Abstract
Objective
Vitamin K-dependent proteins (VKDPs), which require post-translational modification to achieve biologic activity, appear to contribute to thrombus formation, vascular calcification and vessel stiffness. Whether VKDP activity is prospectively associated with incident cardiovascular disease has not been studied.
Approach and Results
VKDP activity was determined by measuring circulating Des-gamma-carboxy Prothrombin (DCP) concentrations in a random sample of 709 multi-ethnic adults free of cardiovascular disease drawn from the Multi-Ethnic Study of Atherosclerosis. Lower DCP concentrations reflect greater VKDP activity. Subjects were followed for risk of ischemic cardiovascular disease (coronary heart disease, stroke, and fatal cardiovascular disease) over 11.0 years of follow up. A total of 75 first ischemic CVD events occurred during follow up. The incidence of ischemic cardiovascular disease increased progressively across DCP quartiles, with event rates of 5.9 and 11.7 per 1000 person-years in the lowest and highest quartiles. In analyses adjusted for traditional cardiovascular risk factors and measures of vitamin K intake, a doubling of DCP concentration was associated with a 1.53 (95% confidence interval, 1.09-2.13; p=0.008) higher risk of incident ischemic cardiovascular disease. The association was consistent across strata of participants with diabetes, hypertension, renal impairment, and low vitamin K nutritional intake.
Conclusions
In this sample of middle-aged and older adults, VKDP activity was associated with incident ischemic cardiovascular events. Further studies to understand the role of this large class of proteins in cardiovascular disease is warranted.
Keywords: Vitamin K, prothrombin, phylloquinone, cardiovascular disease
Introduction
Vitamin K-dependent proteins (VKDPs) are a large class of proteins unified by their reliance on post-translational modification to achieve biologic activity. To date, nineteen VKDPs have been described, with important roles in coagulation, platelet function, and vascular biology. Prothrombin, the most well described VKDP, is produced within the liver, and circulates systemically until stimulation by a platelet plug, facilitating thrombus formation. Matrix Gla protein (MGP) 1 is produced within vascular smooth muscle cells, and inhibits vascular calcification by binding extracellular calcium2. Growth arrest specific factor-6 (Gas-6) is produced within platelets and the vascular wall and affects thrombus formation and cell survival3. Periostin4, expressed in the cardiac ventricle, has a role in ventricular hypertrophy5, valvular function and atherosclerosis6.
Produced in an inactive form, all VKDPS obtain biologic activity through the conversion of a glutamic acid residue into glutamate, a complex process requiring vitamin K hydroquinone as a cofactor and regulated by γ-carboxylase enzyme within the endoplasmic reticulum. In this process, Vitamin K hydroquinone is converted to vitamin K epoxide, which in turn is recycled back to vitamin K hydroquinone by vitamin K epoxide reductase (VKOR). In addition to adequate vitamin K, enzyme activity and polymorphisms affect the carboxylation process, suggesting that the post-translational modification of such proteins have multiple levels of control.
Given the pleiotropic biologic effects of this large class of proteins, and emerging evidence for their role in cardiovascular physiology, we investigated whether VKDP activity was associated with cardiovascular disease in a well-characterized sample of adults drawn from across the United States. Since prothrombin rapidly undergoes carboxylation before being secreted from the liver, we measured circulating Des-gamma-carboxy Prothrombin (DCP) concentrations to indicate lower VKDP activity.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Baseline characteristics stratified by DCP quartiles are presented in table I. Participants with higher DCP concentrations (i.e., lower VKDP activity) tended to be older, with higher BMI and cholesterol medication usage, with lower renal function and less overall physical activity. The rates of diabetes and hypertension were similar, but hs-CRP concentrations increased across the DCP quartiles. As expected, increased phylloquinone concentrations, reflecting greater dietary vitamin K intake, were associated with lower DCP concentrations, reflecting greater VKDP activity. The weighted correlation between DCP and phylloquinone concentrations was inverse, as expected, but modest (r=-0.11;p= 0.006). A total of 84% of the cohort participants had a DCP >2 ng/ml (considered the threshold for VKDP inactivity) while 52% had a phylloquinone concentration <1 nmol/L (considered the threshold for inadequate vitamin K intake). In keeping with previous reports suggesting higher dietary vitamin K intake in those of Chinese descent7, the majority of Chinese participants (55%) were in the lowest DCP quartile, compared to only 15% of white participants.
