Abstract
The heart is one of the first organs to form and function during embryonic development. It is comprised of multiple cell lineages, each integral for proper cardiac development, and include cardiomyocytes, endothelial cells, epicardial cells and neural crest cells. The molecular mechanisms regulating cardiac development and morphogenesis are dependent on signaling crosstalk between multiple lineages through paracrine interactions, cell-ECM interactions, and cell-cell interactions, which together, help facilitate survival, growth, proliferation, differentiation and migration of cardiac tissue. Aberrant regulation of any of these processes can induce developmental disorders and pathological phenotypes. Here, we will discuss each of these processes, the genetic factors that contribute to each step of cardiac development, as well as the current and future therapeutic targets and mechanisms of heart development and disease. Understanding the complex interactions that regulate cardiac development, proliferation and differentiation is not only vital to understanding the causes of congenital heart defects, but to also finding new therapeutics that can treat both pediatric and adult cardiac disease in the near future.
Keywords: signaling, development, congenital, heart, disease, genetics
Introduction
The heart consists of multiple cell lineages, including cardiomyocytes, endothelial cells, fibroblasts, and smooth muscle cells, that together orchestrate a sophisticated network of crosstalk in cardiac development, disease and regeneration [1]. The process of development itself, and the interactions between cell-autonomous and non-autonomous events, are required for cardiac cell proliferation, differentiation, migration, and survival [2]. Growth factors, signaling pathways and transcription factors all play a critical role in facilitating these events. In this review, we focus on the discoveries of recent studies that help unveil the critical role of genetic mutations, signaling pathways and lineage-specific contributions to cardiovascular development, physiology, and disease.
Heart anatomy and function
The heart is a vital, multi-chambered organ that pumps blood to maintain proper pulmonary and systemic circulation, mediating oxygenation of the body’s vital tissues. The mammalian heart is made up of four chambers, the left atrium (LA), left ventricle (LV), right atrium (RA), and right ventricle (RV), each divided by septal and valvular structures [3] (Figure 1). The heart relaxes (dilates) or pumps (contracts) according to electrical signals that stem from the cardiac conduction system. Importantly, it also functions to transport oxygen, nutrients, and signaling molecules to the entire body [3, 4].
Figure 1. Schematic representation of the physiology, major vessels, and circulation of the heart.
The heart structures, major blood vessels, and directions of blood flow are implicated. The oxygenated (red) and deoxygenated (blue) blood exchanges occur through systemic and pulmonary circulations. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Briefly, de-oxygenated blood returning from the body through the inferior and superior vena cava enters the heart through the RA and is pumped to the RV through the tricuspid valve. Subsequently, blood from the RV is propelled into the lungs through the pulmonary arteries, where it becomes enriched with oxygen [3]. Pulmonary veins return oxygenated blood to the LA, which contracts through the mitral valve to fill the LV, the main pumping chamber of the heart, where it is then ejected through the aorta and into the major circulatory network of the body [4]. The process repeats and begins all over again. The contraction of the ventricles is referred to as systole, whereas diastole is the term used to describe the relaxation, or dilation, of the heart muscle [5]. (Figure 1)
Process of cardiac development
The heart is the first fully developed and functional organ in embryogenesis. During gastrulation, a single-layered blastula is re-organized in to three germ layers: a dorsal ectoderm, a ventral endoderm and a mesoderm layer [6]. The heart tissue, consisting of myocardial, endocardial and epicardial cells, is predominantly derived from the mesodermal layer [7]. In addition, ectoderm-derived cardiac neural crest cells are involved in the development of the cardiac cushions of the outflow tract [8].
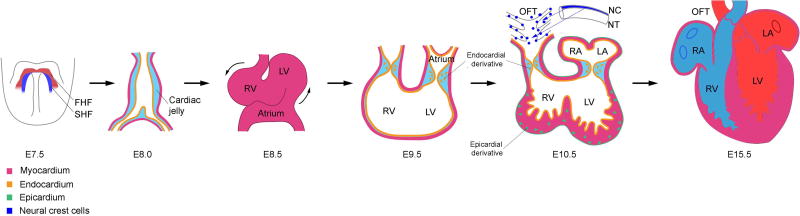
Prospective cardiogenic cells originate from the posterior epiblast, precursor cells derived from mesodermal tissues [9]. At human embryonic day 12 (E12) (E5.5 in mice), the embryo becomes an elongated cylinder, divided in 2 portions: the proximal and distal regions [10]. Subsequently, formation of bilaterally symmetric cardiac primordia occurs in early gestation, at E18 (E7.5 in mice), which is derived from lateral plate mesoderm [10]. These cells then migrate medially and fuse at E20 (E8.0 in mice), generating the initial heart tube, whose composition is comprised of an outer myocardial layer and an inner endocardial layer [10]. The myocardial layer gives rise to cardiomyocytes, the fundamental unit of rhythmic beating; the endocardial layer leads to the development of cardiac valves [11]. By E25 (E10.5 in mice), in response to myocardial signals deposited in the cardiac jelly, the endocardial cells undergo epithelial–mesenchymal transition (EMT), delaminating from the endocardial surface and differentiating into mesenchymal cells, which proliferate and invade the cardiac jelly [11]. This, in turn, gives rise to the endocardial cushion in the atrioventricular (AV) canal and to the outflow tract (OFT), the precursor to future valvular structures [12]. (Figure 2)
Figure 2. Schematic representation of murine heart development.
At E7.5, the cardiac mesodermal cells form the crescent like structure comprised of the first heart field (FHF) and the second heart field (SHF). The FHF cells fuse at the midline, forming the primitive heart tube that mostly contributes to left ventricle (LV) by E8.0. The lumen of this tubule structure is covered by a layer of endocardial cells which is required for the development of atrioventricular (AV) canal and outflow tract (OFT), the precursor cells that give rise to valvular structures. Meanwhile, the SHF cells migrate and integrate into the heart, giving rise to right ventricle (RV), parts of the left and right atrium (LA and RA), and the outflow tract (OFT). After the initiation of heart looping at E8.5, the epicardium expands from the venous pole and covers the entire embryonic heart by E11.5. The epicardial derivatives give rise to multiple cardiac cell lineages including fibroblasts and coronary vascular smooth muscle cells. In addition, cardiac neural crest cells (NCCs) migrate from the neural tube (NT), invade the OFT, mediating the development of endocardium derived cushion tissue and the septation of the myocardial wall. By E15.5, septation is completed, enclosing the ventricular chambers and generating the fully functional four-chambered heart.
At E34 (E11.5 in mice), epicardium, derived from proepicardium, proliferates and covers the entire embryonic heart. It is derived from mesodermal cells positioned dorsal to the initial heart tube and give rise to cardiac fibroblasts, coronary vascular smooth muscle cells, coronary endothelial cells, and even to an additional small population of cardiomyocytes [13, 14]. Finally, migrating neural crest cells (NCC) integrate into the circumpharyngeal ridge and invade endocardium derived cushion tissue in OFT to generate asymmetric growth, allowing for septation of the myocardial wall [15]. In the end, the heart becomes a fully formed, functional organ by E55 (E15.5 in mice) [10]. (Figure 2)
Developmental signals and cardiogenesis
The process of cardiac development is controlled by a complex network of signaling pathways that include NODAL [a member of the transforming growth factor beta superfamily (TGFβ)], bone morphogenic protein (BMP), wingless-type MMTV integration site family member 3 (WNT3A), and fibroblast growth factor (FGF) [16, 17]. Signaling pathway regulation of transcription factors, including the T-box transcription factor eomesodermin, the basic-helix-loop-helix transcription factors mesoderm posterior-1 (MESP1) and Heart and Neural Crest Derivatives Expressed 1/2 (HAND1/2), NK2 Homeobox 5 (NKX2.5), zinc-finger transcription factor GATA, ISL homeobox 1 (ISL1), myocyte enhancer factor 2c (MEF2c), and T-Box (TBX) [2], are also involved in mediating proper development of the heart, the stability of which can be negatively regulated by microRNAs (miRs), a class of small single stranded noncoding RNAs [18, 19].
The cardiac genetic program is initiated following expression of MESP1+ precardiac mesoderm [18]. As MESP1+ progenitors migrate towards the anterolateral plate mesoderm, MESP1 becomes downregulated and a subunit of the SWI/SNF (switch/sucrose non-fermentable) chromatin remodeling complex, SMARCD3, becomes induced, mediating GATA4 binding to enhancer regions of transcription factors required for cardiogenesis, including GATA4, NKX2-5, ISL1, TBX5, and MEF2C [19–21]. These factors each play a key role in the initiation of cardiac development and differentiation. For instance, NKX2.5, the most frequently mutated gene in CHD, is an integral transcriptional regulator that specifies cardiac mesoderm [22–24]. GATA factors too play a central role in early cardiac development; indeed, GATA4/6 double mutant mice lack hearts entirely [25].
The cardiac primitive streak
Signaling networks induce cardiac development through regulation of early mesoderm, generating the cardiac primitive streak, the site where epiblast cells begin to ingress into the embryo and differentiate into the three embryonic germ layers [26]. Regulated by canonical Wnt/β-catenin signaling, eomesodermin expression is induced and increases at the anterior primitive streak, giving rise to both definitive endoderm and cardiac mesoderm [27]. These eomesodermin-positive cells then activate MESP1, which regulates the activation of cardiac and mesodermal genes to mediate migration of mesodermal precursor cells through the primitive streak and become the anterior lateral plate mesoderm [18, 26, 27]. By week 3 of human development (E7.5 in the mouse), these migratory cells become the first and second heart fields (FHF and SHF), the first cell populations of distinct cardiac progenitors to have a defined cardiac fate [28]. (Figure 3)
Figure 3. Differentiation of the myocardial cell lineage during development.
Schematic representation shows the genetic regulation in stagewise commitment of cardiac mesodermal cells. The inhibition of WNT/β-catenin is required for the MESP1 positive cardiac mesoderm to undergo further specification. While the FHF and SHF cells share the expression of some of the same core transcription factors, the two lineages have differences in signaling effects and give rise to distinct myocardial cell types. AV, atrioventricular; SA, sinoatrial.
Development of the first heart field
FHF progenitors are the first to reach the anterolateral plate following mesodermal cell migration, at around E7.5 in mouse development. These cells become spatially organized in a crescent-like shape, now termed the cardiac crescent. FHF progenitors receive BMP2 [29], FGF8 [30], and non-canonical WNT [31] signals from the underlying endoderm to promote their differentiation and to activate TBX5, a regulator of cell fate [25]. TBX5, in turn, interacts with GATA4 and NKX2.5 to drive cardiac muscle development and specification of the LV through induction of several integral cardiac genes, including natriuretic peptide A (Nppa) and the gap junction protein connexin 40 (Gja5) [26, 32, 33]. GATA4 and NKX2.5 repress the hemangiogenic gene program, concomitantly upregulating cardiac-specific genes that include Hand1, Mef2c, and myosin light chain-2v (Myl2, also known as Mlc2v), all components of the necessary machinery required for normal cardiomyocyte structure and function [26, 34, 35]. Ultimately, the FHF cells give rise to the LV free wall, part of the septum, and a portion of the atria [36].
Aberrant regulation of FHF progenitors in the cardiac developmental process leads to severe cardiac defects. In mice, loss of NKX2.5 results in embryonic lethality due to failure of both cardiac looping and left ventricular formation [37]. In humans, mutations in NKX2.5 result in multiple CHDs, including cardiac conduction defects, atrial septal defects, and ventricular septal defects [24]. Similarly, deletion of GATA4 in murine hearts leads to embryonic lethality by E11.5 due to insufficient cardiomyocyte proliferation [38]. Moreover, knock-down of GATA4 causes septation, valvular and functional defects in mouse heart [39–41]. Finally, aberrant expression of TBX5 in the FHF leads to improper positioning (or even absence) of the interventricular septum [33]. (Figure 3)
Development of the second heart field
SHF progenitors give rise to the RV, part of the septum, the outflow tracts, and a portion of the atria [36]. SHF cells are located medially and dorsally to FHF cells. Compared with FHF, SHF progenitors have delayed commitment to the cardiomyocyte lineage. They receive signals from FGF [42], sonic hedgehog [43] and canonical WNT/β-catenin [44] to promote proliferation and multi-lineage differentiation [26]. SHF progenitors contribute to the growth of the heart tube and form the inflow and outflow tracts, ultimately migrating and differentiating into cardiomyocytes, endothelial cells, and smooth muscle cells [45, 46]. SHF is marked by expression of ISL1 [45, 47], although FHF progenitors also transiently express this as well [48]. ISL1 activates FGF and BMP, gene pathways to modulate cardiac progenitor cell proliferation and differentiation [47]. Together with GATA4, ISL1 activates MEF2c to induce expression of HAND2, a transcription factor important for RV development [38, 49, 50]. As SHF progenitors continue to differentiate, NKX2.5 is induced, repressing ISL1 and transitioning progenitor cells from a state of proliferation to one of differentiation [51]. Importantly, direct repression of ISL1 by NKX2.5 is necessary for development of ventricular cardiomyocytes [52] (Figure 3). Significantly, aberrant SHF regulation also leads to severe cardiac developmental defects. ISL1 knockout mice exhibit cardiac looping, RV, and outflow tract abnormalities [47, 53]. HAND2 knockout mice display varying degrees of RV hypoplasia [54].
