Abstract
We examine the effects of state health insurance mandates requiring coverage of screening mammograms. We find evidence that mammography mandates significantly increased mammography screenings by 4.5–25 percent. Effects are larger for women with less than a high school degree in states that ban deductibles, a policy similar to a provision of federal health reform that eliminates cost-sharing for preventive care. We also find that mandates increased detection of early stage in-situ pre-cancers. Finally, we find a substantial proportion of the increased screenings were attributable to mandates that are not consistent with current recommendations of the American Cancer Society.
Keywords: insurance mandates, mammography, breast cancer, quasi-experiment
Recent federal health care reform (the Patient Protection and Affordable Care Act) requires that new or substantially altered private insurance plans cover a variety of preventive health services and prohibits insurance companies from imposing cost-sharing for those services, with the goal of increasing utilization. Mammography, the standard screening test for breast cancer, is one of the most common preventive services used by adult women and played a prominent role in debates about health reform.1 Routine mammography rates among adult women are substantially below the recommended levels of both the United States Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS).
Expanding coverage of mammography through federal health care reform therefore has the potential to increase mammography rates and affect breast cancer outcomes, but there is surprisingly little research showing that adopting more generous insurance coverage will, in fact, increase screening utilization. We provide evidence on this question by studying state experimentation with very similar insurance coverage expansions in the form of benefits mandates. Specifically, from 1987–2000, 42 states adopted laws requiring private insurers within the state to include screening mammography benefits in insurance plans, and six of those states further imposed requirements similar to those in federal health reform that insurance companies may not impose cost-sharing on women who obtain mammograms.2 These policies have not been previously studied using quasi-experimental methods and thus their presence provides researchers a unique opportunity to understand whether mandating insurance coverage and, in some cases, prohibiting cost-sharing for relatively low-cost preventive health services can increase screening utilization and affect breast cancer diagnoses. In so doing, our research also provides valuable insight into the likely effects of federal health reform with respect to mammography and breast cancer outcomes.
To evaluate the effects of the state mandates, we draw on data on mammography screening from the Centers for Disease Control’s 1987–2000 Behavioral Risk Factor Surveillance System (BRFSS). We evaluate the effects of these laws using state and year fixed effects models and augmented triple differences (DDD) models, each with relative strengths and weaknesses. The two-way fixed effects approach takes advantage of variation in the timing of adoption across states. The DDD models augment this approach by using the fact that the mandates specify different benefits for women of different ages, allowing us to estimate models with fixed effects for state, year, and age group, as well as for each of their two-way interactions. We supplement these data with information from the Surveillance Epidemiology and End Results (SEER) cancer registry system to test for effects of mandates on breast cancer diagnoses and in-situ pre-cancer diagnoses using the same approaches.
To preview, we find evidence that state mandates requiring insurance coverage for mammograms significantly increased past year mammography rates among women age 25–74 by between 4.5 and 25 percent, and these effects are driven by insured women. We also find that mandates prohibiting deductibles for mammography—similar to provisions in the recently adopted federal health reform—significantly increased mammography screenings among women with less than a high school degree relative to mandates without such limitations on out-of-pocket costs. These results confirm that mandating insurance coverage for low-cost preventive health services can meaningfully increase utilization rates. They also suggest that federal health reform is likely to further increase mammography screenings. Regarding breast cancer diagnoses, we find that the mandates increased detection of the earliest stage in-situ pre-cancers. Finally, we show that a substantial proportion of the increased screenings are attributable to mandates that are not consistent with current guidelines of the American Cancer Society. This suggests that at least some of the increased screenings may not have been welfare enhancing and is consistent with our findings on in-situ diagnoses, as there is disagreement in the medical community about whether in-situ pre-cancers are likely to progress to more invasive stages.
The paper proceeds as follows: Section I outlines institutional details regarding mammography and the insurance mandates we study, and Section II describes the relevant literature. We describe the research design, data, and empirical approach in Section III, and Section IV presents the results. Section V concludes.
I. Breast Cancer Screening and Institutional Details
Breast cancer is both the most commonly diagnosed cancer and the second leading cause of cancer death among women in the United States: 40,000 women die of breast cancer each year. Early detection of breast cancer through regular screening mammograms is commonly understood to be a key if not the most important determinant of survival. In mammography, a woman’s breasts are placed on a machine that takes low-dose X-ray pictures to check for abnormalities. Screening mammograms are typically given to asymptomatic women to look for suspicious markers. Diagnostic mammograms usually occur among women who have had a previous abnormal screening mammogram (approximately 10 percent of those screened in the early 1990s), have a family history of breast cancer, or have certain symptoms (e.g., presence of lumps in a breast or changes in a nipple or breast). Abnormal screening results can also lead to more invasive procedures such as biopsy.
Cutler (2008) argues that increases in routine cancer screenings such as mammography represent the most important factor behind the reversal in age-adjusted cancer mortality rates that occurred in the 1990s, while Berry et al. (2005) find that the share of the decrease in the rate of breast cancer deaths from 1975 to 2000 due to screening ranged from 28 percent to 65 percent (with treatment accounting for the rest). The increase in population mammography rates was particularly broad-based from 1987 to 2000: screening rates among non-elderly adult women about doubled for women of different age, race/ethnicity, marital status, education, and even household income groups.
The majority of states adopted mammography benefits mandates for qualified private health insurance plans from 1987 to 2000. The modal mandate calls for private insurance plans within the state to cover baseline screening mammograms for 35 to 39 year olds, biennial mammograms for 40 to 49 year olds, and annual mammograms for women age 50 and older. These mandates apply to the insurance companies who sell insurance to private employers (or, in some cases, sell to individuals). Women who have their own employer-related private insurance coverage or who have insurance through employed husbands or others would be affected by these mandates if the firm was not self-insured.
These age-based benefits reflect the age-specific mammography frequency recommendations supported by the American Cancer Society from 1983 until 1991 for asymptomatic women at average risk of getting breast cancer. In 1992 the ACS eliminated the recommendation that 35 to 39 year olds obtain a baseline screening mammogram, and in March 1997 the ACS further revised its recommendations to state that annual screening mammography should begin at age 40.3 In recognition of these changes, some of the mammography mandates adopted in the latter part of our sample period revised pre-existing rules to require plans to cover (or less commonly offer) annual mammography screenings for women age 40 and older.4 Moreover, a handful of states have used different age-based cutoffs in their laws. For example, Wisconsin’s 1990 law requires coverage for two mammograms for women age 45 to 49, provided they have not had one within two years (i.e., this law mandated coverage of nearly biennial mammography beginning at age 45). Texas’ 1987 mandate requires coverage for annual mammograms for all women age 35 and older. Thus, there is substantial age by state by year variation in the frequency of screenings whose coverage is required in state laws that forms the basis of one of our identification strategies below (the fully interacted DDD model).
II. Relevant Literature
Our paper is related to a large literature in economics that has used experimental and quasi-experimental methods to identify causal effects of insurance coverage generosity on use of health services and health outcomes, such as the RAND Health Insurance Experiment (HIE) (Manning et al. 1987), the Oregon Health Insurance Experiment (Finkelstein et al. 2012), and the Massachusetts Health Reform (Kolstad and Kowalski, 2010), all of which examined mammography screenings as a key preventive health care outcome.5 The results of those studies are mixed. Manning et al. (1987) found that cost-sharing deterred participants from obtaining preventive care relative to the ‘free’ plan in the controlled setting of the RAND HIE from 1971 to 1982. Lurie et al. (1987), however, show that mammography rates among women aged 45–64 in the RAND HIE were only around 2 percent, precluding direct tests of cost-sharing on mammography in particular. Regarding a closely related preventive cancer screening – Pap tests for cervical cancer – they found no difference between screening rates for people in the ‘free’ plan versus people randomized to cost-sharing. Finkelstein et al. (2012) study low-income Medicaid-eligible women and find that participants who took-up Medicaid in the state due to winning a lottery in 2008 (i.e., generally moved from no insurance to public insurance) were significantly more likely to get a mammogram in the first year after the program, an effect on the order of 60 percent relative to the control group mean. Notably, there was no cost-sharing for participants in the Oregon plan. In contrast, Kolstad and Kowalski (2010) find no significant change in mammography rates for women in Massachusetts relative to women in other states after the implementation of the state’s mandated health insurance reform in 2006. Thus, the existing quasi-experimental evidence on the role of insurance coverage and cost-sharing in screening mammography is mixed.
We complement these studies in the following ways. First, we examine effects of a different type of policy intervention that specifically targets screening mammography and that in some states mimics key provisions in the federal health reform. Second, we examine effects among a much larger share of the female population (all women 25–74 as opposed to only low-income women in the Oregon case, only 40–64 or 45–64 year old women as in the Oregon and RAND cases, or only women in a single state as in the Oregon and Massachusetts cases). Third, we directly examine effects on cancer diagnoses.
There is little research that estimates the effects of state insurance benefit mandates requiring coverage of mammography. Two public health studies find positive associations between mammography mandates and utilization using purely cross-sectional designs (Mor and Shackleton 2005, Pettibone 2003).6 Dans and Wright (1996) examined claims data for outpatient mammograms for women in Maryland’s Blue Cross Blue Shield plan before and after the state’s 1991 mammography mandate was implemented; they found evidence of a modest increase in overall screening rates. There is, however, no quasi-experimental work that uses the timing of mandate adoption for multiple states while controlling for fixed differences across states or over time.
The absence of a substantial literature on the utilization effects of mammography benefits mandates is striking given that mammography is one of the most commonly mandated benefits (Bunce and Wieske 2008) and over this time period there were unprecedented increases in mammography rates for older women.7 Moreover, other types of state level insurance benefit mandates have been studied extensively by economists. These include: pregnancy benefits, (Gruber 1994a), infertility treatment (e.g., Bitler 2010; Bitler and Schmidt 2012; Schmidt 2007; Bundorf, Henne, and Baker 2007; Buckles 2008), mental health parity (e.g., Pacula and Sturm 2000; Harris, Carpenter, and Bao 2007; Busch and Barry 2008), and overnight hospital stays for newborn deliveries (e.g., Liu, Dow, and Norton 2004; Almond and Doyle 2011).
Researchers have identified a number of considerations for understanding the extent to which any mandated benefits laws should affect outcomes. First, it is commonly argued that mandated benefits laws can cause employers—particularly small firms—to reduce offers of health insurance in response to the rising costs when mandated benefits laws are adopted. While the empirical evidence on this is mixed (Gruber 1994b, Jensen and Gabel 1989, Jensen and Morrisey 1999), any such effects would reduce the potential for benefit mandates to increase utilization. Second, as we noted above, certain insurance plans are exempted from compliance requirements with any state health insurance mandates. The largest of these is the exemption because of ERISA for self-funded insurance plans which generally affects large employers (Buchmueller et al. 2007), though there is very little evidence on how self-insured firms respond to state insurance mandates.8 Butler (2000) estimates that about a third of women have private insurance that would potentially be affected by mandates such as those we study here.
Third, it is possible that benefits mandates do not have much “bite” to the extent that pre-existing private health insurance plans were already covering or offering mammograms. However, available evidence indicates that benefits coverage for these services did not become widespread until the mid-1990s, implying that there was substantial latitude for mammography benefits mandates to affect benefits coverage. A 1986 article in The New York Times lamented that “health insurance plans rarely, if ever, cover screening mammograms” (Brozan 1986). Multiple studies using Health Insurance Association of American (HIAA) employer survey data from the early 1990s indicate that mammograms were covered by less than 70 percent of private and non-self-insured plans, respectively (Sullivan and Rice 1991; McKinney and Marconi 1992). By 1999 the Kaiser/HRET Survey of Employer-Sponsored Health Benefits found that 94 percent of conventional plans and 98 percent of HMO plans were covering mammography screening, suggesting a large increase in mammography coverage over a period of significant mandate adoption (Kaiser/HRET 1999).
