Abstract
Around 19% of U.S. adults smoke cigarettes, and smoking remains the leading avoidable cause of death in this country. Without treatment only ~5% of smokers who try to quit achieve long-term abstinence, but evidence-based cessation treatment increases this figure to 10-30%. The process of smoking cessation comprises different pragmatically defined phases, and these can help guide smoking treatment development and evaluation. This review evaluates the effectiveness of smoking interventions for smokers who are unwilling to make a quit attempt (Motivation Phase), who are willing to make a quit attempt (Cessation Phase), who have recently quit (Maintenance Phase), and who have recently relapsed (Relapse Recovery Phase). Multiple effective treatments exist for some phases (Cessation), but not others (Relapse Recovery). A chronic care approach to treating smoking requires effective interventions for every phase, especially interventions that exert complementary effects both within and across phases, and that can be disseminated broadly and cost-effectively.
Keywords: Tobacco dependence, cigarettes, addiction, relapse
INTRODUCTION
The percentage of adults in the United States who smoke has decreased markedly from nearly 42% in the mid-1960s to around 19% currently (National Center for Health Statistics 2012). However, tobacco dependence remains the leading avoidable cause of death in the U.S., causing close to 20% of all deaths (Danaei et al 2009). Worldwide, tobacco causes nearly 6 million deaths a year and is a major threat to global public health (World Health Organization 2012).
Most smokers want to quit smoking, but only around 10-30% plan to quit in the next 30 days (Carpenter et al 2004, Etter et al 1997, Piper et al in press). When smokers do make a quit attempt, they tend not to use evidence-based treatments, and without treatment only around 5% achieve long-term abstinence (Hughes et al 2004). Even with evidence-based treatment (consisting of counseling and/or medication), only around 10-30% achieve long-term abstinence (Fiore et al 2008). Because of the unique health threat posed by smoking, the development and dissemination of more effective smoking prevention and cessation interventions would have a profound impact on public health.
Many researchers now view tobacco dependence as a chronic disease (Fiore et al 2008). Most smokers need to make repeated quit attempts before quitting permanently, and this realization has led to chronic care models of smoking intervention (e.g., Joseph et al 2011). Such models build in contingencies for patients who relapse and offer appropriate ongoing care to smokers regardless of their willingness to quit or where they are in the quitting process (e.g., recently quit or relapsed).
In this review, we use the Phase-Based Model of Tobacco Use (Baker et al 2011) to guide the evaluation of smoking interventions. This model depicts pragmatically defined phases in the natural history of tobacco dependence that present different challenges and opportunities for intervention (see Figure 1). In theory, these features can guide the development and evaluation of smoking interventions (Baker et al 2011). In some ways, this approach resembles the transtheoretical,“stages of change” model (Prochaska & DiClemente 1983), but that model is based on a specific meta-theory of how people must progress through stages of many types of behavior change. In contrast, the Phase-Based approach is specific to smoking and is simply a framework that focuses the application of any relevant theory (and the testing and analysis of interventions) to particular pragmatically defined phases of cessation. In this review, we will concentrate on cigarette smoking (and not other forms of tobacco use), and we will focus on interventions delivered in clinical, small-group, or individual contexts, rather than on population-based public health interventions (e.g., mass media campaigns, tax increases, and graphic warning labels on cigarettes).
Figure 1.
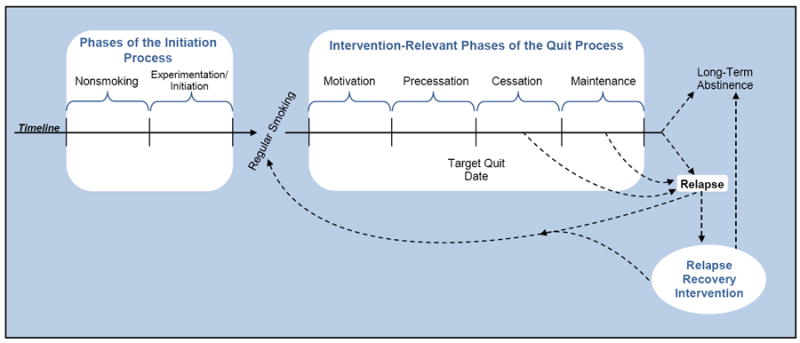
The Longitudinal Phase-Based Model of Tobacco Use Initiation and Cessation
MOTIVATIONAL INTERVENTIONS: MOTIVATING SMOKERS WHO ARE UNWILLING TO QUIT
The Nature of the Interventions and the Relevant Population
Smokers who are not ready to make a quit attempt are in the Motivation Phase (see Figure 1). Perhaps 70-90% of smokers are unwilling to make a quit attempt at a given time (Carpenter et al 2004, Piper et al in press). Such smokers are often reluctant to try to quit because they fear they will fail and because they worry about experiencing negative consequences from quitting such as severe craving for cigarettes, negative affect, and/or weight gain (Fiore et al 2008, Orleans et al 1991). As many as half or more of smokers currently unwilling to make a quit attempt may, however, be willing to engage in an intervention that has a noncessation goal (Carpenter et al 2004). Thus, there is a potentially huge population that might use motivational interventions. Treatments for this phase are evaluated on their ability to reduce smoking heaviness (cigarettes smoked/day) and increase the likelihood of future quit attempts and cessation.
There is evidence that population-based interventions (such as tax increases and warning labels) can increase quit attempts and quitting amongst smokers previously unwilling to quit (Cummings et al 2009). However, our review will focus on clinical interventions for these smokers.
Opportunities and Challenges
One opportunity in the Motivation Phase is that there is considerable time for smokers to learn and practice skills before undergoing cessation related withdrawal. One challenge, though, is convincing patients to participate in treatment when they are not interested in quitting. However, it can be done; in one study, among smokers who refused cessation treatment but expressed an interest in treatment to reduce their smoking, 48.2% attended an initial treatment visit (Piper et al in press).
Two other challenges are relevant to the Motivation Phase and to the other phases discussed below. These challenges involve the demographic groups to which smokers often belong. The first challenge is that smokers are disproportionally likely to have low socioeconomic status (SES) and/or educational attainment. For example, in 2010 in the U.S., 28.9% of adults living below the poverty level smoked versus 18.3% of adults living at or above the poverty level (Centers for Disease Control and Prevention 2011). Low SES is often, of course, associated with such limitations as having poor medical coverage and care, being unable to afford over-the-counter smoking medication, and having a lack of transportation to treatment. Even when treatment is provided at no cost, there is evidence smokers with low educational attainment (less than high school) are less likely to succeed in smoking treatment than are those with more education (Piper et al 2010a).
