Abstract
Background
Endoscopic ultrasound-guided ethanol ablation (EUS-EA) is a recently introduced treatment for pancreatic cystic lesions (PCLs). However, clinical benefits such as survival gain and maintenance of quality of life (QOL) have not been fully established. The aim of this study was to evaluate the clinical benefits of EUS-EA compared with the natural course (NC) of PCLs.
Methods
This retrospective comparative study of patients with PCLs investigated an EUS-EA group (n = 118) and an NC group (n = 428). Propensity score matching (PSM) analysis was applied to minimize the effects of selection bias. The overall survival as the primary outcome and the surgical resection rate and complete remission (CR) rate as the secondary outcomes were evaluated.
Results
Between 84 matched pairs of both groups, there were no significant differences in the baseline clinical characteristics and the mean follow-up duration (78.88 ± 38.86, 75.90 ± 57.46 months, p = 0.694). Overall survival did not differ significantly (194.12 ± 5.60, 247.54 ± 12.70 months, p = 0.235). The surgical resection rate (4.8% versus 26.2%, p < 0.001) was significantly lower in the EUS-EA group. CR was observed only in the EUS-EA group and the CR rate was 32.1%.
Conclusions
EUS-EA for PCLs with low risk of malignancy might not be able to obtain a survival benefit, but showed maintenance of QOL by avoidance of unnecessary surgery, and a certain level of CR when compared to the NC. EUS-EA could be considered a useful treatment option for these, but careful application is needed because of the limited effects in some types of PCLs.
Keywords: endosonography, pancreatic cyst
Introduction
Pancreatic cystic lesions (PCLs) have been more frequently diagnosed than before because of increased health screening and the development of imaging technology.1,2 PCLs comprise a heterogeneous group of diseases, including cystic degeneration of solid tumors, benign cystic lesions such as serous cystic neoplasms (SCN), pseudocysts and premalignant lesions such as mucinous cystic neoplasm (MCN), intraductal papillary mucinous neoplasm (IPMN) and solid pseudopapillary tumor.3 Although accurate diagnosis is very important for proper management, the diagnostic accuracy with computed tomography or magnetic resonance imaging is only 60–70%, and with cystic fluid analysis through an endoscopic ultrasound it is only about 80%.4–7
Although there is no complete consensus among experts, surgery is necessary if the possibility of malignancy is high. Recently, endosonography-guided ethanol ablation (EUS-EA) has been attempted as a nonsurgical treatment for PCLs with low risk of malignancy – namely, no high-risk stigmata, no definitive mural nodule or main duct feature on endoscopic ultrasound.8 Feasibility and efficacy of EUS-EA have been confirmed through several studies.9–12 The complete remission (CR) rate of PCLs through EUS-EA is reportedly 34.3–45.1%,9,11–13 and the CR rate using ethanol and paclitaxel is reportedly 47.6–78.6% without severe complications.14–16 The long-term follow-up results suggest that the treatment effect is also well maintained.9,14
However, there are still some limitations in previous studies that must be overcome to ensure the validity and utility of EUS-EA for PCLs with low risk of malignancy. First, it is difficult to determine what clinical benefits they would have in practice because patients received EUS-EA for a number of PCLs with indolent courses such as SCN or pseudocyst.9 Second, the definite therapeutic effects of EUS-EA were proven,11 but it is difficult to confirm the clinical benefits of EUS-EA compared to a surveillance strategy. It is necessary to determine whether the clinical outcomes actually improve the survival gain through the prevention of malignancy, maintenance of quality of life (QOL) through avoidance of unnecessary surgery and achievement of true CR.
In this study, we evaluated the clinical benefits of EUS-EA therapy for PCLs with a low risk of malignancy, and compared these with those of the natural course (NC) of PCLs using a surveillance strategy.
Method
Study design and patients
This retrospective study investigated patients who visited Seoul National University Hospital with PCLs diagnosed through image modalities. Patients’ data, including age, sex, Adult Comorbidity Evaluation-27,17 initial and last size of PCLs, characteristics of PCLs and surgical reports were collected from a retrospective chart review.
Patients were divided into two groups, an NC group that did not undergo surgery for initial treatment and an EUS-EA group that received ethanol ablation therapy. In the NC group, patients who were diagnosed with PCLs from January 1993 to August 2015 were reviewed. In the EUS-EA group, the procedure was performed from June 2006 to June 2015 and the indications of the EUS-EA were as follows: (1) clinically non-pseudocyst PCLs; (2) cystic size was 2–5 cm without communication with the main pancreatic duct. The exclusion criteria were as follows: (1) <1 year follow up; (2) <20 years old; (3) symptomatic PCLs; (4) planned surgery at the time of diagnosis; (5) PCLs associated with genetic disease; (6) clinically suspicious pseudocysts; (7) PCLs with high-risk stigmata (obstructive jaundice, enhanced solid component, or main pancreatic duct more than 10 mm);8 (8) main duct type or mixed type IPMN; (9) cystic degeneration of solid tumors.
This study was approved by the institutional review board (IRB) of the Seoul National University Hospital, Korea (IRB No. H-1606-049-770). The need for informed consent was waived by the IRB.
