Abstract
There is a lack of data analyzing the influence of cardiovascular disease (CVD) risk factor control on graft survival disparities in African-American kidney transplant recipients. Studies in the general population indicate that CVD risk factor control is poor in African-Americans, leading to higher rates of renal failure and major acute cardiovascular events. However, with the exception of hypertension, there is no data demonstrating similar results within transplant recipients. Recent analyses conducted by our investigator group indicate that CVD risk factors, especially diabetes, are poorly controlled in African-American recipients, which likely impacts graft loss. This study protocol describes a prospective interventional clinical trial with the goal of demonstrating improved medication safety and CVD risk factor control in adult solitary kidney transplant recipients at least one-year post-transplant with a functioning graft. This is a prospective, interventional, 6-month, pharmacist-led and technology enabled study in adult kidney transplant recipients with the goal of improving CVD risk factor outcomes by improving medication safety and patient self-efficacy. This papers describes the issues related to racial disparities in transplant, the details of this intervention and how we expect this intervention to improve CVD risk factor control in kidney transplant recipients, particularly within African-Americans.
Keywords: Clinical trial, Kidney transplantation, Cardiovascular disease, Diabetes, Hypertension, African-American, Pharmacist, Technology
Abbreviations
- AA
African-American
- ADA
American Diabetes Association
- BP
Blood pressure
- CRF
Case report form
- CTCAE
Common terminology criteria for adverse events
- CV
Cardiovascular
- CVD
Cardiovascular disease
- DSMP
Data safety monitoring plan
- eGFR
Estimated glomerular filtration rate
- HIPAA
Health Portability and Accountability Act
- HbA1C
Hemoglobin A1c
- IRB
Institutional review board
- LDL
Low density lipoprotein
- MHC
Major histocompatibility complex
- mmHg
Millimeters of mercury
- NIH
National Institutes of Health
- REDCap
Research Electronic Data Capture
- SES
Socioeconomic status
- VA
Veterans Affairs
1. Background
The rates of graft loss for African-American (AA) renal transplant recipients are significantly higher than the rates of graft loss for non-AAs. Based on recent data, AA recipients have a 42% higher risk of graft loss at five years post-transplant and the average kidney transplant functions about half as long in AA patients [1]. Despite nearly 40 years of focused research endeavors into this disparity, little has changed in this racial inequality [1], [2], [3]. These disparities have primarily been attributed to immunologic risks leading to higher rejection rates [4], [5], [6], [7], lower socioeconomic status (SES) [8], [9], medication non-adherence [10], [11], and comorbidities [12], [13], [14].
AA renal transplant recipients have more robust immunologic responses, placing them at higher risk for acute rejection. These include more MHC polymorphisms [15], pre-sensitization to MHC antigens [16], greater HLA mismatches [17], immune hyper-responsiveness [18], and cytokine polymorphisms [19], [20]. Therefore, most of the early work trying to eliminate outcome disparities in AA patients was appropriately focused on reducing acute rejection rates through immunosuppressant pharmacotherapy [21], [22]. Though the acute rejection rate has decreased, the graft loss disparity within the AA patient population remains the same [22], [23], [24]. Studies evaluating the influence of SES and medication adherence on racial disparities in kidney transplant recipients have produced conflicting results, with some studies suggesting SES and medication adherence may influence racial disparities, while other resulted in contradictory findings [25], [26], [27], [28], [29], [30].
In terms of cardiovascular disease (CVD) and CVD risk factors, AA kidney transplant recipients have nearly twice the rate of diabetes [31], [32], [33] and four times the rate of hypertension [12], [33] as compared to non-AA recipients. Data from the general population suggests that both hypertension and diabetes occur at an earlier age, are of a more aggressive phenotype and more likely to lead to end-organ damage in AA patients [34], [35], [36].
Unfortunately, there is a lack of data analyzing the influence of CVD risk factor control on graft survival disparities in AA transplant recipients. Studies in the non-transplant population indicate that CVD risk factor control is poor in AA patients, leading to higher rates of renal failure and CV events [37]. However, with the exception of hypertension [38], there is paucity in data demonstrating similar results within transplant recipients [39].
Recently, using VA data, we have also demonstrated that CVD risk factor control is lower in AA kidney transplant recipients and that it is a substantial explanatory variable for racial disparities [40]. This clinical trial study stems directly from these retrospective studies. Once completed, this trial will provide empirical evidence demonstrating the feasibility and exploring the potential effectiveness of pharmacist-led interventions to improve medication safety and CVD risk factor control within kidney recipients; while also demonstrating the potential improvements in CVD risk factor control more substantially within AA recipients.