Table 1. Baseline characteristics of the case-cohort sample, stratified by DCP quartiles (n=709).
| DCP Q1 | DCP Q2 | DCP Q3 | DCP Q4 | |
|---|---|---|---|---|
| (0.33-2.40) | (2.41-3.47) | (3.48-4.63) | (4.64-20.1) | |
| Variables | n=178 | n=177 | n=177 | n=177 |
| Age, years | 61 (10) | 63 (10) | 62 (10) | 63 (10) |
| Women, n (%) | 92 (52) | 92 (52) | 92 (52) | 81 (46) |
| Race/ethnicity, n (%) | ||||
| White | 43 (24) | 62 (35) | 78 (44) | 85 (48) |
| Chinese | 42 (24) | 17 (10) | 10 (6) | 8 (5) |
| Black | 62 (35) | 60 (34) | 38 (21) | 36 (20) |
| Hispanic | 31 (17) | 38 (21) | 51 (29) | 48 (27) |
| Body mass index | 27 (6) | 28 (6) | 29 (6) | 30 (5) |
| Cigarette smoking status, n (%) | ||||
| Never | 97 (55) | 94 (53) | 84 (47) | 85 (48) |
| Former | 54 (31) | 59 (33) | 68 (38) | 68 (38) |
| Current | 24 (14) | 24 (14) | 25 (14) | 24 (14) |
| Pack-years cigarette smoking | 8 (18) | 11 (19) | 10 (18) | 16 (29) |
| Intentional physical activity total (MET-min/day) | 218 (310) | 234 (379) | 199 (364) | 194 (238) |
| Current alcohol use, n (%) | 96 (69) | 96 (67) | 116 (77) | 93 (61) |
| High school graduate, n (%) | 139 (79) | 149 (84) | 147 (83) | 141 (80) |
| Diabetes | 27 (15) | 21 (12) | 31 (18) | 20 (11) |
| Antihypertension medication, n (%) | 58 (33) | 66 (37) | 75 (42) | 66 (37) |
| Systolic BP (mmHg) | 124 (22) | 128 (22) | 126 (23) | 130 (20) |
| Diastolic BP (mmHg) | 72 (11) | 72 (11) | 71 (12) | 73 (9) |
| LDL-C | 108 (28) | 116 (28) | 119 (33) | 119 (31) |
| HDL-C | 54 (16) | 53 (16) | 50 (14) | 46 (13) |
| Lipid-lowering medication, n(%) | 23 (13) | 24 (14) | 34 (19) | 42 (24) |
| Triglycerides, mg/dL, median (IQR) | 94 (71-136) | 103 (69-148) | 108 (79-155) | 134 (98-188) |
| Hs-CRP, median (IQR), mg/l | 1.1 (0.6-3.3) | 1.8 (0.9-3.7) | 2.8 (1.1-6.6) | 2.6 (1.3-5.0) |
| Estimated GFR | 83 (18) | 81 (20) | 81 (18) | 79 (19) |
| Urinary Albumin /Creatinine (mg/g) | 14 (37) | 20 (56) | 39 (216) | 40 (184) |
| Phylloquinone, (nmol/L) | 1.8 (2.3) | 1.5 (2.2) | 1.2 (1.2) | 1.2 (1.5) |
| Dihydrophylloquinone (nmol/L) | 0.3 (1.1) | 0.2 (0.5) | 0.2 (0.6) | 0.2 (0.8) |
Mean (standard deviations) provided.
Abbreviation: BP = blood pressure; DCP = Des-gamma carboxy Prothrombin; eGFR = estimated glomerular filtration rate based on creatinine measurement; HDL = high-density lipoprotein cholesterol; Hs-CRP = high-sensitivity C-reactive protein; IQR = interquartile range; LDL-C = low-density lipoprotein cholesterol; MET = metabolic equivalent; Q = quartile.
Note: Sample n=709, except: Cigarette smoking n=706; Pack-years of smoking n=701; Intentional physical activity n=706; High school graduate n=706; Diabetes n=708; LDL-C n=701; HDL-C n=708; Triglycerides n=708; estimated GFR n=708; Urinary Albumin/Creatinine n=706; Phylloquinone n=630; Dihydrophylloquinone n=630. Descriptive statistics are not weighted for case-cohort design.