BMP signaling in cardiomyogenesis
BMP signaling is mediated, at least in part, by induction of GATA4, MEF2c, SRF, and NKX2.5 [55, 56]. Specifically, BMP2 and BMP4, secreted by underlying endoderm, induce cardiomyogenesis of the overlying lateral plate mesoderm [29, 57, 58]. Within SHF, BMP is required for upregulation of TBX2 and TBX3 to maintain slow conduction velocity and to reduce proliferation of myocardium within the outflow tract, atrioventricular canal, and sinus horns [59].
Wnt/β-catenin signaling
Canonical WNT/β-catenin signaling is critical for maintaining proliferation of the SHF progenitors [60]. At the same time, this pathway inhibits differentiation of cells toward more terminal lineages [61, 62]. Therefore, in developing mouse hearts, as SHF progenitors migrate into the developing outflow tract, canonical WNT signaling is downregulated, facilitating concomitant activation of cardiomyocyte-specific genes [58, 63].
Interestingly, non-canonical WNT signaling is required to inhibit canonical WNT/β-catenin pathway regulation [64]. Through calcium-dependent pathways, non-canonical WNT signaling is crucial for normal cardiomyocyte specification [65]. For instance, two non-canonical WNT ligands, WNT5A and WNT11, have demonstrated roles in cardiac development; mouse embryos lacking both WNT5a and WNT11 have dramatically reduced numbers of SHF progenitor cells [64].
HOPX signaling
HOPX, a homeodomain-containing transcriptional repressor, is a recently identified regulator of canonical WNT/β-catenin and BMP signaling pathways in cardiac development [58]. HOPX expression initiates in FHF and in SHF derivatives that are exclusively committed to the cardiomyocyte lineage [26]. Notably, HOPX promotes cardiomyocyte differentiation via inhibition of WNT, which is mediated by direct interaction of HOPX with SMAD4, a transcription factor essential for transducing BMP signals [58]. In essence, as SHF cells migrate into the outflow tract and become exposed to increased concentrations of BMP4 and HOPX, canonical WNT/β-catenin signaling is reduced, facilitating continuation of the cardiomyocyte differentiation process [26, 58].
Role of MicroRNAs in Cardiac Differentiation
MicroRNAs (miRs) are single-stranded, noncoding RNA molecules that negatively affect gene expression at the post-transcriptional level, either by guiding mRNA degradation or by preventing protein translation [26, 66]. In cardiogenesis, miRs mediate transcription factor expression to modulate cell fate, proliferation, and function of cardiac cells [2, 67].
Several miRs play a role in cardiac development. For example, miR-1 and miR-133 are regulated by SRF and MEF2 [68, 69]. Indeed, homozygous deletion of miR-1 is embryonic or perinatal lethal, with defects that include ventricular septal defect, heart failure, and dysrhythmias [70]. Overexpression of miR-1 in fetal cardiomyocytes is also deleterious, resulting in thinning of the ventricular wall and heart failure [70]. Like miR-1, deletion of miR-133a in mice also causes severe heart failure due to ventricular septal defects and dilated cardiomyopathy [71].
Embryonic myocardial growth and differentiation
Once cardiac progenitor cells differentiate into cardiomyocytes, additional cardiomyocyte growth is facilitated only through active proliferation of existing cells [26]. Several signaling pathways control proliferation of cardiomyocytes. For example, Hippo/YAP signaling is required to regulate the size of the heart through activation of Hippo pathway kinases (MST1/2 and LATS1/2), which inhibit cardiomyocyte proliferation through inhibition of transcriptional coactivators YAP and TAZ (formally known as WWTR1) [72, 73]. YAP interacts with TEAD1, a transcription factor that drives activation of downstream signaling pathways, including PI3K-AKT [74–76]. YAP likely also upregulates cardiomyocyte proliferation through interaction with β-catenin and direct modulation of WNT signaling [77].
Differentiation of myocardium leads to generation of an inner trabeculated cardiomyocyte layer adjacent to the endocardium, coupled with an outer compact layer of cells. Interestingly, cardiomyocytes in the compact myocardium proliferate more rapidly than those in the trabecular region [78–80]. Changes in signaling pathway regulation and/or gradients of mitogenic or environmental factors may be important factors in this difference in proliferation [78–80]. In addition, cardiomyocyte proliferation during trabeculation may also be tightly regulated by both cell-autonomous and non-autonomous processes; for example, cross-communication between myocardium and endocardium, regulated through NOTCH1, BMP10, ephrin B2 (EFNB2), HAND2, and neuregulin-1 (NRG1) signaling, is critical for proper proliferation and growth of myocardial cells [78–81].
Endocardium and valve development
Cardiac endothelium is required for establishing the cardiac cushion and valve structures during development, as well as for maintaining valvular homeostasis in the adult heart [11, 82]. Valves are highly organized structures generated to withstand the constant blood pressure driven by heart beats; they are critical for proper blood flow. They are comprised of an outer layer of endothelium and an inner mixture of extracellular matrix (ECM) and interstitial cells [11, 83, 84]. During the initial stages of cardiac looping, ECM is rapidly generated in the atrioventricular canal and OFT. Local endocardium then undergoes EMT, proliferating into a pool of mesenchymal cells that populate this newly derived ECM, thereby forming the endocardial cushions [85]. During EMT, paracrine signals, including those from TGFβ, WNT, VEGF, and Notch, mediate induction of cardiac-specific transcription factors, including SNAIL, MSX2, and TBX20, to facilitate cardiac remodeling, cell proliferation and apoptosis of the endocardial cushions and valves [86]. (Figure 4)
Figure 4. Schematic representation of cardiac valve development and the crosstalk between cardiac cell lineages.
In response to myocardial signals, the endocardial cells undergo epithelial–mesenchymal transition (EMT), delaminating from the endocardial surface and differentiating into mesenchymal cells, which proliferate and invade the cardiac jelly to form the endocardial cushion in the atrioventricular (AV) canal and outflow tract (OFT). Signaling from endocardium thus regulates myocardial proliferation and specification, as well as pathological events that include hypertrophic cardiomyopathy (HCM). In later stages of valvular maturation, signaling from endocardium directs remodeling events that include apoptosis and differentiation of mesenchymal cells.
Specifically, Notch determines endocardium competence through binding of Delta/Jagged [87], initiating EMT and regulating expression of EMT related genes, including ACTA2, SNAIL2, SMAD3, and RUNX3 [88]. Accordingly, inhibition of Notch signaling leads to collapsed endocardium, EMT abnormalities and impaired volume in the cushion mesenchyme [88]. In contrast, increased Notch activity induces elevated expression of mesenchyme genes, including SNAIL1/2, TWIST2, and TGFβ2, as well as mediates ectopic EMT in the endocardial chamber [88]. (Figure 4)
Several pathways play a critical role in valvular development. WNT/β-catenin has a demonstrated role in restricting endocardial competence. Indeed, increasing WNT signaling expands the number of endothelial cells, causing abnormally enlarged valves; conversely, impairing WNT signaling blocks cushion formation [89]. BMP and TGFβ signals induce EMT and promote cushion mesenchyme to proliferate [11]. Cushion mesenchyme also locally represses production of VEGF to permit EMT [90]. Intriguingly, both too much and too little VEGF cause similar cardiac phenotypes; diminishing VEGF signaling evokes increased cushion mesenchyme, whereas increased VEGF increases valve size [11]. Regulation of FGF and MAPK signaling, through expression of Scleraxis (SCX), are also important regulators of proper valvular development; loss of SCX leads to aberrant differentiation of valvular cell lineages and disrupted ECM organization [91]. Finally, our group recently demonstrated that the protein tyrosine phosphatase SHP2 is also an important regulator of valvular development through its modulation of the Ras-MAPK signaling cascade in embryonic cardiac endothelium; loss of SHP2 phosphatase activity disrupts AKT mediated Notch and FOXP1 signaling, leading to enlarged and amorphic valves, endocardial cushions, and valve leaflets [92].
Neural crest cells and migration
Neural crest cells (NCCs) migrate to the heart and facilitate remodeling, particularly septation of the developing OFT [93, 94]. Dysregulated gene expression in cardiac NCCs causes OFT and aortic arch defects in the developing heart. For example, disruption of Hippo-Notch signaling results in impaired smooth muscle differentiation [93].
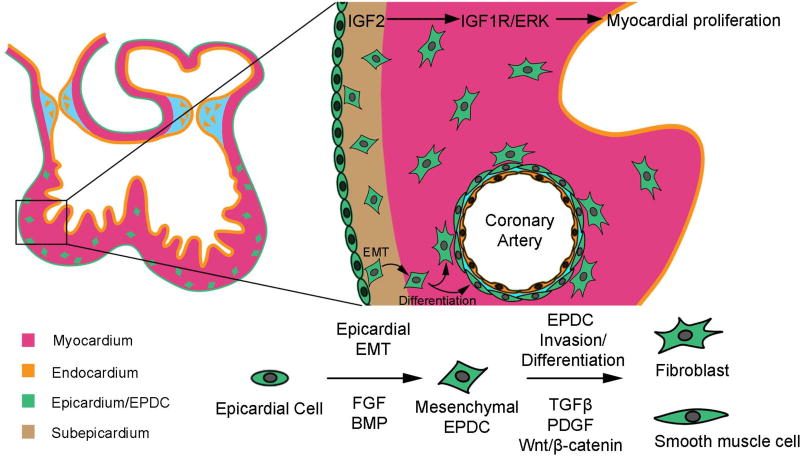
Epicardium
The epicardium is a continuous sheath of cells derived from mesoderm that covers the entire heart and is essential for growth of the compact myocardium [95]. Epicardium secretes IGF2, which activates IGF1R, and subsequently ERK, in cardiomyocytes to induce their proliferation [96, 97]. In mice, the pro-epicardium appears at the venous pole at E9.5. These cells expand and ultimately form a single cell layer of epicardium. Like pre-cardiac mesoderm, FGF and canonical BMP signaling are major players in epicardial specification; however, FGF is thought to be the dominant signal that determines epicardial as opposed to myocardial fate [98]. Intriguingly, the epicardium contains valve progenitors, and participates, at least in part, in the development of atrioventricular valves [99]. In the adult heart, epicardial-derived cells modulate immune responses [100], through Hippo-YAP/TAZ signaling, to prevent fibrosis in myocardial tissue following cardiac injury [101]. (Figure 5)
Figure 5. Schematic representation of signaling pathways regulating the differentiation of epicardium during cardiogenesis.
TGFβ and FGF are the predominant signaling pathways required to promote epithelial–mesenchymal transition (EMT). Mediated by TGFβ, PDGF, and WNT/β-catenin, epicardial derived cells (EDCs) undergo specification and differentiate into cardiac fibroblasts and vascular smooth muscle cells.