Finally, it is natural to ask—given the fairly low cost of low-dose screening mammography ($50–$150 per screening according to Breen and Brown 1994)9—why weren’t all employers and health plans covering these screenings even in the absence of a mandate?10 Note that the population at risk of using a mammogram is very large (both absolutely and relative to other benefits such as infertility and substance abuse treatment): currently, the ACS (USPSTF) recommends that all women age 40 (50) and older get regular screening mammograms. And, even though the direct costs of screening are fairly low, the subsequent costs associated with a positive screening—diagnostic mammography, biopsy, chemotherapy, mastectomy, and other cancer treatments—can be much larger. Like many screening tests, mammograms have a high false positive rate even at a point in time which can lead to high rates of false positives over a woman’s lifetime experience of many screenings. (Upwards of 10 percent of screening mammograms can produce abnormal results. Given that the vast bulk of women screened do not have cancer (remember about 40,000 women are diagnosed each year), the vast bulk of these are false positives.
III. Data Description and Empirical Approach
Our main data on mammography screening come from the Center for Disease Control’s Behavioral Risk Factor Surveillance System (BRFSS). Fielded annually since 1984, the BRFSS has included questions about mammograms in every year since 1987 and is designed to be representative at the state level. Surveys are fielded by the individual states and then sent to CDC to be compiled into a public-use dataset. State participation in the BRFSS increased over the late 1980s; the last state joined in the mid-1990s. In practice, this means that we have an unbalanced panel; because many states adopted laws prior to 1990 we use all available data (i.e., any state/year combination with BRFSS data), though in robustness tests we focus on the subset of states in a balanced panel.11 Our analysis focuses on the period 1987–2000; 42 states adopted or changed mandates over this period.12
The BRFSS breast health questions allow us to create consistent measures of mammography use along several dimensions for women age 18 and older. Specifically, women were asked: “A mammogram is an X-ray of each breast to look for breast cancer. Have you ever had a mammogram?” Women who report ever having had a mammogram are then asked about the timing of their most recent mammogram, as well as the reason for their most recent mammogram.13 We create several outcome variables related to mammography use: first, we identify Ever Had Mammogram as equal to one if the woman reports ever having had a mammogram and zero otherwise. Second, we create Mammogram in the Past Year as equal to one if the woman reports that she had a mammogram within the past year and zero otherwise.14 Third, we create Mammogram in the Past Two Years as equal to one if the woman reports that she had a mammogram within the past two years and zero otherwise. Because the timing of a woman’s most recent mammogram beyond one year is likely to be problematic (Warnecke et al. 1997), we focus on Mammogram in the Past Year as our main outcome of interest. Finally, women are also asked about the reason for their most recent mammogram. We create a variable called Routine Mammogram in the Past Year that equals one if a woman reports she had a mammogram in the last year and also reports that her most recent mammogram was ‘routine’ (as opposed to being due to ‘cancer’ or a ‘problem’). This is the main type of screening that should be affected by the mandates. We also observe (and control for) standard demographic characteristics in the BRFSS, including age, race, education, and marital status. The BRFSS also includes a very basic measure of health insurance coverage: we are able to identify whether the woman is covered by ‘any health plan.’15
To estimate the effects of the mandates on outcomes we use multiple complementary quasi-experimental approaches, including two-way fixed effects models (which control for unrestricted state and year dummies) and augmented triple difference (DDD) models. In the standard state and year fixed effects framework, we use variation in the timing of mandate adoption (still according to the age groups covered by the law) across states to identify the effects of the mammography mandates. In the augmented DDD framework we identify the effects of the mandates using both the mandate timing variation as well as the variation across mandates in the ages of women who are affected by the laws. The identifying assumption in the two-way fixed effects models is that there were no other variables e.g., other public policies that also affected mammography outcomes that were coincident with mandate adoption. The identifying assumption in the DDD model requires that there be no other age-group-specific variable correlated with mandate adoption that also affected outcomes.
Each of these approaches has its strengths and weaknesses. The strength of the DDD approach is that it can unambiguously purge the mandate estimates from confounders that vary at the age group-by-year level (such as changes in national screening guidelines), the age group-by-state level (such as state education campaigns that are always targeted at women of a certain age group), and the state-by-year level (such as the adoption of other state policies and programs targeted at all women in the state). A weakness of the DDD approach, however, is that it possible that the DDD differences out some of the ‘true’ effects of the mammography mandates if there are spillover effects of the laws to women of other age groups. For example, suppose the laws change employer benefits choices for women of all ages (not just the specific ages codified in the state mandate). If so, then women who we think are ‘just untreated’ due to their age being below the minimum law threshold are, in fact, treated by the mammography mandate. If the mandate increases the screening behavior of these younger women who are otherwise ‘ineligible’, the DDD will wrongly difference out part of the real effect of the mandate.
We therefore take multiple additional complementary strategies to test for causal effects of mammography mandates. First, in addition to standard models with state and year fixed effects, we also estimate models that include state-specific linear time trends and, in an additional model, state-specific linear and quadratic trends. This is a standard approach for testing the robustness of key policy relationships in these types of settings (Wolfers 2006); in these models we identify the mandate effects from deviations of mammography outcomes net of smoothly evolving trends in outcomes in each state. Second, we make use of multiple comparison groups. Specifically, we incorporate both younger women (25–34 year olds) and older women (65–74 year olds) into the models (recall the modal mandate targets women age 35 and older). Neither is a perfect comparison group. The younger women age 25–34 have screening rates that are far below the 35–64 year olds (because screening is not recommended for them by any major medical organization unless they have a family history and because they are typically not covered by the mandates), while most of the older women age 65–74 have access to an additional very different set of insurance benefits (i.e., Medicare, which is relatively generous and had two policy changes to mammography reimbursement over our sample period) than do the vast majority of 35–64 year old women in standard private plans. But while neither group is ideal, their combination may help reduce omitted variables bias that may otherwise artificially inflate estimates of the causal effects of mammography mandates (Akosa Antwi, Moriya, and Simon 2013).
We begin with the two-way fixed effects model which we write as:
| (1) |
where Yiast are the various dichotomous screening outcomes for woman i in age group a in state s at time t. Xiast is a vector of individual level demographic controls that includes dummies for 5-year age groups, race/Hispanic ethnicity, education, and marital status. The first three policy variables reflect the mammography mandates which vary at the age, state, and year level.16 While we shorten the variable names for brevity in writing out equation (1), strictly speaking each mandate variable equals the share of the relevant reference window the woman is treated by a mammography mandate at each frequency (baseline, biennial, and annual). Recall that the modal mandate adopted in the late 1980s requires coverage for a baseline screening mammogram for women age 35–39, a biennial mammogram for women age 40–49, and an annual mammogram for women age 50 and older.17 Thus for a state with the modal mandate, the baseline screening mammogram law would be on for women age 35–39, the biennial screening mammogram law would be on for women age 40–49, and the annual screening mammogram law would be on for women age 50 and older.
Zst is a vector of covariates that vary at the state and year level. These include: the unemployment rate; the HMO penetration rate; the number of obstetric beds in the state per 1,000 women age 15–44 (to proxy for state infrastructure for women’s health); the share of women age 15–44 with private health insurance; the share of women age 15–44 who work (or whose spouses work) at private firms of various sizes (<25, 25–99, 100+); the fraction black; the fraction Hispanic; and the fraction urban. The Zst vector also includes controls for other relevant public policies that may be expected to affect outcomes, including: the presence of a state law requiring women to be able to see an OB/GYN without first obtaining a referral from her primary care provider (aka ‘direct access’ laws); the presence of a state low-income screening program through the National Breast and Cervical Cancer Early Detection program; the presence of a state law requiring insurance coverage of cervical cancer screening tests; Medicaid expansions for pregnant women (a proxy for generosity of the states’ public health insurance programs); and welfare reform.18 Dummy variables for each state are captured by Ss and in the two-way fixed effects models control for time-invariant state-specific factors. Dummy variables for each survey year are captured by Tt and in the two-way fixed effects specifications control for period-specific shocks common to all states in any given year.19 Throughout, we cluster the standard errors at the state level (Bertrand, Duflo, and Mullainathan 2004). Regressions are weighted to be population representative, and the main sample is all women aged 25–74 interviewed by the BRFSS in survey years 1987–2000.20
As discussed above, in subsequent models we add state-specific linear time trends and, in an additional model, state-specific linear and quadratic trends in addition to the year and state fixed effects to equation (1). Linear state trends, for example, interact each state fixed effect with a variable called TREND that equals 1 in 1987, 2 in 1988, and so forth. We also estimate a fully interacted triple-differences (DDD) specification which instead of the trends adds to equation (1) a full set of state by age group dummies, a full set of year by age group dummies, and a full set of state by year dummies (thus causing the Zst vector to fall out of this model). The year by age group indicators remove biases common to all women of a particular age group in a given year. The state by age group indicators account for other age-specific state effects. Finally, the full set of state by year interactions account for any other efforts to increase mammography rates in a particular state and year that would be expected to affect women of different ages equally. In the augmented triple difference model, the coefficients of interest, β2 β4, use variation at the age group by state by year level to identify the effects of screening mammography mandates from differences in screening rates for women whose age makes them treated compared to the associated outcomes for women whose age makes them untreated coincident with the timing of policy adoption within each state.
A nontrivial issue is the decision about how to code the mandate [and other policy] variables for the women in the age groups that were not targeted by the laws. For the vast majority of younger women age 25–34, all mandate variables are set equal to zero. The only exception is that one state explicitly mandated screening benefits for women younger than age 35. For the older women age 65–74, however, the decision on how to code the mandate variable is more complex. Many women who are older than age 65 (nearly all of whom are eligible for and are on Medicare) also have supplemental private plans either through a prior employer or a privately purchased ‘Medigap’ plan. These plans could plausibly make the older women treated by the state mandates we study; in fact, a nontrivial number of the mandates explicitly mention Medigap. Super (2002) indicates that, based on data from the 1999 Medicare Current Beneficiaries Survey, about a third of Medicare recipients not in nursing homes have private coverage through a current or former employer (either own or spouse), while another 27 percent have supplemental Medigap coverage (either Medigap alone or Medigap in combination with private employer sponsored coverage). Thus, around half of Medicare beneficiaries are plausibly bound by the state mandates we study (Stanton 2004). Moreover, this is likely to be especially true for our analyses of deductibles prohibitions because although Medicare began covering mammograms in 1991, the program did not explicitly prohibit deductibles until 1998. Since we have no way in our data to identify source of insurance, in our main models we set the mandate variables for 65–74 year old women equal to whatever is true for their 50–64 year old counterparts in that state and year.21
In addition to the specification in equation (1) which we refer to as the ‘expanded mammography mandate’ specification we also estimate an ‘any mammography mandate’ specification that replaces the three policy variables (for baseline, biennial, and annual screening mandates) with a single policy variable that equals the share of the relevant reference window the woman is treated by Any Mammography Mandate. We also consider a ‘scaled mammography mandate’ specification that takes into account the fact that women who are eligible for biennial screenings are treated half as much a women who are eligible for annual screenings and the fact that women who are eligible for baseline screenings (which always includes 35–39 year olds only) are treated one fifth as much as women who are eligible for annual screenings. Thus, the Scaled Mammogram Mandate variable takes on a value of: one for women eligible for annual screenings; 0.5 for women eligible for biennial screenings; and 0.2 for women eligible for a baseline screening (all adjusted for the share of the relevant reference window accordingly). These two specifications (Any and Scaled) are otherwise identical to that reported in equation (1).