2. Methods/Design
2.1. Study design
This is a prospective, clinical trial assessing the potential efficacy of a 6-month, pharmacist-led, technology enabled education intervention on improving medication safety and cardiovascular risk factor control in adult solitary kidney transplant recipients with a secondary aim of assessing if the impact of intervention varies by race. The primary objectives include determining if the study is feasible, as measured proportions of enrolled to approached and completed to enrolled, measuring and comparing, at baseline versus the end of the intervention, the medication safety events, including the number of medication errors, medication non-adherence and medication side effects, in patients enrolled in the study, measuring and comparing, at baseline versus the end of the intervention, cardiovascular disease risk factor control, including hypertension, diabetes and dyslipidemia, in patients enrolled in the study, measuring and comparing, at baseline versus the end of the intervention, patient reported survey results, in patients enrolled in the study and determining if the impact of the intervention is more pronounced in AA recipients, as compared to non-AA recipients. Secondarily, the study seeks to measure episodes of biopsy proven acute rejection, episodes of hospitalization, episodes of acute visits cause (including emergency room and urgent care), episodes of grade 4 or 5 adverse drug events, as defined by the common terminology criteria for adverse events (CTCAE), and episodes of graft loss or death.
2.2. Population
Participants enrolled in this study include adult renal transplant recipients that provide informed consent and meet inclusion and exclusion criteria. The patients will be screened, approached, and consented if they are at least one year post-transplant. Once the patients are enrolled, they will be followed per study protocol and the analysis will occur in an intent-to-treat methodology. To be included in the study, patients must be 18 years or older, have received a first or repeat cadaveric or living donor renal transplant, have an adequate graft function (defined as an estimated glomerular filtration rate [eGFR] of at least 20 mL/min using the 4-variable MDRD equation), and be at least one-year post transplant. The patient must also have documented hypertension (defined as a sitting blood pressure of at least 140/90 mmHg or is receiving anti-hypertensive medication), have documented diabetes mellitus (defined as a hemoglobin A1c of at least 6.5% or is receiving anti-glycemic medication), and be willing to comply with all study visits. Inclusion in the study will not be dependent upon age, gender, or ethnicity. A fairly equal number of males and females are anticipated across the age spectrum (children will be excluded) and the study will be stratified to ensure roughly equal numbers of AA and non-AA kidney transplant recipients. Exclusion criteria includes any patient that has a biopsy proven acute allograft rejection occurring within the past month, or has received an organ transplant other than a kidney. Prisoners will also be excluded from the study.
2.3. Intervention
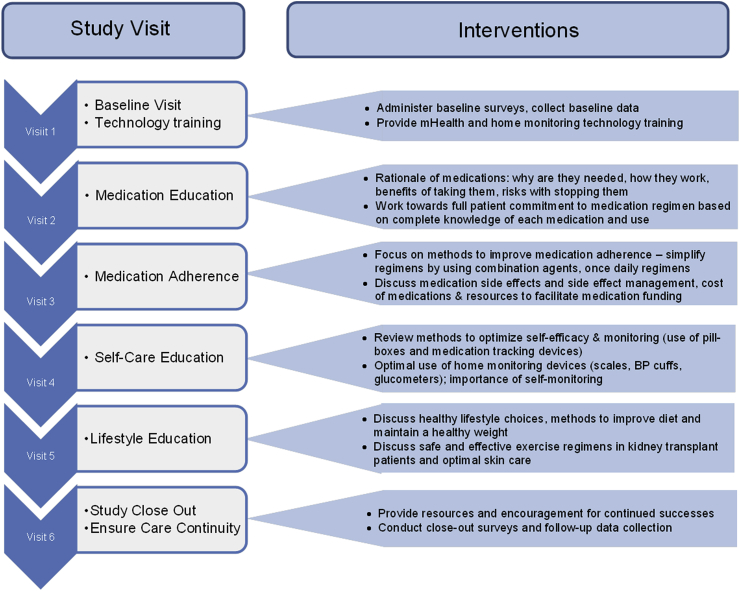
As this is a non-randomized trial, all participants will receive the interventions discussed as followed (no control group). The study intervention will consist of five monthly, face-to-face, pharmacist-led encounters designed to reduce patient-level factors creating barriers for CVD risk factor control, including medication adherence, medication errors, self-efficacy, and lifestyle choices. Fig. 1 outlines the activities that will occur during each session.
Fig. 1.
Legend – Schematic of 6 monthly visits with details of the activities and interventions occurring at each visit.
Visit 1 will be the baseline visit. During this encounter, patients will complete six baseline survey assessments which include a detailed medical history. The six assessment surveys are in the domains of medication adherence, medication side-effects, self-efficacy, health literacy, transplant knowledge, quality of life, depression, and stress/social support (see Table 1). After completion of these surveys, the pharmacist will conduct a thorough medication history, assess the complete medication regimen, and document any identified medication errors. Next, patients will be provided and trained on using a home-based monitoring system and test strips for measuring and documenting home blood pressures and glucose levels. This system works similarly to other home-monitoring systems, but allows the data to be aggregated and reported to providers through a smartphone or mobile device that uploads the data into a HIPAA compliant computer portal. This portal compiles the data and provides reports that track home-based readings, trends, and averages over time. The data will be used at all subsequent visits to facilitate the delivery of the intervention. We have developed processes to mitigate the influence of SES status on being capable of enrolling and completing this study. First, all the devices and testing supplies will be supplied through the study free of charge. Second, if a patient does not have a smartphone to use to sync with the devices, we will provide a tablet to them as well. Finally, we are paying $50 per visit to cover the cost of travel.
