Short abstract
Background
Several studies have shown that scorpion venom peptide BmK AGAP has an analgesic activity. Our previous study also demonstrated that intraplantar injection of BmK AGAP ameliorates formalin-induced spontaneous nociceptive behavior. However, the effect of intrathecal injection of BmK AGAP on nociceptive processing is poorly understood.
Methods
We investigated the effects of intrathecal injection of BmK AGAP on spinal nociceptive processing induced by chronic constrictive injury or formalin. Thermal hyperalgesia and mechanical allodynia were measured using radiant heat and the von Frey filaments test. Formalin-induced spontaneous nociceptive behavior was also investigated. C-Fos expression was assessed by immunohistochemistry. Phosphorylated mitogen-activated protein kinase (p-MAPK) expression was monitored by Western blot assay.
Results
Intrathecal injection of BmK AGAP reduced chronic constrictive injury-induced neuropathic pain behavior and pain from formalin-induced inflammation, accompanied by decreased expression of spinal p-MAPKs and c-Fos protein. The results of combining low doses of different MAPK inhibitor (U0126, SP600125, or SB203580; 0.1 µg for each inhibitor) with a low dose of BmK AGAP (0.2 µg) suggested that BmK AGAP could potentiate the effects of MAPK inhibitors on inflammation-associated pain.
Conclusion
Our results demonstrate that intrathecal injection of BmK AGAP produces a sensory-specific analgesic effect via a p-MAPK-dependent mechanism.
Keywords: BmK AGAP, MAPKs, spinal nociceptive, analgesic effect
Introduction
Many animal-derived drugs have been used in primary health care for hundreds of years.1 Scorpion Buthus martensii Karsch (BmK) is commonly used to treat apoplexy, migraine headache, and pain associated with rheumatism and cancer.2 Scorpion venom is composed of a mixture of various toxic polypeptides with different functions. Researchers have isolated an analgesic-antitumor peptide (BmK AGAP) from the venom of Scorpion Buthus martensii Karsch.3,4 Furthermore, we have demonstrated that BmK AGAP has analgesic activity.3
Sensitization of the peripheral nociceptor induced by inflammation or injury manifests as hyperalgesia, an exaggerated pain response to a noxious stimulus, and/or allodynia, the perception of a non-noxious stimulus as noxious. Peripheral sensitization promotes the firing of small-diameter sensory neurons that transmit information regarding noxious stimuli to the dorsal horn of the spinal cord and augments synaptic function. This in turn induces central sensitization, which could be a major cellular mechanism leading to conversion of acute nociceptive injuries into chronic pain.
Nerve injury can induce painful hyperalgesia accompanied by allodynia.5–7 Several studies have shown that regulation of mitogen-activated protein kinases (MAPKs) contributes to different nociceptive processes and peripheral central sensitization induced by different noxious stimuli.8–13 A recent study demonstrated that BmK AGAP down-regulates the expression of p-p38, phosphorylated Jun N-terminal kinase (p-JNK), and phosphorylated extracellular signal–regulated protein kinase (ERK)1/2 in vitro.14 Our previous study found that intraplantar injection of BmK AGAP ameliorates formalin-induced spontaneous nociceptive behavior, accompanied by decreased expression of peripheral and spinal phosphorylated (p)-MAPKs. Administration of BmK AGAP also inhibits formalin-induced spinal c-Fos expression.15 Thus, it is possible that MAPKs, as downstream effectors, participate in modulating spinal nociceptive processing related to intrathecal injection of BmK AGAP. To test this hypothesis, the present study was designed to determine whether intrathecal injection of BmK AGAP decreases expression of p-MAPKs in spinal nociceptive processing and explore the potential use of BmK AGAP for alleviating acute and chronic pain.
Materials and methods
Animals
Adult male Kunming mice (weight 20–25 g) were provided by the Experimental Animal Center of Jiangsu Province Academy of Traditional Chinese Medicine. Mice were housed in standard transparent plastic cages under a 12-h/12-h light–dark cycle regime and were provided free access to food and water. All experimental protocols were approved by the Animal Care and Use Committee of Jiangsu Province Academy of Traditional Chinese Medicine and were in accordance with the Declaration of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No. 80–23, revised 1996).
Drug application
BmK AGAP was dissolved in 0.9% saline. SB203580 (p38 kinase inhibitor), U0126 (mitogen-activated protein/ERK inhibitor), and SP600125 (JNK inhibitor) were purchased from Biomol Research Laboratories (Plymouth Meeting, PA). All MAPK inhibitors were dissolved in 1% dimethyl sulfoxide. All doses of drugs were determined based on the results of preliminary experiments. The dose of each drug and time points of treatment are indicated in the figure legends.
Drugs were injected intrathecally according to the method described by Hylden and Wilcox.16 In brief, a 28-gauge stainless steel needle attached to a 25-μl Hamilton microsyringe was inserted between the L5 and L6 vertebrae of conscious mice. A sudden slight flick of the tail indicated entry into the subarachnoid space. A volume of 5 μl of drug solution or physiologic saline was injected into the subarachnoid space over a 30-s period, and the injection cannula was left in place for an additional 15 s. Motor function was evaluated by observation of placing or stepping reflexes and righting reflexes 2 min before the nociceptive test. Animals with signs of motor dysfunction were excluded from the experiments.