Procedure steps of EUS-EA
EUS-EA is simultaneously performed with cystic fluid aspiration for PCLs because a split approach has two limitations: (1) two invasive procedures can increase the economic burden and risk of complications; (2) the required waiting period for the procedure because of reduction of cystic size after diagnostic cystic fluid aspiration.
The study patients received EUS-EA from three gastroenterologists who were experts in interventional EUS. Radial-scanning echoendoscope (GF-UM2000, GF-UE260; Olympus Optical Co., Tokyo, Japan) and curvilinear-array echoendoscope (GF-UCT2000, GF-UCT 240, GF-UCT 260; Olympus Optical Co., Tokyo, Japan) with a 7.5 MHz transducer (EU-M 2000 – Olympus Optical Co., Tokyo, Japan; Aloka Alpha 5 and 10 – Hitachi Aloka Medical, Ltd., Tokyo, Japan) were used, and the following parameters were evaluated for PCLs: location, size, locularity, septum, and mural nodules. EUS-EA was conducted through transgastric or transduodenal puncture of the cysts using a curvilinear-array echoendoscope with a 19- or 22-gauge needle (EchoTip Ultra – Cook Endoscopy, Winston-Salem, NC; EZ Shot 2 or 3™ – Olympus Medical, Tokyo, Japan; Expect™ – Boston Scientific, MA, USA). EUS-EA for PCLs was accomplished using the following protocol: (1) the longest diameter was measured; (2) 80% of the cystic fluid was aspirated, after which 99% ethanol was injected and stored in the cyst for 3–5 min; (3) step number 2 was repeated twice; (4) all injected ethanol and remnant cystic fluid was aspirated.
Categorization of PCLs by cystic fluid analysis
The characteristics of PCLs in the EUS-EA group were evaluated by location, size, and measurement of carcinoembryonic antigen (CEA) and amylase level from the aspirated cystic fluid. The PCLs of the EUS-EA group were categorized based on analysis of cystic fluid: SCN (CEA <5 ng/mL, amylase <800 U/L), pseudocyst (CEA <5 ng/mL, amylase ⩾800 U/L), IPMN (CEA ⩾200 ng/mL, amylase ⩾800 U/L), and MCN (CEA ⩾200 ng/mL, amylase <800 U/L). The PCLs that did not meet these criteria (5 ng/mL ⩽ CEA < 200 ng/mL) were categorized as indeterminate cysts.
Study outcome and definition of events
All outcomes were evaluated with propensity score matching (PSM) data. The primary outcome of the study was comparison of overall survival (OS) in both groups. The secondary outcomes were the rate of surgery in both groups, and the CR rate in the EUS-EA group.
The survival status was updated in June 2017. Data from living patients were considered censored for survival analysis. CR was defined as no visible PCLs on repeated imaging studies performed during the follow-up period. The follow-up period was calculated from the time of the diagnosis to the death or most recent outpatient visit.
Statistical analysis
We conducted PSM analysis between the EUS-EA and the NC groups to construct a randomized experiment-like situation and minimize selection bias. A logistic regression model was conducted to calculate the propensity score of the following covariates: age, sex, follow-up duration, initial cystic size, and number of initial worrisome features except size. Based on the propensity score, 1:1 matching was performed between the EUS-EA group and the NC group. For matching, nearest neighbor matching with a caliper width of 0.05 SD was employed.18 The overall balance test showed that the structure of the two groups was similar and the matching was successful (p = 0.983). The L1 of the multivariate imbalance measure on the matching equilibrium was 0.912 before matching and 0.738 after matching. There were no unbalanced covariates with an absolute value >0.25 in the standardized mean difference.
Survival was analyzed using the Kaplan–Meier method and log-rank test. Continuous variables were analyzed by the Student’s t test and categorical variables were analyzed using the Chi-squared test or Fisher’s exact test. p values <0.05 were considered significant. Statistical analyses were performed using SPSS v.23.0 (IBM Corp, Armonk, NY, USA). PSM for SPSS (version 1.0), which was programmed by Felix Thoemmes (Cornell University), was used with the underlying R packages (MatchIt, Rltools, cem).19
Result
Baseline clinical characteristics
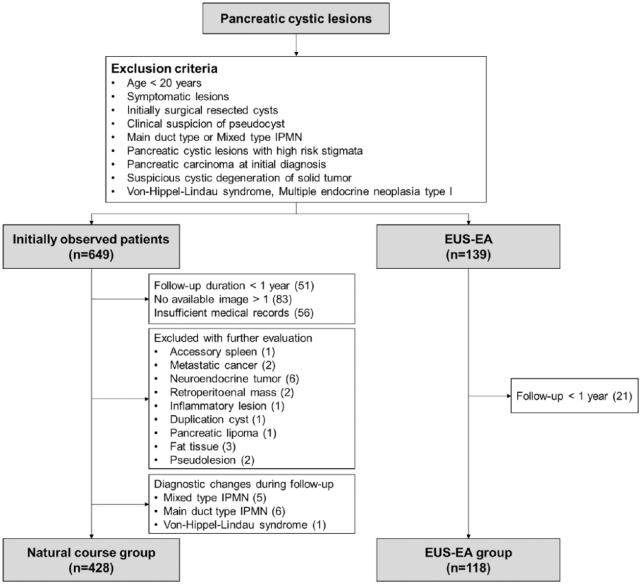
A total of 649 patients were classified as patients in the NC group, while 51 patients with follow-up duration <1 year, 83 patients with only one image, and 56 patients with insufficient medical records were excluded. Finally, 428 patients were included in the NC group, with exclusion of other disease by additional evaluations such as accessory spleen, metastatic cancer, neuroendocrine tumor (NET) and main duct or mixed type IPMN. There were 139 patients who underwent EUS-EA, but 21 patients who were followed up <1 year were excluded (Figure 1).