Finally, we explicitly examined provisions of mandates similar to the federal health reform requirement that insurance plans must not impose cost sharing for obtaining preventive services such as mammograms. Specifically, the relevant provision of the federal health reform law says that mammograms satisfying the USPSTF guidelines from 2002 (mammograms every 1–2 years for women 40 and older) must be covered for non-grandfathered plans without cost-sharing of any kind. We identified six states with mandates that explicitly prohibited deductibles for obtaining a mammogram over our sample period, and we expect that these laws should increase mammography use more than laws without such explicit prohibitions.22 For this model we interact each main mandate variable with an indicator variable equal to one for states that prohibit deductibles, while including the main effect. If this specific provision is meaningful for increasing screening, we expect this interaction term to be positive and statistically significant, particularly for low-educated women (which we use as a proxy for low-income, as the prohibition on deductibles should be more meaningful for low-income women).23
IV. Results
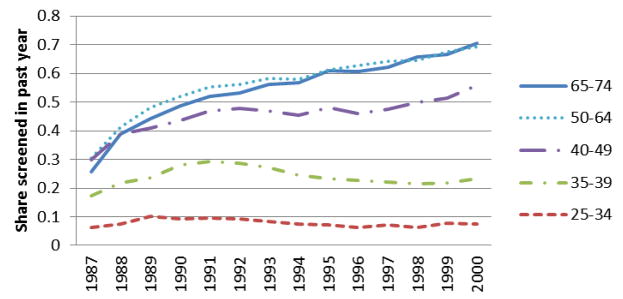
In Figure 1 we show trends in past year mammography use from 1987 to 2000.
Figure 1. Age-Specific Trends in Past Year Mammography.
Figure 1 presents weighted mean share of women of various ages in pooled 1987–2000 BRFSS sample who report having had a mammogram in the previous year.
We present trends for five age groups: 25 to 34 year olds, 35 to 39 year olds (who were usually targeted in provisions calling for baseline mammograms), 40 to 49 year olds (who were usually targeted in provisions calling for biennial mammograms), 50 to 64 year olds (who were usually targeted in provisions calling for annual mammograms), and 65 to 74 year olds. Several features are notable in Figure 1. First, there was almost no increase in recent mammography use for women age 25 to 34 years old. Second, there was a noticeable increase in recent mammography for 35 to 39 year old women from 1987 to until about 1993, after which the rates fell substantially; this is likely attributable in part to the removal of the “baseline” screening mammogram recommendation from the ACS guidelines in 1992. Third, there were steady, long-lasting, and remarkably large increases in mammography use for 40 to 49 year olds, 50 to 64 year olds, and 65–74 year olds. Past year mammography rates among these groups of older women roughly doubled over this period. The patterns in Figure 1 are visually consistent with a role for mammography mandates in increasing mammography use: note that the majority of the legislative action regarding mammography occurred in the 1987–1992 period.
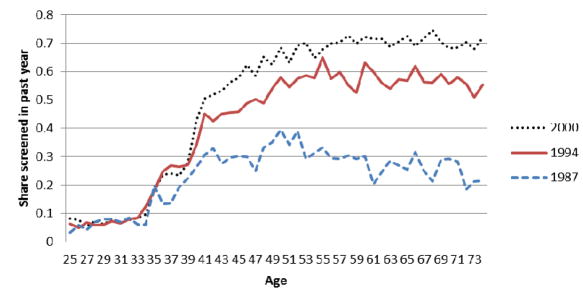
Figure 2 shows these same patterns in a slightly different way. Specifically, we show in Figure 2 the age profile of past year mammography for three different years: 1987 (the first year of our sample), 1994 (the middle of our sample), and 2000 (the last year of our sample).
Figure 2. Age Profile of Past Year Mammography: 1987, 1994, and 2000.
Figure 2 presents share of women of each age who report having had a mammogram in the previous year for BRFSS survey years 1987, 1994, and 2000.
Figure 2 shows that there was a large improvement in recent mammography screening rates for 50–74 year olds between 1987 and 1994 that is, the vertical distance between the lines for 1987 and 1994 at ages 50–74 is large with slightly smaller increases for 40–49 and 35–39 year olds over this same period. From 1994 to 2000, Figure 2 shows essentially no change in screening rates for 35–39 year olds and some modest increase for 40–74 year olds. Again, given that the timing of mandate adoption was mostly between 1987 and 1992 (also visible in Appendix Figures 1–3), the visual patterns in Figure 2 are again consistent with a role for mandates at increasing mammography rates.24
Table 1 presents descriptive statistics of the key health outcomes and the policy variables and shows that, as seen in Figures 1 and 2, mammography rates are strongly increasing with age, and the same is true when we consider whether the woman reports a mammogram in the last year and says her most recent mammogram was routine.25
Table 1. Descriptive Statistics.
Mammogram Outcomes, Mandate Variables, and Breast Cancer Diagnosis Rates, BRFSS and SEER
| Variable | All 25–74 | Age 25–34 | Age 35–39 | Age 40–49 | Age 50–64 | Age 65–74 |
|---|---|---|---|---|---|---|
| BRFSS – Mammography Data | ||||||
| Ever had mammogram | 0.586 | 0.174 | 0.459 | 0.760 | 0.817 | 0.791 |
| Had mammogram w/in past year | 0.379 | 0.080 | 0.241 | 0.469 | 0.583 | 0.566 |
| Had mamm. w/in past year & most recent was routine | 0.334 | 0.058 | 0.200 | 0.416 | 0.528 | 0.509 |
| Had mamm. w/in past year & most recent was not routine | 0.045 | 0.021 | 0.041 | 0.054 | 0.056 | 0.058 |
| Had mammogram w/in past 2 years | 0.486 | 0.115 | 0.344 | 0.636 | 0.709 | 0.687 |
| Means of policy variables for past year outcomes: | ||||||
| Share treated by any mandate, scaled (baseline=.2, biennial=.5, annual=1) | 0.418 | 0.008 | 0.199 | 0.478 | 0.747 | 0.724 |
| Share treated by any mandate | 0.541 | 0.008 | 0.686 | 0.737 | 0.747 | 0.724 |
| Share treated by mandate for baseline screening | 0.080 | 0 | 0.609 | 0 | 0 | 0 |
| Share treated by mandate for biennial screening | 0.116 | 0 | 0 | 0.517 | 0 | 0 |
| Share treated by mandate for annual screening | 0.344 | 0.008 | 0.077 | 0.220 | 0.747 | 0.724 |
| Share treated by any mandate prohibiting deductibles | 0.027 | 0.006 | 0.037 | 0.038 | 0.036 | 0.027 |
| N – BRFSS | 696,761 | 170,352 | 97,610 | 162,580 | 163,195 | 103,024 |
|
| ||||||
| SEER – Cancer Registry Data | ||||||
| Total cancer incidence rate per 100,000 women | 170.5 | 17 | 62.6 | 157.4 | 298.3 | 438 |
| In-situ breast pre-cancer incidence rate per 100,000 women | 32 | 1.6 | 9.5 | 37 | 59.2 | 67.6 |
Notes: Top panel: author calculations from 1987–2000 BRFSS adult females 25–74. Statistics are weighted. N is maximum possible N; a small number of observations are missing for various measures (e.g., individuals who did not answer questions about the timing of their last mammogram are not asked why they had it). Past year outcomes are the share of the prior calendar year (relative to the respondent’s interview date) that a law has been in effect, assuming it first impacted health insurance policies as of January 1 of the year after it was passed. The variable ‘Had mammogram w/in past year’ does not exactly equal the sum of the variables ‘Had mammogram w/in past year & most recent was routine’ and ‘Had mammogram w/in past year & most recent was not routine’ because of a small amount of non-response to the question about the reason for the most recent mammogram. Bottom panel: author calculations from 1985–2000 SEER.
We also show in Table 1 the means of the mandate policy variables. Specifically, we report means of the “share of the previous year” policy variables that take into account the reference windows for past year outcomes. We find that over half of our sample (54.1 percent) is treated by the ‘any mammography mandate’ for one of baseline, biennial, or annual screenings, and this figure is increasing in age. Turning to the alternative approach to measuring all the policies at once which scales baseline mandates to .2, biennial to .5, and annual to 1 and thus defines the share of the year during which screenings are mandated to be covered, we see the average woman age 25–74 is covered for about 40 percent of a year’s screening. Table 1 also shows the share of women treated by mandates for baseline screenings, biennial screenings, and annual screenings, respectively. The majority of women treated by any mammogram mandate are treated by a mandate for an annual mammogram (34.4 of the 54.1 percentage points). Finally, we show that a nontrivial proportion of women in our sample are subject to mandates that explicitly prohibit deductibles for obtaining a mammogram; nearly 5 percent (2.7/54.1) of the mandates prohibit deductibles.
We present the first set of regression results in Table 2 for the Mammogram in the Past Year outcome. Each column of each panel is from a separate model. We present coefficient estimates on the key mandate variables of interest, and in each column we add successively more controls. We present results for the ‘scaled mammography mandate’ specification in the top panel, the ‘any mammography mandate’ specification in the middle panel, and the ‘expanded mammography mandate’ specification in the bottom panel. Column 1 shows estimates from the two-way fixed effects model that controls for: age group dummies; other individual demographic characteristics; state/time varying demographic and economic controls; other state and federal policies; and state, year, and month fixed effects. Column 2 adds linear state trends, Column 3 adds linear and quadratic state trends, and Column 4 replaces the state trends with state by age group, year by age group, and state by year fixed effects and is the fully saturated DDD model.
Table 2. Mammography Insurance Mandates Increased Past Year Mammography.
BRFSS 1987–2000, Adult Women 25–74
| (1) | (2) | (3) | (4) | |
|---|---|---|---|---|
Model is
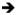
|
State and year fixed effects | (1) + linear state trends | (2) + quadratic state trends | DDD |
| Mandate specification is ↓ | ||||
| Scaled Mandate Specification | ||||
| [Annual=1; Biennial=.5; Baseline=.2] | 0.050*** (0.013) | 0.050*** (0.013) | 0.054*** (0.014) | 0.010 (0.007) |
| Adjusted R squared | 0.21 | 0.21 | 0.21 | 0.22 |
|
| ||||
| Any Mandate Specification | ||||
| Treated by any mammography mandate | 0.037*** (0.007) | 0.037*** (0.007) | 0.040*** (0.007) | 0.005 (0.007) |
| Adjusted R squared | 0.21 | 0.21 | 0.21 | 0.22 |
|
| ||||
| Expanded Mandate Specification | ||||
| Treated by mandate for baseline mammogram | 0.005 (0.008) | 0.003 (0.008) | 0.006 (0.007) | −0.015* (0.009) |
| Treated by mandate for biennial mammogram | 0.035*** (0.009) | 0.035*** (0.009) | 0.038*** (0.009) | 0.011 (0.012) |
| Treated by mandate for annual mammogram | 0.050*** (0.012) | 0.050*** (0.012) | 0.055*** (0.012) | 0.009 (0.007) |
| Adjusted R squared | 0.21 | 0.21 | 0.21 | 0.22 |
Notes: Each panel of each column shows the results from a separate regression model. Sample size for all models is 693,154. The dependent variable in all models is had a mammogram in the past year. Additional controls in all models include: five-year age group dummies; Pap test mandates; state NBCCEDP implementation; laws mandating access to OB/GYNs; Medicare coverage of Pap tests and mammograms for women age 65 and older; race/ethnicity; education; marital status; share of women 15–44 with private health insurance; share of women who work or who have a husband who works at a firm with 24 or fewer employees, 25–99 employees or 100 or more employees; the unemployment rate; welfare reform; the level of HMO penetration (as a share of the population); the number of obstetric beds per 100 women 15–44; the eligibility threshold for Medicaid eligibility for a pregnant woman in the state as a share of the FPL; share urban; share black; share Hispanic; and state, year, and month of interview fixed effects. Models in column 2 add linear state trends. Models in column 3 add quadratic state trends. Models in column 4 replace the trends and state by year controls with state by age group, year by age group, and state by year fixed effects.
significant at 10%;
significant at 5%;
significant at 1%.
Standard errors throughout are clustered at the state level and estimates are weighted.