Preparation of BmK AGAP
BmK AGAP was obtained by inducing the expression of pET28a/SUMOAGAP in Escherichia coli, as described elsewhere. BmK AGAP was dissolved in saline, and its activity was confirmed to be the same as that in the previous study.3
Chronic constrictive injury model
The chronic constrictive injury (CCI) model was developed following the method of Bennett and Xie.17 In brief, mice were anesthetized with sodium pentobarbital (40 mg/kg, intraperitoneal injection). The left sciatic nerve was then exposed at mid-thigh level through a small incision, and a unilateral constriction injury just proximal to the trifurcation was induced with three loose ligatures using 5-0 silk thread (spaced at a 1-mm interval). In sham-operated control animals, the nerve was exposed but not ligated. The incision was closed in layers, and the wound was treated with antibiotics.
Formalin test
The procedure used for the formalin-induced pain model was essentially the same as that reported by Hunskaar and Hole.18 Approximately 30 min before testing, mice were individually placed in perspex observation chambers (10 × 20 × 15 cm) for adaptation. The animals were then removed from the chambers, and 10 µl of 1% formalin in 0.9% saline was injected subcutaneously into the dorsal surface of the right hind paw using a 25-µl Hamilton syringe with a 28-gauge needle. Each mouse was immediately returned to the observation chamber after formalin injection. The total time the mice spent licking and biting the injected paw as well as the number of paw flinches were determined from 0 to 5 min (phase I) and 10 to 40 min (phase II) after formalin injection and were considered indicative of nociception.
Measurement of thermal hyperalgesia
Thermal hyperalgesia was assessed using the paw-withdrawal latency (PWL) test according to the method described by Hargreaves et al.19 In brief, mice were placed in clear plastic chambers (7 × 9 × 11 cm) and allowed to acclimatize to the environment for 1 h before testing. Radiant heat was directed to the plantar surface of each hind paw kept flush against the glass or to the site of injection of solution through the glass plate. The nociceptive endpoint in the radiant heat test was characteristic lifting or licking of the hind paw. The time to endpoint was defined as the PWL period. The radiant heat intensity was adjusted to obtain a basal PWL period of 12–15 s. An automatic 20-s cutoff was used to prevent tissue damage. Thermal stimuli were delivered three times to each hind paw at 5-min intervals.
In the CCI model, withdrawal latencies were measured in the same animal using both the ipsilateral (ligated) and contralateral (unligated) paw. Results are expressed as the PWL difference score: PWL (s) = contralateral latency − ipsilateral latency.
Measurement of mechanical allodynia
Mechanical allodynia was assessed using von Frey filaments (North Coast Medical, Inc., San Jose, CA), starting with 0.31 g and ending with 4.0 g filaments as the cutoff value. Animals were placed in individual plastic boxes (20 × 25 × 15 cm) on a metal mesh floor and allowed to acclimate for 1 h. The filaments were presented, in ascending order of strength, perpendicular to the plantar surface with sufficient force to cause slight bending against the paw and held for 6–8 s. Brisk withdrawal or paw flinching were considered positive responses. The paw-withdrawal threshold (PWT) was determined by sequentially increasing and decreasing the magnitude of the stimulus (the “up-and-down” method). Data were analyzed using the nonparametric method of Dixon, as described by Chaplan et al.20 In the CCI model, withdrawal thresholds were measured in the same animal using both the ipsilateral (ligated) and contralateral (unligated) paw. Behavioral testing was performed by an investigator blinded to the treatment.
Immunohistochemistry
Mice were anesthetized with ketamine/xylazine cocktail (100 mg/kg–5 mg/kg, intraperitoneal injection) and perfused intracardially with saline followed by 4% ice-cold paraformaldehyde in 0.1 M phosphate buffer. The L4-L5 spinal cord were removed, post-fixed in 4% paraformaldehyde for 3 h at room temperature, and subsequently allowed to equilibrate in 30% sucrose in phosphate buffer overnight at 4°C. Next, 30-mm transverse sections were cut using a cryostat. After washing in phosphate-buffered saline (PBS), the tissue sections were blocked in PBS containing 5% normal goat serum and 0.3% Triton X-100 at room temperature for 1 h, followed by incubation with primary polyclonal rabbit anti-pERK1/2 antibody (1:200), primary polyclonal rabbit anti–p-p38 antibody (1:200), primary polyclonal rabbit anti–p-JNK antibody (1:200), or primary polyclonal rabbit anti-Fos antibody (1:1000) at 4°C for 48 h (all antibodies were obtained from Santa Cruz Biotechnology, CA, USA). The sections were then incubated in biotinylated goat anti-rabbit (1:200) secondary antibody at 37°C for 1 h and avidin-biotin-peroxidase complex (1:100) at 37°C for 2 h. Finally, the sections were treated with 0.05% diaminobenzidine for 5–10 min and rinsed with PBS to stop the reaction, mounted on gelatin-coated slides, air dried, dehydrated with 70%–100% alcohol series, cleared with xylene, and coverslipped for microscopic observation. For each animal, the total number of positive neurons in the ipsilateral spinal cord was recorded. All positive neurons were counted without considering the intensity of staining.
Western blot analysis
The spinal cord in the lumbar enlargement was rapidly removed, and the ipsilateral dorsal spinal cord was separated and collected for Western blot analysis. Protein extraction was performed as described previously.15 Proteins (30 µg per sample) were separated on a 10% sodium dodecyl sulfate–polyacrylamide gel and transferred onto nitrocellulose membranes. The membranes were blocked for 2 h in 1% bovine serum albumin (in 0.05% Tween-20 in Tris-buffered saline [TBST]) at room temperature and then immunoblotted overnight at 4°C with primary antibodies. After three washes in TBST, the membranes were incubated with goat anti-mouse IgG-horseradish peroxidase or goat anti-rabbit IgG-horseradish peroxidase secondary antibodies (Santa Cruz) for 2 h at room temperature. The membranes were then washed three times in TBST. Immunoreactive protein bands were detected using the Gel/Chemi Doc System (Bio-Rad).