Table 1.
Patient self-reported surveys administered at baseline and end of study.
| Domain | Sub-Domain | Corresponding Validated Survey |
|---|---|---|
| Medication Related Issues | Medication Adherence | 8-Item Medication Adherence Scale |
| Medication Side Effects | Memphis Side Effect Instrument | |
| Self-Care & Knowledge | Self-Efficacy | Chronic Disease Self-Efficacy Scale |
| Health Literacy | Health Literacy Survey (CHEW) | |
| Transplant Knowledge | MUSC Transplant Knowledge Survey | |
| Psychosocial | Quality of Life | Short Form 12 (SF-12) |
| Depression | Patient Health Questionnaire 8 Item Scale | |
| Stress | Perceived Stress Scale (PSS-Cohen) | |
| Social Support | The Medical Outcomes Study Social Support Survey |
During visit 2, the pharmacist will again review the complete medication regimen, conduct medication reconciliation, and document any medical errors that are identified. The pharmacist will also discuss each medication with the patient, ensuring that they understand the indication, the most common side-effects to watch for, how to manage these side-effects, and the consequences of missed doses. Motivational interviewing techniques will be used to facilitate these discussions. Next the pharmacist will compare the patient's current regimen to the transplant center's approved protocols for diabetes, hypertension, and dyslipidemia. If any protocol deviations are found, the pharmacist will review these with the transplant nephrologist and determine a plan to mitigate these issues.
Visit 3 will entail a review of all medications, including any changes to the regimen and documentation of any medication errors. The pharmacist will then discuss medication adherence, including reasons why adherence is important and tools that can be used to improve adherence. Finally, the pharmacist will work to identify any barriers to medication adherence, such as regimen complexity and financial concerns, and work with the patient and the transplant nephrologist to rectify these issues. Again, motivational interviewing techniques will be used to facilitate these discussions.
During visit 4, the pharmacist will discuss self-care and efficacy with the patient. This includes methods to optimize home-monitoring of glucose and blood pressure levels, how to manage symptoms of chronic disease and medication side-effects, and how to monitor for signs of complications from diabetes and hypertension. Visit 5 will continue with this theme, focusing on life choices including smoking cessation (if applicable), alcohol use, appropriate dietary choices, and appropriate exercise and activity levels. At both of these sessions, the pharmacist will start by reviewing the home-based readings uploaded from mobile devices into the portal and will also review the medication regimen, documenting any medication changes and errors. The interventions delivered during sessions 4 and 5 will be delivered by the pharmacist guided by aspects of the social determinant theory construct of competence; the pharmacist will first identify the subject's beliefs, values, and short-term life goals and then directly link these to the desirable behavior change, most importantly improving upon CV risk factor monitoring and making lifestyle changes (smart dietary choices and improved activity and exercise).
Finally, at visit 6, the pharmacist will complete a study close-out visit, which will include a final review of the medication regimen, documentation of medication changes and errors, and instructions to provide the patient on where to follow-up with issues pertaining to their diabetes, hypertension, or dyslipidemia. The patient will complete a final survey which includes all the questions and domains at the baseline survey. At this point, the patient will have completed the study and no additional follow-up visits or educational interventions will be conducted.
2.4. Data collection and study definitions
2.4.1. Patient surveys
To assess the predominant patient-level barriers preventing optimal medication adherence, medication safety, and CVD risk factor control, and to determine which of the self-reported measures significantly change during the clinical trial, patients will be administered surveys at baseline (Visit 1) and at last follow-up (Visit 6) in the domains of medication-related issues, self-care, health knowledge, and psychosocial issues. Table 1 lists the validated survey instruments that will be used to test each of the major domains and sub-domains. Baseline sociodemographics and baseline and follow-up, medication errors, and clinical outcomes will be collected during this study. This data will allow for the determination of alternative contributing factors to racial disparities in kidney outcomes.
2.4.2. Identifying and defining medication errors
At each visit, the pharmacist will conduct a complete medication regimen review and assessment to identify and document all medication discrepancies and errors. We will utilize a similar methodology as the Covert el al study to identify and characterize medication errors. This characterization of medication errors was based upon previously validated methodology and clinical expertise [41].
2.4.3. CVD risk factor control
Per transplant guidelines, the goal blood pressure (BP) for a patient with hypertension and diabetes or renal disease is < 130/80 mmHg [42]. BPs will be assessed using clinical measurements taken three times, 5 min apart, with the same arm and averaged. These BP values will be used to assess for changes in BP overtime as well as assessing for control. In addition, home BPs, using the ForaCare device, will be measured at routine intervals and recorded (usually once to three time per day, depending on physician discretion). These values will be used by the patient and providers to assess optimal control, and to determine how to optimize BPs during the course of the study.