The results in column 1 of Table 2 with state and year fixed effects (and other covariates) indicate that mammography mandates increased the probability that a woman age 25–74 reports having had a mammogram in the past year, and this finding is insensitive to how we specify the mandate variable. For example, we estimate that the presence of mammography mandate in the ‘scaled’ specification in the top panel of column 1 is associated with a statistically significant 5 percentage point increase in the probability of past year mammography screening. In the bottom panel we find that the presence of a mandate for annual mammography is associated with a 5 percentage point increase in the probability of past year mammography screening. In columns 2 and 3 we find that these relationships are largely unchanged when we add controls for linear and quadratic state trends, respectively. Turning to the fully interacted DDD model in column 4 with a full set of two-way interactions for age, state, and year, we continue to estimate in the ‘scaled’ specification in the top panel that mammography mandates increase the likelihood of reporting a past year mammogram by 1 percentage point, though the estimate is not statistically significant. Taken together, the estimates from the scaled specification in Table 2 fall in the range of 1 to 5.4 percentage point effects, or about a 4.5–24 percent effect relative to the baseline pre-mandate annual mammography rate.26 Using instead the coefficient on annual mandates in the expanded bottom panel, the point estimates vary from .9 to 5.5 percentage points. Measured differently, and given that past year mammography rates increased by about 25.7 percentage points over our time period (see Figure 2), we estimate that mandates for annual mammography account for about 3.5–21.4 percent of the overall increase.
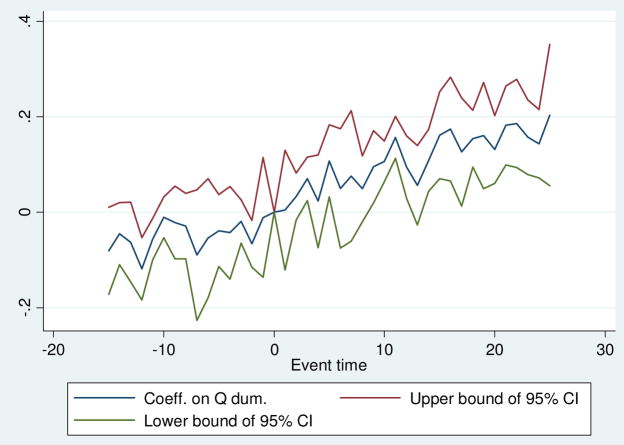
In Figure 3 we present event study estimates of the effect of mandates for annual screening on past year mammography corresponding to the two-way fixed effects model from column 1 of Table 2. This is a commonly used approach for examining the validity of the common trends assumption required for difference-in-differences models, and it also allows us to examine how the effects of insurance mandates vary over time. A challenge is that our institutional setting is not particularly well suited to a classic event study, as there are different types of laws for women of different age groups, and some states amend or repeal their laws in the middle of our sample period. To operationalize the event study, then, we focus attention on 40–64 year old women only, and we also restrict attention to mammography mandates for annual screenings (which explicitly targeted women in this age group over our sample and which the expanded specification in the bottom row of Table 2 shows are the most important types of mandates for increasing screenings). Our analysis sample for the event study is women in states that adopted exactly one mandate for annual screenings for women in this age group and never changed or repealed it, and we further require that states be observed in all years of the sample to guard against composition bias and to allow for meaningful estimates before and after policy adoption.27 We estimate a variant of equation (1) where we replace the annual mandate policy variable into a series of ‘event-time’ or ‘re-centered time’ policy dummy variables capturing the number of quarters in each state relative to the time the state adopted a mandate for annual screening (which we fix at time zero), and we also include state and age-group fixed effects (with standard errors clustered at the state level).
Figure 3. Event Study Estimates of the Effect of Mandates for Annual Screening on Past Year Mammogram.
BRFSS 1987–2000, Adult Women 40–64
Figure 3 shows an event study for past year mammography. Time 0 is the time when each state implemented the first and only annual mandate for women of the relevant ages. The coefficients are those on dummies for each quarter before and after the law change in each state while also controlling for state and 5-year age groups, with the standard errors clustered at the state level, and using BRFSS weights. States are included only if they had only 1 law change and were in the BRFSS data for the whole 1987–2000 sample period.
The results of this exercise are presented in Figure 3.
Figure 3 provides mixed evidence on the important issue of exogenous timing of mandate adoption. On the one hand, there is a notable upward trend prior to mandate adoption, which is inconsistent with the assumption that mandate timing is uncorrelated with unobserved determinants of screening in the classic DD setting. There is, however, evidence that screenings are significantly higher four to six years following mandate adoption, consistent with an important role for mandates. In Appendix Figures 4–6 we show variants of Figure 3 separately for 40–49 and 50–64 year olds. These figures indicate that the pre-trend in Figure 3 is driven by 50–64 year old women; for 40–49 year old women there is no significant pre-trend, and screenings for women in this age group increase significantly within two to four years. Since our data and institutional setting do not produce perfectly clear event studies (particularly for 50–64 year olds), we next present a range of additional robustness and falsification tests to provide important complementary evidence on the effects of mammography mandates.
For the sake of brevity, in all subsequent models for mammograms we only report results from the ‘scaled’ mandate specification, though the full set of results from all specifications (which were very similar) is reported in the Appendix. We present result for the other mammography outcomes in Table 3.
Table 3. Mandates Also Increased Other Mammography Screening Outcomes.
BRFSS 1987–2000, Adult Women 25–74, Scaled Mandate Specification
| (1) | (2) | (3) | (4) | |
|---|---|---|---|---|
Specification is
|
State and year fixed effects | (1) + linear state trends | (2) + quadratic state trends | DDD |
| Outcome is ↓ | ||||
| Mammogram in past year and last one was routine | ||||
| Scaled mandate | 0.050*** (0.013) | 0.050*** (0.013) | 0.055*** (0.014) | 0.016** (0.007) |
| Adjusted R squared | 0.20 | 0.20 | 0.20 | 0.20 |
| N | 691,488 | 691,488 | 691,488 | 691,488 |
|
| ||||
| Mammogram in past two years | ||||
| Scaled mandate | 0.056*** (0.016) | 0.055*** (0.016) | 0.060*** (0.017) | 0.014* (0.008) |
| Adjusted R squared | 0.28 | 0.28 | 0.28 | 0.29 |
| N | 693,154 | 693,154 | 693,154 | 693,154 |
|
| ||||
| Ever had a mammogram | ||||
| Scaled mandate | 0.044*** (0.012) | 0.043*** (0.013) | 0.045*** (0.013) | 0.003 (0.008) |
| Adjusted R squared | 0.33 | 0.33 | 0.33 | 0.34 |
| N | 695,109 | 695,109 | 695,109 | 695,109 |
Notes: Each entry shows the results from a separate regression model. The dependent variable in panel 1 is mammogram in past year and most recent one was routine screening mammogram, that in panel 2 is mammogram in past two years, and that in panel 3 is ever had a mammogram. All specifications in the table report coefficients on the Scaled Mandate variable. The mandate variable for the specification in panel 1 accounts for the share of the year preceding the interview that the law was in effect. The mandate variable for the specification in panel 2 accounts for the share of the two years preceding the interview that the law was in effect. The mandate variable for the specification in panel 3 accounts for whether a mandate has been implemented as of January of the survey year. See notes to Table 2 for additional control variables.
significant at 10%;
significant at 5%;
significant at 1%.
Standard errors throughout are clustered at the state level and estimates are weighted.
The results in the top panel of Table 3 indicate that mandates increased the likelihood that a woman reports she received a mammogram in the last year and that her most recent one was routine by 1.6 to 5.5 percentage points, and these estimates are all statistically significant at the 5 percent level. Having a non-routine mammogram in the last year is not significantly associated with the mandates (not shown in table but available upon request). Since the bulk of any increase in mammograms driven by changes in coverage should be for routine reasons, this supports our interpretation that the mandates increased coverage of screening mammography and that this increased coverage led to more routine mammograms.28 In the middle panel of Table 3, we find that the mandates significantly increased the likelihood a woman reports having had a mammogram within the past two years by approximately 1.4 (column 4) to 6 (column 3) percentage points, and all four of these effects are statistically significant. Finally, in the bottom panel of Table 3 we estimate that mammography mandates significantly increased the likelihood a woman reports ever having had a mammogram by 0.3 (column 4) to 4.5 (column 3) percentage points, with the results from the models in columns 1–3 all being statistically significant.
In Table 4 we provide more direct evidence on the most likely mechanism through which mandates affect utilization: a change in whether mammography is a covered insurance benefit. We begin by ruling out that mandates were associated with changes in health plan coverage; specifically, we estimate the same models as above but where the outcome variable is an indicator for whether the woman currently has any health plan. This is the closest proxy we have to health insurance coverage in the BRFSS; as noted above the overwhelming majority (86 percent) of women with ‘any health plan’ are actually covered by private insurance for women age 25–74 over this time period according to our tabulations of March CPS data. Recall that one possible employer response to rising costs of state mandates is to reduce offers of health insurance to employees; as such, it is possible that mandates such as those we study here could reduce health insurance coverage.
Table 4. Mandates Not Related to Probability a Woman Has a Health Plan and Mandate Effects Driven by Women with a Health Plan.
BRFSS 1991–2000, Adult Women 25–74, Scaled Mandate Specification
| (1) | (2) | (3) | (4) | |
|---|---|---|---|---|
Specification is
|
State and year fixed effects | (1) + linear state trends | (2) + quadratic state trends | DDD |
| Outcome is ↓ | ||||
| Has a Health Plan | ||||
| Scaled mandate | −0.001 (0.006) | 0.001 (0.006) | 0.002 (0.007) | 0.004 (0.007) |
| Adjusted R squared | 0.11 | 0.11 | 0.11 | 0.11 |
| N | 591,650 | 591,650 | 591,650 | 591,650 |
|
| ||||
| Mammogram in past year, 1991–2000 (when health plan questions were asked) | ||||
| Scaled mandate | 0.018 (0.011) | 0.019 (0.012) | 0.020 (0.013) | 0.014 (0.010) |
| Adjusted R squared | 0.22 | 0.22 | 0.22 | 0.23 |
| N | 589, 431 | 589,431 | 589,431 | 589,431 |
|
| ||||
| Mammogram in past year, among those with a health plan | ||||
| Scaled mandate | 0.023** (0.011) | 0.024** (0.011) | 0.025** (0.012) | 0.017* (0.010) |
| Adjusted R squared | 0.23 | 0.23 | 0.23 | 0.23 |
| N | 520,312 | 520,312 | 520,312 | 520,312 |
|
| ||||
| Mammogram in past year, among those without a health plan | ||||
| Scaled mandate | 0.0004 (0.011) | 0.002 (0.012) | 0.004 (0.012) | −0.022 (0.028) |
| Adjusted R squared | 0.11 | 0.11 | 0.11 | 0.12 |
| N | 69,119 | 69,119 | 69,119 | 69,119 |
Notes: Each entry is from a separate regression model. All specifications report coefficients on the Scaled Mandate variable. The dependent variable in panel 1 is an indicator variable for having a health plan, and that in panels 2–4 is mammogram in past year. See notes to Table 2 for additional control variables.
significant at 10%;
significant at 5%;
significant at 1%.
Standard errors throughout are clustered at the state level and estimates are weighted.
In the top panel of Table 4 we show that the insurance mandates are not meaningfully associated with changes in health plan coverage of women.29 In the second panel we simply report the full-sample estimate for the same period over which the health insurance questions were asked (1991–2000) for women with no missing information on health insurance (a very small share fails to report health plan coverage). These models continue to indicate economically significant increases in past year mammography screenings associated with mandate adoption, though the point estimates are smaller than those in Table 2 (recall we lose many states who adopted mandates between 1987 and 1991 for this analysis). In the third and fourth panels we directly examine the past year mammography outcome separately for women with a health plan (third panel) and for women without a health plan (fourth panel). The results indicate that there are statistically significant utilization effects of mandates for women with a health plan in every specification in the third panel of Table 4. In contrast, we find much smaller (very near zero) and/or wrong-signed estimates of the effects of mandates on past year screening for the sample of women without a health plan in the bottom panel of Table 4, and none of these estimates is statistically significant.
