Abstract
Objective
Antimicrobial photodynamic therapy (aPDT) is a controversial approach for endodontic disinfection. The objective of this study was to test the photosensitiser (PS) concentration and assess the optical shielding phenomenon, the use of hydrogen peroxide (H2O2) and minimal energy irradiation to optimise endodontic aPDT for suggesting a protocol for clinical use.
Methods
Different parameters for aPDT were tested. Aqueous solutions of methylene blue (MB) at 50, 100, 150 and 300 μM were tested in vitro for optical shield and reactive oxygen species (ROS) production by the reduction of N,N-dimethyl-4-notrosoaniline (RNO) at 440 nm absorbance when irradiated using a diode laser (660 nm). Ten single-rooted teeth were inoculated with bioluminescent bacteria Pseudomonas aeruginosa for 72 hours to form biofilms. Bioluminescence imaging was used to serially evaluate the minimum energy necessary during endodontic aPDT using MB and a diode laser coupled to an optical fibre for intracanal microbial reduction. In addition, teeth (n=21) infected with Enterococcus faecalis were treated with sequential combinations of endodontic aPDT and H2O2 and the colony-forming unit (CFU) was determined.
Results
ROS production was inversely proportional to the MB concentration in the solution due to quenching of MB. Optical shielding limited light penetration at high MB concentrations. The use of H2O2 before aPDT achieved higher disinfection compared to conventional aPDT or when MB was irradiated in an H2O2 solution. Energy irradiation of 9.6 J achieved a significant reduction and further light delivery did not produce further reduction.
Conclusion
PS concentration of about 50 μM, biofilm pre-treatment with H2O2 for 1 min and energy irradiation around 10 J appear to be an effective protocol for endodontic aPDT.
Keywords: Antimicrobial photodynamic therapy, disinfection, hydrogen peroxide, lasers, methylene blue
INTRODUCTION
One of the main goals of endodontic treatment, whether it is primary treatment, re-treatment or even surgical treatment, is to reduce microbial infection. This is conventionally performed using a combination of mechanical instrumentation and chemical irrigation (1). However, the persistence of microbial infection may result in unsuccessful treatment, which may occur even when procedures are satisfactorily performed (2–4). Reasons for the incomplete control of infection include complexities of the canal system, inefficient instrumentation and/or missed canals (1, 5). Since efficient intracanal disinfection substantially increases the success rate of endodontic treatment, several more effective cleaning protocols have been proposed in literature.
Antimicrobial photodynamic therapy (aPDT) appears to be an effective adjuvant method in endodontic treatment since reports have shown a further degree of microbial reduction in vitro and in vivo when aPDT is used after chemo-mechanical preparation (1–3, 6, 7). This approach involves the combination of a photosensitiser (PS) and a light source (usually a low-intensity laser) to inhibit a broad spectrum of microorganisms through the production of reactive oxygen species (ROS). Absorption of the light excites the PS, which reacts with oxygen to produce ROS that is toxic to microorganisms. Antimicrobial PDT is recognised as a treatment strategy, which is both minimally invasive and minimally toxic (6, 8).
However, there is no consensus on the optimal parameters for aPDT as an endodontic treatment adjuvant. For example, Trindade et al. (9) affirmed that ‘Data suggest the need for protocol adjustments to enhance photodynamic therapy predictability in endodontics’ and Siddiqui et al. (10) stated that ‘Efficacy of aPDT in eliminating E. faecalis from infected root canals remains questionable’. In contrast, Gursoy et al. (11) stated that; ‘aPDT may be used as an adjunctive tool for facilitating the treatment of oral infections’.
Considering these controversies and a clear need for protocol optimisation, this study aimed to evaluate different parameters, such as concentration of PS, time/energy of irradiation and combination of PS with hydrogen peroxide (H2O2) for intracanal microbial reduction in aPDT and to propose a protocol for endodontic aPDT.
METHODS
This study was conducted in accordance with the ethical principles and following the Brazilian norms. The ethics committee of São Leopoldo Mandic Dental Research Center evaluated and approved this study (2012/0076). Written informed consent was obtained from patients who participated in this study.
In vitro analysis
Effect of PS concentration on ROS production
The first parameter tested was the effect of methylene blue (MB) concentration on aPDT efficiency throughout ROS production. We hypothesised that by increasing the number of molecules in an MB solution (i.e. increasing the molarity), more molecules of PS would be available for the photoreaction and could promote intensified ROS production. The results of this experiment would indicate the ideal PS concentration for higher ROS production and the concentration recommended for clinical use during aPDT.