Statistical analysis
GraphPad Prism software (version 5; GraphPad Software Inc., San Diego, CA) was used to perform all statistical analyses. Changes in protein expression and behavioral responses to thermal and mechanical stimuli over time between groups were tested using one-way or two-way analysis of variance with repeated measures followed by Bonferroni post hoc tests, respectively. All data are presented as the mean ± SD. Statistical results were considered significant if P < 0.05.
Results
Intrathecal injection of BmK AGAP in the early phase of neuropathic pain development ameliorates thermal hyperalgesia and mechanical allodynia induced by CCI
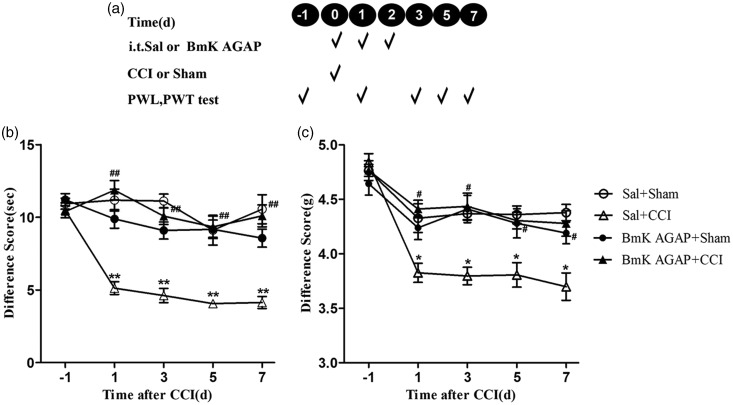
Peripheral nerve injury (i.e., CCI) produced rapid-onset, long-lasting thermal hyperalgesia and mechanical allodynia. To evaluate the effects of BmK AGAP on the development of neuropathic pain, BmK AGAP was intrathecally injected daily for three days during the early phase. The first injection was taken 1 h before CCI. The second and third injections were taken one and two days after CCI; 5 µg BmK AGAP was prepared for each injection (see experimental protocol illustrated in Figure 1(a). Compared with the mice in Sal + Sham group, animals in the Sal + CCI group exhibited significant thermal hyperalgesia in PWL test and mechanical allodynia in PWT test. The induction of thermal hyperalgesia (Figure 1(b)) and mechanical allodynia (Figure 1(c)) was prevented by intrathecal injection of BmK AGAP. At day 1, the PWL increased significantly from 5.12 s to 11.86 s, meanwhile, the paw withdraw threshold increased from 3.83 g to 4.41 g. This effect continued for at least seven days after CCI.
Figure 1.
Intrathecal (i.t.) pre-treatment with BmK AGAP inhibited CCI-induced neuropathic pain. (a) Schematic illustration of pre-treatment experimental protocol. (b) Pre-treatment with BmK AGAP (5 µg) prevented CCI-induced thermal hyperalgesia in the PWL test and (c) mechanical allodynia in the PWT test. *P < 0.05, **P < 0.01, compared with Sal + Sham group; #P < 0.05, ##P < 0.01, compared with Sal + CCI group, n = 8 mice in each group. CCI: chronic constrictive injury; PWL: paw-withdrawal latency; PWT: paw-withdrawal threshold.
Pre-injury intrathecal injection of BmK AGAP reduced the expression of p-MAPKs and spinal Fos induced by CCI
Activated MAPKs in both neurons and spinal glial cells are involved in the mediating spinal nociceptive information.13,21–24 Previous studies have shown that BmK AGAP down-regulated the expression of spinal p-MAPKs both in vitro and in vivo.14,15 Examination of the expression of Fos protein could also be useful in determining the effectiveness of different analgesic regimens.25,26 As such, we wondered if BmK AGAP could modulate CCI-associated increased expression of p-MAPKs and spinal Fos. On the fifth day after CCI (the peak point of pain-associated behavior), L4-L5 spinal vertebrae of some mice were extracted to examine the expression of Fos protein and p-MAPKs (see experimental protocol illustrated in Figure 2(a)). From the results, we can see that CCI induced 2.3-fold increase in spinal Fos protein expression compared with Sham hand paw (Figure 2(b)). The expression of p-ERK, p-JNK, and p-p38 were also increased after CCI (Figure 2(c)). As expected, injection of BmK AGAP (5 µg) significantly attenuated the CCI-induced increased expression of spinal Fos protein (from 349.8 to 145) (Figure 2(b)) as well as the p-MAPKs (from 1.76 to 1.17 for p-ERK, 1.99 to 1.49 for p-JNK, and 1.34 to 0.85 for p-p38) (Figure 2(c)).
Figure 2.
Intrathecal (i.t.) pre-treatment with BmK AGAP (5 µg) inhibited CCI-induced spinal Fos protein expression, accompanied by decreased expression of spinal p-MAPKs. (a) Schematic illustration of the experimental protocol. (b) The representative immunohistochemical staining and quantitative data demonstrating decreased CCI-induced spinal Fos expression by pre-treatment with BmK AGAP. **P < 0.01 compared with saline (Sal)+CCI group; n = 4 mice in each group; scale bar = 200 µm. (c) The representative bands and the quantitative data for demonstrating modulation of spinal p38, p-ERK, and p-JNK expression induced by CCI after pre-treatment with BmK AGAP. The fold-change in the density of each p-MAPK band was normalized to the total MAPK expression for each sample. The fold-change in p-MAPK levels in the Sal + Sham group was set at 1 for quantification. **P < 0.01 or *P < 0.05 compared with Sal + CCI group, ##P < 0.01 compared with Sal + Sham group, n = 4 mice in each group. CCI: chronic constrictive injury; p-MAPK: Phosphorylated mitogen-activated protein kinase; p-ERK: phosphorylated extracellular signal–regulated protein kinase; p-JNK: phosphorylated Jun N-terminal kinase.