The second challenge is that people with psychiatric disorders—and particularly people with serious mental illnesses such as bipolar disorder—are more likely to smoke than people without such disorders. For example, in the U.S. in the early 1990s, 41.0% of people with a current psychiatric disorder smoked and 60.6% of people with current bipolar disorder smoked, but only 22.5% of people who had never had a psychiatric disorder smoked (Lasser et al 2000). This study also estimated that 40.6% of smokers had a current psychiatric disorder. There is evidence that psychiatric comorbidity can hamper the ability to quit smoking, but the strength of the relation between quitting likelihood and disorder varies with type of disorder and recency of symptoms. For instance, psychosis appears to be more strongly associated with decreased quitting likelihood than depression and substance use disorder are (Lasser et al 2000, Piper et al 2010b), but even the latter disorders predict continued smoking if the disorders were recent or are on-going (Fiore et al 2008, Lasser et al 2000). Such relations may be due to smokers with psychiatric diagnoses making fewer quit attempts and/or being less successful in their attempts. Nevertheless, smokers with psychiatric disorders can successfully quit, and evidence-based smoking treatments are effective in such populations (Fiore et al 2008).
Psychosocial and Smoking Medication Motivation Phase Interventions
Motivational interviewing
Motivational interviewing is a collaborative counseling strategy in which the counselor offers patients empathy, and encourages and supports increased motivation for behavior change while respecting patients’ autonomy. Counselors try to increase motivation through nonconfrontational inquiry and feedback that contrasts patients’ current behavior (e.g., smoking) with their valued personal goals (e.g., saving money, being healthy, not harming the health of family members; Hettema et al 2005, Miller & Rollnick 2002). Data show that motivational interviewing increases smokers’ quitting motivation and quit attempts (Fiore et al 2008). Data also suggest that motivational interviewing modestly increases cessation success.
A recent Cochrane meta-analysis (Lai et al 2010) examined 14 studies comprising some 10,000 smokers, many of whom were seen in real-world clinics rather than in research settings. Only 2 of the studies required participants to be initially motivated to quit; the rest of the studies presumably included both smokers motivated and not motivated to quit. The studies most often compared motivational interviewing with brief advice to try to quit. The studies featured fairly brief motivational interviewing counseling, with individual sessions varying between 15 and 45 minutes; 10 of the 14 studies used only a single session of counseling and the maximum number of sessions was 4. Counseling was typically face-to-face (although some studies used phone counseling). Motivational interviewing produced significant, but modest, improvement in abstinence rates assessed 6 months or more after treatment: on average, 11.5 vs. 7.7% of smokers were abstinent in the motivational interviewing and control groups, respectively. Some evidence suggests it is difficult to train counselors in motivational interviewing (Hettema et al 2005), which raises questions about the dissemination potential of this strategy.
Smoking reduction and smoking medications
There is strong evidence that the combination of smoking reduction instruction plus smoking medication increases quitting success amongst smokers previously unmotivated to quit (but does not necessarily increase the number of quit attempts they make and quit rates remain low). In this treatment, smokers who are unwilling to quit are instructed to substantially and persistently reduce their daily smoking (“as much as possible”; Wennike et al 2003), while receiving nicotine replacement therapy (NRT, e.g., nicotine gum; Hughes & Carpenter 2005, Moore et al 2009, Wennike et al 2003). Since these interventions typically comprise both smoking medication and reduction counseling, this literature does not reveal the independent effects of each.
Meta-analyses by the Cochrane Collaboration (Stead & Lancaster 2007) and by Moore and colleagues (Moore et al 2009) compared the effects of NRT versus placebo medication in smokers not interested in quitting who received smoking reduction instruction. NRT produced greater smoking reduction and higher quit rates than did placebo (with effect sizes for abstinence about “2” for relative risk; Moore et al 2009), but absolute quit rates were low (e.g., the Moore meta-analysis found that 6.8% of smokers on NRT vs. 3.3% on placebo attained at least 6 months of sustained abstinence). These studies often involved moderate amounts of behavioral support in addition to medication, with support comprising instructions on using the NRT, how to reduce smoking, problem solving, and encouragement to try to quit smoking (e.g., Moore et al 2009). These studies do not reveal the impact of such support, however, as it was not experimentally manipulated. In the Moore meta-analysis, about one-fifth of smokers initially not interested in quitting were able to reduce their smoking by 50% or more with NRT + behavioral support (Moore et al 2009). Most of the evidence suggests that the combination of NRT + behavioral support does not, however, reliably increase the number of quit attempts participants make (although cf. Carpenter et al 2004).
Some data suggest that smoking reduction per se can boost the likelihood of future cessation. Hughes and Carpenter (Hughes & Carpenter 2006) reviewed nonexperimental, longitudinal studies that generally showed that spontaneous smoking reduction (e.g., by 50% or more) was associated with greater future likelihood of quitting. Glasgow et al. (Glasgow et al 2009) performed perhaps the only experimental study that compared a behavioral smoking reduction intervention with a usual care control. This intervention (which did not involve any smoking medication) yielded primarily nonsignificant effects at 3 and 12 month follow-ups on the percentage of participants reducing their cigarettes per day by at least 50%, but the trends clearly favored this low-contact behavioral intervention (comprising 4 phone calls and newsletters).
CESSATION (AND PRECESSATION) INTERVENTIONS: PREPARING FOR QUITTING, AND QUITTING
The Nature of the Interventions and the Relevant Population
The smoker who is ready and willing to engage in a quit attempt is in the Cessation Phase. The goal of this phase is to help smokers achieve initial cessation and remain abstinent during the first weeks following their target quit day (when withdrawal symptoms are typically most intense; Hughes 2007). This early post-quit period is highly important because most people who ultimately relapse completely (return to regular smoking), smoke one or more cigarettes in the first 2 weeks of their quit attempt (Kenford et al 1994). Smokers in this phase are sometimes given treatments to prepare them for cessation; i.e., precessation treatments that precede the actual quit day. This section evaluates such Precessation Phase interventions in addition to Cessation Phase interventions (i.e., interventions delivered primarily on and after the quit day).
The group of smokers ready to make an immediate quit attempt is notable in several ways. First, this group is relatively small—at any point in time only about 10-30% of smokers are ready to commit to quitting within the next 30 days (Carpenter et al 2004, Piper et al in press). Thus, most smokers are not interested in treatments that stress cessation per se (Prochaska & DiClemente 1983). Second, smokers ready to quit tend to be especially aware of the risks and potential costs of smoking: relative to smokers in general, smokers who report making a recent quit attempt tend to be older (Pierce et al 1989), more concerned about the effects of smoking on their health (Hyland et al 2006), and more likely to have had a medical condition related to smoking (Davila et al 2009). Finally, interest in quitting changes rapidly over time (Hughes et al 2005)—therefore, frequently asking smokers if they would like assistance in quitting may be beneficial because smokers may accept an offer they had recently spurned.