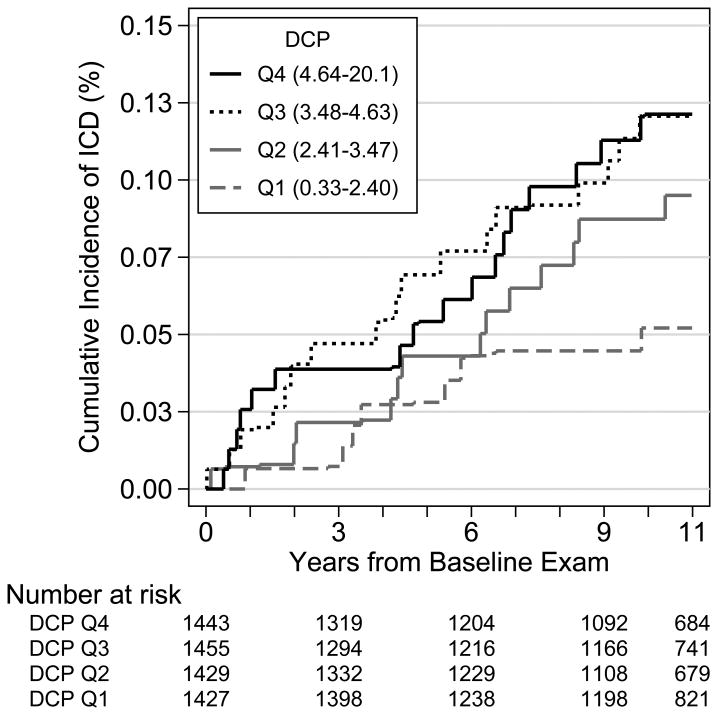
We documented 75 first ischemic CVD events during a median of 11.0 years of follow up, 16 myocardial infarctions, 29 coronary revascularization procedures, 22 fatal and nonfatal strokes, and 8 other fatal CHD events. Unadjusted cumulative incidence rates per DCP quartile are presented in Figure 2. In general, ischemic CVD incidence rates were higher with greater concentration of DCP.
Figure 2. Cumulative Incidence of Ischemic Cardiovascular Disease according to quartiles of Des-gamma carboxy prothrombin (n=709).
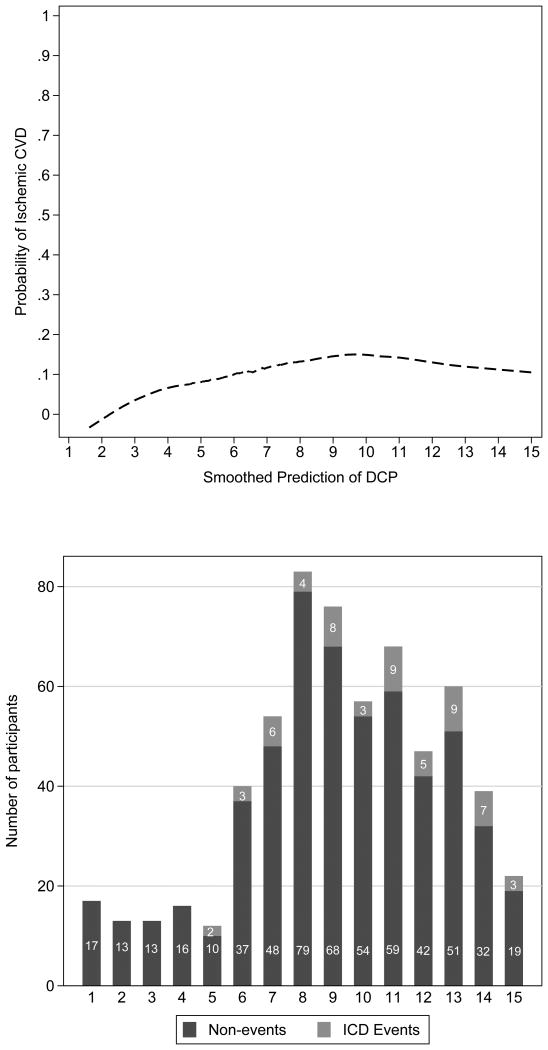
We next assessed the dose-response relationship of DCP with ischemic CVD (Figure 3), which suggested a log-linear association of DCP with CVD incidence. We subsequently performed analyses of log-transformed DCP concentrations with risk, adjusting sequentially for demographics, cardiovascular risk factors, and measures of vitamin K intake. In these analyses (table II), a doubling of circulating DCP concentration was associated approximately 50% higher risk of ischemic CVD. The magnitude of this association was little changed with adjustment for traditional risk factors or phylloquinone concentrations. Given the previously recommended threshold of 2 ng/ml in DCP concentrations to indicate VKDP inactivity, we examined this cutpoint in fully adjusted models, with an adjusted hazard ratio of 3.42 (95% confidence interval, 0.97-12.09; p=0.06).
Figure 3. Locally weighted smoothed scatterplot and histogram of Des-gamma carboxy Prothrombin and Ischemic Cardiovascular Disease (n=617).