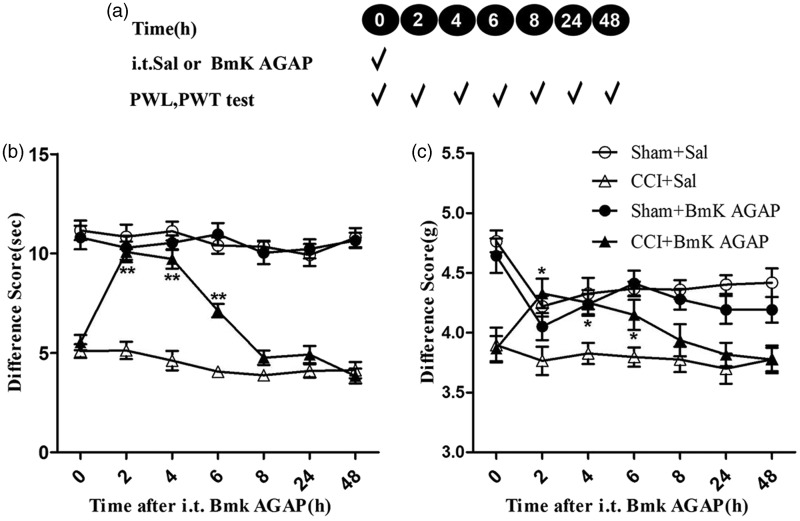
Intrathecal injection of BmK AGAP reversed CCI-induced hyperalgesia
The results of the experiments described above suggested that pre-treatment with BmK AGAP prevents CCI-induced thermal hyperalgesia and mechanical allodynia. We therefore wondered whether BmK AGAP treatment could reverse hyperalgesia induced by CCI. To address this question, mice were given an intrathecal injection of a single dose of BmK AGAP (5 µg) on the fifth day after CCI (see experimental protocol illustrated in Figure 3(a)). This treatment reversed the already established thermal hyperalgesia and mechanical allodynia. The PWL and PWT behavior of the mice was tested 2 h after intrathecal injection of BmK AGAP. We found that compared with the CCI + Sal group, animals in the CCI + BmK AGAP group exhibited significant increase in PWL (from 5.12 s to 10.8 s) and PWT (from 3.77 g to 4.33 g), and this effect lasted at least 6 h (Figure 3(b) and (c)).
Figure 3.
Intrathecal (i.t.) post-treatment with injection of BmK AGAP reversed CCI-induced neuropathic pain. (a) Schematic illustration of the experimental protocol. (b) Post-CCI i.t. injection of BmK AGAP (5 µg) reversed CCI-induced thermal hyperalgesia in the PWL test and (C) mechanical allodynia in the PWT test. *P < 0.05, **P < 0.01 compared with CCI + Sal group, n = 8 mice in each group. CCI: chronic constrictive injury; PWL: paw-withdrawal latency; PWT: paw-withdrawal threshold.
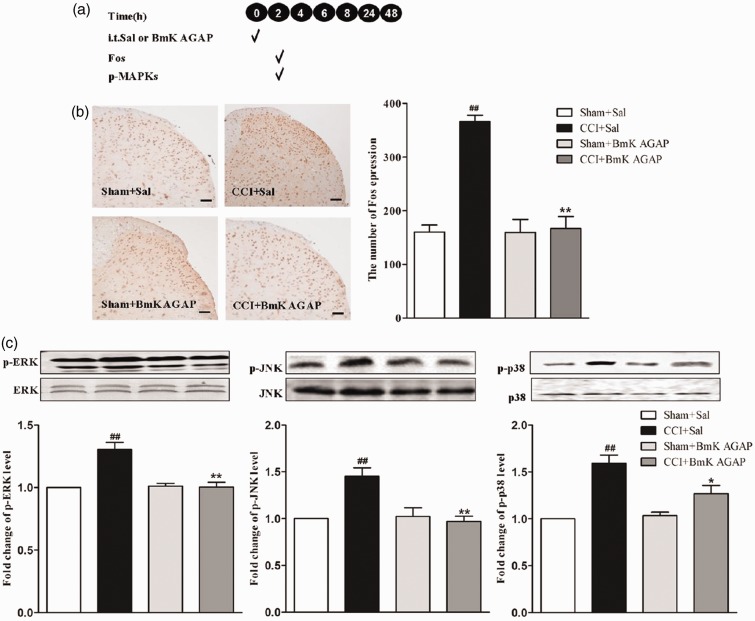
Intrathecal injection of BmK AGAP reversed CCI-induced expression of p-MAPKs and spinal Fos
BmK AGAP prevented CCI-induced pain by attenuating the CCI-induced increased expression of p-MAPKs and spinal Fos protein. The results of the behavior analyses suggest that BmK AGAP reverses hyperalgesia. We then examined whether BmK AGAP reverses the CCI-induced increases in p-MAPKs and spinal Fos simultaneously. We examined the expression (see experimental protocol illustrated in Figure 4(a)) of spinal Fos protein and assayed the phosphorylation of three MAPKs for each treatment in mice spinal cord at 2 h after injection of BmK AGAP. Compared with the untreated controls, the expression of Fos protein decreased markedly from 367 to 167 (Figure 4(b)), and the p-MAPKs also decreased from 1.45 to 0.97 for p-ERK, 1.99 to 1 for p-JNK, and 1.59 to 1.27 for p-p38 (Figure 4(c)) in the BmK AGAP-treated group.