Opportunities and Challenges
The clear opportunity offered by this phase is that the smoker is willing and motivated to make a quit attempt and is often willing to engage in treatment. However, even with treatment, smokers often fail to achieve even short-term abstinence. The following challenges place a smoker at increased risk of cessation failure: (a) daily routines and contexts that expose the smoker to smoking and smoking cues (e.g., living with a smoker and permitting smoking in the house; Farkas et al 1999, Japuntich et al 2011a, Lee & Kahende 2007); (b) low SES, low educational status, or not being married or living with a partner (Japuntich et al 2011a, Lee & Kahende 2007, Piper et al 2010a); (c) severe tobacco dependence (Foulds et al 2006, Ip et al 2012); (d) a pattern of binge drinking prior to the quit attempt (Kahler et al 2009); and (e) certain personality traits and psychiatric disorders (e.g., neuroticism, anxiety disorders; Fiore et al 2008, Piper et al 2011). We are only beginning to understand the mechanisms that link such characteristics with cessation outcomes (Baker et al 2012).
In addition to such person factors, various transitory events and reactions to quitting can derail a quit attempt (leading to lapses or relapse), including (a) stressors or sharp spikes in distress (Shiffman 2005, Shiffman et al 1996a), (b) decreases in quitting-related self-efficacy (Gwaltney et al 2009), (c) exposure to smoking cues (Shiffman et al 1996b), and (d) severe withdrawal symptoms (Bolt et al 2012). Once smokers stop smoking during a quit attempt, they experience withdrawal symptoms that include urges or craving, hunger, restlessness, difficulty concentrating, and negative moods. These symptoms mount rapidly, within a few hours or less of stopping smoking (Hendricks et al 2006), and typically—but not always—last 2 to 4 weeks (Hughes 2007). The intensity of these symptoms (in particular craving) is an important determinant of the quit attempt’s success (Baker et al 2012, Bolt et al 2012).
Psychosocial Interventions
Counseling is the most frequently used psychosocial intervention for smoking and can range in length from a minute or two to over 10 hours. Coding the contents of counseling interventions based on descriptions in research articles typically reveals that counselors use diverse intervention components including problem solving/skills training, relaxation training, contingency management, providing motivational information, offering therapeutic support, and so on. The most frequently provided components—and the counseling components associated with superior abstinence outcomes (Fiore et al 1996)—are skills training and intratreatment support (Fiore et al 2008, Lancaster & Stead 2005). Skills training involves first teaching patients to identify high risk situations and emotional states in which they might be tempted to smoke and then teaching them to execute cognitive and behavioral coping strategies to mitigate the risk (Fiore et al 2008). For example, counselors may help patients change their routines (e.g., make their car smoke free) so as to decrease their exposure to smoking cues, learn the steps involved in problem solving, and identify pleasurable activities to use to distract themselves when cravings arise. Counselors may also offer psychoeducation including information on the nature and typical length of withdrawal and encouragement to remain fully abstinent because a lapse (even one puff)—while not necessarily fatal to the quit attempt—does increase the risk of relapse. Intratreatment support is assumed to involve the counselor offering help, encouragement, and empathy (Fiore et al 2008), but the specific features of intratreatment support, as it has been delivered in studies, are unclear (e.g., to what extent does it refer to stylistic factors vs. content?).
There is considerable evidence that counseling increases long-term cessation success. Recent analyses, however, raise questions about how effective counseling is and whether its effectiveness might be declining (Irvin & Brandon 2000), perhaps because smokers may be becoming harder to treat (as smoking rates have declined, remaining smokers may be particularly refractory; Hughes 2011, Warner & Burns 2003).
Precessation interventions
While many cessation treatments involve a small number of meetings prior to the actual quit day (when smokers attempt to quit completely), relatively little research has evaluated treatments that concentrate on the Precessation Phase and that are designed to prepare smokers to make a quit attempt. The Precessation Phase offers the opportunity for smokers to practice coping skills (e.g., problem solving), practice using smoking medication, and reduce their smoking prior to quitting. The quit day produces a very rapid escalation in disturbing and distracting withdrawal symptoms (some arising within 30 minutes of cessation; Hendricks et al 2006); precessation treatments provide instruction or practice before this onslaught.
While there is reason to believe precessation behavioral counseling could facilitate quit attempts, little research exists on the effects of such counseling when delivered independent of medications. Substantial research has addressed the value of precessation medication use (typically NRT, termed “preloading”), though. Early findings on preloading were positive, but a more recent meta-analysis of eight studies comprising over 2,800 smokers (Lindson & Aveyard 2011) showed only a very modest, non-significant, benefit of precessation NRT use lasting 2-5 weeks on short- and long-term abstinence. However, there was some evidence that even brief 2-5 week pretreatment could decrease smoking rates and tobacco dependence. These findings, and the benefit of medication use during the Motivation Phase, encourage further exploration of longer-term precesssation use of medications.
Brief advice
One common form of cessation counseling is brief advice to quit smoking (Fiore et al 2008). In research studies, a physician usually delivers the advice to quit in a clear, personalized message: e.g., “Quitting smoking is the most important thing you can do for your health, and it is important that you quit.” A Cochrane meta-analysis (Stead et al 2008) analyzed the effect of brief advice versus no advice or usual care across 17 trials, with most studies occurring in the primary care setting. This yielded a relative risk ratio (RR) of 1.66 (95% CI 1.42-1.94) with regard to abstinence determined at 6 months or longer, post-quit. However, this analysis had a generous definition of minimal advice (i.e., a single visit lasting under 20 minutes plus up to one follow-up visit), meaning that “advice” could have included considerable information and instruction. By contrast, a meta-analysis in the 2008 Public Health Service (PHS) Clinical Practice Guideline, Treating Tobacco Use and Dependence restricted advice to interventions lasting up to 5 minutes (Fiore et al 2008). This meta-analysis of 7 studies found advice had a significant, but comparatively smaller, impact on abstinence rates at 5 months (or longer) post-quit relative to no-advice, raising cessation rates from about 8 to 10%.
In sum, brief advice delivered by physicians appears to boost cessation rates. However, many of the studies on this topic were done over 15 years ago, and it is difficult to judge the current relevance of these early studies. In addition, the effects of advice are small in absolute terms; brief advice typically raises quit rates by only 1-3 percentage points (say, from a spontaneous quit rate of about 5% to 7%, for instance). While this increase is small, it nevertheless could be meaningful in terms of public health impact because about 70% of smokers see a physician each year (Tomar et al 1996). In 1992, only about 50% of adult smokers attending a health care visit in the past year received physician advice to quit; by 2009, this figure had climbed to about 65% (National Cancer Institute et al 2010).