Per national transplant guidelines in place at the time this study was conceived, goal lipids are based on pre-defined CVD risk factors and the Framingham risk assessment. Most transplant patients have at least two CVD risk factors, and thus have a goal LDL of <100 md/dL. A subset of patients with diabetes and high CVD risk have a goal LDL of <70 mg/dL. Lipid assessments will be made using fasting blood draws taken at baseline at month 4 and at month 6 to assess control and change in lipoprotein levels over the course of the study [42].
Per ADA Standards of Care, the HbA1C goal for most adults is <7%. Therefore, for the purposes of the trial, patients will have an HbA1C goal of <7%. HbA1C assessments will be made using blood draws take at baseline, month 4 and month 6, to assess control and change in lipoprotein levels over the course of the study [43]. These three CVD risk factor measurements will be assessed at baseline and at the end of follow-up to determine if control improves within patients enrolled in this trial.
2.5. Patient safety
2.5.1. Adequacy of protection against risks
Participation in the study will be voluntary. Subjects who meet inclusion and exclusion criteria for the study will be provided detailed information about the study, including potential benefits and risks, given time to read through the study consent and ask questions. Following this, patients will be asked about their desire to participate in the clinical trial. Patients will be required to complete an informed consent document to ensure they understand the goals, risks, and potential benefits before any study related activities are undertaken.
There should not be any extensive risks to patient safety during the completion of this study as no investigational medications will be used and the study intervention is focused on improving education and monitoring of medications and CVD risk factors. In addition, if there are any medication changes that result from these interventions, they will be made in accordance with national guidelines and transplant center protocols that guide patient care and approved by the patient's transplant nephrologist. Patient clinical outcomes, medication side effects, and adverse events will be closely monitored during each monthly visit, documented, and reviewed by the PI and the Safety Officer as detailed in the DSMP below. In order to protect subjects against any risk regarding loss of personal information, all obligations under the Health Portability and Accountability Act (HIPAA) will be met. Furthermore, any data collected will be stored on the secure network server and behind the MUSC firewall, using the REDCap system. The primary investigator and study team will use electronic CRF forms to gather all study information. Data will only be stored on campus computers under the MUSC secure network. Although it is anticipated that all data will be collected electronically, if there are paper forms, they will be stored within an office, which is a locked and on the MUSC campus. Only study members will have access to these data forms and electronic data elements.
2.5.2. Data safety and monitoring plan (DSMP)
The data safety monitoring plan will include the use of a Safety Officer and the MUSC IRB to monitor the study-related clinical outcomes, medication side effects, and adverse events. Additionally, the DSMP will utilize a statistician to review the data generated by the study and ensure data integrity. Summaries of serious adverse event and patient safety concerns raised by the Safety Officer will be made to the NIH in yearly progress reports unless the nature of a particular event is such that it bears reporting to the NIH immediately. The functions of the Safety Officer are to provide scientific oversight, review all adverse effects or complications related to the study, monitor accrual, review summary reports relating to compliance with protocol requirements, and to provide advice on resource allocation.
The Safety Officer and statistician will review data and safety reports at the following study milestones: once 15 patients have completed the intervention, once 30 patients have completed the intervention, once 45 patients have completed the intervention and once all 60 patients have completed the intervention. The team will also meet on an as needed basis for any unexpected serious adverse events or significant study issues. Data will be provided for these reviews by the investigators on key variables that may indicate harm, including changes in glycemic control, blood pressure, serum lipoproteins, medication errors, side effects and adherence. Study patient clinical events, including hospitalizations, emergency room visits, acute rejections, graft loss and patient death, will also be reviewed.
The biostatistician will evaluate confidentiality and integrity of the database, and the procedures for recording and storing confidential files. The Safety Officer will also review the elements of the research plan to deal with emergencies. At the conclusion of these reviews, the recommendations of the Safety Officer will be reviewed and the PI and investigators will take appropriate corrective actions as needed.
The Safety Officer will have the authority to halt the trial if he perceives that harm is occurring due to the interventions.
The IRB has reviewed and approved the protocol, patient consent forms, ensured protection of patient privacy and safety, and will monitor the study on an ongoing basis. Study-related serious adverse events will be reported to the IRB as they occur. Annual reports to the IRB will indicate accrual rate, serious adverse events and new findings that may influence continuation of the study.
2.6. Data management
The Research Electronic Data Capture (REDCap) system will be used for data management. REDCap is a secure, web based application designed exclusively to support data capture for research studies. REDCap provides an intuitive interface for data entry (with data validation), audit trails for tracking data manipulation and export procedures, automated export procedures for seamless data downloads to common statistical packages (SPSS, SAS, Stata, R, MPlus), procedures for importing data from external sources, and advanced features such as branching logic and calculated fields. The REDCap project (http://www.project-redcap.org) was initiated at Vanderbilt University and includes more than 70 active institutional partners from CTSA, GCRC, RCMI funded institutions, including MUSC, and others through a collaborative international consortium.