Figure 1.
Flow chart of patient selection for the study.
EUS-EA, endoscopic ultrasound-guided ethanol ablation; IPMN, intraductal papillary mucinous neoplasm.
The baseline characteristics of study patients before and after matching are shown in Table 1. There were several significant differences observed before matching in several factors, but after matching there were no significant differences in the age (57.43 ± 12.69, 58.24 ± 12.80 years, p = 0.681), severity of comorbidity (p = 0.153), initial cystic size (23.13 ± 11.11, 22.97 ± 12.00 mm, p = 0.927), number of worrisome features (p = 0.336) or mean follow-up duration (78.88 ± 38.86, 75.90 ± 57.46 months, p = 0.694). Three patients in the EUS-EA group and 12 patients in the NC group died. Seven patients died from non-pancreatic causes; for the others cause of death could not be identified.
Table 1.
Baseline clinical characteristics: before and after matching.
| Before matching | After matching | ||||||
|---|---|---|---|---|---|---|---|
| EUS-EA group (n = 118) | NC group (n = 428) | p value | EUS-EA group (n = 84) | NC group (n = 84) | p value | ||
| Age (years) | 55.69 ± 13.27 | 61.94 ± 10.82 | <0.001 | 57.43 ± 12.69 | 58.24 ± 12.80 | 0.681 | |
| Sex | Male | 42 | 174 | 0.320 | 30 | 29 | 0.872 |
| Female | 76 | 254 | 54 | 55 | |||
| Comorbidity | No | 55 | 203 | 0.001 | 40 | 40 | 0.153 |
| Mild | 45 | 104 | 33 | 21 | |||
| Moderate | 16 | 68 | 10 | 13 | |||
| Severe | 2 | 53 | 1 | 4 | |||
| Initial size (mm) | 26.76 ± 12.01 | 15.61 ± 9.62 | <0.001 | 23.13 ± 11.11 | 22.97 ± 12.00 | 0.927 | |
| Size | <10 mm | 6 | 107 | <0.001 | 6 | 9 | 0.703 |
| <20 mm | 26 | 216 | 26 | 28 | |||
| <30 mm | 37 | 69 | 29 | 23 | |||
| <40 mm | 32 | 23 | 18 | 16 | |||
| ⩾40 mm | 17 | 13 | 5 | 8 | |||
| Location | Head/neck | 42 | 197 | 0.107 | 26 | 30 | 0.204 |
| Body | 47 | 134 | 39 | 28 | |||
| Tail | 29 | 97 | 19 | 26 | |||
| Worrisome features (number) | 0 | 66 | 369 | <0.001 | 58 | 59 | 0.336 |
| 1 | 50 | 46 | 24 | 22 | |||
| 2 | 1 | 10 | 1 | 3 | |||
| 3 | 1 | 3 | 1 | 0 | |||
| Worrisome features13 (category) | Size >30 mm | 49 | 36 | <0.001 | 24 | 23 | 0.864 |
| Thickened wall | 3 | 14 | 1.000 | 2 | 3 | 0.650 | |
| Mural nodule | 2 | 16 | 0.387 | 1 | 2 | 0.560 | |
| MPD dilatation | 1 | 4 | 1.000 | 1 | 1 | 1.000 | |
| Abrupt PD narrowing | 0 | 2 | 1.000 | 0 | 0 | – | |
| Regional LNE | 0 | 3 | 1.000 | 0 | 0 | – | |
| Follow-up duration (months) | 74.23 ± 36.74 | 68.88 ± 51.92 | 0.205 | 78.88 ± 38.86 | 75.90 ± 57.46 | 0.694 | |
| Follow-up after ablation (months) | 51.76 ± 28.18 | 50.39 ± 28.3 | |||||
| Death | 3 | 12 | |||||
| Cause of death | Disease specific death | 0 | 0 | ||||
| Non-pancreatic cause | 2 | 5 | |||||
| Not available | 1 | 7 | |||||
EUS-EA, endoscopic ultrasound-guided ethanol ablation; LNE, lymph node enlargement; MPD, main pancreatic duct; NC, natural course; PD, pancreatic duct.
