Abstract
High-frequency oscillations (HFOs: 100 – 600 Hz) have been widely proposed as biomarkers of epileptic brain tissue. In addition, HFOs over a broader range of frequencies spanning 30 – 2000 Hz are potential biomarkers of both physiological and pathological brain processes. The majority of the results from humans with focal epilepsy have focused on HFOs recorded directly from the brain with intracranial EEG (iEEG) in the high gamma (65 – 100 Hz), ripple (100 – 250 Hz), and fast ripple (250 – 600 Hz) frequency ranges. These results are supplemented by reports of HFOs recorded with iEEG in the low gamma (30 – 65Hz) and very high frequency (500 – 2000 Hz) ranges. Visual detection of HFOs is laborious and limited by poor inter-rater agreement; and the need for accurate, reproducible automated HFOs detection is well recognized. In particular, the clinical translation of HFOs as a biomarker of the epileptogenic brain has been limited by the ability to reliably detect and accurately classify HFOs as physiological or pathological. Despite these challenges, there has been significant progress in the field, which is the subject of this review. Furthermore, we provide data and corresponding analytic code in an effort to promote reproducible research and accelerate clinical translation.
Introduction
High frequency oscillations (HFOs: 30 – 600 Hz) are local field potentials recorded during intracranial electroencephalography (iEEG) and reflect increased neuronal and synaptic synchrony [1]. HFOs have been shown to be associated with both normal and pathologic brain activity in animals and humans [1–3]. Ripple (100 – 250 Hz) [4] and Fast Ripple (250 – 600 Hz) [5] HFOs have been widely proposed as an electrophysiological biomarker of epileptogenic brain tissue, reviewed in [6,7]. HFOs in the gamma frequency range (HFOs: 30 – 100 Hz), however, are believed to play a fundamental physiological role in binding of visual perception [8], information processing [1,9], memory [10], and motor and language functions [10]. Gamma HFOs have also been shown to be increased in the human epileptic brain [11–13] and may also serve as a biomarker. Furthermore, very high frequency oscillations (VHFOs; 600–2,000Hz) were recently described in mesiotemporal structures of patients with temporal lobe epilepsy [14]. The VHFOs in the 1000 – 2000 Hz were more spatially restricted in the brain than lower-frequency HFOs, and better post-surgical outcomes were observed in patients with resection of tissue containing fast ripple HFOs and VHFOs.
While there has been significant progress in automated detection of HFOs, reliably distinguishing normal physiological oscillations from pathological epileptiform oscillations [2,11,15] remains a fundamental challenge in epileptology [16–18].
Originally fast ripple HFOs (250–600 Hz) seen in hippocampal EEG recordings from epileptic rats and patients with mesial temporal lobe epilepsy were described as uniquely pathological HFOs (pHFOs) and were considered to be a highly specific electrophysiological biomarker of epileptic tissue [19,20]. Subsequent human iEEG studies using both microelectrodes and clinical macroelectrodes showed that ripple [12] and gamma frequency HFOs [7, 8, 10, 12] were also increased in human epileptogenic brain tissue. Given the established physiological role of gamma and ripple frequency HFOs, the challenge of how to differentiate pHFOs from normal physiological HFOs (nHFOs) has received considerable attention, and it is now well recognized that pathogenicity cannot be determined simply by HFOs frequency [2].
The anatomic location [2] and underlying mechanism of HFOs [1] has also been suggested to define physiologic or pathologic HFOs. Other studies have considered event-related HFOs evoked by normal physiological tasks as normal, and spontaneous HFOs associated with interictal epileptiform discharges (IEDs) as pathologic events [15,21]. Interestingly, analysis of putative pHFOs and nHFOs show that pHFOs have higher voltages and longer duration compared to physiological nHFOs [15]. Since the task-induced HFOs in the epileptic brain can have spectral properties similar to pHFOs [3,15,22], the rigid dichotomy between pHFOs in epileptic brain and physiological ‘event related’ nHFO in non-epileptic brain requires further study to determine if it is always true.
Here we review recent advances in HFOs detection, classification of normal physiological (nHFOs) and pathological (pHFOs) activity, and the potential for clinical translation of HFOs as a biomarker of the focal human epileptic brain responsible for seizure generation and as a biomarker of the evolution and development of epilepsy (epileptogenesis). We propose that HFOs are a clinically useful biomarker and that interictal HFOs can be used for brain mapping in the intra-operative setting to transform the practice of epilepsy surgery. However, well-designed clinical trials are required to provide needed definitive evidence before wide spread clinical use of these biomarkers and change of current clinical practice. In addition, we have made a large dataset of wide bandwidth recordings from 102 patients and code for detection of HFOs available to promote reproducible research and accelerate clinical translation. (http://msel.mayo.edu/research.html)
HFOs Detection
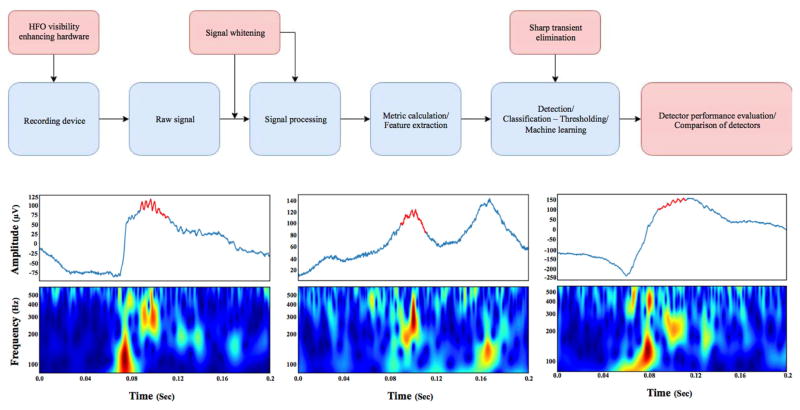
A visual review of HFOs is time-consuming and suffers from reviewer bias, resulting in poor inter-reviewer agreement [23]. This has led to the development of several automated HFOs detectors (Fig. 1) from different laboratories, tuned to particular datasets and HFOs definitions, recently reviewed by Zijlmans et al., [24].
Figure 1. Generic algorithm flow chart of automated HFOs detection.
A) Schematic of automatic HFOs detection. The brain activity is recorded with a wide bandwidth acquisition system. Then, the raw broadband electrophysiologic recording is “whitened” to better highlight the low amplitude HFOs transients. A wide range of features can be extracted to aid classification. After the feature extraction through different methods, the candidate events can be classified using a wide range of classification approaches, like machine learning. The output of these detectors should be evaluated and have comparable outputs from other detectors and laboratories. Red boxes correspond to the advances in HFOs detection and blue boxes are the established flow of HFOs detection. B) Three HFOs detected in a wide bandwidth recording from human brain. Each figure is composed, from top to bottom, of the raw signal (in blue) with the automatically detected HFOs (red) in the signal, and the continuous time frequency image.