To validate the hypothesis, an indirect ROS detection was performed using aqueous solutions of MB at 50, 100, 150 and 300 μM in a 1.0 cm optical path length quartz cuvette.
All chemicals were obtained from Aldrich (Milwaukee, WI, USA) and used without further purification. The indirect ROS assay was based on the oxidation of N,N-dimethyl-4-notrosoaniline (RNO). The cuvette contained L-histidine at 15 mM and the PS used as blank. RNO was then added to achieve a measured absorbance of approximately 1 at 440 nm (concentration of 13.3 μM). Irradiation periods of 30–240 s (energy of 1.2 J every 30 s of irradiation) were maintained using a diode laser (MMOptics, São Paulo, Brazil) that delivered 40 mW out of the fibre, and loss of RNO absorbance was measured every 30 s at 440 nm (12).
Effect of MB concentration on light penetration due to the optical shield phenomenon
To evaluate if an excessive concentration of the PS could promote optical shielding, blocking light due to high absorption at surface and decreasing light penetration, consequently reducing aPDT efficiency in root canals, aqueous solutions of MB at increasing PS concentration: 50, 100, 150 and 300 μM were tested. A quartz cuvette was filled with 5 mL of each solution, and the laser beam (emitting 660 nm, 10 mW) was delivered perpendicular to the cuvette surface. Images were recorded using a digital camera (Pentax k200) and analysed using Image J (National Institute of Health, EUA). A false-coloured image was used to measure light penetration/attenuation compared to the optical length using the tool measurement from Image J software 209/17/19 (13).
Bacterial reduction in root canals
To test the antimicrobial effects of aPDT on a root canal, we attempted to mimic the clinical situation found in an endodontic treatment. Two hypotheses were tested. For both experiments, the methodology for tooth preparation and biofilm growth was similar and was based on previous studies (3, 14).
Ex vivo analysis
Root canals
Thirty freshly extracted human single-rooted teeth (upper central incisors and upper canines), with straight root canals confirmed by radiographic examination, which had been extracted for periodontal reasons, were used in this study. The crowns were removed using a diamond disc, and the roots were shortened to a length of approximately 13 mm. The canals were enlarged to a #30.4 M two files (VDW GmbH, München, Germany) and cleaned with 2.5% sodium hypochlorite (NaOCl) solution between each file. The external root surfaces were sealed with two layers of nail polish to avoid external contamination. The apical foramen was subsequently closed using composite material (Filtek Z 250, 3M, Brazil). The root canals were irrigated with 17% ethylenediaminetetraacetic acid (EDTA) for 2 min followed by irrigation with phosphate-buffered saline (PBS) to remove the smear layer. Prior to inoculation, the specimens were sterilised by autoclaving (3).
Biofilm growth and bacterial strain
The microorganisms studied were Enterococcus faecalis (ATCC 29212) and Pseudomonas aeruginosa (XEN5), which had been genetically engineered to be bioluminescent provided by Xenogen Corp. (Alameda, CA, USA).
Bacteria were grown in brain heart infusion (BHI) broth to form a stationary growth phase suspension of 109 cells/mL (confirmed by transmission spectroscopy at λ=540 nm, T=15% in a glass cuvette with 1 cm of optical path). Each root canal was filled with bacterial suspension and each tooth was subsequently aerobically incubated for 72 h, with shaking (150 rpm), to allow biofilm formation. The BHI broth was changed every 24 h to facilitate biofilm development (13). Scanning electron microscopy (SEM) and bioluminescent imaging were performed to confirm if the biofilm was properly induced (data not shown).
Irradiation parameters
Illumination was performed with a 300 μm-diameter fibre coupled to a diode laser (MMOptics, São Paulo, Brazil). The diode laser delivered 660 nm light at a total power of 40 mW out of the fibre. Spiral movements, from apical to cervical, were manually performed to ensure the uniform diffusion of light inside the canal lumen.
H2O2 combined with aPDT
Previous studies (15, 16) have suggested that the use of H2O2 combined with aPDT provides increased killing of microorganisms. However, there is a need to validate the hypothesis that a different mechanism of killing could occur depending on the sequence used for the antimicrobial effect and how this effect occurs in root canals.
For this experiment, different combination sequences of MB/aPDT and H2O2 were tested, as first proposed in vitro (15).