Figure 4.
Intrathecal (i.t.) post-treatment with BmK AGAP (5 µg) reversed CCI-induced spinal Fos protein expression, accompanied by decreased expression of spinal p-MAPKs. (a) Schematic illustration of the experimental protocol. (b) The representative immunohistochemical staining and quantitative data demonstrating decreased CCI-induced spinal Fos expression by post-treatment with BmK AGAP. **P < 0.01 compared with saline (Sal)+CCI group; n = 4 mice in each group; scale bar = 200 µm. (c) The representative bands and the quantitative data for demonstrating modulation of spinal p-p38, p-ERK, and p-JNK expression induced by CCI after post-treatment with BmK AGAP. The fold-change in the density of each p-MAPKs band was normalized to the total MAPK expression for each sample. The fold-change in p-MAPKs levels in the Sham + Sal group was set at 1 for quantification. **P < 0.01 or *P < 0.05 compared with CCI + Sal group, ##P < 0.01 compared with Sham + Sal group, n = 4 mice in each group. CCI: chronic constrictive injury; p-MAPK: Phosphorylated mitogen-activated protein kinase; p-ERK: phosphorylated extracellular signal–regulated protein kinase; p-JNK: phosphorylated Jun N-terminal kinase.
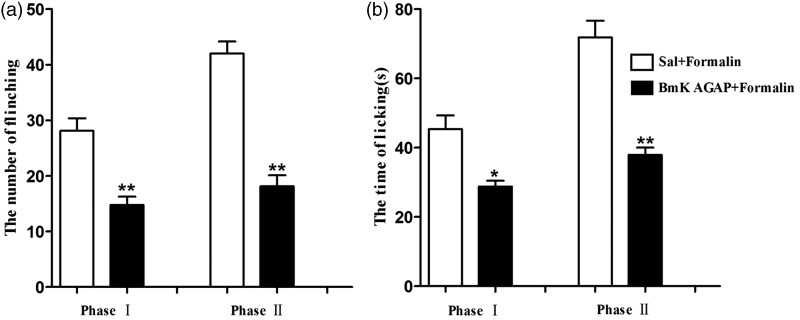
Pre-injury intrathecal injection of BmK AGAP prevents pain associated with formalin-induced inflammation
Formalin injection in mice can produce two phases of nociceptive behavior. The first phase occurs at 0–5 min and the second at 15–30 min after injection. We investigated whether pre-treatment with BmK AGAP could prevent pain associated with formalin-induced inflammation. We observed a similar anti-nociceptive effect compared with CCI model when BmK AGAP was injected in the formalin model. Pre-treatment with an intrathecal injection of BmK AGAP (5 µg) at 30 min before formalin injection significantly reduced the number of flinching responses both at phase I (from 47.5 to 20) and phase II (from 35 to 7.38) (Figure 5(a)). The total time of licking the hind paw also decreased, from 28.1s to 14.8s at phase I and from 42s to 18s at phase II (Figure 5(b)).
Figure 5.
Intrathecal pre-treatment with BmK AGAP inhibited pain associated with formalin-induced inflammation. (a) Pre-treatment with BmK AGAP (5 µg) decreased the number of paw elevations and (b) the time of licking/biting the paws resulting from formalin injection. *P < 0.05 or **P < 0.01 compared with Sal + Formalin group, n = 8 mice in each group.
BmK AGAP down-regulated spinal p-MAPK expression in the formalin model
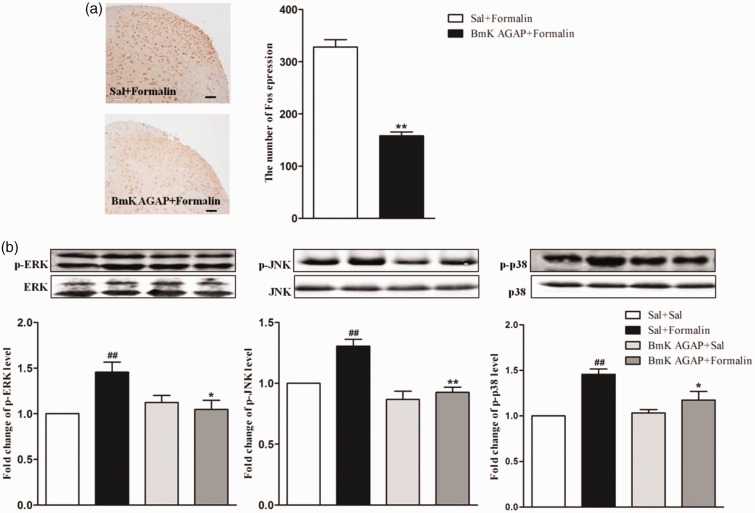
Some studies have shown that the activation of spinal MAPKs mediates inflammatory or injury-induced spinal sensitization and hyperalgesia.27,28 To assess the further effects of BmK AGAP on formalin-induced inflammation, mice were injected intrathecally with BmK AGAP (5 µg) at 30 min before formalin injection. Consistent with behavior, the expression of spinal Fos protein was markedly decreased compared with the untreated control, from 328 to 158 (Figure 6(a)). The expression of p-MAPKs was also inhibition by pre-treatment of BmK AGAP. Specifically, the expression decreased from 1.46 to 1.05 for p-ERK, 1.31 to 0.93 for p-JNK, and 1.46 to 1.18 for p-p38 (Figure 6(b)).