Although the comparison between detectors has been made [25,26], these studies were limited by the testing datasets and the number of compared detectors. Software tools and frameworks for automated comparison of detectors have recently emerged and should prove more robust and reliable [27]. The HFOs-detect initiative (https://github.com/HFOs-detect) contains a number of published detectors and allows for automated and reproducible comparison and feature extraction.
The first HFOs detectors utilized the approach of band pass filtering the raw signal in the frequency band of interest, transformation of the filtered signal into metrics, and representing the signal energy, such as line length or root mean square [28–30]. While this approach works reasonably well, it suffers from relatively high false positive HFOs detection rates because of the oscillatory patterns produced by filtering of sharp transients. Recent detectors tackle this issue by utilizing various approaches such as power band ratio [31,32], multi-channel spatial information [33], and machine learning methods [34].
The differentiation between physiological and pathological HFOs is an unresolved problem that impedes the translation of automated detectors into clinical practice [15,35–37]. However, a couple of recent detectors utilize feature extraction and subsequent clustering to distinguish pathological from physiological HFOs and identify HFOs occurring simultaneously with interictal epileptiform discharges (IEDs) or coupled with slow waves during sleep [38–40].
Further improvements of HFOs signal quality rely on improvements in EEG acquisition [41,42]. Recording in a shielded environment and utilization of specialized hardware amplifiers can improve signal-to-noise ratio and improve the performance of HFOs detectors [33]. Furthermore, the use of techniques to compensate for the one-over-frequency character of EEG can enhance the visibility of HFOs in both time and frequency domain and further improve detectability of HFOs [27].
Classification of pathological HFOs and physiological HFOs
The mammalian brain generates a wide range of activity; the HFOs have received significant attention because of their possible role in the visual cortex binding percepts into an integrated visual perception and in the hippocampus for information encoding and memory. Distinguishing between these physiological HFOs and pathological HFOs associated with epilepsy and seizure generation remains a fundamental challenge of epileptology.
Many earlier studies hypothesized that very high frequency oscillations called fast ripples (>250 Hz) were exclusively pathological. Multiple later studies showed an increase of other frequency bands HFOs in human epileptic tissue as well, both high gamma oscillations [3,11] and ripples [4]. Here we reviewed some of the recent efforts that have been made to distinguish nHFOs from pHFOs in interictal recordings of human studies.
HFOs Rate, Amplitude, and Duration
While early studies were focused on the frequency band of the HFOs oscillations to classify them as pathologic or physiologic, considering the wide range of overlapping pHFOs and nHFOs frequencies [3,4,11,18,42–44], more recent studies have explored other frequency-independent characteristics of HFOs: rate, amplitude, and duration [45–52]. Regions showing a high rate of interictal HFOs have been correlated with pathological epileptic tissue in different studies [2,15,48,52–54]. The rate of HFOs (through various frequency bands) was shown to be higher inside the seizure onset zone (SOZ) than outside (NSOZ) [45,47–49,51,52]. This is supported by studies using different approaches in defining the pHFOs and nHFOs, e.g. considering task-induced HFOs as nHFOs [15,55], or HFOs associated with interictal epileptiform discharges (IEDs) as pHFOs [21,56]. Also, resection of the high-rate HFOs regions was found to be correlated with favorable surgery outcome [45,49,50,52,57,58]. However, it is unclear if the HFOs rate alone is specific enough to distinguish epileptic from non-epileptic regions at the single electrode level that is required to guide epilepsy surgery. The HFOs rate varies in different brain structures [59], types of lesions [60], and also SOZ morphological patterns [61]. These findings should be considered when comparing HFOs rates recorded from different channels.
Additional HFOs features like duration, frequency, and amplitude were studied to assess if they can improve differentiation of pHFOs (as identified by the expert visual review) from physiologic ones and provide additional information about tissue epileptogenicity [15,21,48,51,55,59]. Almost all of these studies found statistically significant differences between two groups, and even correlation with surgery outcome [48], yet there was always a considerable overlap between them. The most consistent finding regarding the difference between characteristics of pathologic and physiologic HFOs, other than rate, was higher amplitudes for pHFOs across different frequency bands [15,21,48,51,55,59].
Other EEG characteristics that may be valuable for localizing pathological tissue is the EEG background activity at the time of HFOs occurrence [16–18, 21]. It was shown that removal of the HFOs occurring on a flat, non-oscillatory background was correlated with better surgery outcomes, while there was no such relation with the resection of the HFOs occurring in an oscillatory background [62]. The channels showing continuous high frequency activity showed no association with SOZ or epileptic lesion, they were instead found to be predominant in the mesial temporal and occipital regions [56,63]. Thus these continuous oscillatory activities were suggested as physiologic patterns specific to certain brain regions.
Association of pHFOs with Interictal Epileptiform Discharges
The association between pHFOs and interictal epileptiform discharges (IEDs) was investigated by various studies [21,22,46,56]. Wang et al., [21] classified neocortical ripples into two subtypes: type I ripples superimposed on an IED, and type II ripples independent of any epileptiform discharges. They found that fast ripples and type I neocortical ripples (found to be more abundant compared to fast ripples) and not the type II ripples were correlated with the SOZ and primary propagation area in neocortical epilepsy. The type I neocortical ripple also were found to be more specifically confined to the seizure onset and propagation regions, and thus a better marker compared to IEDs alone. These authors later reported a significant correlation between the surgery success and the higher resection ratio of the fast ripples and the type I ripples but not type II ripples. [56]
Ren et al., [22] studied the gamma HFOs that precede IEDs and found a strong association between seizure generating brain and gamma HFOs that preceded IEDs, in contrast to IEDs alone. They proposed the gamma HFOs proceeding IEDs may be useful for mapping pathological brain regions.
Anatomical location of HFOs
The anatomical location of HFOs was demonstrated to be a useful biomarker of epileptogenesis. Bragin et al. have shown that, in the intrahippocampal kainate rodent model of mesial temporal lobe epilepsy, interictal ripple frequency HFOs can be found in the dentate gyrus along with fast ripples, an area where ripples normally do not occur in normal rats. Similar to fast ripples, these ripple-frequency HFOs appeared within days to weeks after kainate injection exclusively in rats that later exhibit spontaneous seizures [2,53,64].
Regional differences in the behavior and nature of HFOs have also been reported in human studies, particularly in mesial temporal, motor and occipital structures [15,45,51,55,56,59,65].
Interestingly, it was shown that HFOs are more helpful in distinguishing normal brain regions from epileptic tissue in the mesiotemporal lobe structures than the rest of the brain. The higher rates of physiological ripples with higher amplitudes and frequencies in the occipital lobe prevented good discrimination between normal and epileptic brain tissue in the occipital lobe compared to other regions [59]. The presence of spontaneous physiologic ripples reported in the visual cortex, primary motor cortex [15,21,51,55] and also within the hippocampal structures[66] should be taken into account in HFOs analysis [21,55].