Suspensions of E. faecalis in the stationary phase were incubated for 72 h in the root canal of previously prepared teeth (n=30), as described above. One group of teeth received 50 μM MB aqueous solution for 2 min and was irradiated using the diode laser (energy of 9.6 J). The second group received 50 μM of MB in an H2O2 solution (100 mM) for 2 min and was then irradiated. The third group received a 100 mM solution of H2O2 in the absence of light for 1 min, which was then removed and replaced with 50 μM of MB in an aqueous solution for another 1 min and then irradiated.
To quantify the bacterial reduction, the root canals were irrigated with 1 mL of sterile PBS solution to remove the PS and dried using three sterile paper points (Dentsply Latin America, Petropolis, Brazil), each one left inside the root canal for 1 min. All the three paper points were combined inside a 1.5 mL microcentrifuge tube with PBS and vortexed for 30 s for determining colony forming units (CFU/mL). Aliquots were added to wells of a 96-well plate for serial dilution, streaked on BHI agar plates and incubated for 24 h; the CFUs recovered were calculated.
Real-time analysis of minimal energy irradiation for bacterial reduction in root canal
To analyse the minimal energy necessary kill microorganisms using aPDT, bioluminescent P. aeruginosa was selected. The use of bioluminescent methods to test the efficiency of aPDT allows a real-time analysis and is considered a precise method to determine the minimal energy and time necessary for irradiation (3).
The biofilms P. aeruginosa were grown for 72 h in each tooth (n=10) contained inside its transparent microcentrifuge tube, as described. Imaging was carried out with a low-light intensified camera (Hamamatsu Photonics KK, Bridgewater, NJ, USA). These images served to confirm equal levels of contamination and to obtain the initial signal from the biofilm inside the canal. The use of this bioluminescent imaging system has been described in detail (3, 13, 17), Briefly, bioluminescence signal was accumulated for 2 min at 35 sensitivity level and a maximum setting on the image intensifier control module. Using the ARGUS software (Siemens Medical Solutions, Erlangen, Germany), the luminescence image was presented as a false-colour image superimposed on top of the grey scale reference image. The image-processing component of the software provided mean pixel values from the luminescence images on defined areas covering each tooth on a 256 grey scale. For bioluminescence comparison, all images were recorded at the same bit range. These images served to confirm the level of infection and to obtain the initial signal from the bacteria inside the root canals.
The irradiation procedure was divided into steps alternated by bioluminescence imaging to allow the real-time bacterial reduction follow-up and energy response curve. The initial image (t=0) was acquired after 2 min of PS solution incubation in the dark (pre-irradiation time) to allow MB uptake and diffusion. The MB solution was not removed or refilled during the experiment. Each irradiation was always performed during 1 min, and bioluminescence imaging was performed to obtain the microbial reduction over time/energy.
Statistical analysis
Values are provided as mean and standard deviation. Statistical comparisons within and between groups were performed with t test using Origin software version 8.5 (OriginLab, Northampton, MA, USA) after submitting data to Levenne and Shapiro-Wilk tests to confirm homogeneity and normality. Results were considered significant if P<0.05.
RESULTS
In vitro analysis
Effect of PS concentration on ROS production
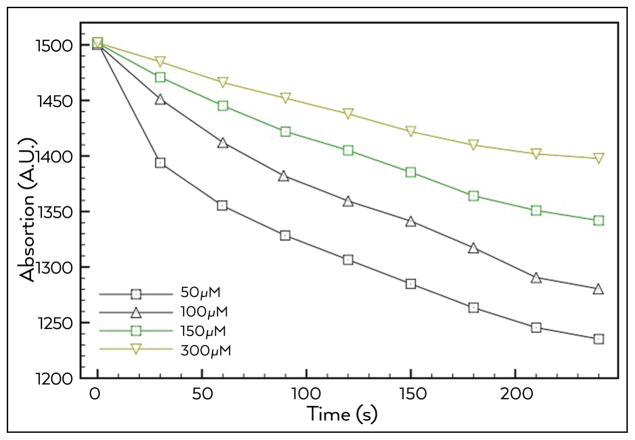
The hypothesis that by increasing the number of molecules in an MB solution, more molecules of PS would be available for the photoreaction and promote an intensification of ROS production was proved wrong. As shown in Figure 1, there were significant differences between the rates of ROS production found at different concentrations of MB solution. Instead, of more ROS being generated with higher concentrations of MB as might have been expected, the opposite result was found, with the rate of RNO oxidation being inversely proportional to the MB concentration. The results indicated that a concentration of 50 μM was the most appropriate rate of MB concentration/ROS production.
Figure 1.