Evaluation of clinical outcomes
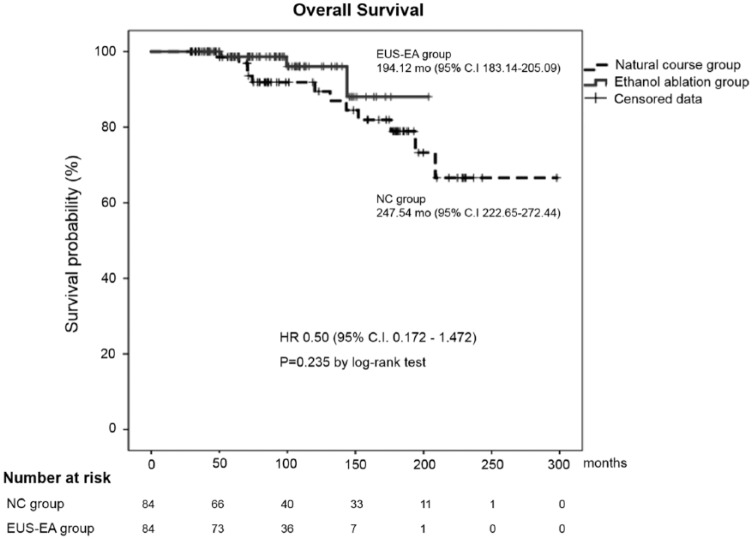
The measured OS was 194.12 ± 5.60 months in the EUS-EA group (95% CI 183.14–205.09) and 247.54 ± 12.704 months (95% CI 222.65–272.44) in the NC group by the Kaplan–Meier analysis, with no significant difference in the log-rank test (p = 0.235) (Figure 2). The rate of surgery was significantly lower in the EUS-EA group than the NC group (4.8% versus 26.2%, p < 0.001). The CR rate was 32.1% (27 of 84) and CR was confirmed only in the EUS-EA group (Table 2). There were no severe adverse events by EUS-EA and all minor adverse events were improved with conservative management.
Figure 2.
Kaplan–Meier curve for overall survival in both groups after matching. This graph shows the OS of both groups and there was no statistically significant difference by the log-rank test (194.12 ± 5.60 versus 247.54 ± 12.70 months, p = 0.235).
EUS-EA, endoscopic ultrasound-guided ethanol ablation; NC, natural course.
Table 2.
Comparison of clinical outcomes of both groups after propensity score matching.
| EUS-EA group (n = 84) | NC group (n = 84) | p value | |
|---|---|---|---|
| Overall survival (month) | 194.12 ± 5.60 | 247.54 ± 12.70 | 0.235 |
| Surgery | 4 (4.8%) | 22 (26.2%) | <0.001 |
| CR | 27 (32.1%) |
CR, complete remission; EUS-EA, endoscopic ultrasound-guided ethanol ablation; NC, natural course.
Review of the patients who underwent surgery
In the NC group, 22 patients (26.2%) underwent surgery and the pathologic diagnoses were as follows: 5 SCN, 3 MCN, 13 IPMN, and 1 pancreatic ductal adenocarcinoma (PDAC). Among mucinous cysts, 62.5% showed low-grade dysplasia or benign, 31.3% showed moderate-grade dysplasia or borderline malignancy, and 6.3% was malignancy. Of the two cysts diagnosed as malignant lesions, PDAC was stage IIA (pT3N0) and intraductal papillary mucinous carcinoma was stage 0 (pTis). The duration from diagnosis to surgery was 32.98 ± 28.17 (4.9–114.47) months. There was no mortality case, but postoperative morbidities were confirmed in nine patients, including four patients with chronic abdominal discomfort or digestive problems, three patients with postoperative infection, two patients with postoperative onset diabetes mellitus, and one patient with postoperative pancreatic juice leakage.
In the EUS-EA group, four patients (4.8%) underwent surgery and the pathological diagnoses were as follows: 1 SCN, 2 MCN (low and intermediate dysplasia) and 1 IPMN with moderate dysplasia. There were no reports describing the ablative effect of EUS-EA in pathologic findings. Two patients were described as having a specific finding, with degeneration with foamy histiocytic infiltration in one, and grayish fibrosis around pericystic parenchyme in the other. There was no recurrence after surgery during the study period (Table 3). In addition, there were no difficult operations due to the severe fibrosis or adhesion and no evidence of dissemination related to EUS-EA. The duration from diagnosis to surgery was 57.5 ± 39.27 (17.97–96.97) months and the duration from EUS-EA to surgery was 20.33 ± 17.61 (6.17–42.93). Among patients who underwent surgery, postoperative chronic dyspepsia was confirmed in three patients. Surgery was performed for various reasons, including constantly or rapidly growing cysts, newly developed risky features, patient concern and planned simultaneous laparotomy for another tumor. A patient who underwent EUS-EA for SCN was found to have liver metastases during follow up and the results of liver biopsy revealed they were metastatic lesions of the NET. Surgery was performed and SCN with traces of EUS-EA adjacent to 1.1 cm of NET (grade 3, pT3N0) without cystic change were identified.
Table 3.