Table 2. Proportional Hazards Cox Regression Model Predicting the Likelihood of Ischemic Cardiovascular Disease by Des-gamma carboxy Prothrombin.
| DCP (quartiles) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Events/N | Q1 (0.33-2.40) |
Q2 (2.41-3.47) |
Q3 (3.48-4.63) |
Q4 (4.64-<20.1) |
DCP (continuous) |
|
| Model 1 | 75/709 | reference | 1.38 | 1.83 | 1.71 | 1.46 |
| (0.62-3.06) | (0.81-4.12) | (0.79-3.70) | (1.08-1.97) | |||
| p=0.428 | p=0.143 | p=0.173 | p=0.013 | |||
| Model 2 | 74/694 | reference | 1.48 | 1.96 | 2.03 | 1.58 |
| (0.62-3.52) | (0.83-4.66) | (0.82-5.04) | (1.13-2.22) | |||
| p=0.378 | p=0.126 | p=0.125 | 0.008 | |||
| Model 3 | 59/617 | reference | 1.46 | 1.82 | 1.87 | 1.53 |
| (0.61-3.50) | (0.78-4.23) | (0.76-4.58) | (1.09-2.13) | |||
| p=0.39 | p=0.163 | p=0.171 | 0.013 | |||
Abbreviation: DCP = des-gamma carboxy Prothrombin; Q = quartile.
Note: Table shows hazard ratio (95% confidence interval). All models are weighted for case-cohort design and use robust standard errors. Model 1: Adjusted for age, gender, and race/ethnicity. Model 2: Adjusted for Model 1 + BMI, cigarette smoking (never, former, current), education (high school graduate), diabetes, systolic blood pressure, antihypertension medication use, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, high-sensitivity C-reactive protein, lipid-lowering medication, estimated glomular filtration rate, albumin and creatinine ratio, intentional physical activity, and current alcohol use. Model 3: Adjusted for Model 2 + phylloquinone, and dihydrophylloquinone.
The association of DCP concentrations and ischemic CVD appeared to be consistent across a broad range of subgroups. We found no significant interaction of DCP with diabetes, hypertension, vitamin K intake, or hs-CRP (all multiplicative interaction terms p > 0.5).
Finally, redefining ischemic CVD to include only cardiac related events, DCP concentrations were associated with a 1.46 (95%CI 1.00-1.06; p=0.047), 1.59(95%CI 1.03-2.44;p=0.035), and 1.53(95%CI 1.00-2.35;p=0.054) odds of incident disease using Model I, II, and III, respectively.
Discussion
In this population of adults followed for 11 years, baseline measures of VKDP activity were associated with incident ischemic cardiovascular disease. Our findings raise awareness of this important class of proteins as a potential contributor to cardiovascular disease.
A large body of evidence relates VKDPs to cardiovascular disease. Early animal studies suggested that warfarin administration, a potent inhibitor of VKOR recycling, induces widespread medial vascular calcification8, 9, otherwise known as Monckeberg calcification. Medial calcification has been associated with vascular stiffness10 and increased mortality11, 12and is prevalent among individuals with diabetes and chronic kidney disease. MGP has received considerable attention as the candidate VKDP protein responsible for the vascular calcification phenotype13. MGP null mice have widespread vascular calcification1, and the circulating form of uncarboxylated MGP has been associated with vascular calcification in clinical studies14-17. Since dietary vitamin K may reduce the inactive form of MGP18, dietary vitamin K intake has been hypothesized as a determinant of vascular calcification. Animal models have shown regression of vascular calcification with high vitamin K diets19, but clinical data have not been uniform, with some studies suggesting a potential benefit of dietary vitamin K20, 21, not supported by others22, 23. A randomized trial of vitamin K for this indication is ongoing24.