ROS production using MB at different concentrations after irradiation with a 660 nm diode laser. Indirect measurement of total ROS formation by reduction of RNO absorbance at 440 nm
Effect of MB concentration on light penetration due to optical shield phenomenon
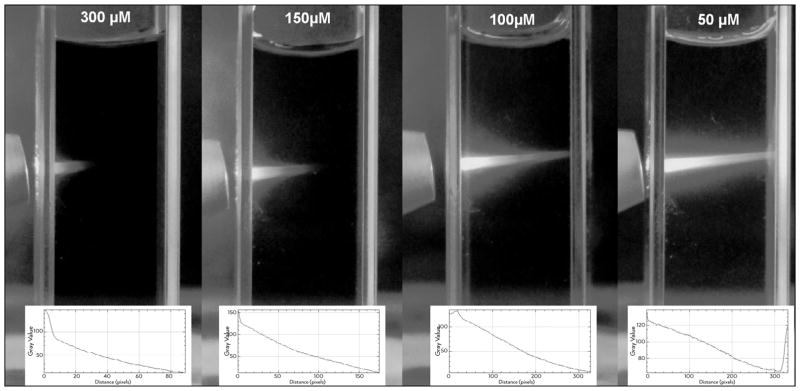
Images were captured and analysed to detect if the presence of optical shielding (Figure 2) would limit light penetration within the MB solution. Note that at increasing molar concentrations of MB, light attenuation also increased. At 50 μM the light passed through the entire optical path (1 cm or 100% of the distance) with no significant attenuation. Light achieved attenuation around 4.5% in the optical path with MB at 100 μM. At 150 μM, the attenuation was 45%, while at 300 μM, the attenuation was 68% of the initial incident light. The results showed that a concentration of 50–100 μM should be indicated more to use in root canal aPDT.
Figure 2.
Images of light penetration at increasing PS concentration in the solution. Original images and penetration/attenuation plot analysis
Based in the in vitro results a concentration of 50 μM for MB was chosen for the ex vivo experiments.
Ex vivo analysis
The addition of 10 μL of a suspension containing 109 cells of E. faecalis or P. aeruginosa into the root canal followed by 72 h incubation at 37°C reliably and reproducibly produced a biofilm that could be imaged and/or quantified. The presence of a microbial biofilm rather than planktonic bacteria was demonstrated by the failure of irrigation with saline, to reduce the bioluminescent signal (data not shown).
Combination with H2O2 to increase aPDI efficiency
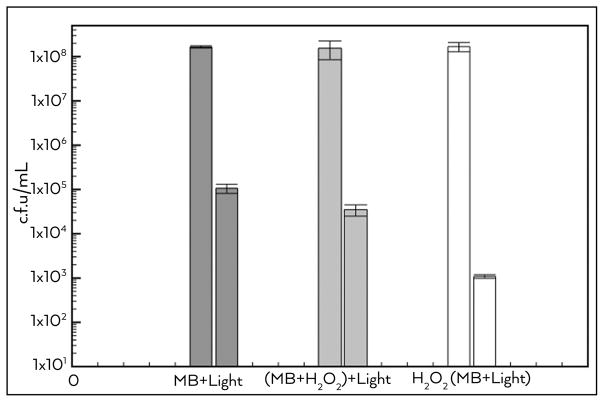
As shown in Figure 3, the bacterial (E. faecalis) inactivation using MB in aqueous solution was about 3 log, and this value was significantly different from all the other groups. The group that received the PS irradiated in H2O2 solution showed a reduction of 3.5 log. Remarkably, the group with the biofilm that was pre-treated with H2O2 for 1 min and then the medium replaced with MB solution before aPDI was performed showed a diminution of 5 log.
Figure 3.
Log (10) CFU before and after endodontic aPDI for each group (n=10). MB+light group received conventional aPDI, while (MB+H2O2)+light group received aPDI using MB in a H2O2 solution and in H2O2+(MB+light) group, the biofilm was pre-treated with H2O2 and then conventional aPDT was provided. Values are mean and bars are standard deviation
Energy irradiation for bacterial reduction
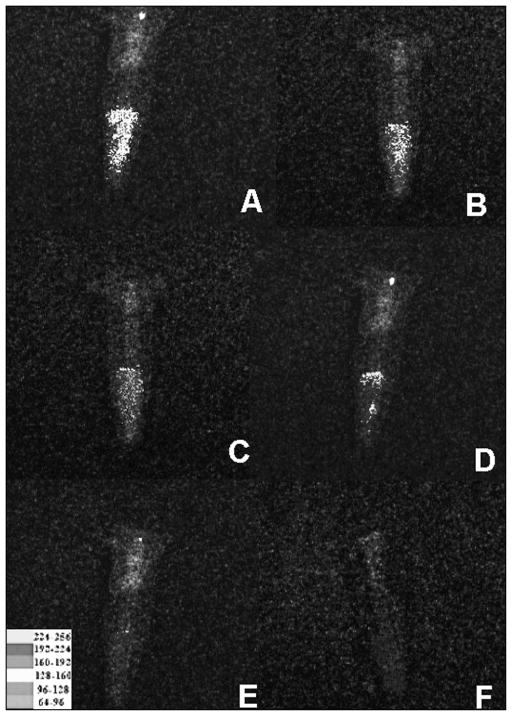
There was an energy-dependent reduction in bioluminescence until a total energy of 9.6 J (4 min) was reached, when further light delivery ceased to have a noticeable effect. The overlaid bioluminescence images of a representative tooth infected with P. aeruginosa 3-day biofilm before treatment and after each step of aPDT are shown in Figure 4.
