Abstract
Oxidative stress (OS) plays an important role in the pathogenesis of a variety of autoimmune diseases (ADs) and many environmental agents participate in this process. Environmental agents, including trichloroethylene (TCE), silica, pristane, mercury, and smoke, are known to induce an autoimmune response, potentially through OS-mediated mechanisms. Here, we focus on unraveling the targets and signaling pathways that have been mechanistically linked with OS, as a result of exposure to these and numerous other environmental agents, and their impact on the immune system in triggering ADs. Antioxidants and molecular targets impeding autoimmunity by targeting specific signaling pathways are also reviewed. The review not only provides an overview of the current knowledge and evidence showing strong associations between environmental exposures, OS, and ADs, but also plausible mechanisms by which OS causes autoimmunity/ADs. We also discuss areas that require additional approaches, such as unraveling specific events/mechanisms leading to such devastating diseases and measures to prevent or attenuate such diseases.
Keywords: Environmental agents, oxidative stress, autoimmunity, trichloroethylene, antioxidants
I. Introduction
Immunotoxicology is generally defined as the study of adverse effects on the immune system resulting from exposure to environmental, occupational, and/or therapeutic agents. Broadly, the effects of these agents could either be immunosuppression or immunostimulation. While a number of environmental/occupational agents are known to cause immunosuppression and have been rather well-studied, the immunostimulatory effects of other agents are relatively less explored despite getting increased attention in recent years, particularly their effects leading to autoimmunity and autoimmune diseases (ADs) and the mechanisms responsible in the disease pathogenesis. Identifying specific events that trigger loss of tolerance leading to autoimmunity is a major challenge and presents as an area of investigation requiring tremendous attention. Mechanisms whereby environmental exposures may contribute to pathogenesis of ADs include epigenetic modifications, systemic inflammation, inflammatory cytokines, and increased oxidative stress (OS). Interestingly, increased OS is associated with several ADs and many environmental agents are known to cause OS (1–6). However, how environmentally-induced OS (due to increased formation of reactive oxygen and nitrogen species) influences the immune system to trigger flares of such ADs is an area which has been gaining momentum in recent years. In this review, we mainly focus on recent research advances with respect to the role of OS in the pathogenesis of various ADs, and the role of environmental/occupational toxicant-induced OS in the initiation and/or progression of ADs.
II. Oxidative stress and autoimmune diseases
ADs such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and scleroderma are chronic and life-threatening disorders. In recent years, increasing evidence has accumulated to suggest that free radical-mediated reactions could play a potential role in the pathogenesis of ADs (1,6,7). Reactive oxygen species (ROS) have the potential to initiate cellular damage to proteins, lipids, and DNA (8–10). In fact, a variety of ROS-mediated modifications of proteins have been reported in ADs (1,7,11,12). Increased protein carbonyls and recognition of ROS-modified human serum albumin by circulating SLE autoantibodies in SLE patients were observed (1,13). Higher levels of anti-oxidized-catalase antibodies are reported in SLE patients, and show strong relation with the SLE disease activity and progression (3,14), suggesting oxidized protein may be a useful biomarker in evaluating the progression of SLE and in elucidating the mechanisms of disease pathogenesis.
Another consequence of OS is increased lipid peroxidation, which promotes the formation of highly reactive lipid peroxidation-derived aldehydes (LPDAs) such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE). These aldehydes can covalently bind to proteins resulting in structural modifications that may elicit an autoimmune response and contribute to disease pathogenesis (1,15,16). Indeed, higher levels of MDA- and HNE-modified proteins have been observed in AD patients (1,5,17–19). Furthermore, higher levels of anti-MDA/anti-HNE protein adduct antibodies were also observed in SLE patients and their levels correlated with SLE disease activity (1). Interestingly, age-related increases in the formation of MDA-/HNE-protein adducts, their corresponding antibodies, and MDA-/HNE-specific immune complexes were also observed in MRL/lpr mice, a widely used animal model for SLE (20). Furthermore, HNE-mouse serum albumin adducts mimic nuclear antigens and cause significant inhibition in ANA binding to nuclear antigens, suggesting that LPDA-modified proteins could be an important source of autoantibodies and CICs in these mice, and thus contribute to AD pathogenesis (20).