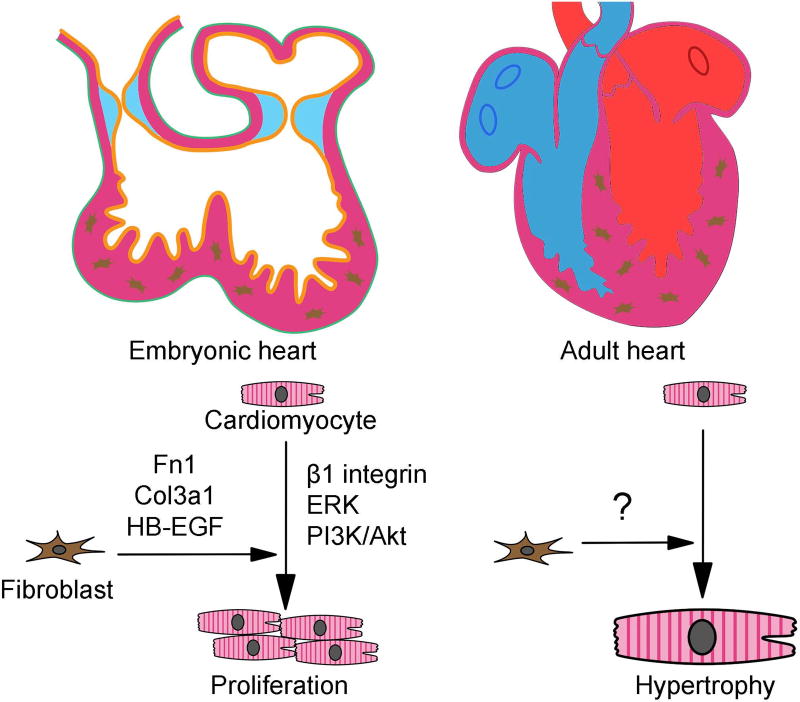
Cardiac fibroblasts
During development, murine embryonic fibroblasts appear at E12.5 and increase in number throughout the myocardial proliferation process [102]. Cardiac fibroblasts promote cardiomyocyte proliferation through expression and activation of several factors, including transcriptional regulators FN1 and COL3a1, growth factors HB-EGF and β1 integrin, as well as upregulation of ERK and PI3K/AKT signaling pathways [102]. Thus, embryonic cardiac fibroblasts synthesize specific ECM components and growth factors to promote myocardial proliferation. Activation of cardiac fibroblasts in adult myocardium typically occurs in response to stress, including hypertrophic cardiomyopathy (HCM) and/or other adult-onset cardiac diseases. (Figure 6)
Figure 6. Distinct roles of cardiac fibroblasts between embryonic and adult stages.
Embryonic cardiac fibroblasts promote cardiomyocyte proliferation through expression and activation of transcriptional regulators FN1 and COL3a1, growth factors HB-EGF and β1 integrin, as well as upregulation of ERK and PI3K/AKT signaling pathways. Activation of cardiac fibroblasts in adult myocardium typically occurs in response to stress, including hypertrophic cardiomyopathy (HCM) and/or other adult-onset cardiac diseases.
Role of macrophages in cardiac development
Macrophages are well known inhabitants of most organs and are necessary to maintain homeostasis, repair, and immunity of the local tissue[103]. The heart, specifically, is populated by a large number of resident macrophages, and their function in response to postnatal pathogenic stimuli, such as inflammation or injury of the heart, has been the primary focus of several previous studies [104]. More recently, however, effort has been placed on identifying a potential function for these cells in neonatal, proliferating hearts; principally, it has been documented that macrophages play a critical role in cardiac regeneration here, likely by promoting angiogenesis [105]. Interestingly, we know little about what role macrophages play in cardiac development. Previously, it was suggested that macrophages were derived from Myb-dependent hematopoietic stem cells delineated from fetal liver and, later on in development, from bone marrow. More recently, however, a group of Myb-independent yolk sac derived macrophages was identified [106]. Subsequently, these cells, identified as early as E8.5 in mice, get recruited to the developing heart by WT1-dependent epicardial signals [100]. At around E13.5, these now classified chemokine (C-C motif) receptor type 2 (CCR2)-negative primitive yolk sac-derived Myb-independent macrophages, but not the CCR2-positive macrophages derived from Myb-dependent fetal liver, are required to exclusively regulate coronary blood vessel patterning in the heart through IGF-dependent signals [107], suggesting that the function of specific subsets of macrophages during cardiogenesis is likely determined by ontogeny. Together, these studies suggest macrophages are integral for the highly coordinated events that occur in the developing heart.
Postnatal Cardiac Growth
Shortly after birth, cardiomyocytes exit the cell cycle and become terminally differentiated, polyploid cells (a single polyploid nucleus in humans or two diploid nuclei in rodents) [26]. They now have a need to become highly specialized for contraction, shifting to oxidative phosphorylation and developing accompanying ultrastructural specializations and changes in gene expression that enable efficient and coordinated cardiomyocyte contraction [26]. Interestingly, the same signaling pathways, transcription factors, and miRs that play a role in the developing heart also modulate postnatal maturation, mediating adult cardiac function and immuno-reactive activities [101, 108]. However, the function of these networks in adult heart is uniquely different from that in development. For example, GATA4, one of central regulators of cardiomyocyte differentiation, significantly alters its chromatin occupancy between fetal and adult stages to activate or suppress different subsets of genes [40]. In addition, re-expression of fetal genes (MHC, ANF, BNP, SERCA2a, etc) is driven by pathological or stress conditions in the adult heart, potentiating onset of myocardial disease, hypertrophy, and heart failure [109].
Several factors are involved in adult cardiomyocyte cell cycle exit. First, mitogenic signaling pathways that drive fetal cardiomyocyte proliferation are attenuated after birth [110]. For example, expression of ERBB2 quickly decreases in cardiomyocytes after birth, reducing the proliferative potency of NRG1 [111]. Second, expression and function of cell cycle machinery is actively inhibited in adult cardiomyocytes. Transcriptional regulators, epigenetic modifiers and miRs actively repress core cell-cycle activators and/or activate cell-cycle inhibitors [26, 112]. Third, activation of targeted signaling pathways, such as p38-MAPK, also inhibits cardiomyocyte proliferation [113, 114]. Finally, increased oxidative stress in postmitotic cardiomyocytes causes DNA damage response-mediated cell-cycle arrest [115].
Congenital Heart Disorders
Congenital heart disorders (CHDs) are the most common type of birth defect, with ~1/100 live births, and are the major cause of birth-related deaths [116]. Moreover, because of major advances in medical and surgical procedures, there are now more adults living with CHD than children [117]. CHD phenotypes range from mild atrial septal defects to severe LV outflow obstructions that are directly attributed to genetic abnormalities. Among numerous CHD phenotypes, abnormal valves, septation defects, and cardiomyopathies are presented in a majority of patients. While recent studies have established a causal relationship between genetic defects to cardiac abnormalities, the underlying molecular mechanisms remain unclear [4]. The aggregate of genetic contributions to CHD are likely to not only underlie structural CHDs, but also contribute to CHD comorbidities as well, including heart failure, arrhythmia, neurocognitive outcomes, and even cancer [117].
Mendelian and inherited forms of CHD have recently been identified using linkage analyses, positional cloning and targeted sequencing of CHD candidate genes [118]. Indeed, dysregulation of multiple integral developmental components lead to CHDs, including aneuploidy, copy number variation, inherited or de novo mutations, dysregulation of transcription factors, and disruption of signaling pathways [117].
Mutations and/or alterations in cardiac transcription factors, including NKX2.5, GATA, TBX and MEF2, have also been implicated in CHD [119–121]. For example, individuals with isolated ASDs, as well as individuals with ASDs along with abnormalities of the conduction system, were identified to have NKX2.5 mutations that were causal to both these defects [23]. In addition, GATA4 mutations have been implicated in at least two families with CHD with cardiac septal defects [122]. Indeed, the GATA4 Del8p23 mutation manifests with a range of CHDs, along with developmental delay [123]. Mutations in TBX5 are likewise implicated in two families with Holt–Oram Syndrome, a disease characterized by upper limb malformations and cardiac septation and conduction defects [124, 125]. Evidence of causality is also demonstrated in heterozygous TBX5 null mice, which have limb abnormalities, septal defects, deformed hearts, and other complex cardiac malformations [33, 126]. Another example, Del22q11 (DiGeorge Sydnrome), is caused by haploinsufficiency in TBX1 [127].
The genetics underlying CHD have identified critical biological pathways involved in CHD, including chromatin remodeling, Notch signaling, cilia function, sarcomere structure and function, and RAS-MAPK signaling [117, 128]. These pathways provide insights into the mechanisms of heart development, as well as identify potential CHD comorbidities, such as the ventricular dysfunction phenotype observed in patients with sarcomeric and RAS-MAPK pathway mutations. Another example is Notch signaling; NOTCH1 mutations, together with its downstream pathway effectors, have been demonstrated in multiple CHD pedigrees [129–132].
New technologies and potential therapeutic approaches
Over the last decade, technological approaches and analytical tools for next-generation sequencing has provided a greater opportunity for us to understand the genetics of complex cardiac diseases. In particular, whole exome sequencing (WES) has allowed identification of mutations that were undefinable through traditional genomic methods, such as de novo variation, variants without clear Mendelian inheritance patterns, variants with marked reduced penetrance, and somatic alterations, among others [26, 133].
In addition, the establishment of inducible pluripotent stem cells (iPSCs) has made it possible to study various disease mutations, now “in a dish.” Here, human somatic cells (blood or skin) can be dedifferentiated and then reprogrammed to generate specified cardiac lineages using a panoply of growth factors to study genetic mutations, developmental differentiation processes, onset of heart disease, cardiac tissue regeneration and even replacement therapy for heart failure patients. We now have the capacity to generate iPSC-derived cardiomyocytes, endothelial cells, cardio-fibroblasts, and smooth muscle cells, all with high efficiency, to determine functional, mechanistic and phenotypic properties of various genetic, developmental, and disease properties [134]. Moreover, there is significant growing interest in developing and understanding the unique roles of various cardiomyocyte subtypes (eg, atrial, ventricular, and nodal), in particular for consideration of therapeutic purposes.
As well, there are limitations to consider when using iPSCs and other technologies. For example, we are limited in understanding mechanisms driving cardiomyocyte maturation [135]; iPSC-derived CMs do not yet become fully differentiated adult cardiomyocytes. Moreover, use of only one cell lineage in vitro to understand the role of the entire functional heart in vivo is indeed limiting (and a bit concerning). In this regard, approaches have been considered to more closely match the in vivo microenvironment, including use of the “heart-on-a-chip” technology, administration of a 3-dimensional (3D) tissue engineering process, mechanical loading precedures, modulation of substrate stiffness on the heart, and electric stimulation of the tissue [26]. In addition, induction of signaling pathways, hormonal supplements, and longer-term differentiation processes in culture have also been considered in understanding the differentiation process of cardiomyocytes in particular. In addition, it may be feasible to combine iPSC technology with three-dimensional printed scaffolds to generate cardiac muscle patches that can be used to treat cardiac disease and/or be used for regeneration of diseased heart tissue [136]. Finally, current advances in genome editing may soon make it feasible to correct genetic anomalies, providing an additional therapeutic opportunity to actually cure CHDs and heart disease in the very near future. [137]. Further studies will be necessary to reproduce, validate, and further advance findings from all these studies.
Conclusions and Future Perspectives
In the past few decades, cardiovascular research efforts have been instrumental in uncovering the role that signaling pathways, transcriptional factors, and genetics play in the cardiomyogenic process. Consequently, we have a better understanding of the regulatory networks needed to drive differentiation and proliferation of various cell lineages involved in cardiac development [26]. Indeed, during the course of writing this review, a new method for time-induced tailored embryonic repair of an autosomal dominant MYBPC3 mutation that causes hypertrophic cardiomyopathy was developed, using CRIPSR-Cas9 technology that targeted metaphase II in the oocyte cell cycle [138]. Compared to previous studies using genome editing in early human embryos, this technique offers significantly increased genomic correction efficiency, with minimal mosaicism, undetectable off-target mutations, and no signs of early developmental defects [138]. Although undesired random DNA repair still occured at considerable frequency using this new methodology, it remains promising that genome editing-based therapeutic approaches can be used to treat and/or cure congenital heart diseases in the near future. Clearly, this needs to be carefully addressed in subsequent studies before proceeding to clinical applications. In summary, the knowledge we have gained in recent years will ultimately enable us to continue to identify new methods and technologies that will advance our understanding of complex genetic and pathological mechanisms, allow for more accurate diagnoses, increase therapeutic precision, and cure many of the cardiac diseases not possible to treat today.