Figure 6.
Intrathecal pre-treatment with BmK AGAP inhibited formalin-induced spinal Fos protein expression, accompanied by decreased expression of spinal phosphorylated mitogen-activated protein kinases (p-MAPKs). (a) The representative immunohistochemical staining and quantitative data demonstrating decreased formalin-induced spinal Fos expression following pre-treatment with BmK AGAP (5 µg) in mice. **P < 0.01 compared with Sal + Formalin group; n = 6 mice in each group; scale bar = 200 µm. (b) The representative bands and the quantitative data for demonstrating modulation of spinal p-p38, p-ERK, and p-JNK expression following injection of formalin after pre-treatment with BmK AGAP (5 µg). The fold-change in the density of each p-MAPKs band was normalized to the total MAPK expression for each sample. The fold-change in p-MAPKs levels in the Sal + Sal group was set at 1 for quantification. **P < 0.01, *P < 0.05 compared with Sal + Formalin group, ##P < 0.01 compared with Sal + Sal group, n = 4 mice in each group. p-ERK: phosphorylated extracellular signal–regulated protein kinase; p-JNK: phosphorylated Jun N-terminal kinase.
BmK AGAP potentiated the effects of MAPK inhibitors on inflammation-associated pain
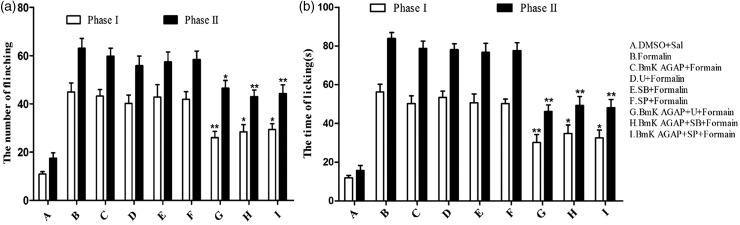
Our results indicated that pre-treatment of mice with BmK AGAP could prevent inflammation-associated pain and this effect is associated with the inhibition of p-MAPKs expression. Theoretically, BmK AGAP might potentiate the effect of MAPK inhibitors with respect to pain-associated behavior. We found that pre-treatment with low dose (0.1 µg dissolved in 5 µl 1% dimethyl sulfoxide for each inhibitor) of p38 MAPK inhibitor SB203580 (SB), MEK inhibitor U0126 (U), or JNK MAPK inhibitor SP600125 (SP) at 10 min before formalin injection had no effect on pain-associated behavior. Just as the MAPK inhibitor groups, there was no significant analgesic effect detected in the group of BmK AGAP (0.2 µg) as well, which was injected at 30 min before formalin injection. Nevertheless, as expected, treatment with BmK AGAP (0.2 µg) 20 min before the injection of inhibitors significantly potentiates the analgesic effect of all the three MAPKs inhibitors. Compared with formalin group, the number of paw elevations was decreased from 44.9 to 26, 28.4, and 29.4 at phase I and from 63 to 46.5, 43, and 44.3 at phase II in BmK AGAP +U+ Formalin group, BmK AGAP +SB+ Formalin group, and BmK AGAP +SP+ Formalin group, respectively (Figure 7(a)). Meanwhile, the total time of paw licking was also reduced from 56.3 s to 30.1 s, 34.8 s, and 32.6 s at phase I and from 83.8 s to 46.1 s, 49.3 s, and 48.1s at phase II in the same co-injection treatment groups, respectively (Figure 7(b)).
Figure 7.
Pre-treatment with low-dose BmK AGAP potentiated the effects of mitogen-activated protein kinase (MAPK) inhibitors on inflammation-associated pain. MAPK inhibitors (U0126, SP600125, or SB203580; 0.1 µg for each inhibitor) were injected at 10 min before formalin injection. BmK AGAP (0.2 µg) was injected at 20 min before the inhibitors. Spontaneous pain was recorded within 0–5 min (phase I) and 15–30 min (phase II) after formalin injection. Combining the inhibitors with BmK AGAP decreased (a) the number of paw elevations and (b) the time spent licking/biting the paws. *P < 0.05, **P < 0.01 compared with Formalin group, n = 8 mice in each group.
Discussion
This study demonstrated that the effect of BmK AGAP on spinal nociceptive processing is mediated via a MAPK-dependent mechanism. The principal findings of our study are as follows: (i) intrathecal injection of BmK AGAP both prevents and reverses CCI-induced thermal hyperalgesia and mechanical allodynia; (ii) CCI-induced increased expression of p-MAPKs and spinal Fos were also prevented or reversed by treatment with BmK AGAP; (iii) BmK AGAP alleviates pain associated with formalin-induced inflammation and modulates formalin-associated increased expression of p-MAPK and spinal Fos; and (iv) BmK AGAP potentiates the effects of MAPK inhibitors on neuropathic and inflammation-associated pain. These findings demonstrate a novel molecular basis of BmK AGAP modulation of spinal nociception.
Central sensitization, characterized by increases in the excitability of neurons and enhancement of responses to nociceptive or/and non-nociceptive stimuli, plays a critical role in the pathogenesis of chronic pain. The induction and maintenance of central sensitization are dependent on maladaptive alterations in the expression, distribution, and activity of ion channels, receptors, and intracellular signal transduction pathways. Central sensitization is also one of the primary causes of behavior hyperalgesia under pathologic conditions and has thus been investigated intensively.29,30 In the present study, CCI produced significant thermal hyperalgesia and mechanical allodynia in mice. A previous study showed that native BmK AGAP and 12 mutants exhibit effective analgesic activity.31 The present study found that pre-injury intrathecal injection of BmK AGAP prevents the induction of thermal hyperalgesia and mechanical allodynia, and this effect lasts at least seven days after CCI. In addition, intrathecal injection of a single dose of BmK AGAP on fifth day after CCI was shown to reverse established thermal hyperalgesia and mechanical allodynia, and the effect lasting up to 8 h. These results indicate that intrathecal administration of BmK AGAP affects both the development and maintenance of neuropathic pain.