Smoking cessation counseling
There is considerable evidence that smoking cessation counseling increases cessation rates over conditions involving very little or no counseling (Fiore et al 2008). However, vital questions remain about the magnitude of such effects, the active elements involved in counseling, and the relationship between intensity and outcome. Table 1 shows a 2008 PHS Guideline meta-analysis of 35 studies with 95 treatment conditions (i.e., study “arms”) with counseling intensity modeled in terms of total minutes of counseling contact time. The table shows a strong relation between contact time and abstinence at 6-months post-quit. However, in general, very high intensity counseling does not differ significantly from less intense counseling. Amongst conditions getting some counseling, intensity differences were found only between those getting 1-3 minutes of counseling versus more (i.e., the confidence intervals for 4-30 minutes, 31-90 minutes, 91-300 minutes, and > 300 minutes all overlap with one another).
Table 1.
Estimated effect sizes and abstinence rates at 5 months (or longer) after the quit day for total amount of counseling contact time.a
| Total amount of contact time | Number of arms | Estimated odds ratio (95% C.I.) | Estimated abstinence rate (95% C.I.) |
|---|---|---|---|
| No minutes | 16 | 1.0 | 11.0 |
| 1-3 minutes | 12 | 1.4 (1.1-1.8) | 14.4 (11.3-17.5) |
| 4-30 minutes | 20 | 1.9 (1.5-2.3) | 18.8 (15.6-22.0) |
| 31-90 minutes | 16 | 3.0 (2.3-3.8) | 26.5 (21.5-31.4) |
| 91-300 minutes | 16 | 3.2 (2.3-4.6) | 28.4 (21.3-35.5) |
| > 300 minutes | 15 | 2.8 (2.0-3.9) | 25.5 (19.2-31.7) |
Meta-analysis results from the PHS Clinical Practice Guideline, Treating Tobacco Use and Dependence. Thirty-five studies generated the estimates.
Another Cochrane meta-analysis (Lancaster & Stead 2005) evaluated the effects of individual counseling with a trained counselor versus minimal counseling. Twenty-two studies were analyzed. “Individual” counseling was defined as sessions of greater than 10 minutes (10 studies involved only a single session), and was typically supplemented with phone follow-up counseling. The control condition, minimal counseling, was defined as up to 10 minutes of counseling, possibly supplemented with self-help material. Results of this meta-analysis showed a relative risk (RR) of 1.39 (95% CIs 1.24-1.57). Thus, there was an effect of counseling, but an additional analysis showed that amongst the “individual” counseling conditions (in which sessions were at least 10 minutes) intensity was unrelated to benefit. Similarly, more intense physician advice to quit does not seem to boost success rates markedly (Aveyard et al 2012, Stead et al 2008).
Therefore, a great deal of research shows counseling does indeed increase long-term abstinence rates. However, it appears much of this effect arises from differences found when comparing very little counseling with moderate or fairly intense counseling; most evidence suggests that once smokers receive 30 minutes or so of counseling, further increases in intensity tend not to produce statistically reliable, commensurate, increases in long-term abstinence rates. Note, however, that evidence will be presented in the review of Maintenance Phase interventions suggesting that very prolonged counseling—with sessions extended for a year—may substantially increase abstinence rates.
Specific populations
Cessation counseling has been shown to be broadly effective with a wide-range of smoker populations, including pregnant smokers, adolescents, those low in SES, and those with psychiatric comorbidities (Fiore et al 2008). These findings are important because a large proportion of smokers are low in SES (Warner & Burns 2003), smokers are more likely than the general population to have psychiatric diagnoses, and there is evidence that smoking medications are not effective with adolescents (for a review see Fiore et al 2008).
Dissemination and quitlines
Cessation counseling has high dissemination potential. For instance, it can be delivered effectively by a wide range of clinicians (physicians, nurses, health counselors; Fiore et al 2008), which means that trained, low-cost providers can be used. Cessation counseling also does not require in-person delivery, permitting use of low-cost, disseminable delivery systems such as telephone quitlines. In 2004, the U.S. Department of Health and Human Services established a national smoking cessation quitline network linking state quitlines via a single portal— 1-800-QUIT-NOW. U.S. state quitlines offer residents (a small percentage have additional eligibility criteria) at least one session of free cessation counseling, and the majority (75% in 2010) also offer free smoking medication (North American Quitline Consortium 2011). Quitline counseling is both clinically effective and cost-effective (Lichtenstein et al 2010, McAfee 2007, Stead et al 2006). In 2010, over half a million U.S. smokers called a state telephone quitline for information or help quitting smoking (North American Quitline Consortium 2011). In fact, smokers are four times more likely to use a quitline than face-to-face cessation counseling (Kaufman et al 2010). Recently, fax-to-quit systems have been implemented that link primary care clinics with state quitlines. In participating clinics, clinic workers can fax state quitlines the contact information of interested smokers, and quitline counselors then proactively call these smokers and offer them services. Thus, recent progress in smoking counseling has not improved its effectiveness, but has made it more easily available.
Effective counseling components
Little is known about the therapeutic contents responsible for counseling efficacy. Cessation counseling interventions often comprise multiple intervention components, making it difficult to assess the effects of any particular one. However, cessation counseling typically comprises two major components—intratreatment support and behavioral skills training/problem solving (Fiore et al 2008, Lancaster & Stead 2005). In one PHS Clinical Practice Guideline meta-analysis, therapeutic content was coded as it was reported in 39 different studies and related to 5-months (or longer) abstinence. Out of multiple components of counseling (e.g., relaxation training, motivational interventions, and extratreatment social support), intratreatment support and skills training were most strongly related to positive outcomes (Fiore et al 1996). However, this evidence is merely suggestive; it is difficult to code type of counseling based on short descriptions by authors, and counseling often comprised multiple components making it difficult to isolate the effects of a particular one. Also, when researchers have directly contrasted skills-based treatments (e.g., “relapse prevention” treatments) with treatments that control for amount of attention or contact (“credible ritual” control treatments), results tend to show no differences in outcomes (e.g., see Carroll 1996). Perhaps the most defensible way to characterize this literature is to say that skills training and intratreatment support do appear to increase abstinence rates over no counseling or less intense counseling, but they are the most common counseling components and to some extent may really be proxies for counseling intensity.
Mechanisms of counseling effects
Very little is known about what mediates the effects of cessation counseling on abstinence outcomes. This issue is best approached via formal mediation analysis, but few studies exist in this area and the existing ones do not yield ready interpretation. There is evidence skills training does result in skill acquisition (Davis & Glaros 1986, Zelman et al 1992) and use of acquired skills in daily life (see McCarthy et al 2010). However, formal attempts to determine whether skill acquisition or real-world skill use mediates the relation between skills training and abstinence have been unrevealing. McCarthy and colleagues (McCarthy et al 2010) compared smokers receiving skills training (along with intratreatment support) with smokers receiving only brief support. The former showed greater levels of coping skill execution in their daily lives, as assessed with ecological momentary assessment (EMA). EMA ratings revealed that skills-based counseling prompted smokers to avoid cigarettes, improved their quitting self-efficacy, reduced how difficult they perceived quitting to be, and offered some protection from feeling demoralized and guilty if they lapsed. However, these effects tended to be small, and the study’s eight 10-minute counseling sessions produced no significant improvement in abstinence outcomes (end of treatment point-prevalence abstinence rates were 29.4% and 25.7% for those who did and did not receive counseling, respectively). Therefore, this study suggests that skills training leads to some skill acquisition and use, but it does not show that these effects mediate long-term abstinence.