Highlights.
heart consists of multiple cell lineages
cellular crosstalk facilitates cardiac development, disease and regeneration
growth factors, signaling pathways and transcription factors play a role in cardiac development
review focuses on role of genetic mutations, signaling pathways and lineage-specific contributions to cardiovascular development, physiology, and disease
Acknowledgments
This work was supported by the National Institutes of Health Grants R01-HL114775 and the Beth Israel Deaconess Medical Center Department of Medicine, Division of Cardiology (to M.I.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors wish to disclose that there are no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Chong JJ, Forte E, Harvey RP. Developmental origins and lineage descendants of endogenous adult cardiac progenitor cells. Stem Cell Res. 2014;13:592–614. doi: 10.1016/j.scr.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–7. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz AM. Physiology of the Heart. fifth. Lippincott Williams & Wilkins; Philaddelphia: 2011. [Google Scholar]
- 4.Lauriol J, Jaffre F, Kontaridis MI. The role of the protein tyrosine phosphatase SHP2 in cardiac development and disease. Semin Cell Dev Biol. 2015;37:73–81. doi: 10.1016/j.semcdb.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuta H, Little WC. The cardiac cycle and the physiologic basis of left ventricular contraction, ejection, relaxation, and filling. Heart Fail Clin. 2008;4:1–11. doi: 10.1016/j.hfc.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rana MS, Christoffels VM, Moorman AF. A molecular and genetic outline of cardiac morphogenesis. Acta Physiol (Oxf) 2013;207:588–615. doi: 10.1111/apha.12061. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407:221–6. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 8.Sylva M, van den Hoff MJ, Moorman AF. Development of the human heart. Am J Med Genet A. 2014;164A:1347–71. doi: 10.1002/ajmg.a.35896. [DOI] [PubMed] [Google Scholar]
- 9.Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136:701–13. doi: 10.1242/dev.017178. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JA, Buck CA. Transcriptional regulation of cardiac development: implications for congenital heart disease and DiGeorge syndrome. Pediatr Res. 2000;48:717–24. doi: 10.1203/00006450-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Lincoln J, Garg V. Etiology of valvular heart disease-genetic and developmental origins. Circ J. 2014;78:1801–7. doi: 10.1253/circj.cj-14-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luxan G, D'Amato G, MacGrogan D, de la Pompa JL. Endocardial Notch Signaling in Cardiac Development and Disease. Circ Res. 2016;118:e1–e18. doi: 10.1161/CIRCRESAHA.115.305350. [DOI] [PubMed] [Google Scholar]
- 13.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–8. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risebro CA, Vieira JM, Klotz L, Riley PR. Characterisation of the human embryonic and foetal epicardium during heart development. Development. 2015;142:3630–6. doi: 10.1242/dev.127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plein A, Fantin A, Ruhrberg C. Neural crest cells in cardiovascular development. Curr Top Dev Biol. 2015;111:183–200. doi: 10.1016/bs.ctdb.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Rivera-Perez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–71. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Arnold SJ, Stappert J, Bauer A, Kispert A, Herrmann BG, Kemler R. Brachyury is a target gene of the Wnt/beta-catenin signaling pathway. Mech Dev. 2000;91:249–58. doi: 10.1016/s0925-4773(99)00309-3. [DOI] [PubMed] [Google Scholar]
- 18.Bondue A, Lapouge G, Paulissen C, Semeraro C, Iacovino M, Kyba M, Blanpain C. Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification. Cell Stem Cell. 2008;3:69–84. doi: 10.1016/j.stem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–11. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Wythe JD, Dang LT, Devine WP, Boudreau E, Artap ST, He D, Schachterle W, Stainier DY, Oettgen P, Black BL, Bruneau BG, Fish JE. ETS factors regulate Vegf-dependent arterial specification. Dev Cell. 2013;26:45–58. doi: 10.1016/j.devcel.2013.06.007. During vascular development, VEGF induces expression of Notch signaling receptor Dll4 in the endothelium to promote arterial specification. However, the regulatory mechanism had not been previously understood. In this manuscript, authors identified regulatory elements of Dll4 driven by VEGF signaling that are mediated by MAPK dependent E26 transformation-specific sequence (ETS) factors. This initial signaling axis is required for arterial specification, whereas later activation of the Notch signaling pathway is necessary for maintenance of arterial identity. While the regulatory function of ETS is still unclear, it may be necessary for the induction of genes that drive additional signaling pathways. This work carefully defines the molecular signaling properties at each step of arterial differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Vliet P, Wu SM, Zaffran S, Puceat M. Early cardiac development: a view from stem cells to embryos. Cardiovasc Res. 2012;96:352–62. doi: 10.1093/cvr/cvs270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–29. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- 23.Benson DW, Silberbach GM, Kavanaugh-McHugh A, Cottrill C, Zhang Y, Riggs S, Smalls O, Johnson MC, Watson MS, Seidman JG, Seidman CE, Plowden J, Kugler JD. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. J Clin Invest. 1999;104:1567–73. doi: 10.1172/JCI8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McElhinney DB, Geiger E, Blinder J, Benson DW, Goldmuntz E. NKX2.5 mutations in patients with congenital heart disease. J Am Coll Cardiol. 2003;42:1650–5. doi: 10.1016/j.jacc.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Zhao R, Watt AJ, Battle MA, Li J, Bondow BJ, Duncan SA. Loss of both GATA4 and GATA6 blocks cardiac myocyte differentiation and results in acardia in mice. Dev Biol. 2008;317:614–9. doi: 10.1016/j.ydbio.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galdos FX, Guo Y, Paige SL, VanDusen NJ, Wu SM, Pu WT. Cardiac Regeneration: Lessons From Development. Circ Res. 2017;120:941–59. doi: 10.1161/CIRCRESAHA.116.309040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Costello I, Pimeisl IM, Drager S, Bikoff EK, Robertson EJ, Arnold SJ. The T-box transcription factor Eomesodermin acts upstream of Mesp1 to specify cardiac mesoderm during mouse gastrulation. Nat Cell Biol. 2011;13:1084–91. doi: 10.1038/ncb2304. In early embryogenesis, epiblast cells undergo epithelial-to-mesenchymal transition to give rise to mesoderm through regulation of NODAL and Smad2/3 signaling. However, the signaling that specifically induces cardiac mesoderm had not been fully elucidated. The authors here identiied that T-box transcription factor Eomesodermin (Eomes) is expressed specifically in cardiac precursor cells. Further, they showed that it cooperates differentially with either low or high doses of NODAL/Smad2/3. In response to low does of NODAL/Smad2/3 signaling, it induces cardiac mesoderm specification through enhanced expression of MESP1, a key transcription factor required for cardiac mesoderm development. In contrast, Eomes exposed to high doses of NODAL/Smad2/3 signaling differentiate to definitive endoderm. Due to the critical role of Eomes and the dose specificity requirements of NODAL and Smad2/3, additional in vivo work should be carried out. Further investigation of this key transcription factor could lead to a greater understanding of the regulatory mechanisms necessary for proper early cardiogenic processes and to the understanding of what determines specific lineage cell fates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spater D, Hansson EM, Zangi L, Chien KR. How to make a cardiomyocyte. Development. 2014;141:4418–31. doi: 10.1242/dev.091538. [DOI] [PubMed] [Google Scholar]
- 29.Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–62. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- 30.Reifers F, Walsh EC, Leger S, Stainier DY, Brand M. Induction and differentiation of the zebrafish heart requires fibroblast growth factor 8 (fgf8/acerebellar) Development. 2000;127:225–35. doi: 10.1242/dev.127.2.225. [DOI] [PubMed] [Google Scholar]
- 31.Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–41. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 32.Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–21. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi JK, Ohgi M, Koshiba-Takeuchi K, Shiratori H, Sakaki I, Ogura K, Saijoh Y, Ogura T. Tbx5 specifies the left/right ventricles and ventricular septum position during cardiogenesis. Development. 2003;130:5953–64. doi: 10.1242/dev.00797. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–80. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- 35**.Iacovino M, Chong D, Szatmari I, Hartweck L, Rux D, Caprioli A, Cleaver O, Kyba M. HoxA3 is an apical regulator of haemogenic endothelium. Nat Cell Biol. 2011;13:72–8. doi: 10.1038/ncb2137. Early embryonic haematopoietic differentiation occurs mainly in yolk sac and in lateral plate mesoderm. However, how haematopoietic progenitor cells themselves are generated remained largely unknown. The authors here evaluated haematopoiesis during yolk sac and lateral plate mesoderm specification. They found that the transcription factor HOXA3 represses expression of a specific set of haematopoietic activators, particularly RUNX1, in lateral plate mesoderm, to maintain endothelial specification. Following inactivation of HOXA3 during the differentation process, RUNX1 expression increases in haemogenic domains, converting the endothelium there into haematopoietic cells. In contrast to lateral plate mesoderm, the yolk sac mesoderm is devoid of HOXA3 expression, allowing concurrent endothelial and haematopoietic differentiation. This work identifies the key regulatory function of transcription factor RUNX1 during embryonic haematopoiesis, as well as indicates the necessity for expression of HOPXA3 in specified compartments for proper progenitor cell differentiation. While the molecular function of RUNX1 and downstream signaling pathways need to be further elucidated, this knowledge benefits our understanding of disorders of the hematopoietic system and delineates the mechanisms that underlie conversion of haematopoietic cells from pluripotent stem cells during development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- 37.Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–66. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 38.Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–31. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pu WT, Ishiwata T, Juraszek AL, Ma Q, Izumo S. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol. 2004;275:235–44. doi: 10.1016/j.ydbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 40*.He A, Gu F, Hu Y, Ma Q, Ye LY, Akiyama JA, Visel A, Pennacchio LA, Pu WT. Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat Commun. 2014;5:4907. doi: 10.1038/ncomms5907. GATA4 is a central cardiac transcriptional regulator. The manuscript here describes how GATA4 binds and participates in establishing active chromatin regions through stimulation of H3K27ac deposition. Together, this facilitates GATA4-driven gene expression. It is intriguing that while GATA4 is conserved and has critical functions in cardiac development, its chromatin occupancy changes markedly between fetal and adult heart, with limited binding site overlap between the two stages. These findings suggest that screening of binding interactions on crhomatin and investigation of potential key players in heart maturation is critical and necessary to ascribe differential function in developing versus adult heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Prendiville TW, Guo H, Lin Z, Zhou P, Stevens SM, He A, VanDusen N, Chen J, Zhong L, Wang DZ, Gao G, Pu WT. Novel Roles of GATA4/6 in the Postnatal Heart Identified through Temporally Controlled, Cardiomyocyte-Specific Gene Inactivation by Adeno-Associated Virus Delivery of Cre Recombinase. PLoS One. 2015;10:e0128105. doi: 10.1371/journal.pone.0128105. Cardiac development is controlled by a complex network of signaling pathways, mediated by evolutionarily conserved transcription factors such as GATA4/6. However, the regulatory network in postnatal heart during maturation is poorly understood. In this work, the authors investigated the stage-specific functions of GATA4 and GATA6 in the postnatal heart by using adeno-associated virus serotype 9 to conduct timed, mosaic gene inactivation by Cre. This method serves as a powerful tool to precisely control the timing and spatial properties for loss of function studies in the heart. This newly established method also delivers means to dissect molecular mechanisms mediating cardiac maturation processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN. Fgf8 is required for anterior heart field development. Development. 2006;133:2435–45. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- 43.Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Dev Biol. 2009;336:137–44. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 46.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, Roberts DJ, Huang PL, Domian IJ, Chien KR. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–7. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 47.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–13. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–7. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131:3931–42. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- 51.Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H, Stennard FA, Wise N, Schaft D, Wolstein O, Furtado MB, Shiratori H, Chien KR, Hamada H, Black BL, Saga Y, Robertson EJ, Buckingham ME, Harvey RP. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–59. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Dorn T, Goedel A, Lam JT, Haas J, Tian Q, Herrmann F, Bundschu K, Dobreva G, Schiemann M, Dirschinger R, Guo Y, Kuhl SJ, Sinnecker D, Lipp P, Laugwitz KL, Kuhl M, Moretti A. Direct nkx2-5 transcriptional repression of isl1 controls cardiomyocyte subtype identity. Stem Cells. 2015;33:1113–29. doi: 10.1002/stem.1923. The cardiac developmental process is regulated by a set of core transcription factors. NKX2.5 and ISL1 are both expressed in second heart field during cardiogenesis and their functions have been well established. Here, the authors showed that upon deletion of NKX2.5, the expression of ISL1 is upregulated, suggesting NKX2.5 functions as a repressor of ISL1 during myocardial differentiation. In embryonic stem cell-derived cardiomyocytes, ISL1 activity promotes NODAL postitive cell specification, resulting in increased HCN4 expression, as determined by electrophysiological properties of the cells. It is worth noting that HCN4 postitive cells are also expressed in first heart field progenitors. While the function of these HCN4 positive cells needs to be further confirmed, this study identifies distinct molecular mechanisms underlying cardiomyocyte subtype specification, which could be beneficial to generating specific myocardial subtypes in in vitro cell differentiation systems in the near future. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Hoffmann AD, Yang XH, Burnicka-Turek O, Bosman JD, Ren X, Steimle JD, Vokes SA, McMahon AP, Kalinichenko VV, Moskowitz IP. Foxf genes integrate tbx5 and hedgehog pathways in the second heart field for cardiac septation. PLoS Genet. 2014;10:e1004604. doi: 10.1371/journal.pgen.1004604. To dissect the regulatory signaling network for development of atrioventricular septation in the second heart field, these authors exerted transcriptional profiling and genome-wide chromosomal binding assays in the context of a Sonic Hedgehog (SHH) mutant background. They identified Gli1 and Ptch1 expression are downregulated, and Foxf1a and Foxf2 are the direct downstream targets of SHH signaling, all necessary actions that mediate atrioventricular septation. This work further demonstrats that Foxf1a is regulated by joint Tbx5 and Hedgehog activity via a cis-regulatory element. While the phenotypic changes of Foxf1a and Foxf2 double heterozygotes are similar to those of a previously described Tbx5-Hedgehog deficiency model, this study expands on the understanding of Tbx5-Hedgehog mediated genetic axis and determines its critical role in atrioventricular septal development. Using this method could facilitate discovery of new risk factors for atrioventricular septal (AVS) defects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Tsuchihashi T, Maeda J, Shin CH, Ivey KN, Black BL, Olson EN, Yamagishi H, Srivastava D. Hand2 function in second heart field progenitors is essential for cardiogenesis. Dev Biol. 2011;351:62–9. doi: 10.1016/j.ydbio.2010.12.023. The second heart field (SHF) is critical for mammalian heart development, giving rise to the right ventricle, part of the septum, the outflow tracts, and a portion of the atria. HAND2 is one of the core regulators of SHF development. In this work, a series of conditional HAND2 loss of function studies were conducted to investigate the role of HAND2 in cardiac development. The authors demonstrated HAND2 is essential for the survival and expansion of SHF progenitors. Alteration of these cellular events in mice led to heart defects similar to those observed in human patients. The function of HAND2 downstream regulated genes and their mechanisms of action have yet to be elucidated. These findings, however, lead to better understanding of myocardium related congenital heart diseases and to identification of genetic factors that are required for proper development of the heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Klaus A, Muller M, Schulz H, Saga Y, Martin JF, Birchmeier W. Wnt/beta-catenin and Bmp signals control distinct sets of transcription factors in cardiac progenitor cells. Proc Natl Acad Sci U S A. 2012;109:10921–6. doi: 10.1073/pnas.1121236109. Myocardial differentiation is regulated by a highly conserved signaling network. While extensive studies have been done to decipher the process by which this occurs, the regulatory mechanisms of myocardial development are still not understood. In mice, disruption of Notch signaling at E9.5 attenuates expression of key second heart field (SHF) transcription factors HAND2, ISL1, and GATA4, as well as BMP signaling in the right ventricle. The authors showed that reintroduction of WNT/β-catenin signaling rescues the Notch loss-of-function phenotypes in SHF, indicating that the Notch-Wnt-Bmp signaling axis is required to induce a specific set of genes (NKX2.5, ISL1, TBX20, MEF2c, and BMP4) critical for SHF cell differentiation. They also identified that WNT and BMP target distinct sets of cardiac-specific transcription factors to maintain normal myocardial differentiation in the outflow tract. Although cardiomyocytes can be obtained from in vitro differentiation, certain subsets of myocardial cells, such as early NODAL positive cells, were still not possible to obtain. This work provides detailed information on how certain myocardial subsets (SHF in this case) can now be identified and generated through activation of specific signaling pathways, providing us with tools needed to delineate these cell functions in vitro, and eventually, to more effectively treat congenital diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lien CL, McAnally J, Richardson JA, Olson EN. Cardiac-specific activity of an Nkx2-5 enhancer requires an evolutionarily conserved Smad binding site. Dev Biol. 2002;244:257–66. doi: 10.1006/dbio.2002.0603. [DOI] [PubMed] [Google Scholar]
- 57.Hutson MR, Zeng XL, Kim AJ, Antoon E, Harward S, Kirby ML. Arterial pole progenitors interpret opposing FGF/BMP signals to proliferate or differentiate. Development. 2010;137:3001–11. doi: 10.1242/dev.051565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58**.Jain R, Li D, Gupta M, Manderfield LJ, Ifkovits JL, Wang Q, Liu F, Liu Y, Poleshko A, Padmanabhan A, Raum JC, Li L, Morrisey EE, Lu MM, Won KJ, Epstein JA. HEART DEVELOPMENT. Integration of Bmp and Wnt signaling by Hopx specifies commitment of cardiomyoblasts. Science. 2015;348:aaa6071. doi: 10.1126/science.aaa6071. The signaling regulatory factors needed to undergo early cardiac progenitor cell specification remain largely unknown. The authors identify HOPX, a transcription factor that is expressed in a subset of cardiac progenitor cells, to have a critical functional role in progenitor cell specification. Through lineage tracing and functional studies, they indicate that HOPX positive precursor cells exclusively give rise to cardiomyocytes. They further demonstrate that HOPX functions to cooperate with BMP to repress WNT signaling, thereby specifically promoting cardiomyogenesis. While the mechanism of WNT signaling inhibition by HOPX-BMP interaction needs to be further explored, it is clear that HOPX is one of the key players in this process. Understanding upstream regulators, downstream targets, and the molecular functions of HOPX can be critical for elucidating the signaling axis mediating specification of early myocardial progenitor cells. HOPX, a factor that plays a critical role in converting other cardiac lineages into cardiomyocytes, may therefore be considered in cardiac regeneration therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paige SL, Plonowska K, Xu A, Wu SM. Molecular regulation of cardiomyocyte differentiation. Circ Res. 2015;116:341–53. doi: 10.1161/CIRCRESAHA.116.302752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A. 2007;104:10894–9. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11:951–7. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:18531–6. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Berg G, Abu-Issa R, de Boer BA, Hutson MR, de Boer PA, Soufan AT, Ruijter JM, Kirby ML, van den Hoff MJ, Moorman AF. A caudal proliferating growth center contributes to both poles of the forming heart tube. Circ Res. 2009;104:179–88. doi: 10.1161/CIRCRESAHA.108.185843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Cohen ED, Miller MF, Wang Z, Moon RT, Morrisey EE. Wnt5a and Wnt11 are essential for second heart field progenitor development. Development. 2012;139:1931–40. doi: 10.1242/dev.069377. Paracrine WNT signaling is a key regulator of heart development. While the role of canonical WNT/β-catenin signaling during cardiogenesis has been well established, in this study, the authors showed how non-canonical WNT signaling, particularly WNT5a and WNT11, is involved at various stages of heart development. At the early cardiac progenitor stage, WNT5a and WNT11 signaling are induced to promote proliferation of MESP1 positive precardiac mesodermal cells, adapting them to a cardiac progenitor cell fate that express cardiac genes NKX2.5 and ISL1. In contrast, in later stages of second heart field development, WNT5a and WNT11 are induced to cooperatively repress canonical WNT/β-catenin signaling, leading to myocardial differentiation. These findings demonstrate the significance of proper timing and activation of signaling pathways during cardiogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruiz-Villalba A, Hoppler S, van den Hoff MJ. Wnt signaling in the heart fields: Variations on a common theme. Dev Dyn. 2016;245:294–306. doi: 10.1002/dvdy.24372. [DOI] [PubMed] [Google Scholar]
- 66.Takaya T, Nishi H, Horie T, Ono K, Hasegawa K. Roles of microRNAs and myocardial cell differentiation. Prog Mol Biol Transl Sci. 2012;111:139–52. doi: 10.1016/B978-0-12-398459-3.00006-X. [DOI] [PubMed] [Google Scholar]
- 67.Ivey KN, Srivastava D. microRNAs as Developmental Regulators. Cold Spring Harb Perspect Biol. 2015;7:a008144. doi: 10.1101/cshperspect.a008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–20. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 69.Liu N, Williams AH, Kim Y, McAnally J, Bezprozvannaya S, Sutherland LB, Richardson JA, Bassel-Duby R, Olson EN. An intragenic MEF2-dependent enhancer directs muscle-specific expression of microRNAs 1 and 133. Proc Natl Acad Sci U S A. 2007;104:20844–9. doi: 10.1073/pnas.0710558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–17. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 71.Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–54. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao Y, Leach J, Wang J, Martin JF. Hippo/Yap Signaling in Cardiac Development and Regeneration. Curr Treat Options Cardiovasc Med. 2016;18:38. doi: 10.1007/s11936-016-0461-y. [DOI] [PubMed] [Google Scholar]
- 73.Lin Z, Pu WT. Strategies for cardiac regeneration and repair. Sci Transl Med. 2014;6:239rv1. doi: 10.1126/scitranslmed.3006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74*.von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, Pu WT. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A. 2012;109:2394–9. doi: 10.1073/pnas.1116136109. The Hippo pathway has been well characterized for its functions in organ size determination during embryogenesis. YAP1 is the downstream target of Hippo signaling. In this study, the role of YAP1 in myocardium was carefully dissected at both the fetal and postnatal stages. During embryogenesis, ablation of YAP1 caused marked, lethal myocardial hypoplasica and decreased cardiomyocyte proliferation. In contrast, fetal activation of YAP1 stimulated cardiomyocyte proliferation. Interestingly, at the postnatal stage, YAP1 gain-of-function mutations were able to induce cardiomyocyte proliferation, but the alteration of YAP1 activity did not seem to affect cell size. The authors found YAP1 binding of TEAD transcription factors is required to induce expression of cell-cycle genes that enhance proliferation in the myocardium. Together, these results suggest that YAP1-TEAD may be a good candidate for future myocardial regeneration strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75*.Lin Z, Zhou P, von Gise A, Gu F, Ma Q, Chen J, Guo H, van Gorp PR, Wang DZ, Pu WT. Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ Res. 2015;116:35–45. doi: 10.1161/CIRCRESAHA.115.304457. It has been recently demonstrated that Hippo-YAP is a key player in regulating cell proliferation and survival of cells during the cardiac maturation process. However, this pathway is inactivated in adult cardiomyocytes, suggesting that this may be a critical reason for the terminal differentation of these cells. Activation of PI3K-AKT, a signaling pathway known for its function in cellular proliferation, survival, and physiological hypertrophy of cardiomyocytes, correlates with Hippo-YAP signaling in cardiac maturation. However, no direct interaction between these two networks had been demonstrated. The authors here showed that PI3Kcb is the linker for the functional activity between Hippo-YAP and PI3K-AKT signaling. The lack of proliferative capacity in adult cardiomyocytes has posed a major obstacle for regenerative medicine, and the molecular mechanisms that cause this have yet to be elucidated. For therapeutic prospects, identification of this link between these two pathways could possibly suggest a role for PI3Kcb in promoting cardiomyocyte proliferation and survival even in differentiated cells, allowing for improved recovery from cardiac injury and attenuation of pathological conditions that occur as a consequence of inactivation of YAP in adult cardiomyocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76*.Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. Development of myocardium is tightly regulated by transcription factors and paracrine signaling networks that include IGF and Hippo pathways. However, the regulatory effects of these pathways in development are poorly understood. IGF and Hippo signaling are both involved in proliferation as well as organ growth during embryogenesis. The authors here showed that YAP, a major target of Hippo signaling, is sufficient to sensitize cardiomyocytes to IGF signaling, thus enhancing proliferation in developing myocardium. While the molecular mechanisms assoicated with YAP-IGF interaction need to be further investigated, this work identifies that effectors controlling postnatal cardiomyocyte proliferation do, in fact, exist, opening doors to researchers in cardiac regenerative medicine to investigate these possiblilities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77**.