Figure 4.
Representative bioluminescence images of 10 teeth infected with 3-day P. aeruginosa biofilms. Initial infection (A); MB in H2O2 solution for 2 min (B); after irradiation with 2.4 J (C); after 4.8 J (D); after 7.2 J (D) and after 9.6 J (F) of total energy
DISCUSSION
Many studies have established that aPDT can be used for endodontic disinfection and is an effective adjuvant when combined with conventional endodontic treatment to reduce the bacterial load in root canal infection (2, 7, 18–20). However, there are also other reports stating that the efficacy of aPDT in endodontic treatments remains questionable or that aPDT cannot significantly improve disinfection when compared to conventional chemo-mechanical preparation with NaOCl (9, 10, 21, 22). Most of the controversy is likely to be due to diverse parameters used in different studies (9, 23).
Despite these controversies, there seems to be a consensus about the use of the phenothiazinium salt MB in a water solution as an endodontic PS due to its worldwide regulatory approved status and non-toxic properties (9, 20, 23). MB solution may be excited by a low power laser emitting at 600–680 nm as a light source; moreover, the use of an optical fibre for root canal irradiation may be facilitated using a laser source.
This study evaluated different parameters for aPDT with regard to MB concentration, minimal energy for intracanal irradiation and use of H2O2 to improve aPDT and proposed an optimised protocol for use in endodontic treatment. According to Fimple et al. (2), increasing the concentration of MB and the light energy fluence (J/cm2) caused an increase in the antibacterial capacity of aPDT. Our results showed that at certain concentrations this statement is true; however, at higher concentrations the presence of MB aggregation and ROS quenching decrease ROS production, and the optical shielding phenomenon could prevent the light from reaching deeper distances. Production of ROS was detected at all PS concentration and each 1, 2 J of energy increased the formation. However, at 50 and 100 μM, the production was more effective than at higher concentrations. Moreover, Carvalho et al. (24) raised an important concern when using a dye in the tooth tissue: the ‘staining/aesthetic disadvantage’. By using low PS concentration the risk of staining the tooth structure is minimised, if not eliminated.
Can optical shielding explain the results with RNO solution? When increasing the PS concentration, the light penetration through the solution decreases. However, in a solution with RNO, this should not cause a large difference because all the light is still absorbed by MB, whether the MB is located close to the surface or is distributed throughout the cuvette, and diffusion ensures that molecules are free to move throughout the whole cuvette. Therefore, an explanation for the reduced production of ROS in a solution is likely to involve concentration-dependent quenching of the MB. Concentration dependent quenching can be either static or dynamic. Static quenching involves aggregation of the MB molecules. MB can form self-quenched dimers that according to Morgounova et al. (25) can reduce the excited state lifetime of MB by three orders of magnitude. A report by McCullagh and Robertson (16) found that at higher concentrations, MB formed dimeric compounds also reducing the efficiency of the system. Dynamic quenching involves reaction of the photoproduced ROS with the MB itself rather than with the bacterial target or with the RNO probe. If MB consumes the ROS, they are unable to react with the bacterial biofilm. This phenomenon is particularly applicable to a PS, such as MB, that is noted for undergoing photobleaching. Photobleaching describes the reaction of the PS itself with the photoproduced ROS leading to PS loss and inactivation of the ROS; this may explain part of the results. This suggests that the production of ROS is affected by the dimerisation and aggregation occurring at a higher MB concentration.
However, optical shielding will be important within the actual root canal cavity. Since the bacterial biofilm is located on the internal walls of the root canal and the light is delivered from the fibre in the middle of the solution/canal, a concentration of MB that is extremely high will result in the ROS being generated in the middle of the solution rather than at the walls or even inside the dentine tubules where the bacteria are actually growing. Sabino et al (6), pointed out that it is important that light reaches all the target area since there is a direct link between optical intensity and energy with microbial reduction.