There is even less evidence on cessation mechanisms that might be activated by other counseling components such as intratreatment support. Our limited understanding, no doubt, serves as a formidable obstacle to the development of more effective counseling interventions and to using counseling components more strategically.
Other psychosocial approaches to cessation
Skills training and intratreatment support are the most extensively used and studied psychosocial smoking cessation strategies. However, other types of psychosocial interventions have been developed and explored. These include mindfulness-based counseling approaches such as Acceptance and Commitment Therapy (Gifford et al 2011, Hernandez-Lopez et al 2009), affectively-focused cognitive behavioral therapy (Haas et al 2004), behavioral activation therapy (MacPherson et al 2010) and motivational interviewing (Hettema et al 2005). These approaches have several key virtues; e.g., they possess strong and clear theoretical rationales and have sufficiently well characterized procedures so they can be reliably implemented and distinguished from other counseling approaches. At present, though, there is too little research to draw firm conclusions about their effectiveness and dissemination potential. Therefore, additional research is needed to establish their efficacies (as sole treatments and as adjuvants), feasibility, patient acceptance, cost-effectiveness, and reach.
Behavioral (noncounseling) approaches
There are behavioral interventions for smoking cessation that do not rely primarily on counseling contents. These interventions include (a) aversive smoking strategies (e.g., rapid smoking which involves taking a puff from a cigarette every few seconds), (b) contingency management, and (c) exposure interventions (in which smokers are repeatedly exposed to smoking cues to extinguish the motivational impact of the cues). There is fairly strong evidence that some types of aversive smoking interventions (e.g., rapid smoking; Gifford & Shoenberger 2009) and contingency management (Heil et al 2008) can reliably enhance abstinence rates. However, the utilization and public health impact of each intervention has been limited by concerns about feasibility and ease of implementation. In addition, there are concerns that aversive smoking might be dangerous (Fiore et al 2008) and that contingency management effects may not be durable once contingencies are discontinued (Dunn et al 2011). While a strong rationale can be advanced for exposure therapy, current research does not permit strong inference regarding its effects (e.g., Perkins 2009).
Contingency management seems especially worthy of additional investigation because it has repeatedly produced substantial increases in abstinence outcomes (e.g., Heil et al 2008, Krishnan-Sarin et al 2006). Contingency management could play a more major role in tobacco cessation efforts and exert a public health impact if modifications could be developed that increase the persistence of its effects and increase its potential for wide scale implementation (e.g., engineering accurate and low-cost ways to determine smoking status and to deliver meaningful consequences rapidly).
Other cessation intervention approaches exist such as relaxation training, hypnosis, and engineering extratreatment social support for a smoker’s quit attempt. However, these have not received sufficient research analysis, or generated sufficiently promising effects, to encourage their clinical use (Fiore et al 2008).
Smoking Medications
There are 7 medications approved by the U.S. Food and Drug Administration (FDA) for smoking cessation: five nicotine replacement products (the nicotine patch, gum, inhaler, nasal spray, and lozenge), as well as bupropion and varenicline (see Table 2). A variety of other agents have shown efficacy for cessation but have not achieved widespread use in the U.S. and have not earned FDA approval as cessation aids: i.e., nortriptyline, clonidine, and cytisine. The current review will focus on the FDA approved medications because these are the most heavily used and enjoy the most research support.
Table 2.
Estimated effect sizes and abstinence rates at 5 months (or longer) after the quit day of FDA approved medications relative to placebo.a
| Medication | Number of arms | Estimated odds ratio (95% C.I.) | Estimated abstinence rate (95% C.I.) |
|---|---|---|---|
| Placebo | 80 | 1.0 | 13.8 |
| Single Agents | |||
| Varenicline (2 mg/day) | 5 | 3.1 (2.5-3.8) | 33.2 (28.9-37.8) |
| Nicotine Nasal Spray | 4 | 2.3 (1.7-3.0) | 26.7 (21.5-32.7) |
| Long-Term Nicotine Gum (>14 weeks) | 6 | 2.2 (1.5-3.2) | 26.1 (19.7-33.6) |
| Nicotine Inhaler | 6 | 2.1 (1.5-2.9) | 24.8 (19.1-31.6) |
| Bupropion SR | 26 | 2.0 (1.8-2.2) | 24.2 (22.2-26.4) |
| Nicotine Patch (6-14 weeks) | 32 | 1.9 (1.7-2.2) | 23.4 (21.3-25.8) |
| Nicotine Patch (>14 weeks) | 10 | 1.9 (1.7-2.3) | 23.7 (21.0-26.6) |
| Nicotine Gum (6-14 weeks) | 15 | 1.5 (1.2-1.7) | 19.0 (16.5-21.9) |
| Combination Therapies | |||
| Nicotine Patch (>14 weeks) + Nicotine Gum or Spray | 3 | 3.6 (2.5-5.2) | 36.5 (28.6-45.3) |
| Nicotine Patch + Bupropion SR | 3 | 2.5 (1.9-3.4) | 28.9 (23.5-35.1) |
| Nicotine Patch + Inhaler | 2 | 2.2 (1.3-3.6) | 25.8 (17.4-36.5) |
Meta-analysis results from the PHS Clinical Practice Guideline, Treating Tobacco Use and Dependence. Eighty-three studies generated the estimates in the entire analysis, which contained some agents not reported on here (e.g., nortriptyline, clonidine).
Table 2 displays the FDA approved smoking medications and evidence on their efficacies from a 2008 PHS Guideline meta-analysis comprising 83 studies. Table 2 also indicates the number of different treatment groups (i.e., study “arms”) across the studies that received the same smoking medication (e.g., patients received varenicline in 5 study arms). Study arms were included in the meta-analysis only where it was possible to eliminate confounds between different smoking medication conditions from the same study (e.g., different medications—including placebo medications—within the same study could not be paired with different intensities of counseling).