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. doi: 10.1126/science.1199010. Hippo signaling is a determinant of organ size during development through its ability to inhibit cell proliferation in Drosophila. The authors here conducted Hippo loss-of-function studies to address the role of Hippo signaling in mammalian cardiogenesis. They found that inactivation of Hippo leads to enlarged hearts due to significant increases in cell proliferation. Importantly, the majority of the mutant animals did not have any cardiac structural defects, despite the increase in proliferation. The authors further showed Hippo signaling represses a subset of WNT/b-catenin signaling genes. Of these, they found that YAP interacts with β-catenin to restrict cell proliferation and to control organ size during normal development. This work greatly increases our understanding of the intrinsic regulatory mechanisms that control organ size and proliferation, and provides a basis for moving forward with future regenerative methods for heart disease treatments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, Shou W, Ballestar E, Esteller M, Rojas A, Perez-Pomares JM, de la Pompa JL. Notch signaling is essential for ventricular chamber development. Dev Cell. 2007;12:415–29. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen H, Shi S, Acosta L, Li W, Lu J, Bao S, Chen Z, Yang Z, Schneider MD, Chien KR, Conway SJ, Yoder MC, Haneline LS, Franco D, Shou W. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131:2219–31. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang W, Chen H, Qu X, Chang CP, Shou W. Molecular mechanism of ventricular trabeculation/compaction and the pathogenesis of the left ventricular noncompaction cardiomyopathy (LVNC) Am J Med Genet C Semin Med Genet. 2013;163C:144–56. doi: 10.1002/ajmg.c.31369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81*.VanDusen NJ, Casanovas J, Vincentz JW, Firulli BA, Osterwalder M, Lopez-Rios J, Zeller R, Zhou B, Grego-Bessa J, De La Pompa JL, Shou W, Firulli AB. Hand2 is an essential regulator for two Notch-dependent functions within the embryonic endocardium. Cell Rep. 2014;9:2071–83. doi: 10.1016/j.celrep.2014.11.021. HAND2 is a critical transcriptional regulator in the development of second heart field, neural crest, and epicardium in embryonic heart. In this study, the ablation of HAND2 in cardiac endothelium was demonstrated to be causal to tricuspid atresia, characterized by a positioning defect of the atrioventricular (AV) cushion and hypoplasia of the myocardial layer in the ventricle. The authors further showed that disruption of the Notch-NRG1 signaling axis mediated by the deletion of HAND2 may be the reason for the defect; reintroduction of HAND2 partially rescued the abnormality induced by impaired upstream Notch-Ephrin activity. This work is the first to suggest that HAND2 is the key regulator of the NRG1 activity modulated by Notch signaling in the developing heart. Moreover it emphasizes the significance of understanding mechanisms regulating crosstalk between the various cardiac lineages during embryogenesis. In this case, endocardium is found to be crucial for the regulation of myocardial differentiation and proliferation. As well, endothelial signaling is also required for mesenchymal cell remodeling in the AV cushion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82*.Anstine LJ, Bobba C, Ghadiali S, Lincoln J. Growth and maturation of heart valves leads to changes in endothelial cell distribution, impaired function, decreased metabolism and reduced cell proliferation. J Mol Cell Cardiol. 2016;100:72–82. doi: 10.1016/j.yjmcc.2016.10.006. The authors identified that cardiac valvular endothelium undergoes regressive changes in morphology, metabolism, proliferation and physiological properties of the heart as a consequence of aging. This observation identifies the critical importance and impact that the age of the valvular structures have on development of disease, a huge cost and social burden worldwide for this, as well as many other disease etiologies. Future genetic studies of valvular endothelium in animal model systems would lead to better understanding of the molecular mechanisms and potential therapeutic approaches that could be taken to offset these effects. As well, the signaling and genetic markers associated with these pathologies need to be identified to combat the effects of aging and diseased heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83*.Yzaguirre AD, Padmanabhan A, de Groh ED, Engleka KA, Li J, Speck NA, Epstein JA. Loss of neurofibromin Ras-GAP activity enhances the formation of cardiac blood islands in murine embryos. Elife. 2015;4:e07780. doi: 10.7554/eLife.07780. Normally, NF1 activity is required for suppression of the overgrowth of neural crest-derived tissues and for the prevention of abnormalities in the developing outflow tract. In this study, authors showed that loss of the GAP function of NF1 leads to ectopic formation of blood islands and to malfunctional endothelium. Functionally, the mechanisms for the loss of GAP activity was caused by an elevated downstream ERK-MAPK activity. In the future, Ras/ERK-MAPK signaling needs to be carefully evaluated as a determinant of erythroid/myeloid cell fate, as well as an integral signaling pathway needed for the proper development of functional endocardium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84*.Aghajanian H, Cho YK, Manderfield LJ, Herling MR, Gupta M, Ho VC, Li L, Degenhardt K, Aharonov A, Tzahor E, Epstein JA. Coronary vasculature patterning requires a novel endothelial ErbB2 holoreceptor. Nat Commun. 2016;7:12038. doi: 10.1038/ncomms12038. The authors identified a novel role for ERBB2 in the coronary endothelial cells in heart development. The disruption of ERBB2-NRP1-Sema3d complex in the endocardium resulted in abnormal connection of the coronary veins to the right atrium and to impaired AKT signaling. This novel role of ERBB2 signaling may lead to further investigation for its possible mechanisms in controlling other endocardial tissue functions, both during cardiac development and in adult heart. In addition, ERBB2 is a common mutation and target for cancer therapies. This research suggests the reasons why, at least in part, ERBB2 inhibition may result in cardiac toxicity during cancer treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MacGrogan D, Luxan G, Driessen-Mol A, Bouten C, Baaijens F, de la Pompa JL. How to make a heart valve: from embryonic development to bioengineering of living valve substitutes. Cold Spring Harb Perspect Med. 2014;4:a013912. doi: 10.1101/cshperspect.a013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.von Gise A, Pu WT. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res. 2012;110:1628–45. doi: 10.1161/CIRCRESAHA.111.259960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garside VC, Chang AC, Karsan A, Hoodless PA. Co-ordinating Notch, BMP, and TGF-beta signaling during heart valve development. Cell Mol Life Sci. 2013;70:2899–917. doi: 10.1007/s00018-012-1197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–7. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- 90.Lambrechts D, Carmeliet P. Sculpting heart valves with NFATc and VEGF. Cell. 2004;118:532–4. doi: 10.1016/j.cell.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 91.Levay AK, Peacock JD, Lu Y, Koch M, Hinton RB, Jr, Kadler KE, Lincoln J. Scleraxis is required for cell lineage differentiation and extracellular matrix remodeling during murine heart valve formation in vivo. Circ Res. 2008;103:948–56. doi: 10.1161/CIRCRESAHA.108.177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92*.Lauriol J, Cabrera JR, Roy A, Keith K, Hough SM, Damilano F, Wang B, Segarra GC, Flessa ME, Miller LE, Das S, Bronson R, Lee KH, Kontaridis MI. Developmental SHP2 dysfunction underlies cardiac hypertrophy in Noonan syndrome with multiple lentigines. J Clin Invest. 2016;126:2989–3005. doi: 10.1172/JCI80396. The majority of Noonan syndrome with multiple lentigines (NSML) patients have characteristic congenital heart disease (CHDs), particularly hypertrophic cardiomyopathy (HCM). It was unclear which cardiac lineage and signaling pathway was responsible for the myocardial hypertrophic phenotype until this work. Moreover, it was unclear whether the HCM phenotype was primary during development or secondary, as a consequence of added pressure overload in the adult. The authors here exerted tissue specific Cre alleles to investigate the effect of SHP2 loss of function mutation in cardiomyocytes, cardiac endothelium, or neural crest cells (NCC). Interestingly, while the cardiomyocyte and NCC specific mutants of SHP2 fail to replicate the myocardial defect in conventional NSML heart, the endothelial NSML mutant was able to recapitulate nearly all of the ubiquitous-expressing NSML cardiac abnormalities. These data indicate that signaling crosstalk between myocardium and endocardium is the key regulator of myocardial differentiation and proliferation in cardiac embryogenesis. Consequently, dysfunction of the crosstalk events during development leads to onset HCM in the adult. Although the underlying mechanism, particularly for trabecular differentiation, needs to be further evaluated, this model well illustrates the complexity of cardiac signaling regulation. This knowledge could promote further understanding of the role of paracrine signaling from endocardium to myocardium needed to facilitate developmental events, as well as to maintain cardiac homeostasis in the adult heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93*.Phillips HM, Mahendran P, Singh E, Anderson RH, Chaudhry B, Henderson DJ. Neural crest cells are required for correct positioning of the developing outflow cushions and pattern the arterial valve leaflets. Cardiovasc Res. 2013;99:452–60. doi: 10.1093/cvr/cvt132. Bicuspid aortic valve (BAV) is a very common type of congenital heart defect, but the regulatory mechanisms underlying its development remain poorly understood. Interestingly, endocardium had been previously thought to contribute to most of the embryonic valvular structures during the developmental process. Here, however, the authors provide evidence to suggest that neural crest cells (NCCs) also are required in early OFT valve formation and regulation. NCCs are known to give rise to multiple cell lineages, integral to the development of the outflow tract (OFT) and for OFT septation. In this study, authors found that the NCCs were critical for proper positioning of the outflow tract cushions, the precursor cells that differentiate to later OFT valvular structures, through regulation of Rho GTPases and ROCK signaling. Further dissection of the ROCK signaling cascade is needed to screen for genetic risk factors that may be causal to valvular disease and/or to identify novel mechanisms or markers that could improve cardiac function in these BAV patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94*.Manderfield LJ, Aghajanian H, Engleka KA, Lim LY, Liu F, Jain R, Li L, Olson EN, Epstein JA. Hippo signaling is required for Notch-dependent smooth muscle differentiation of neural crest. Development. 2015;142:2962–71. doi: 10.1242/dev.125807. Interestingly, in development, Hippo signaling is known to be involved in the regulation of organ size; little, however, was known about whether it may have additional functions and/or regulate cell fate during this process. Here, authors identified that Hippo-Notch signaling functions to regulate smooth muscle differentiation through a Yap-NICD-Rbp-J transcriptional complex in neural crest-derived cardiac tissues. Disruption of Hippo-Notch resulted in impaired smooth muscle differentiation, as well as to disrupted molecular marker expression in the aortic arch artery during embryogenesis. Taken together, these data suggest that the role of Hippo signaling extends far beyond just organ size, and that the pathway regulation of Yap needs to be further evaluated for its potential role in cardiac differentiation and/or cell fate decisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pennisi DJ, Ballard VL, Mikawa T. Epicardium is required for the full rate of myocyte proliferation and levels of expression of myocyte mitogenic factors FGF2 and its receptor, FGFR-1, but not for transmural myocardial patterning in the embryonic chick heart. Dev Dyn. 2003;228:161–72. doi: 10.1002/dvdy.10360. [DOI] [PubMed] [Google Scholar]