Review of the patients who underwent surgery in both patient groups after propensity score matching.
| Age | Sex | Size (mm) | Location | Presumed diagnosis | Duration till surgery (months) | Reason for surgery | Surgical pathology | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | EUS-EA | Operation | ||||||||
| NC group (n = 22) | 62 | M | 31.6 | 50 | Body | 33.6 | Size increasing | SCN | ||
| 50 | F | 15 | 25.3 | Body | 46.9 | Size increasing | BD-IPMN, benign | |||
| 42 | M | 30 | 85 | Tail | 114.5 | Size increasing | MCN | |||
| 57 | F | 37 | 34 | Head | 83.8 | Recurrent pancreatitis | BD-IPMN, MD | |||
| 54 | F | 45 | 48.9 | Tail | 44.4 | Size increasing | MCN, borderline malignancy | |||
| 31 | F | 35 | 40.1 | Tail | 20.1 | Size increasing | SCN | |||
| 52 | M | 20 | 20.5 | Head | 9.2 | Patient’s wish | SCN | |||
| 73 | F | 36.1 | 39.7 | Tail | 10.6 | Mural nodule | BD-IPMN, MD | |||
| 31 | F | 41 | 51 | Body | 26.7 | Size increasing | SCN | |||
| 66 | M | 48.7 | 53 | Head | 50.8 | Mural nodule | BD-IPMN, MD | |||
| 68 | F | 3.3 | 7.5 | Body | 36.8 | Simultaneous surgery with other tumor | BD-IPMN, benign | |||
| 48 | F | 28.1 | 36 | Body | 15.1 | Size increasing | SCN | |||
| 67 | F | 25.3 | 27 | Tail | 69.5 | Enhancing solid portion | PDAC (pT3N0) | |||
| 53 | F | 22 | 21 | Body | 23.8 | Mural nodule | BD-IPMN, LD | |||
| 42 | M | 36 | 33 | Tail | 9.7 | Simultaneous surgery with other tumor | BD-IPMN, MD | |||
| 57 | F | 22 | 21 | Tail | 21.4 | Wall thickening | BD-IPMN, LD | |||
| 69 | M | 34 | 40 | Body | 32.2 | Size increasing | BD-IPMN, LD | |||
| 60 | F | 27 | 35 | Body | 50.2 | Size increasing | MCN | |||
| 69 | M | 48 | 48 | Head | 4.9 | Solid portion | IPMC (pTis) | |||
| 57 | M | 32 | 30 | Tail | 4.9 | Patient’s wish | BD-IPMN, LD | |||
| 64 | M | 12 | 14 | Tail | 6.7 | Size increasing | BD-IPMN, LD | |||
| 62 | F | 20 | 24 | Head | 9.6 | Patient’s wish | BD-IPMN, LD | |||
| EUS-EA group (n = 4) | 72 | M | 36 | 27 | 30 | Body | SCN | 97.0 | Simultaneous surgery with other tumor | SCN |
| 69 | M | 16 | 20 | 25 | Body | IND | 30.1 | Size increasing | BD-IPMN, MD | |
| 34 | F | 50 | 50 | 30 | Head | MCN | 18.0 | Thickened wall | MCN, LD | |
| 46 | F | 15 | 37 | 66 | Tail | IND | 85.0 | Size increasing | MCN, MD | |
BD-IPMN, branch duct type intraductal papillary mucinous neoplasm; EUS-EA, endoscopic ultrasound-guided ethanol ablation; IND, indeterminate cyst; IPMC, intraductal papillary mucinous carcinoma; LD, low-grade dysplasia; MCN, mucinous cystic neoplasm; MD, moderate-grade dysplasia; NC, natural course; PDAC, pancreatic ductal adenocarcinoma; SCN, serous cystic neoplasm.
Treatment response of EUS-guided ethanol ablation for PCLs
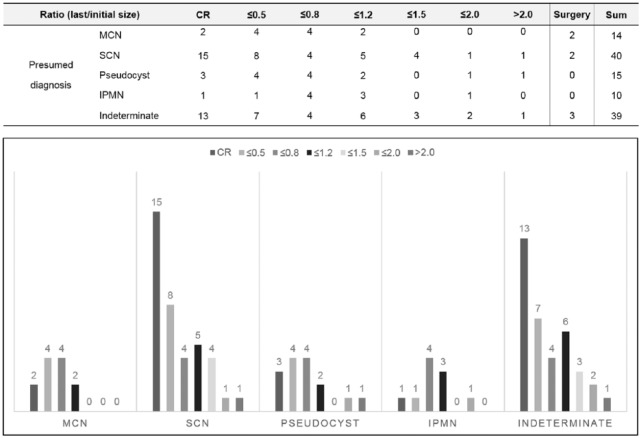
Treatment responses of the EUS-EA group before matching (n = 118) are shown in Figure 3. No patient underwent more than one EUS-EA for the same lesion. The ratio of the last and initial size was calculated to evaluate the treatment response. The CR rate of each PCLs was 13.3% for MCN, 37.5% for SCN, 20.0% for pseudocyst, 10.0% for IPMN and 33.3% for indeterminate cysts. The ratio of over half size reduction was 40.0% for MCN, 57.5% for SCN, 46.7% for pseudocysts, 20.0% for IPMN and 51.3% for indeterminate cysts. There were only three cysts more than twice as large as the initial size after EUS-EA. Seven patients underwent surgery who had been treated with EUS-EA. One patient underwent EUS-EA and was followed up for sustained cyst. Because the size of the cyst slowly increased and a mural nodule was observed, the patient underwent pylorus preserving pancreaticoduodenectomy at 88.6 months after EUS-EA and IPMN-associated invasive carcinoma (pT1N0M0) was diagnosed. All other patients were found to have no malignant portion.