Task and Stimulation Evoked HFOs
Task-induced HFOs
Spontaneous physiological ripples have been identified in the primary visual cortex [21,55] and the primary motor cortex [20]. To investigate physiological HFOs and pathological HFOs, multiple studies have used the task-induced HFOs [15,55,67]. These task-induced HFOs cover a wide range of frequency bands [42,51,53], for instance, task-induced high gamma oscillations recorded in visual cortex [68], auditory cortex [69], motor cortex [70], and language cortex [71]. Physiologic ripples can also be evoked by visual stimuli in the occipital cortex [55], by somatosensory stimuli in the somatosensory cortex [72], and during memory processing in the hippocampus, parahippocampal and specific neocortical areas [3,36,44,49,50,73]. Physiological evoked HFOs in the fast ripple range (at about 600 Hz) were recorded as well during stimulation of the somatosensory cortex [74] and in the animal neocortex [75].
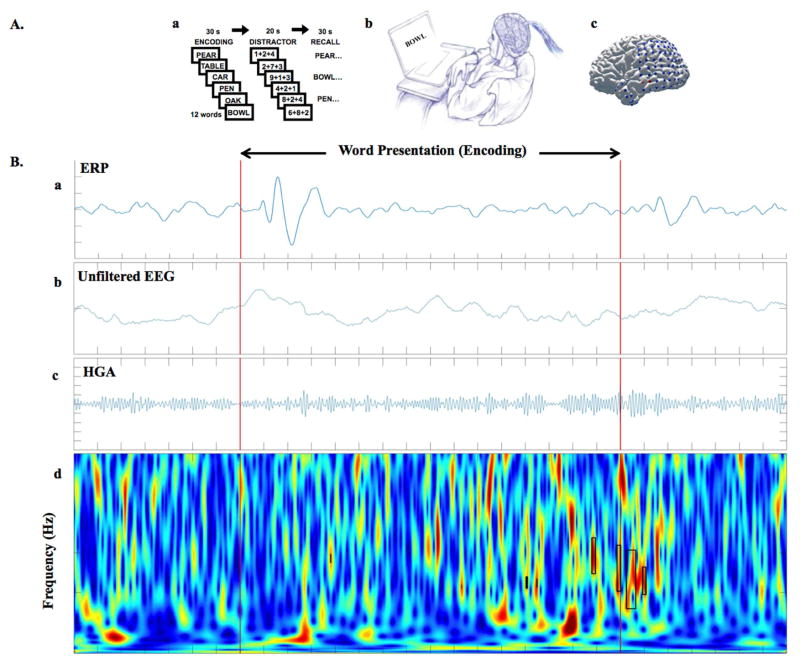
Matsumoto et al. [15] investigated the difference between physiological HFOs and spontaneous pHFOs, assuming task-induced HFOs are physiological, and found significant differences between nHFOs and pHFOs in the spectral power, peak frequency, and duration. Kucewicz et al. [3] used the same protocol to focus on the role of physiological HFOs in human memory processing. The recorded task-induced HFOs demonstrated logical neuroanatomical and biophysical behavior, supporting the notion that they are produced by normal cortical activity [76]. Also, cognitive stimulation was shown to impact ripples in the epileptic hippocampus and non-epileptic hippocampus differently [67]. In recent unpublished work in our lab, we investigated the gamma activity in different regions of the brain during the encoding phase of a verbal memory task, to further investigate properties of these events under cognitive stimulation (Fig. 2). These findings suggest that task-induced HFOs may be useful for brain mapping (Fig. 3).
Figure 2. A) Cognitive task paradigm for studying physiological nHFOs.
Patients with epilepsy who are implanted with subdural and depth electrodes for seizure monitoring as part of the clinical evaluation for drug-resistant epilepsy provide a unique opportunity for neuroscientists to perform cognitive tasks and record the electrophysiological signals directly from the human brain. Areas of active research include investigation of the neural correlates of cognition. These cognitive tasks may help to improve the discrimination of nHFOs from pHFOs. In our study, we used iEEG recorded during a verbal memory task in 11 epilepsy patients to analyze gamma frequency events within and outside the seizure generating brain regions. A. a) Diagram of free recall verbal memory task, A. b) Epileptic patients participating in the study during second phase monitoring doing verbal memory tasks on a laptop, A. c) brain surface of an example patient with implanted grid and strip electrodes the iEEG signal is recording from (unpublished data). B) From top to bottom, B. a) shows the trial-averaged ERP signal for a whole session (300 words presented), plots below are raw data from individual example trial during same session, that subject subsequently recalled the presented word. From top to bottom, the unfiltered EEG (fig 2. B. b), the band-pass filtered signal for high gamma activity (HGA) (fig 2. B. c), and the continuous time frequency image with detected HGAs highlighted with black rectangles (fig 2. B. d). Red lines are stimulus (word presentation) onset and offset (F Khadjevand et al., unpublished).
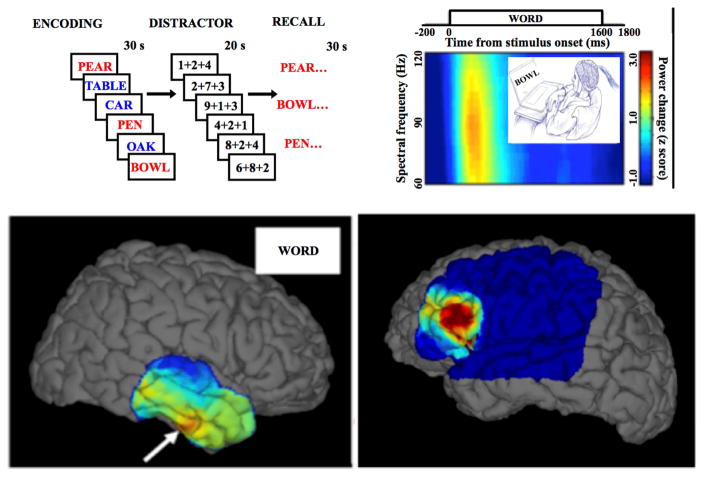
Figure 3. Brain mapping using physiologic task-induced HFOs and pathological HFOs Top, left).
Word lists are presented one-by-one for encoding & subsequent recall. Presentation of words induces high gamma activities (60–120 Hz) in specific brain areas. In this figure subsequently recalled words printed in red and forgotten words presented in blue. Top, right) Spectrogram of local field power during encoding epochs aligned to the presented word. Bottom) Left panel, brain surface maps of high physiological gamma power interpolated over 4×6 temporal cortex grid (White arrow focus of high activation) and in right panel the pathological HFOs over the epileptic region of the brain.
Electrical Stimulation
Van ‘t Klooster et al., investigated the feasibility of stimulation-evoked HFOs in the prediction of the SOZ and the eloquent cortex and found that Single Pulse Electrical Stimulation could elicit fast ripples from the epileptic cortex, but is not capable of distinguishing pathological from physiological ripples [77,78].