Given the pleiotropic biologic roles of VKDPs, it is plausible that VKDPs have a role in CVD independent of any potential effect on vascular calcification. Gas-6, widely expressed in vascular smooth muscle cells and monocytes, has received increasing attention for its role in platelet aggregation, vascular morphology, and atherosclerosis. Post translational change of the N terminus of Gas6 confers the protein the ability to bind to aninonic phospholipids exposed on injured cell surfaces25, and through a family of TAM receptors, Gas6 has multiple downstream effects, including promoting cell survival26, migration27, and remodeling, and potentially reducing atherosclerotic plaque formation28, 29. In addition, Gas6 inhibits the adhesion of leukocytes to endothelial cells30 and reduces plaque inflammation in some31, but not all32, studies. Gas6 deficient mice have defective platelet signaling, but also seem to have a paradoxical protection against thromboembolic disease33. Thus, it is plausible that a generalized state of VKDP inactivity, as measured by increasing DCP concentrations, might also reflect a state of Gas-6 inactivity34, and a consequent pro-atherosclerotic state. Periostin4 is a newly described VKDP, named due to its localization in cortical bone periosteum and the periodontal ligament, with a role in embryonic cardiac development35 and cardiac remodeling5. Periostin knockout (Pn−/−) mice have an increased rate of ventricular rupture after myocardial infarction36, but increased periostin expression is also seen in ventricular hypertrophy and fibrosis37. In addition, prothrombin itself has been linked to CVD, associated with an increased risk of venothrombotic disease38 but not arterial disease39.
In addition to the potential role of VKDPs in thrombus formation and vessel morphology, data suggests an association between VKDPs and inflammation. Administration of high dose dietary vitamin K reduces inflammatory gene expression in animal models40, 41. In our cohort, hs-CRP concentrations increased with greater VKDP inactivity. Given data suggesting that prothrombin is an acute phase reactant, whether increasing DCP concentrations reflect prothrombin production or a carboxylation failure is uncertain, although the association of DCP with ischemic cardiovascular disease was consistent across strata of hs-CRP. In our cohort, the weighted correlation for hs-CRP and DCP was r=0.10, p=0.006.
Our findings add to clinical data linking VKDP activity and cardiovascular disease. In a community-based study of over 4800 subjects, dietary vitamin K intake was inversely associated with incident cardiovascular disease42. Since most American diets meet the recommended daily vitamin K allowances, there are likely dietary independent factors affecting VKDP activity. The function of the gamma carboxylase enzyme, responsible for converting a glutamic acid to glutamate residue, is impaired in kidney disease43 and diabetes44, perhaps accounting for the unexpectedly high prevalence of VKDP inactivity in chronic kidney disease45. In our analysis, the overall correlation between serum phylloquinone and DCP concentrations was low, potentially supporting a role for dietary independent factors, although phylloquinone concentrations may also be more sensitive to very recent vitamin K intake than are DCP levels. Although high doses of dietary vitamin K can improve VKDP activity, whether such treatment will lead to an improvement of outcomes is speculative.
The strengths of our study included a well-characterized multi-ethnic population with adjudicated, prospectively-measured endpoints and detailed phenotyping of cardiovascular risk factors. In addition, measurement of serum phylloquinone concentrations allowed the effect of VKDP activity on cardiovascular disease to be adjusted for nutritional vitamin K. Our study also has important limitations. The correlation between the activity of circulating VKDPs, such as prothrombin, and organ-specific VKDPs, such as periostin or MGP, are not known, and the assumption that DCP concentrations reflect overall VKDP activity cannot be validated without further study. Although this is the largest prospective study of DCP and cardiovascular disease to be performed to our knowledge, we only measured DCP in a subcohort of MESA participants and had limited power to examine associations within subgroups or across components of our composite endpoint. Nevertheless, increasing DCP quartiles had incrementally higher risks of incident CVD, that when examined continuously, had sufficient power to detect a significant overall association between DCP and incident CVD. Further research to understand the role of VKDPs in cardiovascular disease is warranted, including a better understanding of the factors that affect post translational carboxylation of VKDPs, the association between hepatic, platelet, and vascular smooth muscle cell VKDPs, and potential mechanisms of ischemia.
In summary, our analysis suggests that VKDP activity is associated with incident ischemic cardiovascular disease. Our results raise the possibility that dietary or pharmacological improvement of VKDP activity can reduce the incidence of CVD.