Intraplantar injection of formalin is known to induce biphasic spontaneous nociceptive responses in mice. In general, the first-phase response results from formalin-induced high levels of activity in the primary afferents, whereas the second phase is considered a tonic response resulting from inflammation-associated factors.18,32–34 A previous study demonstrated that BmK IT2, a neurotoxic polypeptide, suppresses formalin-induced biphasic nociceptive responses.35 Our previous study demonstrated that intraplantar injection of formalin prior to BmK AGAP prevents or suppress formalin induced inflammation pain in a dose-dependent manner.15 The present data show that intrathecal injection of BmK AGAP prior to formalin injection can also prevent pain resulting from formalin-induced inflammation.
The MAPK family includes ERK1/2, p38, and JNK. These kinases transduce a broad range of extracellular stimuli into diverse intracellular responses by inducing changes in the expression of genes encoding both pro- and anti-nociceptive factors. Activation of intracellular kinase cascades and intranuclear gene expression plays a key role in the development and maintenance of central sensitization. Spinal ERK signaling is activated via phosphorylation, and phosphorylated ERK is considered a marker of pain behavior-associated neuronal sensitization.36 Our previous data also indicated that BmK AGAP prevents inflammation-associated pain accompanied by decreased expression of spinal MAPKs. This finding suggests that MAPK signaling is a good target for analgesia effectors.
The results of the present study suggest that MAPKs signaling mediates the role of BmK AGAP in preventing neuropathic and inflammation-associated pain. We found that CCI induces hyperalgesia and mechanical allodynia accompanied by increased expression of spinal p-MAPKs. Treatment with BmK AGAP prevents and reverses CCI-induced pain behavior and modulates the increased expression of spinal p-MAPKs. Formalin-induced pain and increased expression of spinal p-MAPKs were also inhibited by intrathecal injection of BmK AGAP in the present study. Furthermore, BmK AGAP potentiated the effects of MAPK inhibitors in modulating inflammation-associated pain. These findings indicate that the MAPK pathway mediates the activity of BmK AGAP in anti-nociceptive responses.
Monitoring the expression of Fos protein as a marker of neuronal activation in the central nervous system has been widely used in functional mapping of neuronal circuits in response to various defined stimuli. Moreover, Fos protein expression and the degree of sensitization induced by nociceptive stimuli in spinal cord neurons are positively correlated. In the CCI model used in the present study, we found that pre- and post-injury injection of BmK AGAP prevented and reversed spinal Fos protein expression, respectively. BmK AGAP was also found to suppress formalin-induced Fos expression. The decreased expression of spinal Fos protein as a result of intrathecal injection of BmK AGAP further confirms the anti-nociceptive role of BmK AGAP.
Conclusions
In conclusion, the results of this study indicate that activation of the MAPK signaling pathway in the dorsal spinal cord plays a role in mediating nociceptive behaviors induced by CCI and formalin. Intrathecal injection of the Chinese scorpion venom polypeptide BmK AGAP in mice inhibits the resulting neuropathic and inflammation-associated pain via a MAPK-mediated mechanism. Taken together, our results suggest that intrathecal injection of BmK AGAP could be used for pain relief.
Author Contributions
J-PR, J-LC, and PC designed the research. Q-HM, W-GL, X-TC, Q-L, and H-JY performed the animal experiments. Q-HM, W-GL, J-PR, J-C, Q-F and PC analyzed the data. J-PR, J-C and PC wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81622048, 81302694, 81473377, and 81102834), the project of Quality guarantee system of Chinese herbal medicines (201507002), the Fundamental Research Fund for Central Public Welfare Research Institutes (ZZ08080013), Foundation of Jiangsu Province Institute of Traditional Chinese Medicine (JSBN1302), and grants from Science Foundation for Distinguished Young Scholars of Jiangsu Province (BK20140049).