Effect of Behavioral State on HFOs
Sleep has been proposed as useful for activating HFOs, and to be capable of separating the physiological HFOs from pathological HFOs by different studies [35,59,79–84].
Rate of HFOs during sleep
The rate of HFOs has been shown to be dependent on both the sleep stages and also the accumulated time of sleep [35]. Numerous studies found that the HFOs rate is modulated by the different sleep stages, being highest during NREM slow wave sleep and lowest during REM sleep [35,50,79,82]. Interestingly, the physiologic (events involving only channels with no epileptic activity) and pathologic ripples (events involving only channels in the irritative zone or the SOZ) rates behave differently during the two subtypes of REM sleep. In the brain regions showing epileptic activity, HFOs have higher rate during tonic REM sleep compared to the phasic REM sleep. Suppression of the pHFOs during phasic REM sleep is in line with the fact that EEG desynchronization leads to the suppression of the interictal epileptiform discharges. In contrast, during phasic REM sleep the HFOs rate increases in the brain regions that show no epileptic activity compared to tonic REM sleep. The increase of nHFOs rate during phasic REM sleep may reflect the role of this subtype of REM sleep in memory, learning and dreaming. These findings suggest that the distribution pattern of the HFOs during different REM states may help to identify physiologic and pathologic ripples and improve localization of SOZ, especially in the brain regions with high physiologic HFOs rates, like the hippocampus, and the occipital cortex [83].
As noted before, the rate of HFOs also depends on the total duration of sleep. There is a significant difference in the behavior of pHFOs versus nHFOs across the different sleep cycles. The rate of pathological ripples and fast ripples decreases with time during NREM sleep, while the rate of ripples in channels within the presumably normal brain regions increases with time during REM sleep [35]. The observed association between the behavior of pHFOs and the sleep-homeostatic variations of slow waves may be related to the role of synchronization in the generation of these pathologic events. The increase of phasic REM sleep through sleep cycles may explain the increase in the rate of nHFOs across REM sleep [35]. The contrast between the rate of the two types of ripples found to be largest in the first sleep cycle and Von Ellenrieder et al., [35] pointed out the practical value of this finding by suggesting that the detection of HFOs in epilepsy should be done during the first sleep cycle.
Spread of HFOs during sleep
The stage of sleep not only modulates the rate of HFOs but also determines the extent of their spread [35]. The HFOs field is larger during NREM sleep and more restricted during REM sleep, supporting the notion that HFOs during REM sleep might be more specific to epileptogenicity [35]. Sakuraba et al., [79] suggested HFOs during REM can serve as a specific marker of epileptogenicity, they found that the suppressive effect of REM sleep had been attenuated near the epileptogenic zone, and also resection of the high-rate HFOs channels during REM sleep was associated with favorable surgery outcome.
Coupling of HFOs with slow waves
The coupling of HFOs to the slow waves is different in presumably normal and epileptic brain regions [59,81,82].
The phase of the coupled slow wave
There is a difference in the coupling between the phase of slow waves during sleep and the occurrence of nHFOs and pHFOs [59,82]. HFOs recorded from epileptic regions and epileptic spikes both occur more often before the peak of the deactivated, or down state, of the slow waves during sleep. In other words, this happens during the transition from the ‘up’ to the ‘down’ state of the slow waves. In contrast, physiological HFOs were reported to occur more frequently after the peak of the down state of the slow wave, or in other words during the transition from the ‘down’ to ‘up’ state of the slow waves.
The spectral frequency band of the coupled slow wave
Nonoda et al., [81] showed that the spectral frequency bands of coupled slow waves could be helpful in distinguishing the epileptogenic HFOs from the physiological HFOs. They suggested that pHFOs are coupled with slow waves at 3–4 Hz more preferentially than at 0.5–1 Hz, whereas nHFOs are coupled with the slow wave at 0.5–1 Hz more preferentially than at 3–4 Hz during the slow wave.
Clinical translation of HFOs Biomarkers
Biomarkers are objectively measured signals that characterize normal and pathological biological processes. There is a critical need for epilepsy biomarkers and in particular signatures of ictogenesis (the process underlying the transition of normal brain activity to seizure activity) and epileptogenesis (the process of brain tissue evolution to tissue capable of generating recurrent, unprovoked seizures).
Biomarkers of ictogenesis could be utilized to identify the transition state, or preictal state, from which the pathological seizure discharge arises and lead to new intelligent therapies where stimulation or medications could be adjusted to prevent seizures before they occur. Electroencephalography (EEG) in ictogenesis has been extensively investigated, and there is recent evidence supporting the concept that EEG can be used to track seizure probability [85,86]. There is an evolving literature to support that HFOs activity recorded with iEEG is a potential biomarker of ictogenesis [11,13,87,88]. In the future, the integration of multiple EEG features, including interictal HFOs, in devices that track seizure probability can be used to guide titration of electrical stimulation and drug therapy may prove very effective.
Localization of the brain tissue generating seizures is critical for successful epilepsy surgery and focal brain stimulation. The localization of tissue to be resected often requires fusing multiple signals and includes electroencephalography recorded from scalp and brain, structural & functional MRI, PET, SPECT as well as the semiology of the patient’s habitual seizures. Currently, the gold standard for localizing epileptic brain is to record spontaneous seizures directly using iEEG and many studies have reported on the use of ictal and interictal HFOs to guide surgical resection (recently reviewed). Unfortunately, even after years of seizure freedom post-surgery, patients can suffer epilepsy recurrence. Interestingly, often these patients are seizure free after re-operation that extends the prior resection. The late recurrence of seizures after surgery suggests ongoing epileptogenesis (what W. Penfield call “ripening” of the epileptic focus after injury [89]) such that tissue not involved in seizure generation at the time of initial resective surgery evolves to generate spontaneous, unprovoked seizures. There is evidence from animal models that HFOs are a potential biomarker of epileptogenesis [90], so the possibility that improved brain mapping could identify the resection margin tissue at risk for epileptogenesis, and seizure recurrence is exciting.
Conclusions
In this review, we have presented some of the recent advances in HFOs detection, classification of normal physiological (nHFOs) and pathological (pHFOs) activity, and the potential for clinical translation of HFOs as a biomarker of ictogenesis and epileptogenesis. We believe that wide bandwidth intracranial electrophysiology is poised for clinical translation and could transform the field of epilepsy surgery. However, clinical trials are needed to definitively establish the accuracy of brain mapping using only interictal data (non-seizure data) to replace prolonged (multiple days) iEEG recordings. The focus of clinical translation at this time should be to demonstrate the benefit of interictal brain mapping using HFOs as electrophysiological biomarkers of the epileptogenic brain. Immediate applications include the mapping for the localization of normal and epileptogenic brain, which is critical for planning epilepsy surgery and targeting electrodes for direct brain stimulation. If it proves possible to use wide bandwidth iEEG in the operating room to identify epileptogenic tissue without having to record spontaneous seizures, it will transform epilepsy surgery. The planning for tissue resection or placement of brain stimulation electrodes could be performed as an intra-operative procedure and eliminate multiple days of monitoring.