2.7. Statistical analysis
Data for this clinical trial will be analyzed in a paired intent-to-treat fashion, with each patient acting as their own control, and baseline data being compared to month 6 results (or the last follow-up values carried forward for patients that do not complete the study). Initially, standard descriptive statistics (mean, standard deviation, median, interquartile range and proportions) will be used to assess the baseline characteristics of the study cohort, and proportions will be used to determine feasibility metrics (using proportions of enrolled to approached and completed the study to enrolled). Medication errors will be assessed at baseline and compared to errors at 6 months, using the paired Student's t-test if the data is normally distributed\\ or the paired sample Wilcoxon signed rank test is this assumption is violated.
For the objective of assessing CVD risk factor control, analyses will be conducted by comparing change in values (BPs, A1Cs and lipids) from baseline to end of study with percent of patients deemed at optimal control for each value (as defined above) at the beginning and end of study. Continuous variables will be compared using the paired Student's T-test, with categorical data compared using the McNemar's test. Analyses will be conducted to test intervention effects by race to determine if there are differences between AA vs. non-AA patients using the Breslow Day test in stratified analyses. Additional analyses will include assessing for the effect of relevant covariates on intervention impact and testing for potential mediating and moderating variables using moderator/mediator analysis approaches.
The study is powered based on improving post-transplant hypertension control to goal levels. Hypertension was chosen as it is the predominant CVD risk factor occurring in nearly all kidney transplant recipients (>90%). Grounded on preliminary data, approximately 30% of kidney recipients meet optimal hypertension goals (<130/80). Using this as the baseline rate of control, this study will have 80% power to detect an 18% improvement in rate of optimal BP control (30% baseline, 48% at end of follow-up) using McNemar's test with alpha set at 0.05. Control and degree of change of all CVD risk factors, including BPs, A1Cs and lipids will also be assessed. The secondary objective this study is to compare the improvement in CVD risk factor control between AA and non-AA recipients. We expect to detect a difference in the impact this study intervention has on patients based on race, although the study is not powered to detect a statistically significant difference.
Patient self-reported medication adherence will also be assessed by comparing the adherence score at baseline to that at the end of the intervention using the validated instrument in Table 1. Medication side effects will also be compared in a similar manner, using the Memphis side effect scale. For variables that are captured at each of the six visits, we will also conduct an ANOVA repeated measures assessment. This includes blood pressures and medication error rates.
3. Discussion
Despite decades of research into potential etiologies, racial disparities for graft outcomes within AA kidney transplant recipients continue in both magnitude and scale [1], [2], [3]. Most previous interventions aimed at mitigating these inequalities have focused on improving access and optimizing immunosuppression with the hopes of reducing the known higher immunologic risks within AAs [21], [22]. To date, little attention has been spent on understanding and improving CVD risk factor control as a mechanism to improve racial disparities within transplantation [12]. Our previous research suggests this to be a promising area of research [40]. This clinical trial aims at using technology and pharmacist-led interventions to improve kidney transplant recipients CVD risk factor control and mitigating known disparities in hypertension and diabetes control with AAs.
The study is novel in a number of approaches. It is innovative in its proposed design and implementation. Few studies have focused on improving CVD risk factor control within transplant recipients. The use of pharmacist-led interventions is also novel in its approach to this complex problem [44]. This study design is pioneering in that it focuses on identifying and improving patient-level barriers to CVD risk factor control within kidney transplant recipients. It does so by working towards augmenting outcomes by improving the access and adherence of CVD risk factor medications within transplant patients. The use of home-based monitoring and mHealth technology is innovative and has demonstrated promising results in studies conducted both within and outside the transplant medical discipline. In addition, pharmacist-led interventions have been successful within studies conducted in non-transplant patients, including diabetes [45], hypertension [46], heart failure [47], and dyslipidemia, [48]. However, there are very limited studies addressing this within transplant recipients. Previous studies conducted within our transplant center have demonstrated that pharmacist-led multidisciplinary interventions can improve medication safety and quality outcomes in perioperative transplant patients [49], [50]. Therefore, the proposed pharmacist-led approach to improving outcomes is both innovative and substantiated by previous research.
There are a number of limitations with this study that require discussion. First, although it is prospective, it is not randomized and all patients receive the interventions. While this limits the ability to compare outcomes to a usual care group, it does allow more power to conduct before and after analyses, while also having enough patients in the intervention to conduct race stratified comparisons. Second, the study is only 6 months in duration. While this is long enough to demonstrate improvements in hypertension and diabetes control, it will not have enough follow-up time to demonstrate significant improvements in graft function or survival. Finally, the study focuses on medication safety and patient self-efficacy in a multimodal fashion, but does not implement systems-level interventions, such as improving communication across different patient providers or healthcare facilities. We hope to overcome this issue by educating the patients sufficiently so that they better navigate issues with fragmented care.
In conclusion, the study will provide important and novel information regarding potential interventional methods to improve CVD risk factor control using innovative technology and pharmacist-led interventions. Further, the results of this study should provide sufficient preliminary evidence to determine if these interventions improve CVD risk factor control more substantially in AA kidney transplant recipients; thus offering a promising mechanism to mitigate racial disparities in kidney transplantation.
Acknowledgements
The authors have no acknowledgements.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2017.11.008.