In the Appendix we present the results of several other robustness tests. For example, Appendix Table 14 shows that when we estimate similarly specified models predicting other women’s preventive health behaviors such as clinical breast exams (CBE) (manual examinations of the breast performed by a physician that do not involve X-rays) and Pap tests (the standard screening for cervical cancer) which were not covered by these insurance laws, the mandate estimates are much smaller in magnitude, suggesting the effects we identify are unlikely to be proxying for changes in women’s health more generally.30 Appendix Table 16 shows that our main results for past year mammography are robust to: 1) restricting attention to states constituting a balanced panel in the BRFSS data; 2) replacing our 5-year age group dummy variables with single year of age dummy variables; and 3) separately considering cover from offer mandates (whereby cover mandates have larger and more precisely estimated effects).31 We also performed other robustness exercises not reported in the Appendix. We also estimated models dropping women who were exactly 35, 40, or 50, as some of these women may have received their mammograms before reaching the age when the laws apply; this had no significant effect on our main findings. The Appendix also contains results by race/ethnicity (Appendix Table 17) and by education (Appendix Table 18); we found very broad-based mandate-induced increases in screenings.
Next we present evidence on the effectiveness of provisions in several state mandates that prohibit insurance companies from charging deductibles to women for obtaining mammograms. This type of provision is very similar to one in recently adopted federal health reform, which prohibits all out of pocket costs for eligible individuals obtaining certain preventive health services recommended by the USPSTF, including mammograms. Do mammography mandates that prohibit use of deductibles have larger effects at increasing mammography use than mandates without such provisions, and if so are these effects concentrated among low-income women (who should be more sensitive to limits on out of pocket costs)? We address this question by re-estimating equation (1) but also including interactions between a dummy variable indicating the state has this type of provision and the relevant mandate variables. To conserve space, we only report the coefficients on the scaled mandate variable and its interaction with the variable indicating the state mandate prohibits deductibles. We also only report estimates from the DDD specification (i.e., the specification of column 4 in Tables 2–4), but the patterns are similar using the other parameterizations of the mammography mandate variable and in the less saturated two-way fixed effects models.
In Table 5 we find evidence that these provisions matter, especially for women with low levels of education.
Table 5. Mandates that Prohibit Deductibles Increased Screenings Among Women with Less than a High School Degree Outcome is past year mammogram.
BRFSS 1987–2000, Adult Women 25–74, Scaled Mandate Specification, DDD Models
| (1) | (2) | (3) | (4) | (5) | |
|---|---|---|---|---|---|
Sample is
|
All women | Less than a high school degree | High school degree | Some college | College degree or more |
| Scaled mandate | 0.009 (0.007) | 0.014 (0.027) | −0.001 (0.011) | −0.001 (0.017) | 0.019 (0.017) |
| Scaled mandate * State mandate prohibits deductibles | 0.014* (0.008) | 0.059*** (0.016) | −0.017 (0.021) | 0.013 (0.026) | 0.022 (0.021) |
| Adjusted R-squared | 0.22 | 0.12 | 0.20 | 0.24 | 0.29 |
| N | 693,154 | 86,575 | 236,187 | 190,376 | 178,983 |
Notes: Each column shows the results from a separate DDD regression model. Column 1 sample is all women, column 2 sample is women with less than a high school degree; column 3 sample is women with exactly a high school degree; column 4 sample is women with some college education; and column 5 sample is women with at least a bachelor’s degree. See notes to Table 2 for additional control variables.
significant at 10%;
significant at 5%;
significant at 1%.
Standard errors throughout are clustered at the state level and estimates are weighted.
For the full sample in column 1 we estimate a positive but statistically insignificant main effect and a positive, economically meaningful, and marginally significant interaction coefficient. In columns 2 through 5 we show the results from similar models where we restrict attention to high school dropouts (column 2), women with a high school degree (column 3), women with some college (column 4), and women with at least a college degree (column 5). Prohibitions on deductibles for obtaining mammograms should be expected to have larger effects on low-educated women who are likely to have lower incomes and lower ability to pay such out-of-pocket costs. Indeed, we find in column 2 that, in addition to the positive (but insignificant) main effect of the scaled mammography mandate variable, there is also a large and statistically significant and large positive interaction coefficient, suggesting that for high school dropout women the prohibition on deductibles for obtaining mammograms significantly increased mammography rates over and above the main mandate effect.32 These results suggest that similar rules in federal health reform are likely to further increase screening among low-income women with health insurance coverage.
Next, we provide evidence on the effects of the mandates on breast cancer diagnoses. If screening of asymptomatic women were effective, we might expect to see that the mandate-induced mammograms led to more breast cancers being detected at an early stage than would occur in the absence of screening. To test this, we examine total cancer incidence as well as diagnoses of the earliest stage ‘in-situ’ pre-cancers using data from the Surveillance Epidemiology and End Results (SEER) system, which are registry data on the universe of breast cancer diagnoses (and also on in-situ pre-cancers) within nine areas/states that have been collected since 1973 (SEER Research Data 1973–2012).33 These are the standard cancer diagnosis data used in the field. Returning to the bottom panel in Table 1, we report the overall and age group specific incidence rates per 100,000 women for total cancer incidence and in-situ pre-cancers (not included in the total cancer incidence). The age gradient in diagnoses is clear from the patterns by age group. While the total cancer incidence rate is only 17 per 100,000 for women 25–34, it is 438 per 100,000 for women 65–74. The in-situ pre-cancers are about 19 percent of the overall cancers, with about 32 diagnoses per 100,000.
We examine the effects of mammography mandates on in-situ cancer detections by estimating models where the outcome is the log of the count of the number of in-situ cancers detected for women in each 5-year age group, state, and year, and we include the same right hand side variables as in equation (1) where possible.34 (The SEER data do not include all of the Xs (e.g., education, marital status), and it would also be hard to get appropriate population cells by these other cuts.) We assume a 1-month delay between initial screening and diagnosis, and we control for population as an additional independent variable. These results are presented in Table 6 which follows the format of Table 2 (i.e., state and year fixed effects, linear and quadratic trends, and the fully interacted DDD models) and presents results for the ‘scaled mandate’ specification (top panel), the ‘any mammography mandate’ specification (middle panel), and the ‘expanded mammography mandate’ specification (bottom panel). Unlike the earlier results, we present p-values in parentheses for the usual inference calculations for the key coefficients and present alternative p-values in brackets for the Wild-bootstrap procedure which adjusts for the small number of clusters (Cameron et al. 2008).
Table 6. Mandates Increased Detection of Earliest Stage In-Situ Pre-Cancers.
SEER 1985–2000, Adult Women 25–74
| (1) | (2) | (3) | (4) | |
|---|---|---|---|---|
| DD | DD+linear trends | DD+quadratic trends | DDD | |
| Scaled Mandate Specification | ||||
| Annual=1; Biennial=.5; Baseline=.2 | 0.199 (0.024)** [0.048]** | 0.214 (0.026)** [0.036]** | 0.216 (0.028)** [0.032]** | 0.156 (0.014)** [0.000]*** |
| R-squared | 0.93 | 0.93 | 0.93 | 0.96 |
| N | 1,440 | 1,440 | 1,440 | 1,440 |
|
| ||||
| Any Mandate Specification | ||||
| Treated by any mammography mandate | 0.265 (0.015)** [0.000]*** | 0.283 (0.017)** [0.000]*** | 0.287 (0.018)** [0.000]*** | 0.256 (0.006)*** [0.000]*** |
| R-squared | 0.93 | 0.93 | 0.93 | 0.96 |
| N | 1,440 | 1,440 | 1,440 | 1,440 |
|
| ||||
| Expanded Specification | ||||
| Treated by mandate for baseline mammogram | 0.321 (0.025)** [0.000]*** | 0.333 (0.028)** [0.000]*** | 0.326 (0.032)** [0.000]*** | 0.328 (0.046)** [0.024]** |
| Treated by mandate for biennial mammogram | 0.288 (0.032)** [0.000]*** | 0.304 (0.030)** [0.000]*** | 0.307 (0.028)** [0.000]*** | 0.250 (0.035)** [0.000]*** |
| Treated by mandate for annual mammogram | 0.242 (0.017)** [0.004]*** | 0.262 (0.018)** [0.004]*** | 0.268 (0.019)** [0.016]** | 0.235 (0.004)*** [0.000]*** |
| R-squared | 0.93 | 0.93 | 0.93 | 0.96 |
| N | 1,440 | 1,440 | 1,440 | 1,440 |
Notes: Each entry shows the coefficient from a separate regression model. The dependent variable is one plus the log of the number of breast cancer diagnoses to women in various age groups using SEER-9 data. Though not shown, all models also include various fixed effects (column 1: state and year; column 2: state, year and state specific time trends; column 3: state, year, and a quadratic in state specific time trends; and column 4: state by age group, state by year, and age group by year fixed effects). All models include dummies for the relevant populations of women in the age group. Models in columns 1–4 include all the state-level Xs discussed in the text.
significant at 10%;
significant at 5%;
significant at 1%.
Standard errors throughout are clustered at the state level; p-values for this process are reported in parentheses; p-values calculated using Wild Bootstrap are in brackets.
The results in Table 6 indicate that mammography mandates increased detection of the earliest stage in-situ pre-cancers. Specifically, we find statistically significant increases associated with mandates in all specifications, even after adjusting for the small number of clusters.35 The estimates in the top panel range from 0.156 to 0.216 for a one unit increase in the scaled mandate variable. This means that if the law were to change such that a woman went from living in a state with no mandate to having a value of one for the variable (i.e., living in a state where she were eligible for an annual mammogram), given the log linear model this would lead to increases in the range of 16 to 22 percent in the number of in-situ pre-cancer diagnoses. Taking the values in the bottom panel, implementing an annual mandate would lead to an increase of up to 27 percent in the number of in-situ diagnoses. These estimates are somewhat larger than those we found for screenings. Larger effects on diagnoses compared to screenings may reflect the fact that the most financially constrained women may be the ones who are induced to have mammograms when they gain coverage, and these may be the women who have the highest unmet need for screening.36 We also estimated similar models for total cancer incidence (all diagnoses except in-situ pre-cancers); these results were small in magnitude and inconclusive and are available upon request.
We acknowledge that the welfare implications of these mandate-induced changes in cancer screenings and detection of the earliest stage pre-cancers are not unambiguous. Thus, while the increased screenings documented in Tables 2–5 surely lead to some earlier cancer detection they are also associated with some increase in false positives (and associated harms) that we cannot track with our BRFSS or SEER data. Further, some researchers believe that a share of the increase in in-situ detections documented in Table 6 might not progress and may be treated unnecessarily. Recognizing the importance of this welfare question, we present in Table 7 the results of additional BRFSS-based analyses that essentially ask whether the mandate-induced increases in mammography obtained in Tables 2–5 are consistent with the current recommendations and guidelines of the American Cancer Society. The intuition is that the ACS bases its recommendations, in part, on its own critical evaluation of the state of medical science. As science has evolved, so have the ACS guidelines. At any point in time, then, a state’s insurance mandate is either consistent with current ACS guidelines or inconsistent with current ACS guidelines (it cannot be both).37 This allows us to ask whether the increases in screenings were mainly attributable to mandates that are or are not consistent with current ACS guidelines. A finding that screening increases were due primarily to mandates that are consistent with current ACS guidelines would lend stronger support to the idea that the mandate-induced screenings were welfare enhancing, while the opposite finding would lend more support to the idea that the screening increases had more ambiguous welfare effects.
Table 7. A Substantial Proportion of Increased Screenings are Attributable to Mandates that are Not Consistent with 2014 ACS Recommendations.