To accelerate research and to promote reproducible research, we have made a large dataset of wide bandwidth recordings freely available (http://msel.mayo.edu/research.html). Given the significant progress to date, we anticipate making data and corresponding analytic code freely available will accelerate clinical translation.
Highlights.
The mammalian brain generates physiological and pathological high frequency oscillations (HFOs: 30 – 2000 Hz)
Automated detection of HFOs can be accurate and yield reproducible results
Differentiating physiological and pathological HFOs remains a fundamental challenge
HFOs with pathologic features are biomarkers of epileptogenic brain
Data and code are made available to support reproducible research (http://msel.mayo.edu/research.html)
Acknowledgments
We thank Abigail L. Magee and Dr. Michal T. Kucewicz for helpful comments on the manuscript.
Funding
This work was supported by funding from the National Institutes of Health (NIH: R01-NS092882 and R01-NS063039), Czech Republic Grant Agency (P103/11/0933), and European Regional Development Fund - Project FNUSA - ICRC (CZ.1.05/1.1.00/02.0123).
ABBREVIATIONS
- EEG
Electroencephalography
- iEEG
Intracranial EEG
- HFOs
-
High Frequency Oscillations
Gamma (30 – 100 Hz)
Ripple (100 – 250 Hz)
Fast Ripple (250 – 600Hz)
- VHFOs
Very High Frequency Oscillations (600 – 2000Hz)
- pHFOs
Pathological HFOs
- nHFOs
Physiological/normal HFOs
- HGA
High Gamma Activity
- SOZ
Seizure Onset Zone
- NSOZ
Non Seizure Onset Zone
- IEDs
Interictal Epileptiform Discharges
- REM Sleep
Rapid Eye Movement Sleep
- NREM Sleep
Non Rapid Eye Movement Sleep
- ERP
Event Related Potential
- TF image
Time-Frequency image
- MRI
Magnetic Resonance Imaging
- PET
Positron-Emission Tomography
- SPECT
Single-Photon Emission Computed Tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ Of special interest
▪▪ Of outstanding interest
- 1.Buzsáki G, da Silva FL. High frequency oscillations in the intact brain. Progress in neurobiology. 2012;98(3):241–249. doi: 10.1016/j.pneurobio.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: What is normal and what is not? Epilepsia. 2009;50(4):598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 3.Kucewicz MT, Cimbalnik J, Matsumoto JY, Brinkmann BH, Bower MR, Vasoli V, Sulc V, Meyer F, Marsh WR, Stead SM, Worrell GA. High frequency oscillations are associated with cognitive processing in human recognition memory. Brain. 2014;137(Pt 8):2231–2244. doi: 10.1093/brain/awu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: Simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131(4):928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bragin A, Mody I, Wilson CL, Engel J. Local generation of fast ripples in epileptic brain. Journal of Neuroscience. 2002;22(5):2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worrell GA, Jerbi K, Kobayashi K, Lina J-M, Zelmann R, Le Van Quyen M. Recording and analysis techniques for high-frequency oscillations. Progress in neurobiology. 2012;98(3):265–278. doi: 10.1016/j.pneurobio.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs J, Staba R, Asano E, Otsubo H, Wu J, Zijlmans M, Mohamed I, Kahane P, Dubeau F, Navarro V. High-frequency oscillations (hfos) in clinical epilepsy. Progress in neurobiology. 2012;98(3):302–315. doi: 10.1016/j.pneurobio.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338(6213):334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- 9.Buzsaki G. Rhythms of the brain. Oxford University Press; 2006. [Google Scholar]
- 10.Buzsáki G, Wang X-J. Mechanisms of gamma oscillations. Annual review of neuroscience. 2012;35:203. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127(7):1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- 12.Traub RD, Whittington MA, Buhl EH, LeBeau FE, Bibbig A, Boyd S, Cross H, Baldeweg T. A possible role for gap junctions in generation of very fast eeg oscillations preceding the onset of, and perhaps initiating, seizures. Epilepsia. 2001;42(2):153–170. doi: 10.1046/j.1528-1157.2001.26900.x. [DOI] [PubMed] [Google Scholar]
- 13.Timofeev I, Steriade M. Neocortical seizures: Initiation, development and cessation. Neuroscience. 2004;123(2):299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 14**.Brázdil M, Pail M, Halámek J, Plešinger F, Cimbálník J, Roman R, Klimeš P, Daniel P, Chrastina J, Brichtová E. Very high frequency oscillations: Novel biomarkers of the epileptogenic zone. Annals of Neurology. 2017 doi: 10.1002/ana.25006. This recent paper extends the range of HFOs recorded using iEEG in human subjects to include very fast ripples (VFRs; 500–1,000Hz) and ultrafast ripples (UFRs; 1,000–2,000Hz). VFRs and UFRs were observed only in patients with temporal lobe epilepsy and were recorded exclusively from mesiotemporal structures. When compared to ripple oscillations, significantly better outcomes were observed in patients with a higher percentage of removed contacts containing FRs, VFRs, and UFRs. This study provides evidence that interictal VHFOs are more specific biomarkers for the epileptogenic zone when compared to lower frequency HFOs. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto A, Brinkmann BH, Matthew Stead S, Matsumoto J, Kucewicz MT, Marsh WR, Meyer F, Worrell G. Pathological and physiological high-frequency oscillations in focal human epilepsy. J Neurophysiol. 2013;110(8):1958–1964. doi: 10.1152/jn.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bragin A, Engel J, Jr, Staba RJ. High-frequency oscillations in epileptic brain. Current opinion in neurology. 2010;23(2):151. doi: 10.1097/WCO.0b013e3283373ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Worrell G, Gotman J. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: Clinical studies. Biomarkers. 2011;5(5):557–566. doi: 10.2217/bmm.11.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimbalnik J, Kucewicz MT, Worrell G. Interictal high-frequency oscillations in focal human epilepsy. Current opinion in neurology. 2016;29(2):175. doi: 10.1097/WCO.0000000000000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bragin A, Engel J, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus. 1999;9(2):137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Bragin A, Engel J, Wilson CL, Vizentin E, Mathern GW. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal ka injection. Epilepsia. 1999;40(9):1210–1221. doi: 10.1111/j.1528-1157.1999.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Wang IZ, Bulacio JC, Mosher JC, Gonzalez-Martinez J, Alexopoulos AV, Najm IM, So NK. Ripple classification helps to localize the seizure onset zone in neocortical epilepsy. Epilepsia. 2013;54(2):370–376. doi: 10.1111/j.1528-1167.2012.03721.x. [DOI] [PubMed] [Google Scholar]