Several other OS-responsive mechanisms are also associated with ADs. OS can induce an inflammatory response/AD via activation of enzyme poly (ADP-ribose) polymerase-1 (PARP-1). PARP-1 can module Th17 and Treg cells to cause an imbalance between pro-and anti-inflammatory responses (21). Lack of nuclear factor E2-related factor 2 (Nrf2), which is a major regulator of the antioxidant response, is associated with SLE-like AD (22–25). Also, Nrf2 polymorphism is linked with autoimmune nephritis in SLE patients (26). In T cells from SLE patients and animal models of the disease, glutathione, the main intracellular antioxidant, is depleted and serine/threonine-protein kinase mTOR undergoes redox-dependent activation. Blocking mTOR activation in T cells could, therefore, represent an approach for immunosuppression (6). Furthermore, depletion of glutathione could lead to a pathogenic response and its reversal by NAC may be beneficial, as evident from studies in mouse models and patients with SLE (6). The role of ROS-mediated inflammasome activation in autoimmunity is yet another pathway which needs to be explored (27).
Like ROS, reactive nitrogen species (RNS) could also play a significant role in the pathogenesis of ADs. The potential of ·NO, generated by inducible nitric oxide synthase (iNOS), in disease pathogenesis lies largely in the extent of its production and generation of O2·−, leading to formation of peroxynitrite (ONOO−). ONOO− is a potent nitrating and oxidizing agent which can react with tyrosine residues to form nitrotyrosine (NT; 28–30). In addition, ONOO−-mediated modifications of endogenous proteins and DNA may enhance their immunogenicity, leading to a break in immune tolerance (28,31,32). Accumulating evidence in murine lupus shows increasing iNOS activity with the development and progression of ADs, and studies using competitive inhibitors suggest that iNOS could play a pathogenic role in murine ADs (29,30,33–35). Also, the elevated presence of nitrated proteins has been found in many diseases, including ADs (7,11,32,36). Data from human studies also suggest that overexpression of iNOS and increased production of ONOO− may contribute to glomerular and vascular pathology, as well as the pathogenesis of many other ADs (7,37,38).
III. Environmental agents, oxidative stress, and autoimmunity
The etiology and pathogenesis of ADs are highly complex processes. Both genetic predisposition and environmental factors such as chemicals, smoke, infection, and nutrition are implicated in the pathogenic process (39). Environmental factors have gained much attention in recent years for their role in triggering autoimmunity, with increasing evidence of their influence being apparent from epidemiological and animal studies (39). These factors, including cigarette smoking, crystalline silica, and exposures to trichloroethylene (TCE), perchloroethylene, mercury, pesticides, and pristane are implicated in increasing the risk for ADs (4,40–42). Such factors are considered serious threats in the etiology and progression of ADs such as SLE, rheumatic arthritis, systemic sclerosis, and autoimmune hepatitis. Since increased OS is associated with several ADs and many environmental agents are known to cause OS, deciphering the intricate relationship between OS and ADs could be critical in elucidating key pathogenic mechanisms that could lead to novel interventions for the clinical management of ADs. Our focus, therefore, is to evaluate the impact of environmentally-induced OS in the pathogenesis of ADs, especially SLE because it is a relatively more extensively studied AD. Although numerous agents likely participate in the pathogenesis of ADs, below we present the more prominent ones known to cause OS and participate in the induction/exacerbation/progression of ADs:
Trichloroethylene (TCE)
TCE is a widely used organic solvent which has been implicated in the development of various ADs, such as SLE, systemic sclerosis, fasciitis, and autoimmune hepatitis both from occupational and environmental exposures (26,43–46). Khan et al. (1995) were first to propose and use MRL+/+ mice as an animal model to provide direct evidence of an association between TCE exposure and autoimmunity (47). Khan et al. (2001) were also first to propose the role of OS in TCE-mediated autoimmunity based on the novel observation of anti-MDA antibodies in MRL +/+ mice exposed to TCE (48). Since then, a series of studies have further strengthened the contribution of OS in TCE-mediated autoimmunity (2,34,49–51). TCE-induced OS leads to a variety of reactive oxygen and nitrogen species (RONS)-mediated structural modifications of the endogenous proteins, such as increased formation of MDA- and HNA-protein adducts and carbonylation/nitration of proteins, which could potentially lead to generation of neoantigens. After antigen processing, these neoantigens could elicit an autoimmune response by stimulating T and/or B lymphocytes, especially Th1 and Th17 cells (50,51). Stimulation of splenic lymphocytes from TCE-treated MRL +/+ mice with MDA-adducted mouse serum albumin (MDA-MSA) or HNE-MSA resulted in significant proliferation of CD4+ T cells (50). Furthermore, splenocytes from TCE-treated mice secreted higher levels of IL-17 and IL-21 after stimulation with MDA-MSA or HNE-MSA adducts (51). These studies provide evidence that MDA- and or HNE-modified proteins contribute to TCE-mediated autoimmunity via activation of Th1, Th17 cells (50,51).