- 96*.Li P, Cavallero S, Gu Y, Chen TH, Hughes J, Hassan AB, Bruning JC, Pashmforoush M, Sucov HM. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development. 2011;138:1795–805. doi: 10.1242/dev.054338. Epicardium gives rise to multiple cardiac lineages. However the signaling crosstalk between epicardium and myocardium is poorly understood. In this study, the authors showed that IGF is strongly expressed in the epicardium, and that its paracrine function is critical to the regulation of myocardial proliferation during embryogenesis. IGF loss-of-function resulted in severe hypoplasia in the myocardial compact zone. The importance of IGF signaling is also confirmed by similar phenotypes induced by deletion of IGF receptor in the myocardium. While further epicardial-specific IGF deletion would confirm the epicardial-assoicated myocardial responses to IGF, this work clearly demonstrates the importance of signaling crosstalk between cardiac lineages during cardiogenesis. Moreover, IGF signaling may be considered a potential growth factor necessary for the induction of adult cardiomyocyte proliferation, serving as a potential regenerative approach for recovery of heart in response to injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97*.Huang Y, Harrison MR, Osorio A, Kim J, Baugh A, Duan C, Sucov HM, Lien CL. Igf Signaling is Required for Cardiomyocyte Proliferation during Zebrafish Heart Development and Regeneration. PLoS One. 2013;8:e67266. doi: 10.1371/journal.pone.0067266. In contrast to mammalian hearts, cardiomyocytes in adult zebrafish undergo robust proliferation to compensate for the loss of myocardial cells following cardiac injury. However, the paracrine signaling mechanisms underlying this regeneration capacity is poorly understood. The authors showed that IGF2 expression, which is required for myocardial proliferation during normal development, is also upregulated in response to cardiac injury and mediates proliferation of adult cardiomyocytes in zebrafish. In contrast, however, it is likely that the transduction of IGF signaling may be more limited in the adult mammalian heart; while, like zebrafish, it is required in embryogenesis, the metabolic, structural and genetic differences between fish and mammals suggest its role in adult heart may be to induce hypertrophy, not proliferation. Further genetic and pharmacological studies on mouse models are needed to further elucidate the role of IGF signaling in mammalian myocardial regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Wijk B, van den Berg G, Abu-Issa R, Barnett P, van der Velden S, Schmidt M, Ruijter JM, Kirby ML, Moorman AF, van den Hoff MJ. Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways. Circ Res. 2009;105:431–41. doi: 10.1161/CIRCRESAHA.109.203083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99*.Wessels A, van den Hoff MJ, Adamo RF, Phelps AL, Lockhart MM, Sauls K, Briggs LE, Norris RA, van Wijk B, Perez-Pomares JM, Dettman RW, Burch JB. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev Biol. 2012;366:111–24. doi: 10.1016/j.ydbio.2012.04.020. Epicardium and epicardial derived cells (EPDCs) are known to give rise to multiple cardiac lineages, particularly cardiac fibroblasts during cardiogenesis. In this study, the authors carefully conducted lineage tracing experiments to identify EPDC-derived cells. They found that while EPDCs contribute to a majority of the embryonic cardiac fibroblasts, these fibroblasts do not populate the trabecular myocardial tissue until E17. Moreover, the authors asked whether EPDCs contribute to the valvular fibroblast (or valve interstitial cell) population. In contrast to what was previously reported, they found that a considerable number of valvular fibroblasts, particularly in lateral atrioventricular cushions, were in fact epicardially derived. While the mechanism regulating the migration pattern of epicardial-derived fibroblasts in the myocardium remains to be determined and additional methods need to be assessed to confirm the EPDC contribution to valvular fibroblasts, this study provides interesting and important findings regarding the contribution of this lineage to the leaflet mesenchyme of the cardiac valve. Indeed, these cells may have very different origins with distinct signaling networks critical for normal cardiac valve development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100*.Stevens SM, Gise A, VanDusen N, Zhou B, Pu WT. Epicardium is required for cardiac seeding by yolk sac macrophages, precursors of resident macrophages of the adult heart. Dev Biol. 2016;413:153–9. doi: 10.1016/j.ydbio.2016.03.014. In this study, fetal yolk sac derived macrophages were found to be specifically recruited to the developing heart by the mesothelial epicardium. This subset of macrophages is involved in injury response and is known to have a beneficial role in cardiac repair. In the embryonic heart, transcription factor WT1 in epicardium is a key regulator in the recruitment of fetal yolk sac derived macrophages. While the regulatory cascade, particularly the extracellular components in this process, remain to be explored, this work prompts further examination of the organ-specific signaling pathways necessary for recruitment of macrophages during development, as well as in postnatal stages of the heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101**.Ramjee V, Li D, Manderfield LJ, Liu F, Engleka KA, Aghajanian H, Rodell CB, Lu W, Ho V, Wang T, Li L, Singh A, Cibi DM, Burdick JA, Singh MK, Jain R, Epstein JA. Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction. J Clin Invest. 2017;127:899–911. doi: 10.1172/JCI88759. This study is the first to indicate that the epicardial Hippo-YAP/TAZ signaling pathway is critical for modulating immune responses to prevent fibrosis, post myocardial infarction. Although the role of epicardial derived lineages and Hippo-YAP/TAZ signaling has been well established in cardiogenesis, their functions in the postnatal heart remained unknown. Here, the authors identified this signaling pathway, stemming from epicardium, is required for the regulation of T-regulatory cells in post injury events. While further investigation of myocardial cytokine modulation and interaction of local immune cells is needed, this research warrents futher understanding of the development and function of the cardiac immune system and its role in cardiac regeneration and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–44. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 104.Swirski FK, Robbins CS, Nahrendorf M. Development and Function of Arterial and Cardiac Macrophages. Trends Immunol. 2016;37:32–40. doi: 10.1016/j.it.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–92. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 107*.Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ. Primitive Embryonic Macrophages are Required for Coronary Development and Maturation. Circ Res. 2016;118:1498–511. doi: 10.1161/CIRCRESAHA.115.308270. The role of macrophages has been extensively studied in adult tissues. However, the ontogeny and function of macrophages in the developing heart remain largely unknown. In this study, the authors identified two groups of macrophages, CCR2− primitive yolk sac derived macrophages and CCR2+ fetal liver monocyte derived macrophages, both of which are required to populate the embryonic heart. They found that only CCR2− macrophages are required for normal coronary blood vessel formation, through specific regulation of IGF signaling. While the authors have yet to explore the role of embryonic CCR2+ macrophages in the development of trabeculation and endocardium, this work provides valuable insight into yolk sac macrophage development and its role in establishing coronary structures in the fetal heart. Together with other recent studies, this work suggests that the function of specific subsets of macrophages is determined by ontogeny, particularly during cardiogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108**.Ding J, Chen J, Wang Y, Kataoka M, Ma L, Zhou P, Hu X, Lin Z, Nie M, Deng ZL, Pu WT, Wang DZ. Trbp regulates heart function through microRNA-mediated Sox6 repression. Nat Genet. 2015;47:776–83. doi: 10.1038/ng.3324. MicroRNAs (miRNAs) mediate development and function of the heart during embryogenesis. However, little is known about their role in modulating physiological functions in heart maturation in the adult. Here, authors show that the RNA-binding protein Trbp represses Sox6 through miR-208a to regulate normal function of the postnatal heart. It is interesting that the correction in expression of a single miRNA, and its transcription factor target, completely normalize postnatal cardiac function, suggesting that miRNAs can play a significant and critical role in cardiac biology. Futher work is needed, however, to confirm their significance and to identify additional miRNAs involved in cardiac homeostasis, heart maturation, and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seidman CE, Seidman JG. Identifying sarcomere gene mutations in hypertrophic cardiomyopathy: a personal history. Circ Res. 2011;108:743–50. doi: 10.1161/CIRCRESAHA.110.223834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110**.Lin Z, Guo H, Cao Y, Zohrabian S, Zhou P, Ma Q, VanDusen N, Guo Y, Zhang J, Stevens SM, Liang F, Quan Q, van Gorp PR, Li A, Dos Remedios C, He A, Bezzerides VJ, Pu WT. Acetylation of VGLL4 Regulates Hippo-YAP Signaling and Postnatal Cardiac Growth. Dev Cell. 2016;39:466–79. doi: 10.1016/j.devcel.2016.09.005. During development, Hippo signaling mediates the regulation of organ size. Recently Hippo-YAP signaling was also shown to be involved in the proliferation of the fetal heart. Here, the authors found that acetylation of VGLL4 serves as a switch to activate Hippo-YAP signaling through TEAD. During heart maturation, however, the level of VGLL4 acetylation is downregulated, resulting in a more modest proliferative capacity of adult cardiomyocytes. This work focuses on the newly discovered function of Hippo-YAP pathway and unveils its upstream regulator. In the future, the regulators and cofactors of Hippo signaling are worthy of being investigated. This knowledge will give us insight into the cardiac maturation process, which is currently poorly understood, as well as identify potential mechanisms that allow adult myocardium to continue dividing, particularly following stress and/or injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111*.D'Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17:627–38. doi: 10.1038/ncb3149. In postnatal mammalian heart, the proliferation capacity of cardiomyocytes is gradually shut down, terminally differentiating cardiomyoctyes and posing a major issue for cardiac repair after injury. In this study, the oncogene ERBB2 was found to be involved in the regulation of postnatal myocardial proliferation through NRG1 signaling. The authors showed that in mice, a loss-of-function model of myocardial ERBB2 leads to reduced proliferation and dysfunction in the heart, whereas constitutively active ERBB2 signaling causes enlarged cell size, dedifferentiation, and significantly increased proliferation of cardiomyocytes, even in the adult heart after cardiac injury. The therapeutic purposes for this finding remains unclear; activation of ERBB2 in adults is still considered a risk factor for cardiomegaly and for development of cancer. However, this data may identify possible cell-cycle control mechanisms in the postnatal myocardium, and may therefore help identify possible approaches for treatment of cardiac injury. [DOI] [PubMed] [Google Scholar]
- 112*.Aix E, Gutierrez-Gutierrez O, Sanchez-Ferrer C, Aguado T, Flores I. Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation. J Cell Biol. 2016;213:571–83. doi: 10.1083/jcb.201510091. Heart disease remains the top cause of death worldwide, largely due to the very limited proliferation capacity of adult cardiomyocytes in response to cardiac injury. To explore the mechanism of the postnatal transition between proliferation and cell-cycle arrest, the authors here investigated the role of telomere shortening in response to cellular events. They identify that telomere dysfunction/shortening results in premature cardiomyocyte cell-cycle arrest. Moreover, they find that the upregulation of the cell-cycle inhibitor and tumor suppressor p21 is likely responsible for these effects. These findings are validated in Terc and p21 knock-out mice. It is interesting that disruption of telomerase does not appear to have any significant effect on cardiac phenotype in embryogenesis, suggesting that other, perhaps additional or parallel mechanisms may be involved in maintaining the rapid cell proliferation of these cells during development. This work give us insight into the molecular mechanisms that regulate cell-cycle control during cardiac maturation, a process which is still poorly understood in the heart. Moreover, these data provide us with targets to consider for potential regenerative therapies, for improving recovery of patients following cardiac injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–87. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:15546–51. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115*.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–79. doi: 10.1016/j.cell.2014.03.032. Mammalian heart is unable to regenerate damaged myocardial tissue due to the lack of proliferative capacity in adult cardiomyocytes. It is the major obstacle for developing methods of treating adult-based cardiac injury. The authors here showed that postnatal myocardium gradually undergoes cell-cycle arrest, along with elevated activity of reactive oxygen species, oxidative DNA damage, and DNA damage response markers induced by increased environmental oxygen. In mice, inhibition of these signaling pathways allows for a longer period of postnatal myocardial proliferation, suggesting these factors may contribute to the overall maturation of adult cardiomyocytes. Importantly, inhibition of these components also attenuates the severity of cardiac injury, producing more cardiomyocytes to compensate for the loss of myocardium. While the downstream genetic cascade remains unknown, this work emphasizes the importance of understanding the role of downstream cardiac signaling responses to metabolic and environmental changes in the heart. It also provides the basis for investigating further possible cell-cycle arrest effects in adult cardiomyocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weismann CG, Gelb BD. The genetics of congenital heart disease: a review of recent developments. Curr Opin Cardiol. 2007;22:200–6. doi: 10.1097/HCO.0b013e3280f629c7. [DOI] [PubMed] [Google Scholar]