Figure 3.
Final treatment response of EUS-guided ethanol ablation for PCLs according to presumed diagnosis by cystic fluid analysis: ratio of last size and initial size.
CR, complete remission; IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasm; SCN, serous cystic neoplasm.
The comparison data for the two groups after PSM for the change in size are as follows: the size reduction differed significantly between groups [p < 0.001, 67.9% (57 of 84) in EUS-EA group, 15.5% (13 of 84) in NC group]. However, size growth showed no significant difference (p = 0.264): 6.0% (5 of 84) in the EUS-EA group, while it was 10.7% (9 of 84) in the NC group.
Discussion
Surgery for PCLs is the only curative and the most important treatment, but it can lead to a deterioration in QOL. Some patients underwent unnecessary surgery because of the ambiguity of exact surgical indications. As understanding of the NC and malignant potential of PCLs develops, the management has gradually become more conservative.8,20,21 Several studies of feasibility and safety of EUS-EA have been conducted and have shown a certain amount of CR (Table 4). However, there has been no comparative analysis of how EUS-EA affects clinical benefits such as survival gain and maintenance of QOL. This study showed that EUS-EA might not be able to provide survival gain, but it is able to provide clinical benefits including maintenance of QOL by avoidance of unnecessary surgery and a certain level of CR for PCLs with low risk of malignancy.
Table 4.
Literature review of previous studies for EUS-guided ablation.
| Author (year) | N | Ablative agents | Median follow up (months) | CR (%) | CR criteria | Severe complication |
|---|---|---|---|---|---|---|
| Gan and colleagues (2005)12 | 23 | E | 6–12 | 34.8 | Disappear | 0 |
| Dimaio and colleagues (2011)13 | 13 | E | 13.4 | 38.5 | Disappear | 0 |
| Oh and colleagues (2011)16 | 47 | E + P | 21.7 (2–44) | 61.7 | <5% volume | 0 |
| Caillol and colleagues (2012)10 | 13 | E | 26 (4–118) | 84.6 | Disappear | 0 |
| DeWitt and colleagues (2014)15 | 21 | E + P | 27 (17–42) | 47.6 | <5% volume | 4 |
| Park and colleagues (2016)9 | 91 | E | 40 (13–117) | 45.1 | Disappear | 0 |
| Gomez and colleagues (2016)22 | 23 | E | 45.8 (14.7–90.8) | 8.7 | Disappear | 1 |
| Choi and colleagues (2017)14 | 158 | E + P | 69 (48–81) | 72.2 | <5% volume | 1 |
CR, complete remission; E, ethanol; EUS, endoscopic ultrasound; P, paclitaxel.
Treatment of PCLs with EUS-EA did not significantly improve survival compared with the NC group in this study. Survival in these patients seems to be determined by the severity of the accompanying comorbidities, because patients whose cause of death could be identified were found to have died because of worsened underlying disease or newly developed fatal disease. Moderate to severe levels of comorbidity were found in 40% of the mortality cases, which was significantly higher than that of the whole patients. These results are in line with those of previous studies that showed most of the PCLs without any risk factors were unlikely to cause mortality or morbidity because of their benign disease course and slow-growing features.1,23–25 PCLs with no suspicious features had a lower risk of pancreatic malignancy in recent studies than in previous studies based on surgical series. The similar level of surgical mortality and malignancy detection rate in the surveillance group has significant implications.1,23 Therefore, it is very important to consider the patient’s QOL when treating PCLs.
The QOL of patients with PCLs may have been determined by morbidity caused by surgery, psychological anxiety for malignancy, and medical economic burden. The long-term adverse events associated with postoperative morbidity were significantly less in the EUS-EA group than in the NC group. We estimated the average economic burden, including the procedure or surgery fee, hospitalization fee, and surveillance fee per patient, of the EUS-EA group to be $3146 (USD) and that of the NC group to be $4005 for the follow-up period of 7 years after diagnosis based on the Korean medical price and our medical policy (p = 0.102). Therefore, management of PCLs including EUS-EA seems to reduce the medical economic burden while also reducing the possibility of the patient suffering from morbidity due to surgery. We believe that EUS-EA can be considered a minimally invasive treatment option in the management of PCLs with low risk of malignancy and clearly be helpful to the patient by preventing deterioration of QOL by reducing unnecessary surgery.