The contribution of protein oxidation (carbonylation and nitration) in the induction of TCE-induced autoimmunity has also been explored (2,34,52). The modification of proteins may alter immunogenicity of self-antigens (converting them to neoantigens), and may lead to an autoimmune response by stimulating T cells (especially activation of Th1 cells; 34). TCE treatment in iNOS-null female MRL+/+ mice still resulted in increased serum ANA and anti-dsDNA, but the increases were less pronounced compared to that in TCE-treated MRL+/+ mice (35), suggesting a role for nitrosative stress. These results support an association between protein oxidation and induction/exacerbation of autoimmune responses, and present a potential mechanism by which oxidatively modified proteins could contribute to TCE-induced autoimmune response (2,34,35).
Silica
Among the environmental factors that contribute to the onset of such ADs as scleroderma, rheumatoid arthritis, and SLE, silica exposure is important due to its widespread exposure (53). Lupus-prone female NZBWF1 mice develop SLE-like disease when exposed to crystalline silica (54). Furthermore, silica exposure resulted in increased ANA formation in non-autoimmune mice and rats (41,55,56). The mechanisms of silica-mediated autoimmunity are not clearly known yet. However, interaction of macrophages with silica and asbestos causes increased ROS production. Silica can also induce transcription of pro-inflammatory cytokines, stimulate T cell responses, decrease Treg cells, increase OS, and induce apoptosis (40). All of these parameters could potentially contribute to an autoimmune response.
Smoke
Smoke can play a pathogenic role in certain ADs as it may trigger development of autoantibodies and alter pathogenic mechanisms linked to an imbalance of the immune system. Smoke, by provoking OS, may contribute to SLE by dysregulating DNA methylation and upregulating immune genes, thereby leading to autoreactivity. Further support for the role of smoking in ADs is evident from studies demonstrating a higher risk for developing SLE in current smokers compared with non-smokers and ex-smokers (57–59).
Pristane
Pristane is a mineral oil component which has been associated with rheumatoid arthritis and SLE (60). Exposure to pristane in susceptible mouse strains causes SLE-like disease that is characterized by increased ANA and immune complex-mediated glomerulonephritis (60). In Balb/c mice, pristane exposure led to increased ROS formation (61). In another study, pristane administration in C57BL/6J mice was shown to induce macrophage activation, OS (increased lipid peroxidation and reduced glutathione levels), and skewed Th1/Th2 response, which was attenuated by chloroquine (62). These studies provide support for a potential role of OS in pristane-mediated autoimmunity. However, more detailed evaluations are needed to firmly establish the role of OS in pristane-mediated ADs.
Mercury (Hg)
Hg exposure is associated with high levels of ANA and SLE (63,64), but the severity of the disease induced by Hg exposure appears mild compared to that of idiopathic SLE (65). Hg induces OS by depleting thiol-containing and other cellular antioxidants (66). In human T lymphocytes, both methylmercury and inorganic Hg induce alterations in mitochondrial function and glutathione (GSH) depletion (67), resulting in ROS generation and activation of apoptotic signaling pathways (68).
IV. Antioxidants impede AD activity
As the role of environmental factors and OS in the pathogenesis of human ADs has become more established, there has also been a surge to develop preventive and therapeutic measures. Among them, N-acetylcysteine (NAC) has found prominence in both human and experimental studies. NAC reduces SLE activity by blocking the mTOR pathway in T cells from SLE patients. NAC also reverses expansion of CD4−CD8− T cells, stimulates Foxp3 expression in CD4+CD25+ T cells, and reduces anti-DNA antibody production (52,69,70). Resveratrol is another powerful antioxidant, which provided protection against pristane-induced lupus (71,72). Similarly, antroquinonol, with antioxidant activity, is known to prevent the transformation of mild lupus nephritis into higher-grade nephritis in a murine lupus model (73). It inhibits production of RONS and enhances Treg suppression via increasing activation of Nrf2, a master regulator of the antioxidant response (73). Another antioxidant, epigallactocatechin-3-gallate, which is a major bioactive polyphenol present in green tea with free radical scavenging activity, prevents lupus nephritis development in mice (74) by activating Nrf2 signaling, decreasing NLRP3 inflammasome activation, and increasing systemic Treg cell activity (74). Vitamin E, quercetin, and curcumin have also been utilized for their anti-inflammatory role and amelioration of autoimmune responses (75). The data from different antioxidants are promising therapeutic options, but the efficacy of specific agents needs further evaluation.