- 22.Ren L, Kucewicz MT, Cimbalnik J, Matsumoto JY, Brinkmann BH, Hu W, Marsh WR, Meyer FB, Stead SM, Worrell GA. Gamma oscillations precede interictal epileptiform spikes in the seizure onset zone. Neurology. 2015;84(6):602–608. doi: 10.1212/WNL.0000000000001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spring AM, Pittman DJ, Aghakhani Y, Jirsch J, Pillay N, Bello-Espinosa LE, Josephson C, Federico P. Interrater reliability of visually evaluated high frequency oscillations. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2017;128(3):433–441. doi: 10.1016/j.clinph.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 24**.Zijlmans M, Worrell GA, Dümpelmann M, Stieglitz T, Barborica A, Heers M, Ikeda A, Usui N, Le Van Quyen M. How to record high-frequency oscillations in epilepsy: A practical guideline. Epilepsia. 2017 doi: 10.1111/epi.13814. This review provides practical and technical guidance for researchers and clinicians on recording, evaluation, and interpretation of HFOs: ripples, fast ripples, and very high frequency oscillations; emphasizing the importance of low noise recording to minimize artifacts. They further discuss the challenges of overcoming various sources of artifacts in MEG and iEEG recordings. [DOI] [PubMed] [Google Scholar]
- 25.Zelmann R, Mari F, Jacobs J, Zijlmans M, Dubeau F, Gotman J. A comparison between detectors of high frequency oscillations. Clinical Neurophysiology. 2012;123(1):106–116. doi: 10.1016/j.clinph.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salami P, Levesque M, Gotman J, Avoli M. A comparison between automated detection methods of high-frequency oscillations (80–500 hz) during seizures. J Neurosci Methods. 2012;211(2):265–271. doi: 10.1016/j.jneumeth.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roehri N, Pizzo F, Bartolomei F, Wendling F, Bénar C-G. What are the assets and weaknesses of hfo detectors? A benchmark framework based on realistic simulations PloS one. 2017;12(4):e0174702. doi: 10.1371/journal.pone.0174702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staba RJ, Wilson CL, Bragin A, Fried I, Engel J. Quantitative analysis of high-frequency oscillations (80–500 hz) recorded in human epileptic hippocampus and entorhinal cortex. Journal of neurophysiology. 2002;88(4):1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- 29.Gardner AB, Worrell GA, Marsh E, Dlugos D, Litt B. Human and automated detection of high-frequency oscillations in clinical intracranial eeg recordings. Clinical neurophysiology. 2007;118(5):1134–1143. doi: 10.1016/j.clinph.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crépon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, Adam C, Le Van Quyen M. Mapping interictal oscillations greater than 200 hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2009;133(1):33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- 31.Burnos S, Hilfiker P, Sürücü O, Scholkmann F, Krayenbühl N, Grunwald T, Sarnthein J. Human intracranial high frequency oscillations (hfos) detected by automatic time-frequency analysis. PLoS One. 2014;9(4):e94381. doi: 10.1371/journal.pone.0094381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birot G, Kachenoura A, Albera L, Bénar C, Wendling F. Automatic detection of fast ripples. Journal of neuroscience methods. 2013;213(2):236–249. doi: 10.1016/j.jneumeth.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Fedele T, van ‘t Klooster M, Burnos S, Zweiphenning W, van Klink N, Leijten F, Zijlmans M, Sarnthein J. Automatic detection of high frequency oscillations during epilepsy surgery predicts seizure outcome. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2016;127(9):3066–3074. doi: 10.1016/j.clinph.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Amiri M, Lina JM, Pizzo F, Gotman J. High frequency oscillations and spikes: Separating real hfos from false oscillations. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2016;127(1):187–196. doi: 10.1016/j.clinph.2015.04.290. [DOI] [PubMed] [Google Scholar]
- 35*.von Ellenrieder N, Dubeau F, Gotman J, Frauscher B. Physiological and pathological high-frequency oscillations have distinct sleep-homeostatic properties. NeuroImage: Clinical. 2017;14:566–573. doi: 10.1016/j.nicl.2017.02.018. This study investigated the rate and spread of HFOs in different sleep stages across different sleep cycles, and demonstrated different behaviors of the physiologic and pathologic high frequency oscillations. They found 1) As the nights advanced the rate of pHFOs during NREM sleep decreased while the rate of nHFOs during REM sleep increased, 2) There was higher rates and larger field of high frequency oscillations during NREM, and lower rates of HFOs in a more restricted field during REM, 3) Given the highest contrast between the rate of pHFOs and nHFOs the first cycle of sleep proposed to be the best time to study HFOs in epilepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van’t Klooster M, van Klink N, van Blooijs D, Ferrier C, Braun K, Leijten F, Huiskamp G, Zijlmans M. Evoked versus spontaneous high frequency oscillations in the chronic electrocorticogram in focal epilepsy. Clinical Neurophysiology. 2017;128(5):858–866. doi: 10.1016/j.clinph.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Bruder JC, Dümpelmann M, Piza DL, Mader M, Schulze-Bonhage A, Jacobs-Le Van J. Physiological ripples associated with sleep spindles differ in waveform morphology from epileptic ripples. International journal of neural systems. 2016:1750011. doi: 10.1142/S0129065717500113. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Sha Z, Sencer A, Aydoseli A, Bebek N, Abosch A, Henry T, Gurses C, Ince NF. Exploring the time–frequency content of high frequency oscillations for automated identification of seizure onset zone in epilepsy. Journal of neural engineering. 2016;13(2):026026. doi: 10.1088/1741-2560/13/2/026026. [DOI] [PubMed] [Google Scholar]
- 39.Jrad N, Kachenoura A, Merlet I, Bartolomei F, Nica A, Biraben A, Wendling F. Automatic detection and classification of high frequency oscillations in depth-eeg signals. IEEE Transactions on Biomedical Engineering. 2016 doi: 10.1109/TBME.2016.2633391. [DOI] [PubMed] [Google Scholar]
- 40.Huang L, Ni X, Ditto WL, Spano M, Carney PR, Lai Y-C. Detecting and characterizing high-frequency oscillations in epilepsy: A case study of big data analysis. Open Science. 2017;4(1):160741. doi: 10.1098/rsos.160741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gliske SV, Irwin ZT, Davis KA, Sahaya K, Chestek C, Stacey WC. Universal automated high frequency oscillation detector for real-time, long term eeg. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2016;127(2):1057–1066. doi: 10.1016/j.clinph.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.de la Prida LM, Staba RJ, Dian JA. Conundrums of high-frequency oscillations (80–800 hz) in the epileptic brain. Journal of clinical neurophysiology: official publication of the American Electroencephalographic Society. 2015;32(3):207. doi: 10.1097/WNP.0000000000000150. This invited review article is focused on the underlying, not well-understood complex neuronal events, underlying pathological HFOs (80–800 Hz). Discussing the main issues in the recording, analysis, and interpretation of HFOs in the epileptic brain, they provided a list of recommendations to improve obtaining comparable HFOs signals in clinical and basic epilepsy research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanco JA, Stead M, Krieger A, Stacey W, Maus D, Marsh E, Viventi J, Lee KH, Marsh R, Litt B, Worrell GA. Data mining neocortical high-frequency oscillations in epilepsy and controls. Brain. 2011;134(Pt 10):2948–2959. doi: 10.1093/brain/awr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kucewicz MT, Berry BM, Kremen V, Brinkmann BH, Sperling MR, Jobst BC, Gross RE, Lega B, Sheth SA, Stein JM, Das SR, et al. Dissecting gamma frequency activity during human memory processing. Brain. 2017 doi: 10.1093/brain/awx043. [DOI] [PubMed] [Google Scholar]