Table 2 reveals substantial consistency in the effects of the single agents; in general, relative to placebo, the single agents doubled the likelihood of point-prevalence abstinence at 5 or more months after the target quit day. Varenicline stood out amongst the single agents as it almost tripled a smoker’s likelihood of successful cessation. Individual trials comparing varenicline with other individual agents support the notion that varenicline produces higher long-term smoking abstinence rates (e.g., Jorenby et al 2006, Nides et al 2008). Table 2 also shows that compared to most single agents, combination medications tended to produce higher long-term abstinence rates. This effect was particularly pronounced for combination NRT; i.e., long-term nicotine patch (>14 weeks) plus either nicotine gum or nasal spray. A focused analysis directly comparing the nicotine patch with other medications showed that only varenicline and combination NRT were statistically superior to the patch by itself. In addition, recent well-powered individual clinical trials support the relative superiority of medication combinations (i.e., nicotine patch + nicotine lozenge and bupropion + nicotine lozenge) over single medication treatments (e.g., Piper et al 2009). For instance, in one study (Smith et al 2009), the combination of bupropion plus nicotine lozenge resulted in a 6-month abstinence rate of about 30% which was superior to the abstinence rates for bupropion alone, the nicotine patch alone, and the nicotine lozenge alone (which were all under 20%).
Smoking medication and counseling effects
Meta-analyses have found that the FDA-approved smoking medications augment the benefits of counseling, and vice versa, with counseling typically involving skills training and/or intratreatment support (based on the analyzed articles’ descriptions of the counseling). A meta-analysis for the 2008 PHS Guideline compared the effects of counseling alone versus the same level of counseling supplemented with FDA approved smoking medication. There were 9 studies in the analysis, which contributed 11 counseling-only arms (the reference or control condition), and 13 counseling + medication arms. The results showed that counseling alone (of varying intensities) produced an estimated 5-month (or longer) abstinence rate of 14.6%, while the combination of counseling + medication produced an abstinence rate of 22.1%. Conversely, a PHS Clinical Practice Guideline meta-analysis of a different set of studies compared the effects of smoking medication alone versus medication + counseling. This analysis showed that across 18 studies, counseling (of varying intensities) raised estimated 5-month (or longer) abstinence rates from 21.7% to 27.6% (Fiore et al 2008). These findings underscore practice guideline recommendations that cessation treatments combine counseling and medication whenever possible (e.g., Fiore et al 2008).
Mediation of smoking medication effects
In contrast to the situation with cessation counseling, research has shed light on the mechanisms that account for the effect of smoking medications on abstinence. Several smoking medications have been shown to increase abstinence rates because they reduce smokers’ craving to smoke in the immediate post-cessation period (Bolt et al 2012, Piper et al 2008). In fact, greater suppression of craving accounts, in part, for the superiority of combination NRT versus single medications (Bolt et al 2012). However, craving reduction accounts for only a portion of the benefit derived from medication and other mechanisms have not yet been identified.
Smoking medication effects in real-world settings
Some recent studies have failed to find that smoking medications are effective in real-world use (Ferguson et al 2012a), raising questions about the effects of these medications outside of highly controlled clinical trials. Medications might exert stronger effects in highly controlled trials for various reasons (e.g., these trials may more strongly encourage adherent medication use or may recruit smokers more motivated to quit smoking—which could interact with medication condition). Several types of studies are highly relevant to the evaluation of medication effects in real world settings. Effectiveness trials examine the effects of the medication in randomized clinical trials that strongly mimic contexts of real world use (e.g., participants are recruited via real world recruitment channels, the level of counseling and participation inducements are similar to real world contexts, and real world clinical personnel deliver the therapies). In general, such trials strongly support the effectiveness of smoking medications (McAfee et al 2008, Smith et al 2009).
The second type of study uses retrospective cross sectional, or longitudinal, surveys that relate smokers’ reports of quitting with their reports of use of smoking medication. The nicotine patch, gum, and lozenge have been approved for over-the-counter (OTC) use in many countries, and smokers in such survey studies typically get their medication via that route. Some of these studies show that use of smoking medication is not related to higher rates of quitting (e.g., Pierce & Gilpin 2002). However, other survey studies do show benefit of medication use (Kasza et al 2012). Moreover, research consistently shows that smokers who use OTC medication differ substantially from those who do not (e.g., smokers who use OTC medication are more highly tobacco dependent; Borland et al 2012, Hughes et al 2011). A recent, careful analysis of survey studies concluded that no strong inferences could be drawn at present from this literature (Hughes et al 2011).
Finally, formal experiments have been conducted that are intended to reproduce the features of OTC use. In general, such studies show that those who receive OTC medication quit at higher levels than those who do not (for a review see Fiore et al 2008). However, some of these studies used methods that may not, in fact, reflect the context of real world OTC use: e.g., meetings with research personnel, and receiving free medication (Walsh 2008). Therefore, while it appears that OTC NRT use can increase abstinence rates, further research—especially better experimental, controlled research that mimics OTC use (Hughes et al 2011)—is needed to achieve a high level of confidence regarding this topic. This topic is of great importance because OTC NRT use constitutes the most commonly used cessation aid in the U.S., being used by about one-third of smokers trying to quit (Shiffman et al 2008a). However, it is important to bear in mind that in clinical use, in which the smoker is enrolled in some sort of treatment program (e.g., via a quitline or clinician), the available data overwhelmingly support the effectiveness of cessation medications.
Smoking medication safety and tolerability
As with any medication, choosing amongst smoking medications requires consideration of the benefits and risks of the various alternatives, with attention to each person’s medical and psychiatric status. In general, NRT, including combination NRT, is quite safe, with adverse events or reactions (e.g., nausea, skin or mouth irritation) posing modest risks (Fiore et al 2008, Piper et al 2009). A meta-analysis of 120 studies showed no significant increase in the risk of serious health effects with the use of NRT (Mills et al 2010).
Bupropion and varenicline pose greater safety concerns. In 2010, on the basis of postmarketing surveillance, the FDA issued a black-box warning for both varenicline and bupropion concerning serious neuropsychiatric symptoms such as hostility, agitation, depressed mood, and suicidal thoughts and behavior. The FDA advised that clinicians discuss these symptoms with their patients, weigh the risks of such symptoms for patients with serious psychiatric illness, monitor patients for the symptoms, and discontinue the medication in the event of their occurrence. Also, a recent drug-safety communication from the FDA noted that varenicline may also be associated with a small increased risk of cardiovascular events, including heart attack, and it called for physicians to weigh the risks and benefits of using varenicline in patients with cardiovascular disease. In addition, bupropion can lower the seizure threshold and should not be used in patients who have a history of a seizure disorder or who drink heavily. These concerns must be weighed against the facts that both varenicline and bupropion have been used safely with many thousands of patients (Fiore et al 2008), and these medications reliably increase smoking cessation rates, enabling many people to escape the serious disease risks of smoking.