V. Conclusions and future directions
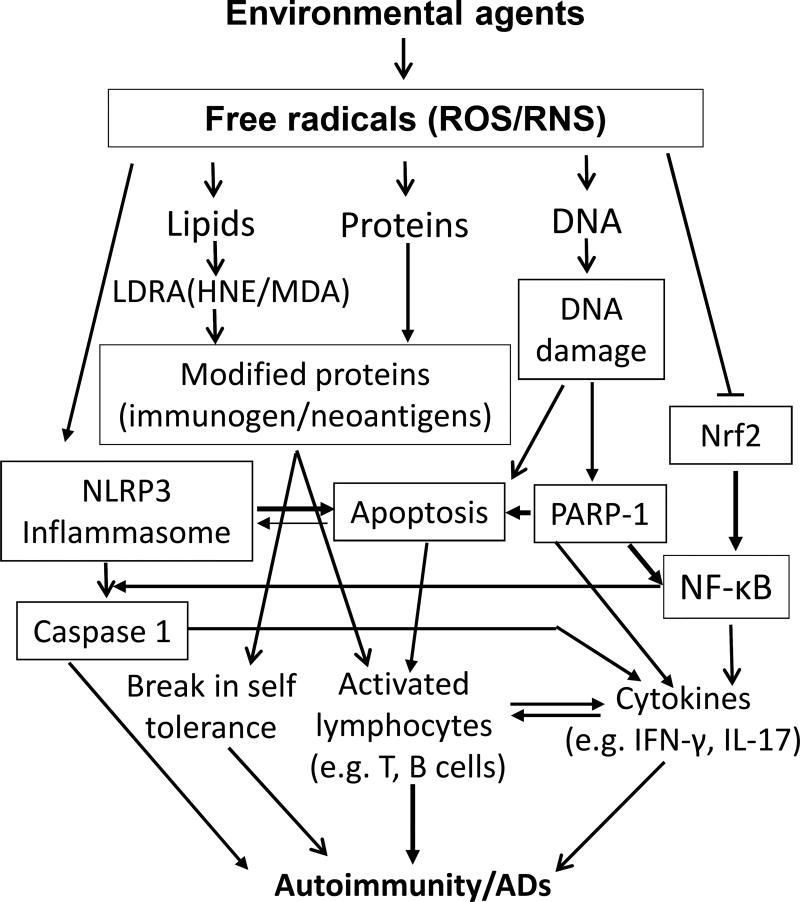
Oxidative stress plays an important role in the pathogenesis of ADs and many environmental agents participate in this process. Agents, including TCE, silica, pristane, mercury, and smoke, are known to induce an autoimmune response, potentially through OS-mediated mechanisms (Fig. 1). Unraveling the impact of OS as a result of exposure to these and numerous other agents on the immune system in triggering flares of such ADs is an area which deserves more attention. New detailed studies to unravel the distinct pathways by which OS contributes to autoimmunity, especially the redox proteome, blocking/inhibiting OS-specific signaling pathways, knocking out/down target genes, and exploring epigenetic involvement, will also reveal critical mechanisms in OS-induced autoimmunity.
Fig.1.
Plausible mechanisms of oxidative stress (OS)-induced autoimmunity/autoimmune diseases (ADs). Environmental agents (EAs) induce excessive free radicals (ROS/RNS) that can potentially cause damage to molecules including lipids, proteins and DNA, resulting in lipid-derived reactive aldehydes (MAD/HNE) or ROS/RNS modified proteins and oxidative DNA damage. The modified proteins serve as neoantigens which can activate lymphocytes and reduce self-tolerance leading to autoimmunity. Oxidative DNA damage can lead to ADs by directly or indirectly (PARP-1 activation) inducing apoptosis. EA-mediated OS can also contribute to the development of ADs through activation of NLRP3 inflammasome, NF-κB, caspase-1, and IL-1β signaling pathways.
Highlights.
There is a clear link between oxidative stress and autoimmune diseases.
Many environmental agents lead to autoimmune diseases by inducing oxidative stress.
Oxidative stress markers such as MDA-/HNE-adducts and oxidized/nitrated proteins are correlated with SLE disease activity.
TCE-mediated autoimmunity is attenuated by both an antioxidant (NAC) and iNOS gene knockout, further supporting the role of oxidative stress.
Antioxidants can attenuate SLE disease activity by down regulating NLRP3 inflammasome activation and activating Nrf2 signaling.