BRFSS 1987–2000, Women age 25–74, Expanded Mandate Specification
| (1) | (2) | (3) | (4) | |
|---|---|---|---|---|
Model is
|
State and year fixed effects | (1) + linear state trends | (2) + quadratic state trends | DDD |
| Mandate specification is ↓ | ||||
| Expanded Mandate Specification | ||||
| Mandate is consistent with current [2014] ACS guideline for annual | 0.055*** (0.011) | 0.054*** (0.012) | 0.059*** (0.012) | 0.006 (0.007) |
| Mandate is not consistent with current [2014] ACS guideline for annual | 0.012 (0.007) | 0.014** (0.007) | 0.021*** (0.007) | 0.046*** (0.009) |
| Adjusted R squared | 0.21 | 0.21 | 0.21 | 0.22 |
| N | 693,154 | 693,154 | 693,154 | 693,154 |
Notes: Each column shows the results from a separate regression model. The dependent variable in all models is had a mammogram in the past year. See notes to Table 2 for additional control variables. All models also include a variable indicating that the mandate is not consistent with current ACS guideline for baseline screening and a variable indicating that the mandate is not consistent with the current ACS guideline for biennial screening. Models in column 2 add linear state trends. Models in column 3 add quadratic state trends. Models in column 4 replace the trends with state by age group, year by age group, and state by year fixed effects.
significant at 10%;
significant at 5%;
significant at 1%.
Standard errors throughout are clustered at the state level and estimates are weighted.
We present the results from this analysis in Table 7 using the ‘expanded’ specification. Note that there are no mandates for baseline or biennial screening that are consistent with current ACS guidelines, so we only show the coefficient estimates for the mandate variable that requires annual screening (separately for annual mandates that are and are not consistent with current ACS guidelines).38
The results in Table 7 show an important role for annual mandates that are consistent with today’s ACS guidelines, but we also find an important role for annual mandates that are not consistent with today’s ACS guidelines. For example, the coefficients on the variable indicating the mandate is not consistent with current ACS guidelines regarding annual screening in the bottom row of Table 7 are all positive, and the estimates in columns 2–4 are all statistically significant. Moreover, in the fully saturated specification of column 4 the associated coefficient on the variable indicating the mandate is consistent with current ACS guidelines regarding annual screening in that same specification is much smaller and statistically insignificant. Overall, the results in Table 7 indicate that a substantial proportion – and in some cases the vast majority – of the increases in mammography due to mandates for annual screenings are not consistent with the state of current medical science as espoused in today’s guidelines of the ACS. This suggests that at least from the perspective of current knowledge, not all the mandate-induced increases in mammograms were likely to have been beneficial at the population level.39
V. Conclusion
Our results suggest that state laws requiring private insurers to cover screening mammograms played an important role at increasing the rates of past year mammography over an unprecedented period of improved preventive health behaviors among women from 1987 to 2000. Specifically, we estimate that a mandate requiring coverage of an annual mammogram significantly increased the likelihood a 25–74 year old woman reported getting screened in the past year by about 0.9–5.5 percentage points, or about 5–25 percent. These results hold up to numerous validation checks and robustness analyses. Moreover, we show that mandates specifically prohibiting deductibles were particularly effective at increasing mammography screenings among low-educated women (the group for whom such provisions are most likely to matter). We also find that the mandates led to increases in detection of the earliest stage in-situ pre-cancers. Finally, we find that at least some of the increased screenings are attributable to mandates that are not consistent with current recommendations of the American Cancer Society. This suggests that some of the mandate-induced increases in screenings were plausibly not welfare enhancing.
Given that nearly all states have already adopted mammography mandates, what are the public policy implications of our study in general and specifically with respect to the federal health reform? There are several. First, there is still wide variation in the ages of women who are targeted by these laws. Moreover, as noted above, most states’ existing recommendations are not in accordance with current recommendations from the ACS or the USPSTF. Specifically, the majority of state mandates still cover annual screening mammograms for women age 50 and older, despite that the ACS now recommends annual mammograms for women beginning at age 40 and the USPSTF now recommends biennial mammograms for women beginning at age 50. If a greater scientific consensus were to be reached regarding the most appropriate screening frequencies for women of different ages, policies could be amended accordingly.
Second, recently adopted federal health care reform has the potential to further increase screening rates because the state mandates are not binding for firms that self-insure under well-known provisions of ERISA. Since most self-insured firms will have to comply with the federal reform’s requirement that no cost-sharing can be imposed on mammography, it is possible that women whose insurance is from a self-insured organization will see increases in the generosity of insurance coverage for mammography.
Also, a minority of state mandates include provisions prohibiting insurance companies from imposing deductibles for obtaining a mammogram. Our estimates suggest that these deductible prohibitions led to an even larger increase in screenings for women with low education. Since federal health reform prohibits these out of pocket costs for any new or substantially revised private insurance plans, this further suggests potential for public policy to increase screening rates among low-income women.40
Finally, it is highly plausible that people may not have known about the provision in federal health reform requiring no deductibles for preventive care (even if the provision existed), and the federal change (and earlier state changes) may have increased awareness of this benefit due to widespread news coverage about the provision and changes in how plans present information about coverage of preventive care. This too suggests a potential meaningful role for federal health reform to affect mammography screenings and breast cancer diagnosis outcomes, thus speaking to the relevance of this research.
Supplementary Material
Acknowledgments
We thank Brian Asquith, Charles Hardy, Bhavanna Mannam, Ian Salas, Kathleen Wong, and Melody Yang for excellent research assistance. We are grateful to Kathleen Adams, Laura Argys, Cathy Bradley, David Neumark, Edward Norton, Barak Richman, Lucie Schmidt, Lara Shore-Sheppard, Kosali Simon, Madeline Zavodny, and numerous conference and seminar participants for many useful discussions and comments. Two anonymous referees and the Editor also greatly improved the paper. David Howard generously shared NBCCEDP data. Bitler worked on this paper while visiting the Federal Reserve Bank of San Francisco. We are grateful to the American Cancer Society (Grant #RSGI-11-003-01-CPHPS) and the UCI School of Medicine’s Institute for Clinical Translational Science for grant funding. The UCI ICTS project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR001414. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. All errors are our own. The views in this paper are solely the responsibility of the authors and should not necessarily be interpreted as reflecting the views of the Federal Reserve Bank of San Francisco, the Board of Governors of the Federal Reserve System, or the American Cancer Society.
Footnotes
In part this was due to controversy among the USPSTF, the ACS, and other major medical organizations regarding the appropriate age at which women should begin obtaining mammograms as well as how frequently screening should occur. All interested parties, however, agree that women age 50 to 74 should have regular mammograms.
Firms which purchase insurance are directly affected by these mandates; self-insured firms are not required to comply with state insurance mandates due to the well-known exemption provisions of the Employee Retirement Income Security Act (ERISA).
Notably, there is not uniform agreement across major medical organizations with respect to these recommendations. The USPSTF, for example, did not recommend routine screening mammography (every 1–2 years) for women age 40 and older until 2002. Prior to 2002, the USPSTF only recommended screening mammograms at this frequency for women age 50 and older and in 2009 revised their recommendations to only include regular screenings for all women age 50 and older. We take no stance on which organization’s recommendations are the most scientifically or clinically valid, and such discussions are well beyond the scope of this paper. For our triple difference models described below, it is important to note that our estimates of the effects of mandates will rely only on variation at the state by age group by year level coincident with the timing of mandate adoption; any recommendations from major medical organizations will be absorbed by the age group times year interactions since, although the recommendations themselves are age-based, they are nationwide (i.e., not state-specific) guidelines.
This discussion highlights (and Appendix Figures 1–3 make visually apparent) that the state by year by age group identifying variation in the mandates is only weakly correlated with variation in screening recommendations of ACS and USPSTF. That is, the failure of most states to consistently update their laws in response to changes in national screening guidelines from ACS and USPSTF (which are themselves contradictory) provides us substantial variation for disentangling the independent effect of insurance-based eligibility for mammography screening from the effect of guidelines on mammography utilization in one of our identification strategies. For an analysis of the effects of such guidelines in the US and Canada, see Kadiyala and Strumpf (2011a, b). For an analysis of guideline adherence, see Phillips et al. (1998).
Trivedi et al. (2008) show that voluntary choices by plans to impose cost sharing for screening mammography were associated with reduced screening rates, though they do not address unobserved heterogeneity.
A handful of studies have evaluated changes in Medicare reimbursement policy for screening mammography. Kelaher and Stellman (2000) find that when Medicare Part B began covering biennial mammography in 1991, past two year mammography rates for Medicare eligible women significantly increased relative to younger women who were not eligible for Medicare. Kadiyala and Strumpf (2012) find a substantial increase in cancer detection at age 65 (when all women who are not already eligible for Medicare become Medicare-eligible), most of which is among cancers with established screening tests.
Public health studies of the increasing trend in mammography over the 1980s and 1990s discuss the role of mammography mandates as a seemingly well-documented determinant of the improvement in women’s preventive health. Nelson et al. (2002), for example, write that “[e]ducational campaigns directed toward health care practitioners and the general public, state mandates for insurance coverage of mammograms, and programs for providing mammography services to low-income women have all played a role in increasing breast cancer screening in nearly all states.”
A recent piece of indirect evidence suggesting the importance of the ERISA exemption is Akosa Antwi, Moriya, and Simon (2013) who find that state policies that did not affect ERISA plans had smaller effects than did federal policies that did not exempt ERISA plans.
We are not aware of good estimates of how the costs of mammography have changed over time. Mammography technology, however, seems not to have changed substantially over the period we study, in part motivating our choice to study this period (i.e., our study period is before the widespread adoption of computer-aided detection and magnetic resonance imaging for mammography) (Fenton et al. 2010).
Ideally we would observe the marginal premium cost of adding mammograms to an insurance policy. Evidence from a 2000 Texas Department of Insurance report on the cost of mandates suggests that the Texas mandate for mammography screening was responsible for 0.6 percent of total premium costs (Albee et al. 2000). The Departments of Health and Human Services, Labor, and the Treasury estimated a slightly larger figure (1.5 percent) for all preventive care services (not just mammography) required to be covered with no cost sharing under the Affordable Care Act for non-grandfathered plans (Federal Register 2010). These figures are similar in magnitude to the analogous premium shares for three of the five mandates identified as “expensive” in Gruber (1994b) (alcohol treatment, chiropractor services, and continuation of health insurance coverage).
The number of states in the balanced panel changes depending on the first year of the panel. This is because the mammography questions were only asked as part of a women’s health module in 1988 (questions in modules of the BRFSS are not administered by all states). The 15 states observed in all years from 1987 to 2000 are: California, Illinois, Indiana, Kentucky, Maine, Maryland, Montana, Nebraska, New Hampshire, New Mexico, New York, North Carolina, South Carolina, Washington, and Wisconsin. Another 18 states appear in 13 of the 14 years of the sample, and another 7 states appear in 12 of the 14 years.
We stop our sample in 2000 because: 1) there was a significant change in reimbursement by Medicare for digital mammography in 2000; and 2) there was a federal law passed in 2000 regarding funding for breast cancer treatments for low-income uninsured women the Breast and Cervical Cancer Prevention and Treatment Act (BCCPTA). Specifically, the BCCPTA gives states the option to use their Medicaid programs to cover breast cancer treatments for previously uninsured women who were screened through the National Breast and Cervical Cancer Early Detection Program (NBCCEDP). We control for state implementation of the NBCCEDP program in all specifications.
Beginning in 1989, the survey eliminated an introductory screener question about whether the respondent had heard of a mammogram (this screener was preceded by text informing women that a mammogram was an X-ray of the breast to detect cancer). After this, the introduction to the question about lifetime mammography use included a sentence defining a mammogram. We code women in the early waves who report that they had not ever heard of a mammogram as also not ever having had a mammogram (this is a very small share of women).
Item non-response is fairly low for these questions. We omit observations with a “don’t know” or “refused” response to the mammogram questions.