Contributor Information
Andrew J. Cole, Email: coleand@musc.edu.
Reginald W. Johnson, II, Email: johnsreg@musc.edu.
Leonard E. Egede, Email: legede@mcw.edu.
Prabhakar K. Baliga, Email: baligap@musc.edu.
David J. Taber, Email: taberd@musc.edu.
Funding
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK099440.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2010 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration. Healthcare Systems Bureau, Division of Transplantation; 2011. [Google Scholar]
- 2.Opelz G., Mickey M.R., Terasaki P.I. Influence of race on kidney transplant survival. Transpl. Proc. 1977;9:137–142. [PubMed] [Google Scholar]
- 3.Terasaki P.I., Opelz G., Mickey M.R. Summary of kidney transplant data, 1977-factors affecting graft outcome. Transpl. Proc. 1978;10(2):417–421. [PubMed] [Google Scholar]
- 4.Ciancio G., Burke G.W., Suzart K., Mattiazzi A., Vaidya A., Roth D., Kupin W., Rosen A., Johnson N., Miller J. The use of daclizumab, tacrolimus and mycophenolate mofetil in African-American and Hispanic first renal transplant recipients. Am. J. Transpl. 2003;3(8):1010–1016. doi: 10.1034/j.1600-6143.2003.00181.x. [DOI] [PubMed] [Google Scholar]
- 5.Neylan J.F. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 kidney transplant study group. Transplantation. 1998;65(4):515–523. doi: 10.1097/00007890-199802270-00011. 27. [DOI] [PubMed] [Google Scholar]
- 6.Podder H., Podbielski J., Hussein I., Katz S., Buren C., Kahan B.D. Sirolimus improves the two-year outcome of renal allografts in African-American patients. Transpl. Int. 2001;14(3):135–142. doi: 10.1007/s001470100315. [DOI] [PubMed] [Google Scholar]
- 7.Weber M., Deng S., Arenas J., Aradhye S., Grossman R., Shaw L., Naji A., Barker C., Brayman K.L. Decreased rejection episodes in African-American renal transplant recipients receiving mycophenolate mofetil/tacrolimus therapy. Transpl. Proc. 1997;29(8):3669–3670. doi: 10.1016/s0041-1345(97)01067-1. [DOI] [PubMed] [Google Scholar]
- 8.Butkus D.E., Meydrech E.F., Raju S.S. Racial differences in the survival of cadaveric renal allografts. overriding effects of HLA matching and socioeconomic factors. N. Engl. J. Med. 1992;327(12):840–845. doi: 10.1056/NEJM199209173271203. [DOI] [PubMed] [Google Scholar]
- 9.Curtis J.J. Kidney transplantation: racial or socioeconomic disparities? Am. J. Kidney Dis. 1999;34(4):756–758. doi: 10.1016/S0272-6386(99)70404-X. [DOI] [PubMed] [Google Scholar]
- 10.Schweizer R.T., Rovelli M., Palmeri D., Vossler E., Hull D., Bartus S. Noncompliance in organ transplant recipients. Transplantation. 1990;49(2):374–377. doi: 10.1097/00007890-199002000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Kalil R., Heim-Duthoy K., Kasiske B. Patients with a low income have reduced renal allograft survival. Am. J. Kidney Dis. 1992;20(1) doi: 10.1016/s0272-6386(12)80318-0. 63. [DOI] [PubMed] [Google Scholar]
- 12.Cosio F.G., Dillon J.J., Falkenhain M.E., Tesi R.J., Henry M.L., Elkhammas E.A., Davies E.A., Bumgardner G.L., Ferguson R.M. Racial differences in renal allograft survival: the role of systemic hypertension. Kidney Int. 1995;47(4):1136–1141. doi: 10.1038/ki.1995.162. [DOI] [PubMed] [Google Scholar]
- 13.Cosio F.G., Pesavento T.E., Kim S., Osei K., Henry M., Ferguson R.M. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002;62(4):1440–1446. doi: 10.1111/j.1523-1755.2002.kid582.x. [DOI] [PubMed] [Google Scholar]
- 14.Cosio F.G., Hickson L.J., Griffin M.D., Stegall M.D., Kudva Y. Patient survival and cardiovascular risk after kidney transplantation: the challenge of diabetes. Am. J. Transpl. 2008;8(3):593–599. doi: 10.1111/j.1600-6143.2007.02101.x. [DOI] [PubMed] [Google Scholar]
- 15.Leffell M.S., Steinberg A.G., Bias W.B., Machan C.H., Zachary A.A. The distribution of HLA antigens and phenotypes among donors and patients in the UNOS registry. Transplantation. 1994;58(10):1119–1130. [PubMed] [Google Scholar]
- 16.Takemoto S., Terasaki P.I., Cecka J.M., Cho Y.W., Gjertson D.W. Survival of nationally shared, HLA-matched kidney transplants from cadaveric donors. N. Engl. J. Med. 1992;327(12):834–839. doi: 10.1056/NEJM199209173271202. [DOI] [PubMed] [Google Scholar]
- 17.Rebellato L.M., Arnold A.N., Bozik K.M., Haisch C.E. HLA matching and the united network for organ sharing allocation system: impact of HLA matching on African-American recipients of cadaveric kidney transplants. Transplantation. 2002;74(11):1634–1636. doi: 10.1097/00007890-200212150-00024. [DOI] [PubMed] [Google Scholar]