Adherence to smoking medications
Smokers tend not to use smoking medications adherently (i.e., they use less medication, for less time, than is recommended; Balmford et al 2011). Nonadherence seems to be especially common with NRT products, particularly in OTC use (Balmford et al 2011). However, even with varenicline, people often use less than is prescribed or recommended (Balmford et al 2011; although Catz et al 2011 report adherence rates of 80% with varenicline). Failure to use these products adherently is correlated with cessation failure (Balmford et al 2011, Catz et al 2011, Shiffman et al 2008b). Because nonadherence is common, and related to cessation failure, researchers have increasingly focused on characterizing its level of occurrence, its association with cessation, and methods to reduce it. A recent important finding in this area is that nonadherence appears to be due, in part, to people starting to fail in their quit attempt; i.e., they start to smoke while taking medication and this may cause them to stop taking the medication (Balmford et al 2011, Catz et al 2011). Thus, nonadherence and cessation failure may be reciprocally related. It is important to note that some evidence suggests that even if smokers have begun to smoke during their quit attempt, if they continue to take their medication (in this case the nicotine patch) it may help them re-establish abstinence (Ferguson et al 2012b). The identification of cost effective methods that increase adherence to cessation medication remains a vital research goal.
Smoking medications and special populations
Smoking medications have not been shown to be efficacious for very young or adolescent smokers (Fiore et al 2008). Also, there is little evidence that smoking medications are effective in pregnant smokers and such medications could place the fetus at risk (Fiore et al 2008). This underscores the pressing need to develop more effective treatments for pregnant and postpartum smokers because of the health risks of smoking for the mother, the fetus, and, later, for the child. While many smokers can quit during pregnancy, they very frequently relapse postpartum, even with treatment (Fiore et al 2008).
Medication use strategies
There are many smoking medications and many use strategies have been tried (e.g., combinations of medications, different lengths of use; see Table 2). However, at present there is little solid basis for recommending one type of smoking medication versus another for a smoker. Some authors suggest using a patient’s prior experience with a medication or a patient’s preferences when recommending a type of medication, or they recommend adjusting treatment based on the patient’s on-going experience with the medication (Bittoun 2006, Hughes 2008). Some tentative inroads are being made towards personalized medication use, however. First, decision tree analysis has found that patients who are low in nicotine dependence and have strong environmental prods to smoke are not significantly helped by combination NRT, whereas other types of patients are (Loh et al 2012). A second suggestive finding involves a gene cluster on chromosome 15 that increases risk of nicotine dependence. Smokers who have particular “risk” genetic variants in this region face heightened risk of smoking cessation failure if they are given placebo, but no increased risk of failure if given smoking medication (Chen et al 2012). Smokers without these risk variants do as well with placebo as with medication, suggesting that smokers with these risk variants should be given medication, and, perhaps, that those without these variants should be given more intense counseling. The above findings are tentative and certainly require systematic replication before their clinical use. Development of sound medication algorithms is important because it could significantly boost smoking medication effectiveness, without requiring the discovery of new medications.
Development of new medications
Numerous agents and strategies have been tested as smoking medications, and many have not yet been successful: e.g., buspirone, nicotine vaccines, mecamylamine, fluoxetine, rimonabant. However, drug development efforts continue, and there is evidence the agent cytisine (derived from the golden acacia plant)—a nicotine receptor mixed agonist like varenicline—may be an effective cessation agent (West et al 2011).
Cost-effectiveness of Cessation Treatments
Multiple studies have demonstrated the cost-effectiveness of smoking cessation treatments, both counseling and medications, using varying assumptions regarding costs and efficacy. Cessation treatments are cost-effective with regard to multiple metrics, including cost per quitter, cost per quality life year saved, and per member per month costs as a health plan benefit (e.g., Cromwell et al 1997, Fiore et al 2008, Knight et al 2010). Cessation treatments are also quite cost-effective relative to treatments of smoking-related diseases (e.g., treatments for chronic obstructive pulmonary disease; Zimovetz et al 2011). Further, smoking cessation treatment is cost-effective relative to other, common preventive interventions. The cost of saving a life by use of smoking cessation treatment has been estimated at $3,539, which compares favorably with $5,200 for hypertension screening for men (ages 45-54), and $4,100 for cervical screening for women (ages 34-39: Fiore et al 2008). Other studies have shown that over 7 to 10 years, a health care system or insurer will recoup almost all their investment in smoking cessation treatments, and that quitters will gain on average 7 additional years of life (Fiore et al 2008). The cost-effectiveness of cessation treatment is even higher in certain populations such as pregnant women where cessation reduces the risk of low birth-weight and of fetal and infant mortality (Fiore et al 2008). Such data have encouraged health insurance plans to offer cessation treatment as a covered benefit and have encouraged government funding for both cessation counseling and medications via quitlines.
MAINTENANCE INTERVENTIONS: SUSTAINING ABSTINENCE
The Nature of the Interventions and the Relevant Population
The smoker who has remained essentially abstinent (except, perhaps, for occasional lapses) for the first 2 to 3 weeks following the target quit day can be considered to have moved from the Cessation Phase to the Maintenance Phase (Baker et al 2011). By this point, quitters’ withdrawal symptoms have typically improved (Hughes 2007), and the goal of this phase is to help smokers remain abstinent by avoiding lapsing and progressing from a lapse to a relapse.
Most smokers who ultimately relapse have their first cigarette quite early in the quit attempt. Perhaps 70% or so of those who relapse within the first 6 months start smoking within the first 2 weeks (Kenford et al 1994), but escalation to relapse per se often unfolds slowly and stretches out over weeks with smoking occurring at a gradually accelerating pace (Kirchner et al 2012). The representative time course of relapse was captured in an observational internet study of smokers planning to quit smoking within 3 months (N = 2431; Zhou et al 2009). This study found that almost 80% of smokers making a quit attempt reported relapsing in the first 3 months, but 60% of the remaining smokers relapsed between months 3 and 6. The relapse rate amongst smokers who maintained abstinence for at least 6 months decreased to less than 20% for the following 12 months. Thus, initial smoking early in the quit attempt may set the stage for ultimate relapse, but even months of abstinence do not guarantee lasting success.
Opportunities and Challenges
Quitters in this phase have already remained largely abstinent for several weeks, perhaps reflecting their good coping skills, strong motivation, and/or favorable environmental contexts. In addition, the worst of their tobacco withdrawal is generally over which should bode well for their continued success. However, despite these advantages, many smokers relapse during the Maintenance Phase, and, in general, maintenance interventions produce only modest benefit.
Smokers who are 2 to 3 weeks into a quit attempt may have experienced the worst of withdrawal, but clearly they still face challenges that often derail their abstinence. The leading threats include: decreased self-efficacy (especially after lapses: Gwaltney et al 2009, Kirchner et al 2012); the premature cessation of either medication or counseling (Tonstad et al 2006); poor medication adherence (Stapleton et al 1995); frequent exposure to smoking cues (Loh et al 2012, Shiffman et al 1996b); and unexpected exacerbations of withdrawal craving that may occur weeks after the quit day (Piasecki et al 2003). How these events unfold as a process is not yet clear. What is clear is that smoking a cigarette after the quit day starts a cascade of events that typically progresses to full relapse, including a plunge in self-efficacy, demoralization and withdrawal from treatment. As Kirchner and colleagues write: “…when lapses degrade smokers’ confidence and make them feel like giving up, more lapses soon follow, perhaps because the next lapse seems inevitable, and avoiding it or coping with the temptation to lapse seems too difficult or pointless” (Kirchner et al 2012, p. 193).