- 117.Zaidi S, Brueckner M. Genetics and Genomics of Congenital Heart Disease. Circ Res. 2017;120:923–40. doi: 10.1161/CIRCRESAHA.116.309140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118**.McKean DM, Homsy J, Wakimoto H, Patel N, Gorham J, DePalma SR, Ware JS, Zaidi S, Ma W, Patel N, Lifton RP, Chung WK, Kim R, Shen Y, Brueckner M, Goldmuntz E, Sharp AJ, Seidman CE, Gelb BD, Seidman JG. Loss of RNA expression and allele-specific expression associated with congenital heart disease. Nat Commun. 2016;7:12824. doi: 10.1038/ncomms12824. The authors utilized next generation RNA sequencing analyses of tissues from congenital heart diseases (CHD) to identify candidates of pathogenic genetic mutations. While molecular mechanisms have yet to be determined and validated in animal models, this pioneer research provides us with potentially important gene candidates that can be used to confirm association in CHDs. Currently, whole genomic sequencing, which has become cheaper and easier to use in research, is used to identify additional mutations and regulatory elements involved in the development of CHDs. This approach is instrumental in the discovery of novel genes, which in time, can be used to identify targets and test therapeutics for the treatment of CHDs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prendiville T, Jay PY, Pu WT. Insights into the genetic structure of congenital heart disease from human and murine studies on monogenic disorders. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a013946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCulley DJ, Black BL. Transcription factor pathways and congenital heart disease. Curr Top Dev Biol. 2012;100:253–77. doi: 10.1016/B978-0-12-387786-4.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bruneau BG. Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb Perspect Biol. 2013;5:a008292. doi: 10.1101/cshperspect.a008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, Matsuoka R, Cohen JC, Srivastava D. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–7. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- 123.Pehlivan T, Pober BR, Brueckner M, Garrett S, Slaugh R, Van Rheeden R, Wilson DB, Watson MS, Hing AV. GATA4 haploinsufficiency in patients with interstitial deletion of chromosome region 8p23.1 and congenital heart disease. Am J Med Genet. 1999;83:201–6. [PubMed] [Google Scholar]
- 124.Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, Soults J, Grayzel D, Kroumpouzou E, Traill TA, Leblanc-Straceski J, Renault B, Kucherlapati R, Seidman JG, Seidman CE. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15:30–5. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 125.Li QY, Newbury-Ecob RA, Terrett JA, Wilson DI, Curtis AR, Yi CH, Gebuhr T, Bullen PJ, Robson SC, Strachan T, Bonnet D, Lyonnet S, Young ID, Raeburn JA, Buckler AJ, Law DJ, Brook JD. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet. 1997;15:21–9. doi: 10.1038/ng0197-21. [DOI] [PubMed] [Google Scholar]
- 126*.Nadadur RD, Broman MT, Boukens B, Mazurek SR, Yang X, van den Boogaard M, Bekeny J, Gadek M, Ward T, Zhang M, Qiao Y, Martin JF, Seidman CE, Seidman J, Christoffels V, Efimov IR, McNally EM, Weber CR, Moskowitz IP. Pitx2 modulates a Tbx5-dependent gene regulatory network to maintain atrial rhythm. Sci Transl Med. 2016;8:354ra115. doi: 10.1126/scitranslmed.aaf4891. TBX5 and PITX2 are integral genes involved in heart development. Mutations in TBX5 result in Holt-Oram syndrome, patients of which have pacemaker and other cardiac defects. It was still unclear whether aberrant TBX5 expression led to arrhythmias. In this study, the authors found that TBX5 directly regulates PITX2 to mediate expression of membrane effector genes. Deletion of TBX5 in mice directly led to atrial fibrillation, confirming a role for this transcription factor in arrhythmias. While the upstream activator of TBX5 is unknown, it is important to recognize that this work provides a causal link between genetic mutations and phenotypic consequences. In further studies, combining the knowledge of disease risk factors and genetic mechanisms will establish prediction models for congenital and acquired cardiac disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, Bradley A, Baldini A. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- 128*.Burnicka-Turek O, Steimle JD, Huang W, Felker L, Kamp A, Kweon J, Peterson M, Reeves RH, Maslen CL, Gruber PJ, Yang XH, Shendure J, Moskowitz IP. Cilia gene mutations cause atrioventricular septal defects by multiple mechanisms. Hum Mol Genet. 2016;25:3011–28. doi: 10.1093/hmg/ddw155. In this study, the authors carefully dissected the molecular mechanisms of cilia gene mutations causing atrioventricular septal defects (AVSDs). They found that whereas all these mutations lead to AVSD, the underlying mechanisms for each mutation are distinct. In mice, the MKS1 mutation interrupts second heart field hedgehog signaling, while DNAH11 affects laterality, implicating multiple roles for cilia in the developing heart. Although the causality between cilia gene mutations and human AVSD needs to be further addressed, this research reminds us of the important roles of cilia during cardiogenesis and establishes a model to predict AVSD based on associated cilia gene mutations. These data could be useful for potentiation of precision medicine in the future. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129*.Dina C, Bouatia-Naji N, Tucker N, Delling FN, Toomer K, Durst R, Perrocheau M, Fernandez-Friera L, Solis J, investigators P, Le Tourneau T, Chen MH, Probst V, Bosse Y, Pibarot P, Zelenika D, Lathrop M, Hercberg S, Roussel R, Benjamin EJ, Bonnet F, Lo SH, Dolmatova E, Simonet F, Lecointe S, Kyndt F, Redon R, Le Marec H, Froguel P, Ellinor PT, Vasan RS, Bruneval P, Markwald RR, Norris RA, Milan DJ, Slaugenhaupt SA, Levine RA, Schott JJ, Hagege AA, France MVP, Jeunemaitre X, M.N. Leducq Transatlantic Genetic association analyses highlight biological pathways underlying mitral valve prolapse. Nat Genet. 2015;47:1206–11. doi: 10.1038/ng.3383. Several common congenital heart diseases (CHDs) are considered idiopathic, with no known genetic mutation. However, technological advances and additional research efforts have helped correlate mutations with phenotypic consequence and their driving mechanistic causes. As an example, the genetic cause of nonsyndromic mitral valve prolapse (MVP) was identified here using novel sequencing technology and screening methodology. The authors carried out genome-wide association studies in nearly 4000 individuals. They narrowed down 6 candidate genes that could be responsible for the defects and subsequently conducted in vivo experimentation to validate the GWAS results. The authors identified TNS1 as a risk factor for MVP, and both zebrafish and mouse experiments showed that TNS1 loss-of-function caused abnormal valvular phenotypes, similar to those found in MVP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–4. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 131*.Preuss C, Capredon M, Wunnemann F, Chetaille P, Prince A, Godard B, Leclerc S, Sobreira N, Ling H, Awadalla P, Thibeault M, Khairy P, M.L. consortium. Samuels ME, Andelfinger G. Family Based Whole Exome Sequencing Reveals the Multifaceted Role of Notch Signaling in Congenital Heart Disease. PLoS Genet. 2016;12:e1006335. doi: 10.1371/journal.pgen.1006335. The role of Notch signaling pathway in heart development and CHD is well established in mouse models. However, the mutations that disrupt Notch signaling in many left-ventricular outflow tract obstructions (LVOTO) have not been fully evaluated. The authors utilized whole exome sequencing on families with LVOTOs, and discovered a caustic link between a group of rare mutations in conserved members of Notch signaling and congenital heart defect patients. While this study is limited by the requirement for large sample sizes and the difficulties associated with filtering environmental factors, it provides valuable insights that can help future understanding of critical biological pathways and genetic causes involved in these and many other types of CHDs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Andreassi MG, Della Corte A. Genetics of bicuspid aortic valve aortopathy. Curr Opin Cardiol. 2016;31:585–92. doi: 10.1097/HCO.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 133.Kaltman JR, Burns KM, Pearson GD. Perspective on Congenital Heart Disease Research. Circ Res. 2017;120:898–900. doi: 10.1161/CIRCRESAHA.116.310334. [DOI] [PubMed] [Google Scholar]
- 134**.Zhou H, Dickson ME, Kim MS, Bassel-Duby R, Olson EN. Akt1/protein kinase B enhances transcriptional reprogramming of fibroblasts to functional cardiomyocytes. Proc Natl Acad Sci U S A. 2015;112:11864–9. doi: 10.1073/pnas.1516237112. The evolutionary conserved transcription factors GATA4, HAND2, MEF2, and TBX5 (GHMT) have been shown to be key players involved in cardiomyocyte differentiation. In this work, the authors found that activation of AKT significantly increases the efficiency of reprogramming of fibroblasts into cardiomyocytes through presence of GHMT. While physiological studies should be conducted to further strengthen this discovery, the authors demonstrate that fibroblasts can be a source for new cardiomyocytes and that they could be used for thereapeutics. Importantly, the mechanism that underlies this process in fibroblasts appears to be AKT signaling; AKT facilitates the fibroblast-derived cardiomyocytes to develop binuclear or multinuclear phenotypes, indicating AKT signaling is integral to the cardiomyocyte maturation process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135*.Guo Y, VanDusen NJ, Zhang L, Gu W, Sethi I, Guatimosim S, Ma Q, Jardin BD, Ai Y, Zhang D, Chen B, Guo A, Yuan GC, Song LS, Pu WT. Analysis of Cardiac Myocyte Maturation Using CASAAV, A Platform for Rapid Dissection of Cardiac Myocyte Gene Function In Vivo. Circ Res. 2017 doi: 10.1161/CIRCRESAHA.116.310283. Researchers are facing numerous challenges in studying cardiomyocyte maturation, including not yet knowing all the signaling and transcription factors involved, as well as not yet having all the technical expertise needed to properly address the questions at hand. Here, the authors utilized a novel CRISPR/Cas9-AAV9-based somatic mutagenesis (CASAAV) method to identify a new candidate gene RYR2 that is critical for T-tubule maturation. According to the presented data, CASAAV is a powerful tool that can be used to dissect postnatal heart maturation. Interestingly, using this method, mosaic knockout of Jph2, which is considered to be important in postnatal cardiac maturation, shows no major cell-autonomous role in T-tubule maturation, indicating that the CASAAV mosaic model is valuable in ruling out organ level secondary effects in this process. Indeed, this technology may be a feasible method to use in re-evaluating several of the genes previously thought to be critical in the heart maturation process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136**.Gao L, Kupfer ME, Jung JP, Yang L, Zhang P, Da Sie Y, Tran Q, Ajeti V, Freeman BT, Fast VG, Campagnola PJ, Ogle BM, Zhang J. Myocardial Tissue Engineering With Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circ Res. 2017;120:1318–25. doi: 10.1161/CIRCRESAHA.116.310277. Traditional 3-dimensional (3D) printing techniques are not capable of building structures sufficient to harbor a fully functional and contractile cardiac tissue. The authors here utilized newly developed multiphoton-excited 3D printing technology to construct a cardiac extracellular matrix-like scaffold, with higher resolution to facilitate the integration of seeded cardiac cells. Combined with induced pluripotent stem cell (IPSC) technology, they were able to produce functional cardiac patches that contain IPSC derived cardiomyocytes, smooth muscle cells, and endothelial cells. They were able to further test these in context of cardiac injury mouse models to unveil their efficacy and potency in prevetion of cardiac tissue damage. While the survival rate of cardiac cells, particularly cardiomyocytes, is relatively low at 4 weeks after implantation, this cardiac patch model has great potential for future optimization and use in cardiac disease treatment. Indeed, it may be possible to obtain artificial cardiac tissue with higher cell viability and electromechanical properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen IY, Matsa E, Wu JC. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat Rev Cardiol. 2016;13:333–49. doi: 10.1038/nrcardio.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138**.Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, Koski A, Ji D, Hayama T, Ahmed R, Darby H, Van Dyken C, Li Y, Kang E, Park AR, Kim D, Kim ST, Gong J, Gu Y, Xu X, Battaglia D, Krieg SA, Lee DM, Wu DH, Wolf DP, Heitner SB, Belmonte JCI, Amato P, Kim JS, Kaul S, Mitalipov S. Correction of a pathogenic gene mutation in human embryos. Nature. 2017 doi: 10.1038/nature23305. CRIPSR-Cas9 technology exerts homologous-directed repair (HDR) to selectively edit targeted locations in the genome, greatly improving the efficiency of gene targeting, particularly in human cells. However, concerns remain to be addressed, including handling of off-target mutations, mosaicism of targeted and untargeted cells, and the undesired activation of the non-homologous end joining (NHEJ) repair mechanism. In this study, the authors conducted CRIPSR-Cas9 genome editing in early human embryos to correct an autosomal dominant MYBPC3 mutation that causes hypertrophic cardiomyopathy. They found that administration of CRISPR components during metaphase II of the oocyte cell cycle resulted in significantly increased HDR genomic correction efficiency, with 72.4% WT allele observed in blastocysts of CRISPR mutation-corrected groups as compared to only 47.4% in the non-corrected control groups. Importantly, they found minimal mosaicism, undetectable off-target mutations, and no signs of developmental defects. While the undesired NHEJ mutation did occur at considerable frequency (27.6%), posing a major obstacle for clinical usage of this method at this juncture, this study still provides us with invaluable understanding for how we can potentially use this technology to target the DNA-repair mechanism in early human embryos. Further work is needed to optimize the conditions of this method, which could shed light on clinical approaches that could treat/cure the genetic causes of several types of congenital heart disease in the near future. [DOI] [PubMed] [Google Scholar]