References
- 1.Ling CQ, Yue XQ, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med 2014; 12: 331–335. [DOI] [PubMed] [Google Scholar]
- 2.Shao J, Zhang R, Ge X.Yang B, Zhang J. Analgesic peptides in buthus martensii karsch: a traditional Chinese animal medicine the analgesic peptides in scorpion venoms. Asian J Tradit Med 2007; 2: 45–50. [Google Scholar]
- 3.Cao P, Yu J, Lu W.Cai X, Wang Z, Gu Z, Zhang J, Ye T, Wang M. Expression and purification of an antitumor-analgesic peptide from the venom of Mesobuthus martensii Karsch by small ubiquitin-related modifier fusion in Escherichia coli. Biotechnol Prog 2010; 26: 1240–1244. [DOI] [PubMed] [Google Scholar]
- 4.Liu YF, Ma RL, Wang SL.Duan ZY, Zhang JH, Wu LJ, Wu CF. Expression of an antitumor-analgesic peptide from the venom of Chinese scorpion Buthus martensii karsch in Escherichia coli. Protein Expr Purif 2003; 27: 253–258. [DOI] [PubMed] [Google Scholar]
- 5.Devor M. The pathophysiology of damaged peripheral nerves In:Wall PD andMelzack R (eds) Textbook of pain. London: Churchill Livingstone, 1994, pp. 79–100. [Google Scholar]
- 6.Woolf CJ. Central sensitizationuncovering the relation between pain and plasticity. Anesthesiology 2007; 106: 864–867. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol 2001; 429: 23–37. [DOI] [PubMed] [Google Scholar]
- 8.Ji R-R, Baba H, Brenner GJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 1999; 2: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 9.Jin SX, Zhuang ZY, Woolf CJ.Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci 2003; 23: 4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawasaki Y, Kohno T, Zhuang ZY.Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci 2004; 24: 8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obata K andNoguchi K.. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci 2004; 74: 2643–2653. [DOI] [PubMed] [Google Scholar]
- 12.Song XS, Cao JL, Xu YB.He JH, Zhang LC, Zeng YM. Activation of ERK/CREB pathway in spinal cord contributes to chronic constrictive injury-induced neuropathic pain in rats. Acta Pharmacol Sin 2005; 26: 789–798. [DOI] [PubMed] [Google Scholar]
- 13.Ji RR, Gereau RW, Malcangio M.Strichartz GR. MAP kinase and pain. Brain Res Rev 2009; 60: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Cai X, Ye T.Huo J, Liu C, Zhang S, Cao P. Analgesic-antitumor peptide inhibits proliferation and migration of SHG-44 human malignant glioma cells. J Cell Biochem 2011; 112: 2424–2434. [DOI] [PubMed] [Google Scholar]
- 15.Mao Q, Ruan J, Cai X.Lu W, Ye J, Yang J, Yang Y, Sun X, Cao J, Cao P. Antinociceptive effects of analgesic-antitumor peptide (AGAP), a neurotoxin from the scorpion Buthus martensii Karsch, on formalin-induced inflammatory pain through a mitogen-activated protein kinases–dependent mechanism in mice. Plos One 2013; 8: e78239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hylden JLK andWilcox GL.. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 1980; 67: 313. [DOI] [PubMed] [Google Scholar]
- 17.Bennett GJ andXie YK.. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33: 87–107. [DOI] [PubMed] [Google Scholar]
- 18.Hunskaar S andHole K.. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 1987; 30: 103–114. [DOI] [PubMed] [Google Scholar]
- 19.Hargreaves K, Dubner R, Brown F.Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 20.Chaplan SR, Bach F, Pogrel J.Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 21.Daulhac L, Mallet C, Courteix C.Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol Pharmacol 2006; 70: 1246–1254. [DOI] [PubMed] [Google Scholar]
- 22.Katsura H, Obata K, Miyoshi K.Kondo T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Sakagami M, Noguchi K. Transforming growth factor-activated kinase 1 induced in spinal astrocytes contributes to mechanical hypersensitivity after nerve injury. Glia 2008; 56: 723–733. [DOI] [PubMed] [Google Scholar]
- 23.Yamanaka H, Obata K, Kobayashi K.Dai Y, Fukuoka T, Noguchi K. Activation of fibroblast growth factor receptor by axotomy, through downstream p38 in dorsal root ganglion, contributes to neuropathic pain. Neuroscience 2007; 150: 202–211. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang Z.Z-Y, Gerner P, Woolf CJ.Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005; 114: 149–159. [DOI] [PubMed] [Google Scholar]
- 25.Coggeshall RE. Fos, nociception and the dorsal horn. Prog Neurobiol 2005; 77: 299. [DOI] [PubMed] [Google Scholar]
- 26.Munglani R Fleming BG andHunt SP.. Remembrance of times past: the significance of c-fos in pain. Br J Anaesth 1996; 76: 1–4. [DOI] [PubMed] [Google Scholar]
- 27.Dai Y, Iwata K, Fukuoka T.Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci 2002; 22: 7737–7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweitzer SM, Peters MC, Ma JY.Kerr I, Mangadu R, Chakravarty S, Dugar S, Medicherla S, Protter AA, Yeomans DC. Peripheral and central p38 MAPK mediates capsaicin-induced hyperalgesia. Pain 2004; 111: 278–285. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda H, Heinke B, Ruscheweyh R.Sandkühler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 2003; 299: 1237–1240. [DOI] [PubMed] [Google Scholar]
- 30.Melzack R, Coderre TJ, Katz J.Vaccarino AL. Central neuroplasticity and pathological pain. Ann N Y Acad Sci 2001; 933: 157. [DOI] [PubMed] [Google Scholar]
- 31.Ma R, Cui Y, Zhou Y.Bao Y, Yang W, Liu Y, Wu C, Zhang J. Location of the analgesic domain in Scorpion toxin BmK AGAP by mutagenesis of disulfide bridges. Biochem Biophys Res Commun 2010; 394: 330–334. [DOI] [PubMed] [Google Scholar]
- 32.Shibata M, Ohkubo T, Takahashi H.Inoki R. Modified formalin test: characteristic biphasic pain response. Pain 1989; 38: 347–352. [DOI] [PubMed] [Google Scholar]
- 33.Taylor BK Peterson MA andBasbaum AI.. Persistent cardiovascular and behavioral nociceptive responses to subcutaneous formalin require peripheral nerve input. J Neurosci 1995; 15: 7575–7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tjolsen A, Berge OG, Hunskaar S.Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain 1992; 51: 5–17. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Pang XY, Jiang F.Bai ZT, Ji YH. Anti-nociceptive effects induced by intrathecal injection of BmK AS, a polypeptide from the venom of Chinese-scorpion Buthus martensi Karsch, in rat formalin test. J Ethnopharmacol 2008; 117: 332–338. [DOI] [PubMed] [Google Scholar]
- 36.Gao YJ andJi RR.. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? TOPAINJ 2009; 2: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]