- 45.Iimura Y, Jones K, Hattori K, Okazawa Y, Noda A, Hoashi K, Nonoda Y, Asano E, Akiyama T, Go C, Ochi A, et al. Epileptogenic high-frequency oscillations skip the motor area in children with multilobar drug-resistant epilepsy. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2017;128(7):1197–1205. doi: 10.1016/j.clinph.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs J, Vogt C, LeVan P, Zelmann R, Gotman J, Kobayashi K. The identification of distinct high-frequency oscillations during spikes delineates the seizure onset zone better than high-frequency spectral power changes. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2016;127(1):129–142. doi: 10.1016/j.clinph.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 47.Malinowska U, Bergey GK, Harezlak J, Jouny CC. Identification of seizure onset zone and preictal state based on characteristics of high frequency oscillations. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2015;126(8):1505–1513. doi: 10.1016/j.clinph.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Pail M, Řehulka P, Cimbálník J, Doležalová I, Chrastina J, Brázdil M. Frequency-independent characteristics of high-frequency oscillations in epileptic and non-epileptic regions. Clinical Neurophysiology. 2017;128(1):106–114. doi: 10.1016/j.clinph.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Burnos S, Frauscher B, Zelmann R, Haegelen C, Sarnthein J, Gotman J. The morphology of high frequency oscillations (hfo) does not improve delineating the epileptogenic zone. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2016;127(4):2140–2148. doi: 10.1016/j.clinph.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Dumpelmann M, Jacobs J, Schulze-Bonhage A. Temporal and spatial characteristics of high frequency oscillations as a new biomarker in epilepsy. Epilepsia. 2015;56(2):197–206. doi: 10.1111/epi.12844. [DOI] [PubMed] [Google Scholar]
- 51.Alkawadri R, Gaspard N, Goncharova II, Spencer DD, Gerrard JL, Zaveri H, Duckrow RB, Blumenfeld H, Hirsch LJ. The spatial and signal characteristics of physiologic high frequency oscillations. Epilepsia. 2014;55(12):1986–1995. doi: 10.1111/epi.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Cho JR, Koo DL, Joo EY, Seo DW, Hong SC, Jiruska P, Hong SB. Resection of individually identified high-rate high-frequency oscillations region is associated with favorable outcome in neocortical epilepsy. Epilepsia. 2014;55(11):1872–1883. doi: 10.1111/epi.12808. This study proposed a patient-individualized approach of identifying high-rate HFOs regions to plan the neocortical resection, demonstrating a correlation between these regions and surgery success. [DOI] [PubMed] [Google Scholar]
- 53.Staba RJ. Normal and pathological high-frequency oscillations. Jasper’s Basic Mechanisms of the Epilepsies. 2012:202. [Google Scholar]
- 54.Staba RJ, Stead M, Worrell GA. Electrophysiological biomarkers of epilepsy. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2014;11(2):334–346. doi: 10.1007/s13311-014-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagasawa T, Juhász C, Rothermel R, Hoechstetter K, Sood S, Asano E. Spontaneous and visually driven high-frequency oscillations in the occipital cortex: Intracranial recording in epileptic patients. Human brain mapping. 2012;33(3):569–583. doi: 10.1002/hbm.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56**.Wang S, So NK, Jin B, Wang IZ, Bulacio JC, Enatsu R, Dai S, Chen Z, Gonzalez-Martinez J, Najm IM. Interictal ripples nested in epileptiform discharge help to identify the epileptogenic zone in neocortical epilepsy. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2017;128(6):945–951. doi: 10.1016/j.clinph.2017.03.033. This study classified HFOs based on their association with interictal epileptiform discharges and demonstrated that neocortical ripples superimposed on epileptiform discharges along with fast ripples might localize SOZ, and were associated with surgery success. [DOI] [PubMed] [Google Scholar]
- 57.Haegelen C, Perucca P, Chatillon CE, Andrade-Valenca L, Zelmann R, Jacobs J, Collins DL, Dubeau F, Olivier A, Gotman J. High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia. 2013;54(5):848–857. doi: 10.1111/epi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okanishi T, Akiyama T, Tanaka S, Mayo E, Mitsutake A, Boelman C, Go C, Snead OC, 3rd, Drake J, Rutka J, Ochi A, et al. Interictal high frequency oscillations correlating with seizure outcome in patients with widespread epileptic networks in tuberous sclerosis complex. Epilepsia. 2014;55(10):1602–1610. doi: 10.1111/epi.12761. [DOI] [PubMed] [Google Scholar]
- 59.von Ellenrieder N, Frauscher B, Dubeau F, Gotman J. Interaction with slow waves during sleep improves discrimination of physiologic and pathologic high-frequency oscillations (80–500 hz) Epilepsia. 2016;57(6):869–878. doi: 10.1111/epi.13380. [DOI] [PubMed] [Google Scholar]
- 60.Ferrari-Marinho T, Perucca P, Mok K, Olivier A, Hall J, Dubeau F, Gotman J. Pathologic substrates of focal epilepsy influence the generation of high-frequency oscillations. Epilepsia. 2015;56(4):592–598. doi: 10.1111/epi.12940. [DOI] [PubMed] [Google Scholar]
- 61.Ferrari-Marinho T, Perucca P, Dubeau F, Gotman J. Intracranial eeg seizure onset-patterns correlate with high-frequency oscillations in patients with drug-resistant epilepsy. Epilepsy Res. 2016;127:200–206. doi: 10.1016/j.eplepsyres.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Kerber K, Dumpelmann M, Schelter B, Le Van P, Korinthenberg R, Schulze-Bonhage A, Jacobs J. Differentiation of specific ripple patterns helps to identify epileptogenic areas for surgical procedures. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2014;125(7):1339–1345. doi: 10.1016/j.clinph.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 63.Melani F, Zelmann R, Mari F, Gotman J. Continuous high frequency activity: A peculiar seeg pattern related to specific brain regions. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2013;124(8):1507–1516. doi: 10.1016/j.clinph.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bragin A, Wilson CL, Almajano J, Mody I, Engel J. High-frequency oscillations after status epilepticus: Epileptogenesis and seizure genesis. Epilepsia. 2004;45(9):1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- 65.Jacobs J, Banks S, Zelmann R, Zijlmans M, Jones-Gotman M, Gotman J. Spontaneous ripples in the hippocampus correlate with epileptogenicity and not memory function in patients with refractory epilepsy. Epilepsy & Behavior. 2016;62:258–266. doi: 10.1016/j.yebeh.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 66.Girardeau G, Zugaro M. Hippocampal ripples and memory consolidation. Current opinion in neurobiology. 2011;21(3):452–459. doi: 10.1016/j.conb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 67.Brazdil M, Cimbalnik J, Roman R, Shaw DJ, Stead MM, Daniel P, Jurak P, Halamek J. Impact of cognitive stimulation on ripples within human epileptic and non-epileptic hippocampus. BMC neuroscience. 2015;16(47) doi: 10.1186/s12868-015-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asano E, Nishida M, Fukuda M, Rothermel R, Juhász C, Sood S. Differential visually-induced gamma-oscillations in human cerebral cortex. Neuroimage. 2009;45(2):477–489. doi: 10.1016/j.neuroimage.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edwards E, Soltani M, Deouell LY, Berger MS, Knight RT. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. Journal of neurophysiology. 2005;94(6):4269–4280. doi: 10.1152/jn.00324.2005. [DOI] [PubMed] [Google Scholar]