Some studies have evaluated the use of H2O2 in combination with aPDT to improve the microbial reduction. A previous in vitro study tested different sequences of H2O2 (15). In the present study, this combination was tested for the first time in an infected tooth model for endodontic treatment. Confirming the in vitro results, our results showed that the use of MB in an H2O2 solution did improve the antimicrobial effects of PDT. Moreover, the use of the H2O2 solution before (rather than during) aPDT of E. faecalis intracanal biofilms achieved a statistically significant highest microbial killing.
As hypothesised earlier, the H2O2 solution may pre-treat the biofilm allowing better PS penetration, and the higher PS concentration inside the bacteria/biofilm could explain the better antimicrobial effect and not only the increase of oxygen available in the environment (15).
A real-time method using bioluminescent bacteria to evaluate the antimicrobial effects allows a quantitative comparison of sequential steps of treatment. Also, the bioluminescence method is a non-invasive technique; therefore, sequential images could be obtained for each sample, allowing statistical analysis without any inter-sample variation. The method is an alternative to traditional in vitro culture methods using paper point sampling and quantitative culture and has been used in different studies to evaluate infections and bacterial reduction in vitro and in animal models (3, 6, 13, 17, 26, 17, 27).
In this study, the use of bioluminescent imaging allowed determination of the minimum energy necessary for a significant bacterial reduction inside the root canal. The irradiation parameters are one of the most controversial subjects in endodontic PDT, ranging from an irradiation time of 30 s to 10 min or a total energy of 1.2 J to 70 J (28–31). Since energy is the result of power by time, and particularly because the variance of power output among the equipment available in the world market is huge, the use of total energy instead of time is recommended.
In a pilot study (data not shown), an incremental irradiation of 1.2 J (30 s) using the 660 nm low-power laser coupled to a 300 μm optical fibre did not show a significant bioluminescent signal reduction at each step; therefore, an irradiation of 2.4 J (1 min) was chosen. Irradiation of the PS with 2.4 J of energy promoted approximately 57% of signal reduction, an additional irradiation (4.8 J) resulted in reduction of 79%, 7.2 J caused a 93% decrease and after 9,6 J no remaining bioluminescence was detected. Different authors also used irradiation energies of around 10 J. Nunes et al. (21) achieved E. faecalis biofilm reduction of 99.9% (3 log) by using 8 J, and Sabino et al. (6) achieved a 3.5 log reduction against Candida albicans endodontic infection in curved root canals using 12 J of total energy, similar energies were used in this study.
The controversy in the parameters for endodontic aPDT make it difficult for the dentist to use this technique in the daily routine, and the lack of an accepted protocol makes it difficult to compare the results found in literature.
CONCLUSION
In conclusion, based in the results found in this study and supported by literature (2, 6, 7, 13, 19, 29), we recommend the following protocol for endodontic aPDT:
Use of a low-power laser emitting at 660 nm, preferably coupled to an optical fibre or diffusor
Use of a phenothiazinium salt, such as MB, as a PS at concentrations of about 50 μM
Before irradiation, a pre-treatment with H2O2 solution for 1 min improves the aPDT efficiency
Irradiation with a minimum energy of 10 J (which means around 2–4 min of irradiation using equipment with a power output of 40 to 100 mW)
HIGHLIGHTS.