Supplementary Material
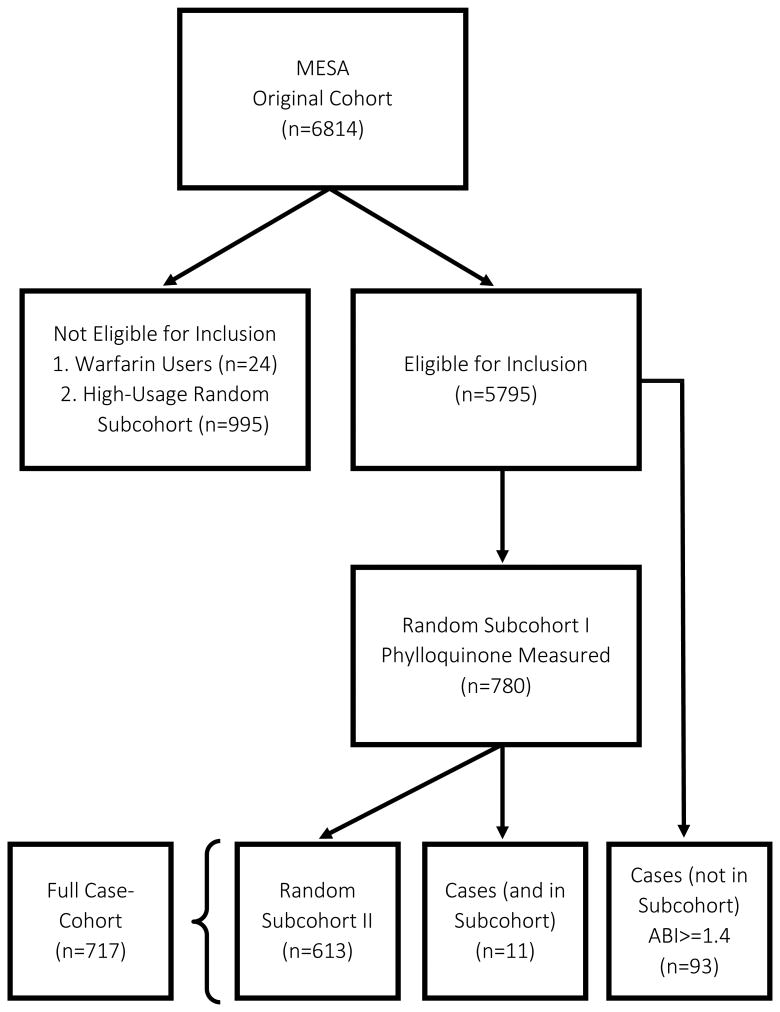
Figure 1. Participant selection for the MESA weighted cohort design.
Significance.
-
-
Vitamin K dependent protein activity, as measured by circulating Des-gamma-carboxy Prothrombin (DCP) concentrations, is associated with incident ischemic cardiovascular disease.
Acknowledgments
Sources of Funding: This research was supported by a Normon S. Coplon grant from Satellite Health Care (JD) and contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Abbreviations
- VKDP
Vitamin K dependent protein
- MESA
Multi Ethnic Study of Atherosclerosis
- DCP
Des-gamma-carboxy prothrombin
- MGP
Matrix Gla protein
- Gas-6
Growth Arrest Specific factor 6
- LDL
Low density lipoprotein
- HDL
High density lipoprotein
Footnotes
All authors have contributed to this manuscript and have reviewed and agreed to its content.
Disclosures: None of the authors have any disclosures to report.
References
- 1.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix gla protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 2.Schurgers LJ, Spronk HM, Skepper JN, Hackeng TM, Shanahan CM, Vermeer C, Weissberg PL, Proudfoot D. Post-translational modifications regulate matrix gla protein function: Importance for inhibition of vascular smooth muscle cell calcification. Journal of thrombosis and haemostasis : JTH. 2007;5:2503–2511. doi: 10.1111/j.1538-7836.2007.02758.x. [DOI] [PubMed] [Google Scholar]
- 3.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 4.Coutu DL, Wu JH, Monette A, Rivard GE, Blostein MD, Galipeau J. Periostin, a member of a novel family of vitamin k-dependent proteins, is expressed by mesenchymal stromal cells. The Journal of biological chemistry. 2008;283:17991–18001. doi: 10.1074/jbc.M708029200. [DOI] [PubMed] [Google Scholar]
- 5.Stansfield WE, Andersen NM, Tang RH, Selzman CH. Periostin is a novel factor in cardiac remodeling after experimental and clinical unloading of the failing heart. The Annals of thoracic surgery. 2009;88:1916–1921. doi: 10.1016/j.athoracsur.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, Yozu R, Shukunami C, Hiraki Y, Kudo A, Ogawa S, Fukuda K. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and mmp production in humans and rodents. The Journal of clinical investigation. 2010;120:2292–2306. doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shea MK, Holden RM. Vitamin k status and vascular calcification: Evidence from observational and clinical studies. Advances in nutrition. 2012;3:158–165. doi: 10.3945/an.111.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:1400–1407. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 9.Price PA, Faus SA, Williamson MK. Warfarin-induced artery calcification is accelerated by growth and vitamin d. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:317–327. doi: 10.1161/01.atv.20.2.317. [DOI] [PubMed] [Google Scholar]
- 10.Jouni H, Rodeheffer RJ, Kullo IJ. Increased serum n-terminal pro-b-type natriuretic peptide levels in patients with medial arterial calcification and poorly compressible leg arteries. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:197–202. doi: 10.1161/ATVBAHA.110.216770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with niddm. Diabetes care. 1994;17:1252–1256. doi: 10.2337/diacare.17.11.1252. [DOI] [PubMed] [Google Scholar]
- 12.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 13.Schurgers LJ, Uitto J, Reutelingsperger CP. Vitamin k-dependent carboxylation of matrix gla-protein: A crucial switch to control ectopic mineralization. Trends in molecular medicine. 2013;19:217–226. doi: 10.1016/j.molmed.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: A preliminary report. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:568–575. doi: 10.2215/CJN.07081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rennenberg RJ, de Leeuw PW, Kessels AG, Schurgers LJ, Vermeer C, van Engelshoven JM, Kemerink GJ, Kroon AA. Calcium scores and matrix gla protein levels: Association with vitamin k status. European journal of clinical investigation. 2010;40:344–349. doi: 10.1111/j.1365-2362.2010.02275.x. [DOI] [PubMed] [Google Scholar]