- 18.Kerman R.H., Kimball P., Van Buren C.T., Lewis R.M., Kahan B.D. Possible contribution of pretransplant immune responder status to renal allograft survival differences of black versus white recipients. Transplantation. 1991;51(2) doi: 10.1097/00007890-199102000-00013. 338. [DOI] [PubMed] [Google Scholar]
- 19.McDaniel D., Barber W., Nguyan C., Rhodes S., May W., McDaniel L., Vig P., Jemeson L., Butkus D. Combined analysis of cytokine genotype polymorphism and the level of expression with allograft function in African-American renal transplant patients. Transpl. Immunol. 2003;11(1):107–119. doi: 10.1016/S0966-3274(02)00171-5. [DOI] [PubMed] [Google Scholar]
- 20.Hutchings A., Purcell W.M., Benfield M.R. Increased costimulatory responses in African-American kidney allograft recipients. Transplantation. 2001 Mar 15;71(5):692–695. doi: 10.1097/00007890-200103150-00021. [DOI] [PubMed] [Google Scholar]
- 21.Young C.J., Kew C. Health disparities in transplantation: focus on the complexity and challenge of renal transplantation in African Americans. Med. Clin. N. Am. 2005;89:1003–1031. doi: 10.1016/j.mcna.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Malat G.E., Culkin C., Palya A., Ranganna K., Kumar M.S. African American kidney transplantation survival: the ability of immunosuppression to balance the inherent pre- and post-transplant risk factors. Drugs. 2009;69(15):2045–2062. doi: 10.2165/11318570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Hricik D.E., Anton H.A.S., Knauss T.C., Rodriguez V., Seaman D., Siegel C., Valente J., Schulak J.A. Outcomes of African American kidney transplant recipients treated with sirolimus, tacrolimus, and corticosteroids. Transplantation. 2002;74(2):189–193. doi: 10.1097/00007890-200207270-00008. [DOI] [PubMed] [Google Scholar]
- 24.Pilch N.A., Taber D.J., Moussa O., Thomas B., Denmark S., Meadows H.B. Prospective randomized controlled trial of rabbit antithymocyte globulin compared with IL-2 receptor antagonist induction therapy in kidney transplantation. Ann. Surg. 2014;259:888–893. doi: 10.1097/SLA.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 25.Pallet N., Thervet E., Alberti C., Emal-Aglaé V., Bedrossian J., Martinez F., Roy C., Legendre C. Kidney transplant in black recipients: are African Europeans different from African Americans? Am. J. Transpl. 2005;5(11):2682–2687. doi: 10.1111/j.1600-6143.2005.01057.x. [DOI] [PubMed] [Google Scholar]
- 26.Yeates K., Wiebe N., Gill J., Sima C., Schaubel D., Holland D., Hemmelgarn B., Tonelli M. Similar outcomes among Black and White renal allograft recipients. J. Am. Soc. Nephrol. 2009;20(1):172–179. doi: 10.1681/ASN.2007070820. PMCID: PMC2615721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakkera H.A., O'Hare A.M., Johansen K.L., Hynes D., Stroupe K., Colin P.M., Chertow G.M. Influence of race on kidney transplant outcomes within and outside the department of veterans affairs. J. Am. Soc. Nephrol. 2005;16(1):269–277. doi: 10.1681/ASN.2004040333. [DOI] [PubMed] [Google Scholar]
- 28.Isaacs R.B., Nock S.L., Spencer C.E., Connors A.F., Wang X.Q., Sawyer R., Lobo P.I. Racial disparities in renal transplant outcomes. Am. J. Kidney Dis. 1999;34(4):706–712. doi: 10.1016/S0272-6386(99)70397-5. [DOI] [PubMed] [Google Scholar]
- 29.Isaacs R.B., Conners A., Jr., Nock S., Spencer C., Lobo P. Noncompliance in living-related donor renal transplantation: the united network of organ sharing experience. Transpl. Proc. 1999;31(4A):19S–20S. doi: 10.1016/s0041-1345(99)00117-7. [DOI] [PubMed] [Google Scholar]
- 30.Chisholm-Burns M.A., Spivey C.A., Rehfeld R., Zawaideh M., Roe D.J., Gruessner R. Immunosuppressant therapy adherence and graft failure among pediatric renal transplant recipients. Am. J. Transpl. 2009;9(11):2497–2504. doi: 10.1111/j.1600-6143.2009.02793.x. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigo E., Fernández-Fresnedo G., Valero R., Ruiz J.C., Piñera C., Palomar R., González-Cotorruelo J., Gómez-Alamillo C., Arias M. New-onset diabetes after kidney transplantation: risk factors. J. Am. Soc. Nephrol. 2006;17(12 suppl 3):S291–S295. doi: 10.1681/ASN.2006080929. [DOI] [PubMed] [Google Scholar]
- 32.Cowie C.C., Port F.K., Rust K.F., Harris M.I. Differences in survival between black and white patients with diabetic end-stage renal disease. Diabetes Care. 1994;17(7):681–687. doi: 10.2337/diacare.17.7.681. [DOI] [PubMed] [Google Scholar]