Acknowledgments
This work was supported by Grant ES016302 from the National Institute of Environmental Health Sciences (NIEHS), National Institute of Health (NIH), and it contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Wang G, Pierangeli SS, Papalardo E, Ansari GA, Khan MF. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity. Arthritis Rheum. 2010;62:2064–2072. doi: 10.1002/art.27442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang G, Cai P, Ansari GA, Khan MF. Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response. Toxicology. 2007;229:186–193. doi: 10.1016/j.tox.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Shobaili HA, Robaee AA, Alzolibani AA, Rasheed Z. Immunological studies of reactive oxygen species damaged catalase in patients with systemic lupus erythematosus: correlation with disease activity index. Immunol Invest. 2013;42:191–203. doi: 10.3109/08820139.2012.751396. [DOI] [PubMed] [Google Scholar]
- 4.Cooper GS, Makris SL, Nietert PJ, Jinot J. Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Perspect. 2009;117:696–702. doi: 10.1289/ehp.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frostegard J, Svenungsson E, Wu R, Gunnarsson I, Lundberg IE, Klareskog L, Horkko S, Witztum JL. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum. 2005;52:192–200. doi: 10.1002/art.20780. [DOI] [PubMed] [Google Scholar]
- 6.Perl A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat Rev Rheumatol. 2013;9:674–686. doi: 10.1038/nrrheum.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan PE, Sturgess AD, Davies MJ. Increased levels of serum protein oxidation and correlation with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2005;52:2069–2079. doi: 10.1002/art.21130. [DOI] [PubMed] [Google Scholar]
- 8.Biemond P, Swaak AJ, Koster JF. Protective factors against oxygen free radicals and hydrogen peroxide in rheumatoid arthritis synovial fluid. Arthritis Rheum. 1984;27:760–765. doi: 10.1002/art.1780270706. [DOI] [PubMed] [Google Scholar]
- 9.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliwell B, Gutteridge JM. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984;1:1396–1397. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 11.Oates JC, Christensen EF, Reilly CM, Self SE, Gilkeson GS. Prospective measure of serum 3-nitrotyrosine levels in systemic lupus erythematosus: correlation with disease activity. Proc Assoc Am Physicians. 1999;111:611–621. doi: 10.1046/j.1525-1381.1999.99110.x. [DOI] [PubMed] [Google Scholar]
- 12.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 13.Kurien BT, Scofield RH. Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev. 2008;7:567–573. doi: 10.1016/j.autrev.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Shobaili HA, Rasheed Z. Immunological studies of oxidized superoxide dismutase in patients with systemic lupus erythematosus. Correlation with disease induction and progression. Saudi Med J. 2012;33:1177–1184. [PubMed] [Google Scholar]
- 15.Khan MF, Kaphalia BS, Ansari GAS. Time-dependent autoimmune response of dichloroacetyl chloride in female MRL +/+ mice. Immunopharmacol Immunotoxicol. 1997;19:265–277. doi: 10.3109/08923979709007662. [DOI] [PubMed] [Google Scholar]
- 16.Khan MF, Wu X, Boor PJ, Ansari GAS. Oxidative modification of lipids and proteins in aniline-induced splenic toxicity. Toxicol Sci. 1999;48:134–140. doi: 10.1093/toxsci/48.1.134. [DOI] [PubMed] [Google Scholar]
- 17.Ben Mansour R, Lassoued S, Elgaied A, Haddouk S, Marzouk S, Bahloul Z, Masmoudi H, Attia H, Aïfa MS, Fakhfakh F. Enhanced reactivity to malondialdehyde-modified proteins by systemic lupus erythematosus autoantibodies. Scand J Rheumatol. 2010;39:247–253. doi: 10.3109/03009740903362511. [DOI] [PubMed] [Google Scholar]