- 16.Dalmeijer GW, van der Schouw YT, Vermeer C, Magdeleyns EJ, Schurgers LJ, Beulens JW. Circulating matrix gla protein is associated with coronary artery calcification and vitamin k status in healthy women. The Journal of nutritional biochemistry. 2013;24:624–628. doi: 10.1016/j.jnutbio.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Delanaye P, Krzesinski JM, Warling X, Moonen M, Smelten N, Medart L, Pottel H, Cavalier E. Dephosphorylated-uncarboxylated matrix gla protein concentration is predictive of vitamin k status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC nephrology. 2014;15:145. doi: 10.1186/1471-2369-15-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boxma PY, van den Berg E, Geleijnse JM, Laverman GD, Schurgers LJ, Vermeer C, Kema IP, Muskiet FA, Navis G, Bakker SJ, de Borst MH. Vitamin k intake and plasma desphospho-uncarboxylated matrix gla-protein levels in kidney transplant recipients. PloS one. 2012;7:e47991. doi: 10.1371/journal.pone.0047991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schurgers LJ, Spronk HM, Soute BA, Schiffers PM, DeMey JG, Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin k in rats. Blood. 2007;109:2823–2831. doi: 10.1182/blood-2006-07-035345. [DOI] [PubMed] [Google Scholar]
- 20.Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL. Vitamin k supplementation and progression of coronary artery calcium in older men and women. The American journal of clinical nutrition. 2009;89:1799–1807. doi: 10.3945/ajcn.2008.27338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braam LA, Hoeks AP, Brouns F, Hamulyak K, Gerichhausen MJ, Vermeer C. Beneficial effects of vitamins d and k on the elastic properties of the vessel wall in postmenopausal women: A follow-up study. Thrombosis and haemostasis. 2004;91:373–380. doi: 10.1160/TH03-07-0423. [DOI] [PubMed] [Google Scholar]
- 22.Maas AH, van der Schouw YT, Beijerinck D, Deurenberg JJ, Mali WP, Grobbee DE, van der Graaf Y. Vitamin k intake and calcifications in breast arteries. Maturitas. 2007;56:273–279. doi: 10.1016/j.maturitas.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Villines TC, Hatzigeorgiou C, Feuerstein IM, O'Malley PG, Taylor AJ. Vitamin k1 intake and coronary calcification. Coronary artery disease. 2005;16:199–203. doi: 10.1097/00019501-200505000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Krueger T, Schlieper G, Schurgers L, et al. Vitamin k1 to slow vascular calcification in haemodialysis patients (vitavask trial): A rationale and study protocol. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29:1633–1638. doi: 10.1093/ndt/gft459. [DOI] [PubMed] [Google Scholar]
- 25.Stenflo J. Contributions of gla and egf-like domains to the function of vitamin k-dependent coagulation factors. Critical reviews in eukaryotic gene expression. 1999;9:59–88. [PubMed] [Google Scholar]
- 26.Nakano T, Kawamoto K, Higashino K, Arita H. Prevention of growth arrest-induced cell death of vascular smooth muscle cells by a product of growth arrest-specific gene, gas6. FEBS letters. 1996;387:78–80. doi: 10.1016/0014-5793(96)00395-x. [DOI] [PubMed] [Google Scholar]
- 27.Fridell YW, Villa J, Jr, Attar EC, Liu ET. Gas6 induces axl-mediated chemotaxis of vascular smooth muscle cells. The Journal of biological chemistry. 1998;273:7123–7126. doi: 10.1074/jbc.273.12.7123. [DOI] [PubMed] [Google Scholar]
- 28.Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of ps liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. Journal of biochemistry. 2000;127:411–417. doi: 10.1093/oxfordjournals.jbchem.a022622. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Gerbod-Giannone MC, Seitz H, Cui D, Thorp E, Tall AR, Matsushima GK, Tabas I. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the mer receptor. The Journal of biological chemistry. 2006;281:6707–6717. doi: 10.1074/jbc.M510579200. [DOI] [PubMed] [Google Scholar]
