, Meazza R, Berndt A, et al. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99(5):1659. doi: 10.1182/blood.v99.5.1659. [DOI] [PubMed] [Google Scholar]
- 49.Schliemann C, Gutbrodt KL, Kerkhoff A, Pohlen M, Wiebe S, Silling G, et al. Targeting interleukin-2 to the bone marrow stroma for therapy of acute myeloid leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Cancer Immunol Res. 2015;3(5):547–56. doi: 10.1158/2326-6066.CIR-14-0179. [DOI] [PubMed] [Google Scholar]
- 50.Probst P, Kopp J, Oxenius A, Colombo MP, Ritz D, Fugmann T, et al. Sarcoma eradication by doxorubicin and targeted TNF relies upon CD8+ T cell recognition of a retroviral antigen. Cancer Research. 2017 doi: 10.1158/0008-5472.CAN-16-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.