- 18.D'souza A, Kurien BT, Rodgers R, Shenoi J, Kurono S, Matsumoto H, Hensley K, Nath SK, Scofield RH. Detection of catalase as a major protein target of the lipid peroxidation product 4-HNE and the lack of its genetic association as a risk factor in SLE. BMC Med Genet. 2008;9:62–69. doi: 10.1186/1471-2350-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grune T, Michel P, Sitte N, Eggert W, Albrecht-Nebe H, Esterbauer H, Siems WG. Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Radic Biol Med. 1997;23:357–360. doi: 10.1016/s0891-5849(96)00586-2. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Li H, Khan MF. Differential oxidative modification of proteins in MRL+/+ and MRL/lpr mice: Increased formation of lipid peroxidation-derived aldehyde-protein adducts may contribute to accelerated onset of autoimmune response. Free Rad Res. 2012;46:1472–1481. doi: 10.3109/10715762.2012.727209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad SF, Zoheir KM, Bakheet SA, Ashour AE, Attia SM. Poly(ADP-ribose) polymerase-1 inhibitor modulates T regulatory and IL-17 cells in the prevention of adjuvant induced arthritis in mice model. Cytokine. 2014;68:76–85. doi: 10.1016/j.cyto.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Kamanli A, Naziroglu M, Aydilek N, Hacievliyagil C. Plasma lipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochem Funct. 2004;22:53–57. doi: 10.1002/cbf.1055. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Stein TD, Johnson JA. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics. 2004;18:261–272. doi: 10.1152/physiolgenomics.00209.2003. [DOI] [PubMed] [Google Scholar]
- 24.Ma Q, Battelli L, Hubbs A. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am J Pathol. 2006;168:1960–1774. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoh K, Itoh K, Enomoto A, Hirayama A, Yamaguchi N, Kobayashi M, Morito N, Koyama A, Yamamoto M, Takahashi S. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 26.Cordova EJ, Velazquez-Cruz R, Centeno F, Baca V, Orozco L. The Nrf2 gene variant, −653G/A, is associated with nephritis in childhood-onset systemic lupus erythematosus. Lupus. 2010;19:1237–1242. doi: 10.1177/0961203310367917. [DOI] [PubMed] [Google Scholar]
- 27.Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352. doi: 10.3389/fphys.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan MF, Wu X, Kaphalia BS, Boor PJ, Ansari GAS. Nitrotyrosine formation in splenic toxicity of aniline. Toxicology. 2003;194:95–102. doi: 10.1016/j.tox.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg JB, Granger DL, Pisetsky DS, Seldin MF, Misukonis MA, Mason SN, Pippen AM, Ruiz P, Wood ER, Gilkeson GS. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J Exp Med. 1994;179:651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci USA. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurien BT, Hensley K, Bachmann M, Scofield RH. Oxidatively modified autoantigens in autoimmune diseases. Free Radic Biol Med. 2006;41:549–556. doi: 10.1016/j.freeradbiomed.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Ohmori H, Kanayama N. Immunogenicity of an inflammation-associated product, tyrosine nitrated self-proteins. Autoimmun Rev. 2005;4:224–229. doi: 10.1016/j.autrev.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Karpuzoglu E, Ahmed SA. Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: implications for immunity, autoimmune diseases, and apoptosis. Nitric Oxide. 2006;15:177–186. doi: 10.1016/j.niox.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Wang G, Wang J, Ma H, Khan MF. Increased nitration and carbonylation of proteins in MRL+/+ mice exposed to trichloroethene: potential role of protein oxidation in autoimmunity. Toxicol Appl Pharmacol. 2009;237:188–195. doi: 10.1016/j.taap.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G, Wakamiya M, Wang J, Ansari GA, Khan MF. iNOS null MRL+/+ mice show attenuation of trichloroethene-mediated autoimmunity: contribution of reactive nitrogen species and lipid-derived reactive aldehydes. Free Radic Biol Med. 2015;89:770–776. doi: 10.1016/j.freeradbiomed.2015.10.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan F, Ali R. Antibodies against nitric oxide damaged poly L-tyrosine and 3-nitrotyrosine levels in systemic lupus erythematosus. J Biochem Mol Biol. 2006;39:189–96. doi: 10.5483/bmbrep.2006.39.2.189. [DOI] [PubMed] [Google Scholar]
- 37.Nagy G, Koncz A, Fernandez D, Perl A. Nitric oxide, mitochondrial hyperpolarization, and T cell activation. Free Radic Biol Med. 2007;42:1625–1631. doi: 10.1016/j.freeradbiomed.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wanchu A, Khullar M, Deodhar SD, Bambery P, Sud A. Nitric oxide synthesis is increased in patients with systemic lupus erythematosus. Rheumatol Int. 1998;18:41–43. doi: 10.1007/s002960050055. [DOI] [PubMed] [Google Scholar]