One might be concerned that this ‘any health plan’ measure is picking up some women who have Medicaid and should not be affected by the mandates. We have examined data from the March Current Population Surveys for 1988–2000 to see what share of health care coverage is from private insurance. For women age 25–74, 86 percent of those with any health coverage in the CPS had private coverage. The share for most subgroups of interest is also at least 86 percent (e.g., high school graduates age 25–74 (87 percent), women with some college age 25–74 (91 percent), college graduates age 25–74 (96 percent), and non-Hispanic white women age 25–74 (90 percent)). For non-Hispanic blacks and Hispanics age 25–74, the relevant figure is above 70 percent. Even for high school dropouts age 25–64, 61 percent of those with any health coverage had private coverage.
There is a great deal of variation across states in the language regarding when the laws are supposed to take effect. Some states set a date after which “all policies sold or renewed after that date” must comply with the mandate, while others state that benefits must be changed effective immediately. We have coded plans as taking effect January 1 of the year after the year in which they are passed, with the logic that most policies are negotiated in the fall to take effect at the beginning of the following calendar year. Note the BRFSS questions introduce a ‘reference window’ problem as they report use over a period stretching back across time; for details, see the Appendix. Briefly, each mandate variable represents the share of the window over which use is measured that the law was in effect.
Our policy data come from the National Cancer Institute’s State Cancer Legislative Database (SCLD) (NCI, 2005). SCLD tracks every piece of legislation pertaining to different types of cancers, including breast cancer. We used a SCLD-produced table showing every state’s mammography mandate activity that included information on substantive revisions to the state laws, the year and quarter of law adoption, and the age groups and mammography frequency described in the law. To verify the information in the SCLD table we next consulted the actual text of each state’s laws by calling up individual records in SCLD. Discrepancies were discussed between the two authors.
Our information on the state rollout of the NBCCEDP program comes from personal correspondence with David Howard. The NBCCEDP was created by the 1990 Breast and Cervical Cancer Mortality Prevention Act. This program provides federal funds for cancer screening of low-income uninsured women, and states began participating at various times from 1991–1996. Note that we set the NBCCEDP variable equal to zero for all women age 65 and older as well as for all women 25–39, as the program was specifically targeted to women 40 and older but under age 65. Our information on direct access laws comes from Baker and Chan (2007). In a small number of states insurers must cover mammograms for women upon a physician’s recommendation. We include a separate control for women in states with these laws in the Z vector. Results in the two-way fixed effects models are robust to excluding the Z vector.
We also include month of interview dummies throughout (though not shown in the equation) to account for idiosyncratic month effects (e.g., October is Breast Cancer Awareness Month).
Though not shown in equation (1), we also control for the Medicare reimbursement changes to mammography described earlier and for Medicare reimbursement for cervical cancer screening
Note that there is the same issue with direct access laws as there is for the mandate variables with respect to how we treat 65–74 year old women; we follow the same decision rule as described above and code the direct access laws for the oldest women in our sample as equal to the same as for the younger women in the same state and year. Results using the alternative assumption (i.e., that the mandate variable is set equal to zero for all 65–74 year old women) produced very similar results to the ones we present which code these older women as experiencing the mandates faced by women 50–64. These results are in Appendix Tables 2–9. We also note that due to explicit differences in the language of Colorado and Ohio statutes we treat 65 year olds in those two states the same as 64 (not 66) year olds with respect to policy coding choices.
The six states are: Colorado, DC, Louisiana, Maryland, Ohio, and Oklahoma.
We use education instead of income because the BRFSS only offers information on income in large ranges, and these ranges have changed over time. Education, in contrast, is consistently measured over our entire sample period.
Again, we note that Medicare had two changes to mammography reimbursement over this time period, so the increases for the 65–74 year old women in both Figures 1 and 2 could be driven in part by Medicare changes as well as mammography mandates that affect some share of these older women.
Information on descriptive statistics regarding demographic variables is presented in Appendix Table 1.
Coefficients on the demographic controls generally have the expected signs. Older women are more likely to be screened. Women with low education are less likely to be screened than those with more education. Married women are more likely than others to be screened. Conditional on all of these things plus the various interactions, Hispanics and black non-Hispanics are more likely than white non-Hispanics to report being screened.
This is due to the nature of the timing of policy adoption relative to the nature of the timing of the BRFSS sample. The policy variation for 50–64 year old women occurs near the beginning of the sample period, while the policy variation for 40–49 year old women occurs near the end of the sample period; thus, requiring states to be observed for the entire 1987–2000 period is especially important for meaningful pre-period estimates for 50–64 year old women and meaningful post-period estimates for 40–49 year old women. Separate event studies for these two age groups are shown in the Appendix.
While some share of women whose most recent mammogram was not routine might have also had a routine one in the last year, it seems unlikely that the most recent one would be for routine reasons if a previous recent one was diagnostic. Also note that any causal effect on diagnostic use would be a very small share of the effect on screenings, as only a small share of screenings result in diagnostic mammograms.
We also estimated models of the likelihood of private health insurance using the March CPS. These models similarly returned no consistent significant evidence that the mammography mandates were associated with changes in private health insurance coverage and are presented in Appendix Table 11.
The small estimated increases for CBE and Pap tests may also reflect spillover effects of mammography mandates if, for example, a woman who is induced by the mandate to get a mammogram also finds out she needs a CBE or Pap test. In Appendix Table 15 we show that the relationship between mammography mandates and mammography use is very similar when we restrict attention to the sampled years in which we observe the other outcomes.
Cover mandates require privately sold plans to include coverage of mammography while offer mandates only require that insurers offer at least one such plan to an employer. We would typically expect the effects of offer mandates to be weaker than cover mandates (i.e., have smaller or no effects on utilization) since the latter should much more strongly reduce barriers to screening mammography for those privately insured women who did not have coverage previously. If there were no incentives to adjust coverage decisions besides the text of the laws, employers in offer states who did not wish to add the coverage could simply choose plans which did not include the “offered” coverage of mammograms. In practice, the bulk of our results pertain to cover mandates because they are far more common in our setting than are offer mandates: only three states ever had an offer mandate for screening mammography which did not subsequently become a cover mandate over our sample period. Measured differently, the proportion of women in our sample subject to any type of mammography mandate is 54.1 percent; 49.9 percent of these are cover mandates. That is, mandates to ‘cover’ represent over 90 percent of the women subject to any mandate.
Note that there is a strong education gradient in the presence of health insurance: while 34 percent of women age 25–64 with less than a high school degree do not have a heath plan, only 16 (6) percent of women in this age group with a high school degree (some college) lack a health plan. The fact we estimate larger effects of deductibles prohibitions for less educated women suggests the true effect for this group is likely even larger.
We study 1985–2000. The 9 states in SEER are: Georgia, Connecticut, Michigan, Hawaii, Iowa, New Mexico, California, Washington, and Utah. Note that when the National Cancer Institute (NCI) refers to total cancer incidence, it generally excludes the earliest stage in-situ cancers but includes a very small number of unstaged cancers (National Cancer Institute, 2013). These earliest stage ‘in situ’ diagnoses are independently interesting and potentially important in our context, and so we analyze them separately. ‘In-situ’ refers both to ductal carcinoma in situ (DCIS) and to the less common lobular carcinoma in situ (LCIS). Erbas et al. (2006) discuss uncertainty about what share of DCIS tumors will progress to invasive breast cancer. Regarding uncertainty about LCIS, the American Cancer Society’s “Breast Cancer Facts and Figures 2011–2012” report indicates that “many oncologists believe … that LCIS is not a true cancer, but an indicator of increased risk for developing invasive cancer in either breast” (p1).
For the small number of cells with zero cancer detections we add one because the log of zero is not defined. Note that we combine black, white, and other race women together for this analysis because there are some SEER sites with very small populations of black and other race women and thus the ‘zero cancer detections’ problem is substantially worse if we consider race groups separately. Alternatively, models restricted only to whites returned very similar results. We also estimated fixed-effects Poisson models which returned qualitatively similar results for the ‘any’ and ‘expanded’ mandate specifications and are available in Appendix Table 19. We present the log counts models for ease of interpretation and because these models allow us to adjust p-values for the small number of clusters (Cameron et al. 2008). We do so with Mammen (1993) weights and imposing the null hypothesis.
We also considered estimating models of breast cancer mortality, but we chose not to examine deaths for several reasons. First, there is usually a long and variable lag between mammography and breast cancer death that has changed considerably over time as treatment technologies have changed. This means there is not a clear econometric strategy that consistently yields a particular lag structure for linking particular types of diagnoses to later expected declines in mortality across our time period. Second, when a person has breast cancer, there are usually multiple mammograms involved [e.g., an initial screening one and subsequent diagnostic ones] which additionally complicates decisions about how to appropriately attribute mandate-induced screenings to breast cancer deaths. We leave this important question to future work.
Of course, the confidence intervals around the estimates in Table 6 include the effect sizes from the screening analyses presented earlier.
Note that ideally we would examine an alternative dependent variable indicating whether each mammogram was consistent or not consistent with the ACS guidelines. Unfortunately, women are only asked about their most recent mammogram, and we are unable to create meaningful variables for consistency with ACS guidelines without having each woman’s entire mammography history at each age in each year.
Note that we cannot do the variant of this test using current USPSTF guidelines because there are no annual mandates that are consistent with current USPSTF guidelines, which means none of the increase we find is driven by guidelines that are consistent with current knowledge as specified by USPSTF. Another interesting exercise would be to separate the mandate variables into mandates that are and are not consistent with the ACS guidelines in place at the time of interview (as opposed to current ACS guidelines). We present those results in Appendix Table 20; they generally suggest that most of the mandate-induced screenings were consistent with the state of science as the ACS interpreted it when the women were being screened. We think that the analysis of consistency with the current ACS guidelines provides a more direct commentary on possible welfare effects.
We note that there is lack of agreement within the medical community about whether the ACS guidelines (or even the USPSTF guidelines) are too aggressive. To the extent that this is true, this might suggest even less alignment between best medical practice as is known now and the mandates which drove increased screenings.
We note that that copays and coinsurance can - and do - exist even in the presence of a provision whereby individuals do not have to meet the deductible before obtaining preventive services (which is true of 75–90 percent of workers with health insurance according to a 2009 Kaiser Family Foundation Annual Survey). Thus, the total out-of-pocket costs in a zero deductible plan for preventive health services might still be nontrivial. Since the federal health reform requires zero out-of-pocket costs, not just elimination of deductibles, our results on cost-sharing remain highly relevant for predicting the likely effects of federal reform. For example, the Kaiser Family Foundation Survey for 2011 suggests 23 percent of workers faced changes in cost-sharing due to ACA, and 31 percent of workers were in plans which changed what services were considered preventative. This is relevant as ACA in addition to stating that preventive services must be covered without cost-sharing imposed explicit rules as to what services were preventive: those with a grade of B or higher from USPSTF.