- 30.Avanzi GC, Gallicchio M, Bottarel F, et al. Gas6 inhibits granulocyte adhesion to endothelial cells. Blood. 1998;91:2334–2340. [PubMed] [Google Scholar]
- 31.Clauser S, Meilhac O, Bieche I, Raynal P, Bruneval P, Michel JB, Borgel D. Increased secretion of gas6 by smooth muscle cells in human atherosclerotic carotid plaques. Thrombosis and haemostasis. 2012;107:140–149. doi: 10.1160/TH11-05-0368. [DOI] [PubMed] [Google Scholar]
- 32.Tjwa M, Bellido-Martin L, Lin Y, et al. Gas6 promotes inflammation by enhancing interactions between endothelial cells, platelets, and leukocytes. Blood. 2008;111:4096–4105. doi: 10.1182/blood-2007-05-089565. [DOI] [PubMed] [Google Scholar]
- 33.Angelillo-Scherrer A, de Frutos P, Aparicio C, Melis E, Savi P, Lupu F, Arnout J, Dewerchin M, Hoylaerts M, Herbert J, Collen D, Dahlback B, Carmeliet P. Deficiency or inhibition of gas6 causes platelet dysfunction and protects mice against thrombosis. Nature medicine. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 34.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for axl, sky, and mer receptor tyrosine kinases. The Journal of biological chemistry. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 35.Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mechanisms of development. 2001;103:183–188. doi: 10.1016/s0925-4773(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 36.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circulation research. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pohjolainen V, Rysa J, Napankangas J, Koobi P, Eraranta A, Ilves M, Serpi R, Porsti I, Ruskoaho H. Left ventricular periostin gene expression is associated with fibrogenesis in experimental renal insufficiency. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27:115–122. doi: 10.1093/ndt/gfr279. [DOI] [PubMed] [Google Scholar]
- 38.Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698–3703. [PubMed] [Google Scholar]
- 39.Smiles AM, Jenny NS, Tang Z, Arnold A, Cushman M, Tracy RP. No association of plasma prothrombin concentration or the g20210a mutation with incident cardiovascular disease: Results from the cardiovascular health study. Thrombosis and haemostasis. 2002;87:614–621. [PubMed] [Google Scholar]
- 40.Ohsaki Y, Shirakawa H, Miura A, Giriwono PE, Sato S, Ohashi A, Iribe M, Goto T, Komai M. Vitamin k suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor kappab through the repression of ikkalpha/beta phosphorylation. The Journal of nutritional biochemistry. 2010;21:1120–1126. doi: 10.1016/j.jnutbio.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Ohsaki Y, Shirakawa H, Hiwatashi K, Furukawa Y, Mizutani T, Komai M. Vitamin k suppresses lipopolysaccharide-induced inflammation in the rat. Bioscience, biotechnology, and biochemistry. 2006;70:926–932. doi: 10.1271/bbb.70.926. [DOI] [PubMed] [Google Scholar]
- 42.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The rotterdam study. The Journal of nutrition. 2004;134:3100–3105. doi: 10.1093/jn/134.11.3100. [DOI] [PubMed] [Google Scholar]
- 43.Kaesler N, Magdeleyns E, Herfs M, Schettgen T, Brandenburg V, Fliser D, Vermeer C, Floege J, Schlieper G, Kruger T. Impaired vitamin k recycling in uremia is rescued by vitamin k supplementation. Kidney international. 2014;86:286–293. doi: 10.1038/ki.2013.530. [DOI] [PubMed] [Google Scholar]
- 44.Doyon M, Mathieu P, Moreau P. Decreased expression of gamma-carboxylase in diabetes-associated arterial stiffness: Impact on matrix gla protein. Cardiovascular research. 2013;97:331–338. doi: 10.1093/cvr/cvs325. [DOI] [PubMed] [Google Scholar]
- 45.Holden RM, Morton AR, Garland JS, Pavlov A, Day AG, Booth SL. Vitamins k and d status in stages 3-5 chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:590–597. doi: 10.2215/CJN.06420909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.