This article evaluates the effects of antimicrobial photodynamic therapy (aPDT) associated with endodontic treatment in vitro
Different protocols, such as PS concentration, use of H2O2, assessment of optical shielding phenomenon and minimal energy irradiation, were tested to optimise endodontic antimicrobial photodynamic therapy to suggest a protocol for clinical use
The use of H2O2 before antimicrobial photodynamic therapy achieved higher disinfection than conventional antimicrobial photodynamic therapy
Energy irradiation of 9.6 J achieved a significant reduction on intracanal bacterial load
Photosensitiser concentration of about 50 μM, biofilm pre-treatment with H2O2 for 1 min and energy irradiation around 10 J appear to be an effective protocol for endodontic antimicrobial photodynamic therapy.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of São Leopoldo Mandic Dental Research Center.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.S.G.; Design - A.S.G., M.R.H.; Supervision - M.R.H.; Resources -A.S.G.; Materials - A.S.G., M.R.H.; Data Collection and/or Processing - A.S.G.; Analysis and/or Interpretation - A.S.G., M.R.H.; Literature Search - A.S.G.; Writing Manuscript - A.S.G.; Critical Review - M.R.H.; Other - A.S.G., M.R.H.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Kosarieh E, Khavas SS, Rahimi A, Chiniforush N, Gutknecht N. The comparison of penetration depth of two different photosensitizers in root canals with and without smear layer: An in vitro study. Photodiagnosis Photodyn Ther. 2016;13:10–4. doi: 10.1016/j.pdpdt.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Fimple JL, Fontana CR, Foschi F, Ruggiero K, Xiaoging Song, Pagonis TC, et al. Photodynamic treatment of endodontic polymicrobial infection in vitro. J Endod. 2008;34(6):728–34. doi: 10.1016/j.joen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcez AS, Nunez SC, Lage-Marques JL, Hamblin MR, Ribeiro MS. Photonic real-time monitoring of bacterial reduction in root canals by genetically engineered bacteria after chemomechanical endodontic therapy. Braz Dent J. 2007;18(3):202–7. doi: 10.1590/s0103-64402007000300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosa TP, Signoretti FG, Montagner F, Gomes BPFA, Jacinto RC. Prevalence of Treponema spp. in endodontic retreatment-resistant periapical lesions. Braz Oral Res. 2015:29. doi: 10.1590/1807-3107BOR-2015.vol29.0031. [DOI] [PubMed] [Google Scholar]
- 5.Gomes BP, Pinheiro ET, Gade-Neto CR, Sousa ELR, Ferraz CCR, Zaia AA, et al. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004;19(2):71–6. doi: 10.1046/j.0902-0055.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 6.Sabino CP, Garcez AS, Nunez SC, Ribeiro MS, Hamblin MR. Real-time evaluation of two light delivery systems for photodynamic disinfection of Candida albicans biofilm in curved root canals. Lasers Med Sci. 2015;30(6):1657–65. doi: 10.1007/s10103-014-1629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcez AS, Nunez SC, Hamblim MR, Suzuki H, Ribeiro MS. Photodynamic therapy associated with conventional endodontic treatment in patients with antibiotic-resistant microflora: a preliminary report. J Endod. 2010;36(9):1463–6. doi: 10.1016/j.joen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Muhammad OH, Chevalier M, Rocca JP, Brulat-Bouchard N, Medioni E. Photodynamic therapy versus ultrasonic irrigation: interaction with endodontic microbial biofilm, an ex vivo study. Photodiagnosis Photodyn Ther. 2014;11(2):171–81. doi: 10.1016/j.pdpdt.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Trindade AC, De Figueiredo JA, Steier L, Weber JB. Photodynamic therapy in endodontics: a literature review. Photomed Laser Surg. 2015;33(3):175–82. doi: 10.1089/pho.2014.3776. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui SH, Awan KH, Javed F. Bactericidal efficacy of photodynamic therapy against Enterococcus faecalis in infected root canals: a systematic literature review. Photodiagnosis Photodyn Ther. 2013;10(4):632–43. doi: 10.1016/j.pdpdt.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Gursoy H, Ozcakir-Tomruk C, Tanalp J, Yilmaz S. Photodynamic therapy in dentistry: a literature review. Clin Oral Investig. 2013;17(4):1113–25. doi: 10.1007/s00784-012-0845-7. [DOI] [PubMed] [Google Scholar]
- 12.Tegos GP, Anbe M, Yang C, Demidova TN, Satti M, Pawel Mroz, et al. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob Agents Chemother. 2006;50(4):1402–10. doi: 10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcez AS, Fregnani ER, Rodriguez HM, Nunez SC, Sabino CP, Suzuki H, et al. The use of optical fiber in endodontic photodynamic therapy. Is it really relevant? Lasers Med Sci. 2013;28(1):79–85. doi: 10.1007/s10103-012-1073-8. [DOI] [PubMed] [Google Scholar]