Despite expected advantages with guaranteed feasibility and safety of EUS-EA, some experts have a negative view of the real long-term efficacy of EUS-EA because of the low CR ratio in mucinous neoplasm.22 Moreover, controversies exist regarding the potential for missing proper surgery for premalignant lesions.26,27 The PSM comparative analysis in this study produced the following meaningful findings. First, it seems possible to manage PCLs without unnecessary surgery by providing additional treatment options (4.8% versus 26.2%, p < 0.001). In fact, 22.7% of SCN and 36.4% of mucinous cysts with low-grade dysplasia did not require surgery according to the surgical pathology of the NC group. In our opinion, unnecessary surgery was more likely to be performed in the NC group because there are only two options in the treatment of PCLs: surgery or surveillance. Second, the results of surgical pathology suggest that the treatment strategy with EUS-EA showed no tendency to miss the appropriate treatment opportunity for malignant transformed PCLs. It seems possible to opt for surgery in cases that the cystic size continues to grow or high-risk features emerge after EUS-EA. Third, a certain level of CR can be expected from appropriate indicated PCLs. Patients with PCLs reaching CR may be able to increase the interval of examination and relieve the psychological pressure regarding malignant transformation.
However, it is difficult to use EUS-EA as an alternative treatment for surgery for three reasons. First, the effects on some types of PCLs has been shown to be low based on data from this and previous studies. The results of defining the CR using the volume criteria show that the success rate is high, but the actual volume decreased to <5%, indicating that about one-third of the length was sustained. Therefore, follow up is not terminated even for PCLs with CR based on volume criteria after EUS-EA. Second, it has not been confirmed that EUS-EA can actually prevent malignant transformation. DeWitt and colleagues reported in molecular-level studies that EUS-guided ablation with ethanol and paclitaxel may eliminate mutant DNA in PCLs,15 but further molecular-level and pathologic data are needed to reach a conclusion. Third, EUS-EA will proceed with uncertain diagnosis at the time of the procedure. Depending on the presumed diagnosis, which is confirmed afterwards by cystic fluid analysis, it is possible to predict the response after EUS-EA and the decision about the necessity for surgery or close follow up. If a center is available with confocal laser endomicroscopy, this will provide more accurate information about the diagnosis and help to determine whether or not to perform the procedure.28,29 Considering these aspects, it is more reasonable for EUS-EA to act as an affordable and feasible treatment option with its own indications rather than as an alternative to surgery.
This study has several limitations. First, there are definite limitations to application of the data for a comparative study because this was a retrospective observational study. However, PSM was performed to minimize differences in baseline clinical and lesion characteristics between study groups. Second, the results in this study are based on the presumed diagnosis of PCLs by imaging studies or cystic fluid analysis. It is important to note that decision-making should be made under uncertain situations in real practice. Third, the evaluation of the EUS-EA response was conducted by comparing the size of the first and the last image. We did not analyze whether the size increased after decline, recurrence after CR, or growth rate. Fourth, QOL was not quantitatively assessed, and the exact causal relationship between QOL and postoperative morbidity of the patients who underwent surgery was not investigated in our study. In order to compensate for this, we have also discussed the economic aspects of EUS-EA as closely as possible to suggest that it has a positive effect on the maintenance of QOL.
There are several strengths of this study. First, we focused on the clinical implications of EUS-EA rather than the success of the procedure itself, unlike previous studies. Second, as far as we know, this is the first comparative study of long-term clinical benefits such as survival gain, avoidance of surgery and CR in a large number of patients. The prospective randomization comparisons of EUS-EA that could respond to CR and surveillance without treatment seemed to be ethically unacceptable. Third, the outcomes are more robust based on the PSM method than those reflected in previous single-arm studies. Fourth, CR criteria were strictly defined and analyzed because of the slow-growing nature of PCLs and limited ways to verify the absence of a viable portion after EUS-EA.
In conclusion, EUS-EA might not be able to provide a survival benefit, but could be able to generate clinical benefits including maintenance of QOL by avoidance of unnecessary surgery and a certain level of CR in patients with PCLs with low risk of malignancy. Based on the first comparison data for treatment of PCLs, EUS-EA could be considered a useful treatment option for PCLs with lower risk of malignancy and a complementary treatment option for patients with high risk of surgery, but careful application is needed because of the limited effects when applied to some types of PCLs.
Footnotes
Author contributions: Jin Ho Choi: acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis. Sang Hyub Lee: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision. Young Hoon Choi: analysis and interpretation of data. Jinwoo Kang: analysis and interpretation of data. Woo Hyun Paik: critical revision of the manuscript for important intellectual content. Dong-Won Ahn: acquisition of data, critical revision of the manuscript for important intellectual content. Ji Kon Ryu: critical revision of the manuscript for important intellectual content, study supervision. Yong-Tae Kim: critical revision of the manuscript for important intellectual content, analysis and interpretation of data, study supervision.
Funding: This study was supported by the Seoul National University College of Medicine Research Fund (2015).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Jin Ho Choi, Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Sang Hyub Lee, Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul, 03083, Korea.
Young Hoon Choi, Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Jinwoo Kang, Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Woo Hyun Paik, Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Dong-Won Ahn, Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea.