- 70.Darvas F, Scherer R, Ojemann JG, Rao R, Miller KJ, Sorensen LB. High gamma mapping using eeg. Neuroimage. 2010;49(1):930–938. doi: 10.1016/j.neuroimage.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, Lenz FA, Crone NE. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128(7):1556–1570. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- 72.Hashimoto I. High-frequency oscillations of somatosensory evoked potentials and fields. Journal of Clinical Neurophysiology. 2000;17(3):309–320. doi: 10.1097/00004691-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Crepon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, Adam C, Le Van Quyen M. Mapping interictal oscillations greater than 200 hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2010;133(Pt 1):33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- 74.Curio G, Mackert B-M, Burghoff M, Neumann J, Nolte G, Scherg M, Marx P. Somato-topic source arrangement of 600 hz oscillatory magnetic fields at the human primary somatosensory hand cortex. Neuroscience letters. 1997;234(2):131–134. doi: 10.1016/s0304-3940(97)00690-3. [DOI] [PubMed] [Google Scholar]
- 75.Kandel A, Buzsáki G. Cellular–synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. Journal of Neuroscience. 1997;17(17):6783–6797. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stacey W. Abby…Normal? A new gold standard for identifying normal high frequency oscillations Epilepsy Curr. 2015;15(4):211–212. doi: 10.5698/1535-7511-15.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van ‘t Klooster MA, van Klink NE, van Blooijs D, Ferrier CH, Braun KP, Leijten FS, Huiskamp GJ, Zijlmans M. Evoked versus spontaneous high frequency oscillations in the chronic electrocorticogram in focal epilepsy. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2017;128(5):858–866. doi: 10.1016/j.clinph.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 78.Asano E. Don’t chase all of them. Elsevier; 2017. High-frequency oscillations are under your control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakuraba R, Iwasaki M, Okumura E, Jin K, Kakisaka Y, Kato K, Tominaga T, Nakasato N. High frequency oscillations are less frequent but more specific to epileptogenicity during rapid eye movement sleep. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2016;127(1):179–186. doi: 10.1016/j.clinph.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 80.Piza DL, Bruder JC, Jacobs J, Schulze-Bonhage A, Stieglitz T, Dümpelmann M. Differentiation of spindle associated hippocampal hfos based on a correlation analysis. Abs. :5501–5504. doi: 10.1109/EMBC.2016.7591972. [DOI] [PubMed] [Google Scholar]
- 81*.Nonoda Y, Miyakoshi M, Ojeda A, Makeig S, Juhasz C, Sood S, Asano E. Interictal high-frequency oscillations generated by seizure onset and eloquent areas may be differentially coupled with different slow waves. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2016;127(6):2489–2499. doi: 10.1016/j.clinph.2016.03.022. This study investigated when distinguishing nHFOs from pHFOs, considering the spectral frequency bands of the coupled slow wave would be helpful. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82*.Frauscher B, von Ellenrieder N, Ferrari-Marinho T, Avoli M, Dubeau F, Gotman J. Facilitation of epileptic activity during sleep is mediated by high amplitude slow waves. Brain. 2015;138(6):1629–1641. doi: 10.1093/brain/awv073. This study demonstrated that pHFOs’ density is highest during the transition from the ‘up’ to the ‘down’ state of the slow wave, a period of high synchronization, and based on this finding proposed that epileptic discharges are more associated with synchronization than with excitability. And also discussing the differential pattern of peaking during slow wave cycle may help to distinguish nHFOs from pHFOs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frauscher B, von Ellenrieder N, Dubeau F, Gotman J. Eeg desynchronization during phasic rem sleep suppresses interictal epileptic activity in humans. Epilepsia. 2016;57(6):879–888. doi: 10.1111/epi.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bruder JC, Dumpelmann M, Piza DL, Mader M, Schulze-Bonhage A, Jacobs-Le Van J. Physiological ripples associated with sleep spindles differ in waveform morphology from epileptic ripples. Int J Neural Syst. 2016:1750011. doi: 10.1142/S0129065717500113. [DOI] [PubMed] [Google Scholar]
- 85.Cook MJ, O’Brien TJ, Berkovic SF, Murphy M, Morokoff A, Fabinyi G, D’Souza W, Yerra R, Archer J, Litewka L. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: A first-in-man study. The Lancet Neurology. 2013;12(6):563–571. doi: 10.1016/S1474-4422(13)70075-9. [DOI] [PubMed] [Google Scholar]
- 86**.Brinkmann BH, Wagenaar J, Abbot D, Adkins P, Bosshard SC, Chen M, Tieng QM, He J, Muñoz-Almaraz F, Botella-Rocamora P. Crowdsourcing reproducible seizure forecasting in human and canine epilepsy. Brain. 2016;139(6):1713–1722. doi: 10.1093/brain/aww045. Conducted a seizure forecasting competition on kaggle.com using open access chronic ambulatory intracranial electroencephalography from five canines with naturally occurring epilepsy and two humans undergoing prolonged wide bandwidth intracranial electroencephalographic monitoring, this provided open access to long duration recordings and provided access to the winning algorithms. An example of reproducible research with shared data and code. They found that the overall performance of multiple contestants on unseen data was better than a random predictor, and concluded that seizure forecasting in canine and human epilepsy is feasible. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pearce A, Wulsin D, Blanco JA, Krieger A, Litt B, Stacey WC. Temporal changes of neocortical high-frequency oscillations in epilepsy. J Neurophysiol. 2013;110(5):1167–1179. doi: 10.1152/jn.01009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80–200 hz) during seizures: Intracellular correlates. Journal of neurophysiology. 2003;89(2):841–852. doi: 10.1152/jn.00420.2002. [DOI] [PubMed] [Google Scholar]
- 89.Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. 1954 [Google Scholar]
- 90.Bragin A, Wilson C, Engel J. Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: A hypothesis. Epilepsia. 2000;41(s6) doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]