- 39.Long H, Yin H, Wang L, Gershwin ME, Lu Q. The critical role of epigenetics in systemic lupus erythematosus and autoimmunity. J Autoimmun. 2016;74:118–138. doi: 10.1016/j.jaut.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Barbhaiya M, Costenbader KH. Environmental exposures and the development of systemic lupus erythematosus. Curr Opin Rheumatol. 2016;28:497–505. doi: 10.1097/BOR.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollard KM, Hultman P, Kono DH. Toxicology of autoimmune diseases. Chem Res Toxicol. 2010;23:455–466. doi: 10.1021/tx9003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang G, Wang J, Ansari GAS, Khan MF. Autoimmune potential of perchloroethylene: Role of lipid-derived aldehydes. Toxicol Appl Pharmacol. 2017;333:76–83. doi: 10.1016/j.taap.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byers VS, Levin AS, Ozonoff DM, Baldwin RW. Association between clinical symptoms and lymphocyte abnormalities in a population with chronic domestic exposure to industrial solvent-contaminated domestic water supply and a high incidence of leukaemia. Cancer Immunol Immunother. 1988;27:77–81. doi: 10.1007/BF00205762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kilburn KH, Warshaw RH. Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ Res. 1992;57:1–9. doi: 10.1016/s0013-9351(05)80014-3. [DOI] [PubMed] [Google Scholar]
- 45.Kondraganti S, König R, Boor PJ, Khan S, Bhupendra S, Kaphalia BS, Khan MF, Ansari GAS. Mechanistic evaluation of trichloroethene-mediated autoimmune hepatitis-like disease in female MRL+/+ mice. The Open Toxicology Journal. 2012;5:1–10. [Google Scholar]
- 46.Lockey JE, Kelly CR, Cannon GW, Colby TV, Aldrich V, Livingston GK. Progressive systemic sclerosis associated with exposure to trichloroethylene. J Occup Med. 1987;29:493–496. [PubMed] [Google Scholar]
- 47.Khan MF, Kaphalia BS, Prabhakar BS, Kanz MF, Ansari GAS. Trichloroethene-induced autoimmune response in female MRL +/+ mice. Toxicol Appl Pharmacol. 1995;134:155–160. doi: 10.1006/taap.1995.1179. [DOI] [PubMed] [Google Scholar]
- 48.Khan MF, Wu X, Ansari GAS. Anti-malondialdehyde antibodies in MRL+/+ mice treated with trichloroethene and dichloroacetyl chloride: possible role of lipid peroxidation in autoimmunity. Toxicol Appl Pharmacol. 2001;170:88–92. doi: 10.1006/taap.2000.9086. [DOI] [PubMed] [Google Scholar]
- 49.Wang G, Ansari GA, Khan MF. Involvement of lipid peroxidation-derived aldehyde-protein adducts in autoimmunity mediated by trichloroethene. J Toxicol Environ Health A. 2007;70:1977–1985. doi: 10.1080/15287390701550888. [DOI] [PubMed] [Google Scholar]
- 50.Wang G, König R, Ansari GAS, Khan MF. Lipid peroxidation-derived aldehyde-protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4+ T cells. Free Radic Biol Med. 2008;44:1475–1482. doi: 10.1016/j.freeradbiomed.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang G, Wang J, Fan X, Ansari GA, Khan MF. Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: dose- and time-response studies in female MRL+/+ mice. Toxicology. 2012;292:113–122. doi: 10.1016/j.tox.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang G, Wang J, Ma H, Ansari GA, Khan MF. N-Acetylcysteine protects against trichloroethene-mediated autoimmunity by attenuating oxidative stress. Toxicol Appl Pharmacol. 2013;273:189–195. doi: 10.1016/j.taap.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farhat SC, Silva CA, Orione MA, Campos LM, Sallum AM, Braga AL. Air pollution in autoimmune rheumatic diseases: a review. Autoimmun Rev. 2011;11:14–21. doi: 10.1016/j.autrev.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Tsao BP, Wallace DJ. Genetics of systemic lupus erythematosus. Curr Opin Rheumatol. 1997;9:377–937. doi: 10.1097/00002281-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Baechler EC, Gregersen PK, Behrens TW. The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol. 2004;16:801–807. doi: 10.1016/j.coi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Satoh M, Weintraub JP, Yoshida H, Shaheen VM, Richards HB, Shaw M, Reeves WH. Fas and Fas ligand mutations inhibit autoantibody production in pristane-induced lupus. J Immunol. 2000;165:1036–1043. doi: 10.4049/jimmunol.165.2.1036. [DOI] [PubMed] [Google Scholar]