Ji Kon Ryu, Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Yong-Tae Kim, Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
References
- 1. Gaujoux S, Brennan MF, Gonen M, et al. Cystic lesions of the pancreas: changes in the presentation and management of 1,424 patients at a single institution over a 15-year time period. J Am Coll Surg 2011; 212: 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol 2010; 8: 806–811. [DOI] [PubMed] [Google Scholar]
- 3. Stark A, Donahue TR, Reber HA, et al. Pancreatic cyst disease: a review. JAMA 2016; 315: 1882–1893. [DOI] [PubMed] [Google Scholar]
- 4. Jang DK, Song BJ, Ryu JK, et al. Preoperative diagnosis of pancreatic cystic lesions: the accuracy of endoscopic ultrasound and cross-sectional imaging. Pancreas 2015; 44: 1329–1333. [DOI] [PubMed] [Google Scholar]
- 5. Khashab MA, Kim K, Lennon AM, et al. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas 2013; 42: 717–721. [DOI] [PubMed] [Google Scholar]
- 6. Cho CS, Russ AJ, Loeffler AG, et al. Preoperative classification of pancreatic cystic neoplasms: the clinical significance of diagnostic inaccuracy. Ann Surg Oncol 2013; 20: 3112–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007; 102: 2339–2349. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12: 183–197. [DOI] [PubMed] [Google Scholar]
- 9. Park JK, Song BJ, Ryu JK, et al. Clinical outcomes of endoscopic ultrasonography-guided pancreatic cyst ablation. Pancreas 2016; 45: 889–894. [DOI] [PubMed] [Google Scholar]
- 10. Caillol F, Poincloux L, Bories E, et al. Ethanol lavage of 14 mucinous cysts of the pancreas: a retrospective study in two tertiary centers. Endosc Ultrasound 2012; 1: 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeWitt J, McGreevy K, Schmidt CM, et al. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc 2009; 70: 710–723. [DOI] [PubMed] [Google Scholar]
- 12. Gan SI, Thompson CC, Lauwers GY, et al. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc 2005; 61: 746–752. [DOI] [PubMed] [Google Scholar]
- 13. DiMaio CJ, DeWitt JM, Brugge WR. Ablation of pancreatic cystic lesions: the use of multiple endoscopic ultrasound-guided ethanol lavage sessions. Pancreas 2011; 40: 664–668. [DOI] [PubMed] [Google Scholar]
- 14. Choi JH, Seo DW, Song TJ, et al. Long-term outcomes after endoscopic ultrasound-guided ablation of pancreatic cysts. Endoscopy 2017; 49: 866–873. [DOI] [PubMed] [Google Scholar]
- 15. DeWitt JM, Al-Haddad M, Sherman S, et al. Alterations in cyst fluid genetics following endoscopic ultrasound-guided pancreatic cyst ablation with ethanol and paclitaxel. Endoscopy 2014; 46: 457–464. [DOI] [PubMed] [Google Scholar]
- 16. Oh HC, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology 2011; 140: 172–179. [DOI] [PubMed] [Google Scholar]
- 17. Piccirillo JF, Feinstein AR. Clinical symptoms and comorbidity: significance for the prognostic classification of cancer. Cancer 1996; 77: 834–842. [PubMed] [Google Scholar]
- 18. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ho DE, Imai K, King G, et al. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 2007; 15: 199–236. [Google Scholar]
- 20. Vege SS, Ziring B, Jain R, et al. American Gastroenterological Association Institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015; 148: 819–822. [DOI] [PubMed] [Google Scholar]
- 21. Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 2013; 45: 703–711. [DOI] [PubMed] [Google Scholar]
- 22. Gomez V, Takahashi N, Levy MJ, et al. EUS-guided ethanol lavage does not reliably ablate pancreatic cystic neoplasms (with video). Gastrointest Endosc 2016; 83: 914–920. [DOI] [PubMed] [Google Scholar]
- 23. Wu BU, Sampath K, Berberian CE, et al. Prediction of malignancy in cystic neoplasms of the pancreas: a population-based cohort study. Am J Gastroenterol 2014; 109: 121–129. [DOI] [PubMed] [Google Scholar]
- 24. Ahn DW, Lee SH, Kim J, et al. Long-term outcome of cystic lesions in the pancreas: a retrospective cohort study. Gut Liver 2012; 6: 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pausawasdi N, Heidt D, Kwon R, et al. Long-term follow-up of patients with incidentally discovered pancreatic cystic neoplasms evaluated by endoscopic ultrasound. Surgery 2010; 147: 13–20. [DOI] [PubMed] [Google Scholar]
- 26. Lekkerkerker SJ, Besselink MG, Busch OR, et al. Long-term follow-up of neoplastic pancreatic cysts without high-risk stigmata: how often do we change treatment strategy because of malignant transformation? Scand J Gastroenterol 2016; 51: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 27. Malleo G, Marchegiani G, Borin A, et al. Observational study of the incidence of pancreatic and extrapancreatic malignancies during surveillance of patients with branch-duct intraductal papillary mucinous neoplasm. Ann Surg 2015; 261: 984–990. [DOI] [PubMed] [Google Scholar]
- 28. Napoléon B, Lemaistre AI, Pujol B, et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy 2015; 47: 26–32. [DOI] [PubMed] [Google Scholar]
- 29. Kadayifci A, Atar M, Yang M, et al. Imaging of pancreatic cystic lesions with confocal laser endomicroscopy: an ex vivo pilot study. Surg Endosc 2017; 31: 5119–5126. [DOI] [PubMed] [Google Scholar]