- 33.U S Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-stage Renal Disease in the United States. [Google Scholar]
- 34.Banerji M.A. Diabetes in African Americans: unique pathophysiologic features. Curr. Diabetes Rep. 2004;4(3):219–223. doi: 10.1007/s11892-004-0027-3. [DOI] [PubMed] [Google Scholar]
- 35.Crook E.D. Diabetic renal disease in African Americans. Am. J. Med. Sci. 2002;323(2):78–84. doi: 10.1097/00000441-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Egede L.E., Dagogo-Jack S. Epidemiology of type 2 diabetes: focus on ethnic minorities. Med. Clin. North Am. 2005;89(5):949–975. doi: 10.1016/j.mcna.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Ferdinand K.C. Coronary heart disease and lipid-modifying treatment in African American patients. Am. Heart J. 2004;147(5):774–782. doi: 10.1016/j.ahj.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Scantlebury V., McCauley J., Woods H., Shapiro R., Irish W., McMichael J., Jordan M., Vivas C., Fung J.J., Starzl T.E. Posttransplant hypertension in Blacks versus nonBlacks. Transpl. Proc. 1993;25(4):2456–2457. PMCID: PMC2972903. [PMC free article] [PubMed] [Google Scholar]
- 39.Gaston R.S. Factors affecting renal allograft survival in African Americans. Blood Purificat. 1996;14(4):327–333. doi: 10.1159/000170281. [DOI] [PubMed] [Google Scholar]
- 40.Taber D.J., Hunt K.J., Fominaya C.E., Payne E.H., Gebregziabher M. Impact of cardiovascular risk factors on graft outcome disparities in black kidney transplant recipients. Hypertension. 2016;68:715–725. doi: 10.1161/HYPERTENSIONAHA.116.07775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Covert K.L., Mardis C.R., Fleming J.N., Pilch N.A., Meadows H.B. Development of a predictive model for drug-related problems in kidney transplant recipients. Pharmacotherapy. 2017;37:159–169. doi: 10.1002/phar.1886. [DOI] [PubMed] [Google Scholar]
- 42.Kasiske B.L., Zeier M.G., Craig J.C., Ekberg H., Garvey C.A. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transpl. 2009;9(suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 43.American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2013;36(suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chisholm M.A., Mulloy L.L., Jagadeesan M., Martin B.C., DiPiro J.T. Effect of clinical pharmacy services on the blood pressure of African-American renal transplant patients. Ethn. Dis. 2002;12(3):392–397. [PubMed] [Google Scholar]
- 45.Leal S., Glover J.J., Herrier R.N., Felix A. Improving quality of care in diabetes through a comprehensive pharmacist-based disease management program. Diabetes Care. 2004;27(12):2983–2984. doi: 10.2337/diacare.27.12.2983. [DOI] [PubMed] [Google Scholar]
- 46.Green B.B., Cook A.J., Ralston J.D., Fishman P.A., Catz S.L., Carlson J., Carrell D., Tyll L., Larson E.B., Thompson R.S. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control. J. Am. Med. Assoc. 2008;299(24):2857–2867. doi: 10.1001/jama.299.24.2857. PMCID: PMC2715866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gattis W.A., Hasselblad V., Whellan D.J., O'Connor C.M. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the pharmacist in heart failure assessment recommendation and monitoring (PHARM) study. Arch. Intern Med. 1999;159(16):1939. doi: 10.1001/archinte.159.16.1939. [DOI] [PubMed] [Google Scholar]
- 48.Ellis S.L., Carter B.L., Malone D.C., Billups S.J., Okano G.J., Valuck R.J., Barnette D.J., Sintek C.D., Covey D., Mason B. Clinical and economic impact of ambulatory care clinical pharmacists in management of dyslipidemia in older adults: the IMPROVE study. Pharmacotherapy. 2000;20(12):1508–1516. doi: 10.1592/phco.20.19.1508.34852. [DOI] [PubMed] [Google Scholar]
- 49.Taber D.J., Pilch N.A., McGillicuddy J.W., Bratton C.F., Chavin K.D., Baliga P.K. Improved patient safety and outcomes with a comprehensive interdisciplinary improvement initiative in kidney transplant recipients. Am. J. Med. Qual. 2013;28:103–112. doi: 10.1177/1062860612450309. [DOI] [PubMed] [Google Scholar]
- 50.Taber D.J., Pilch N.A., McGillicuddy J.W., Bratton C.F., Lin A., Chavin K.D., Baliga P.K. Improving the perioperative value of care for vulnerable kidney transplant recipients. J. Am. Coll. Surg. 2013;216:668–676. doi: 10.1016/j.jamcollsurg.2012.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.