- 57.Ghaussy NO, Sibbitt WL, Jr, Qualls CR. Cigarette smoking, alcohol consumption, and the risk of systemic lupus erythematosus: a case-control study. J Rheumatol. 2001;28:2449–2453. [PubMed] [Google Scholar]
- 58.Hardy CJ, Palmer BP, Muir KR, Sutton AJ, Powell RJ. Smoking history, alcohol consumption, and systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 1998;57:451–455. doi: 10.1136/ard.57.8.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perricone C, Versini M, Ben-Ami D, Gertel S, Watad A, Segel MJ, Ceccarelli F, Conti F, Cantarini L, Bogdanos DP, Antonelli A, Amital H, Valesini G, Shoenfeld Y. Smoke and autoimmunity: The fire behind the disease. Automimmun Rev. 2016;15:354–374. doi: 10.1016/j.autrev.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Layland LE, Wulferink M, Dierkes S, Gleichmann E. Drug-induced autoantibody formation in mice: triggering by primed CD4+CD25− T cells, prevention by primed CD4+CD25+ T cells. Eur J Immunol. 2004;34:36–46. doi: 10.1002/eji.200324406. [DOI] [PubMed] [Google Scholar]
- 61.Minhas U, Das P, Bhatnagar A. Role of reactive intermediates in the immunopathogenesis of the pristane-induced Balb/c model of lupus. Lupus. 2011;20:1421–1425. doi: 10.1177/0961203311418791. [DOI] [PubMed] [Google Scholar]
- 62.Ouyang Q, Huang Z, Wang Z, Chen X, Ni J, Lin L. Effects of pristane alone or combined with chloroquine on macrophage activation, oxidative stress, and TH1/TH2 skewness. J Immunol Res. 2014;2014:613136. doi: 10.1155/2014/613136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Somers EC, Richardson BC. Environmental exposures, epigenetic changes and the risk of lupus. Lupus. 2014;23:568–576. doi: 10.1177/0961203313499419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil: a cross-sectional study. Environ Res. 2010;110:345–354. doi: 10.1016/j.envres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pollard KM, Kono DH. Requirements for innate immune pathways in environmentally induced autoimmunity. BMC Med. 2013;11:100. doi: 10.1186/1741-7015-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 67.Shenker BJ, Mayro JS, Rooney C, Vitale L, Shapiro IM. Immunotoxic effects of mercuric compounds on human lymphocytes and monocytes. IV. Alterations in cellular glutathione content. Immunopharmacol Immunotoxicol. 1993;15:273–290. doi: 10.3109/08923979309025999. [DOI] [PubMed] [Google Scholar]
- 68.Guo TL, Miller MA, Shapiro IM, Shenker BJ. Mercuric chloride induces apoptosis in human T lymphocytes: evidence of mitochondrial dysfunction. Toxicol Appl Pharmacol. 1998;153:250–257. doi: 10.1006/taap.1998.8549. [DOI] [PubMed] [Google Scholar]
- 69.Lai ZW, Hanczko R, Bonilla E, Phillips PE, Perl A. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatism. 2012;64:2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang J, Yang X, Zou H, Li M. Oxidative Stress and Treg and Th17 Dysfunction in Systemic Lupus Erythematosus. Oxid Med Cell Longev. 2016;2016:9. doi: 10.1155/2016/2526174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh N, Singh U, Nagarkatti P, Nagarkatti M. Resveratrol ameliorates lupus by epigenetic modulation of Foxp3 and IL-17 genes resulting in differential regulation of regulatory T cells and Th17 cells via activation of aryl hydrocarbon receptor (P5129) J Immunol. 2013;190(1 Supp):137–142. [Google Scholar]
- 72.Wang ZL, Luo XF, Li MT, Xu D, Zhou S, Chen HZ, Gao N, Chen Z, Zhang LL, Zeng XF. Resveratrol possesses protective effects in a pristane-induced lupus mouse model. PLoS One. 2014;9:e114792. doi: 10.1371/journal.pone.0114792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai PY, Ka SM, Chang JM. Antroquinonol differentially modulates T cell activity and reduces interleukin-18 production, but enhances Nrf2 activation, in murine accelerated severe lupus nephritis. Arthritis Rheum. 2012;64:232–242. doi: 10.1002/art.33328. [DOI] [PubMed] [Google Scholar]
- 74.Tsai PY, Ka SM, Chang JM, Chen HC, Shui HA, Li CY, Hua KF, Chang WL, Huang JJ, Yang SS, Chen A. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic Biol Med. 2011;51:744–754. doi: 10.1016/j.freeradbiomed.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 75.Ortona E, Maselli A, Delunardo F, Colasanti T, Giovannetti A, Pierdominici M. Relationship between redox status and cell fate in immunity and autoimmunity. Antioxid Redox Signal. 2014;21:103–122. doi: 10.1089/ars.2013.5752. [DOI] [PubMed] [Google Scholar]