JEL classification: I1, J8, K32
Contributor Information
Marianne P. Bitler, Department of Economics, University of California Davis, 1 Shields Avenue, Davis, CA 95616
Christopher S. Carpenter, Department of Economics, Vanderbilt University, 2301 Vanderbilt Place, PMB 351819, Nashville, TN 37235-1819
References
- Akosa Antwi Y, Moriya A, Simon K. Effects of Federal Policy to Insure Young Adults: Evidence from the 2010 Affordable Care Act’s Dependent Coverage Mandate. American Economic Journal Economic Policy. 2013;5(4):1–28. [Google Scholar]
- Albee S, Blount E, Lee T, Litow M, Sturm M. Cost Impact Study of Mandated Benefits in Texas: Report # 1. Milliman & Robertson, Inc., for the Texas Department of Insurance; 2000. [accessed 3/9/2009]. http://www.tdi.state.tx.us/reports/documents/benefits1_00.pdf. [Google Scholar]
- Almond D, Doyle JJ. After Midnight: A Regression Discontinuity Design in Length of Postpartum Hospital Stays. American Economic Journal: Economic Policy. 2011;3(3):1–34. [Google Scholar]
- Baker L, Chan J. Laws Requiring Health Plans to Provide Direct Access to Obstetricians and Gynecologists, and Use of Cancer Screening by Women. Health Services Research. 2007;42(3):990–1006. doi: 10.1111/j.1475-6773.2006.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema DF, Feuer EJ. Effect of Screening and Adjuvant Therapy on Mortality from Breast Cancer. New England Journal of Medicine. 2005;352(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- Bertrand M, Duflo E, Mullainathan S. How Much Should We Trust Difference-In-Differences Estimates? Quarterly Journal of Economics. 2004;119(1):249–275. [Google Scholar]
- Bitler M. Utilization of Infertility Treatments: The Effects of Insurance Mandates. Mimeo. 2008 doi: 10.1007/s13524-011-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitler M, Schmidt L. Utilization of Infertility Treatments: The Effects of Insurance Mandates. Demography. 2012 doi: 10.1007/s13524-011-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen N, Brown M. The Price of Mammography in the United States: Data from the National Survey of Mammography Facilities. Milbank Quarterly. 1994;72(3):431–450. [PubMed] [Google Scholar]
- Brozan N. Cost of Mammography is a Major Deterrent. The New York Times. 1986 Sep 22; [Google Scholar]
- Buchmueller T, Cooper P, Jacobson M, Zuvekas S. Parity for Whom? Exemptions and the Extent of State Mental Health parity Legislation. Health Affairs. 2007;26(4):483–487. doi: 10.1377/hlthaff.26.4.w483. [DOI] [PubMed] [Google Scholar]
- Buckles Kasey. Stopping the Biological Clock: Infertility Treatments and the Career-Family Tradeoff. Mimeo 2007 [Google Scholar]
- Bundorf, Kate M, Henne Melinda, Baker Laurence. Mandated Health Insurance Benefits and the Utilization and Outcomes of Infertility Treatments. NBER WP 12820 2007 [Google Scholar]
- Bunce V, Wieske J. [accessed 10/31/2008];“Health Insurance Mandates in the States 2008” report from Council for Affordable Health Insurance. 2008 available at: http://www.cahi.org/cahi_contents/resources/pdf/HealthInsuranceMandates2008.pdf.
- Butler P. ERISA Preemption Manual for State Health Policy Makers. Washington, DC: Kaiser Family Foundation; 2000. [Google Scholar]
- Busch S, Barry C. New Evidence on the Effects of State Mental Health Mandates. Inquiry. 2008;45:308–322. doi: 10.5034/inquiryjrnl_45.03.308. [DOI] [PubMed] [Google Scholar]
- Cameron A, Gelbach J, Miller D. Bootstrap-Based Improvements for Inference with Clustered Errors. The Review of Economics and Statistics. 2008;90(3):414–427. [Google Scholar]
- Cutler D. Are We Finally Winning the War on Cancer? Journal of Economic Perspectives. 2008;22(4):3–26. doi: 10.1257/jep.22.4.3. [DOI] [PubMed] [Google Scholar]
- Dans P, Wright A. Effect of a Legislative Mandate on Mammography Use and Coding Practices in Maryland. Radiology. 1996;198:647–660. doi: 10.1148/radiology.198.3.8628850. [DOI] [PubMed] [Google Scholar]
- Erbas B, Prevenzano E, Armes J, Gertig D. The Natural History of Ductal Carcinoma In Situ of the Breast: A Review. Breast Cancer Research and Treatment. 2006;97(2):135–44. doi: 10.1007/s10549-005-9101-z. [DOI] [PubMed] [Google Scholar]
- Federal Register. Interim Final Rules for Group Health Plans and Health Insurance Issuers Relating to Coverage of Preventive Services Under the Patient Protection and Affordable Care Act. Federal Register. 2010;75(137):41726–60. [PubMed] [Google Scholar]
- Fenton J, Foote S, Green P, Baldwin L-M. Diffusion of Computer-Aided Mammography after Mandated Medicare Coverage. Archives of Internal Medicine. 2010;170(11):987–989. doi: 10.1001/archinternmed.2010.104. [DOI] [PubMed] [Google Scholar]
- Finkelstein Amy, Taubman Sarah, Wright Bill, Bernstein Mira, Gruber Jonathan, Newhouse Joseph P, Allen Heidi, Baicker Katherine the Oregon Health Study Group. The Oregon Health Insurance Experiment: Evidence from the First Year. Quarterly Journal of Economics. 2012;127(3):1057–1106. doi: 10.1093/qje/qjs020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J. The Incidence of Mandated Maternity Benefits. American Economic Review. 1994a;84(3):622–641. [PubMed] [Google Scholar]
- Gruber J. State Mandated Benefits and Employer Provided Insurance. Journal of Public Economics. 1994b;55(3):433–464. [Google Scholar]
- Harris K, Carpenter C, Bao Y. The Effects of State Parity Laws on the Use of Mental Health Care. Medical Care. 2006;44(6):499–505. doi: 10.1097/01.mlr.0000215813.16211.00. [DOI] [PubMed] [Google Scholar]
- The Henry J. Kaiser Family Foundation and Health Research Educational Trust. Employer Health Benefits: 1999 Annual Survey. 1999 accessed online at: http://www.aone.org/hret/publications/content/1999.pdf.
- Jensen G, Gabel J. State Mandated Benefits and the Small Firm’s Decision to Offer Insurance. Journal of Regulatory Economics. 1992;4:379–404. [Google Scholar]
- Jensen G, Morrissey M. Employer-Sponsored Health Insurance and Mandated Benefits Laws. Milbank Quarterly. 1999;77(4):425–59. doi: 10.1111/1468-0009.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiyala S, Strumpf E. How Effective is Population-Based Cancer Screening? Regression Discontinuity Estimates from the U.S. Guideline Screening Initiation Ages. SSRN working paper. 2011a doi: 10.1515/fhep-2014-0014. [DOI] [PubMed] [Google Scholar]
- Kadiyala S, Strumpf E. Are United States and Canadian Cancer Screening Rates Consistent with Guideline Information Regarding the Age of Screening Initiation? International Journal for Quality in Health Care. 2011b;23(6):611–620. doi: 10.1093/intqhc/mzr050. [DOI] [PubMed] [Google Scholar]
- Kadiyala S, Strumpf E. How Does Health Insurance Impact Health? The Case of Medicare and Cancer Detection. SSRN working paper 2012 [Google Scholar]
- Kaiser Family Foundation and Health Research & Educational Trust. Employer Health Benefits: 2011 Annual Survey. 2011 available online at: http://ehbs.kff.org/?page=charts&id=2&sn=30&ch=2218.
- Kaiser Family Foundation and Health Research & Educational Trust. Employer Health Benefits: 2009 Annual Survey. 2009 available online at: http://ehbs.kff.org/pdf/2009/7936.pdf.
- Kelaher M, Stellman J. The Impact of Medicare Funding on the Use of Mammography Among Older Women: Implications for Improving Access to Screening. Preventive Medicine. 2000;31:658–664. doi: 10.1006/pmed.2000.0759. [DOI] [PubMed] [Google Scholar]
- Kolstad Jonathan, Kowalski Amanda. The Impact of Health Care Reform on Hospital and Preventive Care: Evidence from Massachusetts. NBER Working Paper #16102. 2010 doi: 10.1016/j.jpubeco.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dow W, Norton E. The Effect of Drive-Through Delivery Laws on Postpartum Length of Stay and Hospital Charges. Journal of Health Economics. 2004;23(1):129–155. doi: 10.1016/j.jhealeco.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Lurie Nicole, Manning Willard G, Peterson Christine, Goldberg George A, Phelps Charles A, Lillard Lee. Preventive Care: Do We Practice What We Preach? American Journal of Public Health. 1987;77(7):801–804. doi: 10.2105/ajph.77.7.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney M, Marconi K. Legislative Interventions to Increase Access to Screening Mammography. Journal of Community Health. 1992;17(6):333–349. doi: 10.1007/BF01323996. [DOI] [PubMed] [Google Scholar]
- Mammen Enno. Boostrap and Wild Bootstrap for High Dimension Linear Models. The Annals of Statistics. 1993;21(1):255–285. [Google Scholar]
- Manning Willard, Newhouse Joseph P, Duan Naihua, Keeler Emmett B, Leibowitz Arleen. Health Insurance and the Demand for Medical Care: Evidence from a Randomized Experiment. American Economic Review. 1987;77(June):251–277. [PubMed] [Google Scholar]
- Mor V, Shackleton R. The Effects of State Mandated Coverage of Mammography. Abstracts of the AcademyHealth Meetings. 2005;2005 available at: http://gateway.nlm.nih.gov/MeetingAbstracts/ma?f=103623574.html and through personal correspondence with V. Mor. [Google Scholar]
- National Cancer Institute. [Last accessed 10/18/2013];SEER Stat Fact Sheets: Breast. 2013 available at: http://seer.cancer.gov/statfacts/html/breast.html#survival.
- National Cancer Institute. [accessed 10/31/2008];State Laws Requiring Third-Party Payers to Offer or Provide Coverage for Screening Mammograms. 2005 table available at http://www.scld-nci.net/Data/mam_6_30_05.pdf.
- Nelson David, Bland Shayne, Powell-Griner Eve, Klein Richard, Wells Henry, Hogelin Gary, Marks James. State Trends in Health Risk Factors and Receipt of Clinical Preventive Services Among US Adults During the 1990s. Journal of the American Medical Association. 2002;287(20):2659–2667. doi: 10.1001/jama.287.20.2659. [DOI] [PubMed] [Google Scholar]
- Pacula R, Sturm R. Mental Health Parity Legislation: Much Ado About Nothing? Health Services Research. 2000;35(1Pt2):263–275. [PMC free article] [PubMed] [Google Scholar]
- Pettibone K. Mandating Cancer Screening Reimbursement Coverage: Does it Improve Women’s Rates of Breast and Cervical Cancer Screening Use? Abstracts of the AcademyHealth Meetings. 2003;2003 available at: http://gateway.nlm.nih.gov/MeetingAbstracts/ma?f=102275235.html. [Google Scholar]
- Phillips KA, Kerlikowske K, Baker L, Chang SW, Brown ML. Factors Associated with Women’s Adherence to Mammography Screening Guidelines. Health Services Research. 1998;33(1):29–53. [PMC free article] [PubMed] [Google Scholar]
- Schmidt L. Effects of Infertility Insurance Mandates on Fertility. Journal of Health Economics. 2007;26(3):431–446. doi: 10.1016/j.jhealeco.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Data (1973–2012) National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; SEER Research Data 1973–2012 -- ASCII Text Data: Surveillance, Epidemiology, and End Results (SEER) Program. ( www.seer.cancer.gov) released April 2015, based on the November 2014 submission. [Google Scholar]
- Stanton M. Agency for Healthcare Research and Quality (AHRQ) Research In Action Issue #17. 2004. Employer-Sponsored Health Insurance: Trends in Cost and Access. Dated September 2004. [Google Scholar]
- Sullivan C, Rice T. The Health Insurance Picture in 1990. Health Affairs. 1991 Summer;:104–115. doi: 10.1377/hlthaff.10.2.104. [DOI] [PubMed] [Google Scholar]
- Super N. Medigap: Prevalence, Premiums, and Opportunities for Reform. National Health Policy Forum Issue Brief No. 782. 2002 Dated September 9, 2002. [PubMed] [Google Scholar]
- Trivedi A, Rakowski W, Ayanian J. Effect of Cost Sharing on Screening Mammography in Medicare Health Plans. The New England Journal of Medicine. 2008;358(4):375–383. doi: 10.1056/NEJMsa070929. [DOI] [PubMed] [Google Scholar]
- Warnecke R, Sudman S, Johnson T, O’Rourke D, Davis A, Jobe J. Cognitive Aspects of Recalling and Reporting Health-related Events: Papanicolaou Smears, Clinical Breast Exams, and Mammograms. American Journal of Epidemiology. 1997;146(11):982–992. doi: 10.1093/oxfordjournals.aje.a009226. [DOI] [PubMed] [Google Scholar]
- Wolfers J. Did Unilateral Divorce Laws Raise Divorce Rates? A Reconciliation and New Results. American Economic Review. 2006;96(5):1802–1820. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.