- 14.Garcez AS, Ribeiro MS, Tegos GP, Nunez SC, Jorge AOC, Hamblin MR. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg Med. 2007;39(1):59–66. doi: 10.1002/lsm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcez AS, Nunez SC, Baptista MS, Daghastanli NA, Itri R, Hamblin MR, et al. Antimicrobial mechanisms behind photodynamic effect in the presence of hydrogen peroxide. Photochem Photobiol Sci. 2011;10(4):483–90. doi: 10.1039/c0pp00082e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCullagh C, Robertson PK. Photo-dynamic biocidal action of methylene blue and hydrogen peroxide on the cyanobacterium Synechococcus leopoliensis under visible light irradiation. J Photochem Photobiol B. 2006;83(1):63–8. doi: 10.1016/j.jphotobiol.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Hamblin MR, O’Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75(1):51–7. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Garcez AS, Arantes-Neto JG, Sellera DP, Fregnani ER. Effects of antimicrobial photodynamic therapy and surgical endodontic treatment on the bacterial load reduction and periapical lesion healing. Three years follow up. Photodiagnosis Photodyn Ther. 2015;12(4):575–80. doi: 10.1016/j.pdpdt.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Silva Garcez A, Nunez SC, Lage-Marques JL, Jorge AOC, Ribeiro MS. Efficiency of NaOCl and laser-assisted photosensitization on the reduction of Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(4):e93–8. doi: 10.1016/j.tripleo.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Javed F, Romanos GE. Does photodynamic therapy enhance standard antibacterial therapy in dentistry? Photomed Laser Surg. 2013;31(11):512–8. doi: 10.1089/pho.2012.3329. [DOI] [PubMed] [Google Scholar]
- 21.Nunes MR, Mello I, Franco GC, de Medeiros JMF, dos Santos SSF, Habitanite SM, et al. Effectiveness of photodynamic therapy against Enterococcus faecalis, with and without the use of an intracanal optical fiber: an in vitro study. Photomed Laser Surg. 2011;29(12):803–8. doi: 10.1089/pho.2011.2995. [DOI] [PubMed] [Google Scholar]
- 22.Souza LC, Brito PR, de Oliveira JC, Alves FRF, Moreira EJL, Sampaio-Filho Helio R, et al. Photodynamic therapy with two different photosensitizers as a supplement to instrumentation/irrigation procedures in promoting intracanal reduction of Enterococcus faecalis. J Endod. 2010;36(2):292–6. doi: 10.1016/j.joen.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 23.de Oliveira BP, Aguiar CM, Camara AC. Photodynamic therapy in combating the causative microorganisms from endodontic infections. Eur J Dent. 2014;8(3):424–30. doi: 10.4103/1305-7456.137662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.dos Carvalho ES, Mello I, Albergaria SJ, Habitante SM, Lage-Marquez JS, Raldi DP. Effect of chemical substances in removing methylene blue after photodynamic therapy in root canal treatment. Photomed Laser Surg. 2011;29(8):559–63. doi: 10.1089/pho.2010.2922. [DOI] [PubMed] [Google Scholar]
- 25.Morgounova E, Shao Q, Hackel BJ, Thomas DD, Ashkenazi S. Photoacoustic lifetime contrast between methylene blue monomers and self-quenched dimers as a model for dual-labeled activatable probes. J Biomed Opt. 2013;18(5):56004. doi: 10.1117/1.JBO.18.5.056004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin RM, Bhayana B, Hamblin MR, Dai T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg Med. 2016 doi: 10.1002/lsm.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhu Y, Chen J, Wang Y, Sherwood ME, Murray CK. Antimicrobial blue light inactivation of Candida albicans: in vitro and in vivo studies. Virulence. 2016;7(5):536–45. doi: 10.1080/21505594.2016.1155015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asnaashari M, Mojahedi SM, Asadi Z, Azari-Marhabi S, Maleki A. A comparison of the antibacterial activity of the two methods of photodynamic therapy (using diode laser 810nm and LED lamp 630nm) against Enterococcus faecalis in extracted human anterior teeth. Photodiagnosis Photodyn Ther. 2016;13:233–7. doi: 10.1016/j.pdpdt.2015.07.171. [DOI] [PubMed] [Google Scholar]
- 29.Foschi F, Fontana CR, Ruggiero K, Riahi R, Vera A, Doukas AG. Photodynamic inactivation of Enterococcus faecalis in dental root canals in vitro. Lasers Surg Med. 2007;39(10):782–87. doi: 10.1002/lsm.20579. [DOI] [PubMed] [Google Scholar]
- 30.Stojicic S, Amorim H, Shen Y, Haapasalo M. Ex vivo killing of Enterococcus faecalis and mixed plaque bacteria in planktonic and biofilm culture by modified photoactivated disinfection. Int Endod J. 2013;46(7):649–59. doi: 10.1111/iej.12041. [DOI] [PubMed] [Google Scholar]
- 31.Soukos NS, Chen PS, Morris JT, Ruggiero K, Abernethy AD, Som S. Photodynamic therapy for endodontic disinfection. J Endod. 2006;32(10):979–84. doi: 10.1016/j.joen.2006.04.007. [DOI] [PubMed] [Google Scholar]