Multiple literature reviews have documented the need for more effective relapse prevention/maintenance interventions (e.g., Agboola et al 2010, Brandon et al 2007, Carroll 1996). Unfortunately, few studies have explored novel approaches to maintenance, which has limited progress, and limits the scope of this review.
Psychosocial Maintenance Interventions
Extended maintenance counseling
Several studies suggest that very extended counseling (which generally involves both intratreatment support and skills training) may reduce relapse risk (Hall et al 2004, Killen et al 2008, Mermelstein et al 2003). Thus, while the meta-analyses of cessation studies reviewed above showed that the effects of counseling plateaued once counseling exceeded a half hour or so, some research suggests that dramatically longer counseling (extending out to a year) will maintain cessation success. Perhaps the strongest evidence of this arises from research by Hall and colleagues. In one recent study (Hall et al 2009), older (≥ age 50) smokers were offered a standard 12-week cessation treatment: viz. five group counseling sessions, and bupropion plus nicotine gum. Then, regardless of their smoking status, all participants were randomized to one of four conditions: (a) no extended treatment (i.e., the control condition); (b) extended NRT (40 additional weeks of nicotine gum to be used as needed); (c) extended CBT for cessation (11 individual 20-40 minute sessions during the year after the quit date); or (d) extended CBT plus extended NRT (nicotine gum). Both the conditions that comprised extended CBT did consistently well across the 2-year follow-up period (e.g., at 2 years, 55% achieved point prevalence abstinence who received CBT-alone as did 45% who received CBT plus NRT; 40% were abstinent who received extended nicotine gum, and 36% were abstinent in the control condition). However across the 2 years of follow-up time points, only the CBT-alone condition differed reliably from the control condition. Hall and colleagues obtained similar positive findings regarding extended counseling in an earlier study (Hall et al 2004), and another study reported significantly elevated smoking abstinence rates over a 2-year period when comparing smokers offered the opportunity every 6 months to receive smoking medication plus repeated counseling calls versus smoking medication alone (Ellerbeck et al 2009), although these were secondary analyses. The need for very prolonged counseling is consistent with evidence that the effects of moderately long-term supportive counseling tend to vanish as soon as it is discontinued (e.g., Brandon et al 1987, Killen et al 2008). It appears that counseling must continue for a very long time in order to shepherd the smoker past the point of high relapse risk.
In sum, ongoing counseling over the course of a year does appear to increase long-term abstinence rates meaningfully, and these increases appear to be persistent. While these findings are impressive and encouraging, their translation into general practice may be limited because of cost and because many smokers may be unwilling to commit so much time and effort to quitting.
Hall’s extended CBT for cessation comprised multiple elements, and it is unknown which are most responsible for the boost in maintenance. These elements included counseling contents targeted at increasing motivation, coping with negative moods, providing social support, controlling weight (e.g., via exercise), and coping with withdrawal. A formal mediation analysis performed by Hall and her colleagues (Hendricks et al 2010) suggested that a treatment-induced increase in self-efficacy was most strongly related to long-term abstinence (versus, say, perceived social support). However, particular intervention components must be systematically, experimentally contrasted with one another to strongly implicate particular mechanisms and components (e.g., Baker et al 2011).
Relapse prevention booklets
Effects similar to those achieved with person-to-person skills training can perhaps be obtained using written or computerized interventions. In particular, a set of readings (Forever Free™: Brandon et al 2000, Brandon et al 2004, Brandon et al 2007) that focus heavily on skills training has repeatedly been found to decrease the future likelihood of relapse amongst smokers who recently quit. In one study, among smokers who recently quit, 12% of those mailed booklets reported that they were smoking at the 12 month follow-up versus 35% of those not mailed booklets (Brandon et al 2000). The effectiveness of these booklets, therefore, suggests that skills training contributes to maintaining abstinence. These booklets are of special note because they appear to sustain abstinence rates via a highly feasible and economic route—they can be made available via mailings or on-line access (in fact, they are available at no cost at www.smokefree.gov/resources.aspx).
Smoking Medications
The weight of the evidence suggests that extending the use of smoking medication enhances long-term smoking abstinence (Agboola et al 2010, Schnoll et al 2010, Tonstad et al 2006), and that termination of smoking medication (NRT in this particular meta-analysis) increases the likelihood of relapse (Medioni et al 2005). Severe tobacco dependence leads to severe tobacco withdrawal and heightened risk of lapsing and relapsing (Baker et al 2012, Japuntich et al 2011a), but there is substantial evidence that smoking medications can neutralize these risks. For instance, more intense (combination) versus single-agent smoking medication significantly reduces withdrawal-associated craving and this effect mediates the impact of smoking medications on 8-week abstinence (Bolt et al 2012). Similarly, high dose nicotine patch therapy, relative to placebo, reduces the likelihood that craving will precipitate lapses, approximately doubles the time from one lapse to the next (slows the rate of progression), and decreases the likelihood that a lapser will ultimately relapse (Ferguson & Shiffman 2010, Kirchner et al 2012; also see Japuntich et al 2011b). In sum, smoking medications used for extended periods of time (up to 6 months or even a year) and higher dose or combination medications especially, seem to help smokers maintain long-term abstinence. The evidence suggests that smoking medication facilitates abstinence by mitigating factors such as craving that are related to tobacco dependence. However, the effects of prolonged medication tend to be modest; these modest effects must be weighed against the costs, relatively low adherence, and side effects of such medication regimens.
RELAPSE RECOVERY INTERVENTIONS: CONVERTING RELAPSE INTO LONG-TERM SUCCESS
The Nature of the Interventions and the Relevant Population
In the Relapse Recovery phase, a person has returned to smoking after a quit attempt. As noted earlier, relapse is modal amongst those trying to quit. Even when smokers receive considerable counseling plus smoking medication, the great majority relapse by 6 months after the quit attempt (e.g., 65% in Piper et al 2009). Thus, there is a need for effective treatments for people who have relapsed—treatments that limit their smoking, keep them engaged in treatment, and promote a return to abstinence. Unfortunately, very little research has focused on this population.
Opportunities and Challenges
Recently relapsed smokers may enter relapse recovery treatment with some decided strengths. One is that some of their tobacco dependence may have diminished during the prior period of abstinence. Also, smokers who relapse tend to smoke fewer cigarettes per day than they did before they quit, even with no training or encouragement to do so and this may promote later successful cessation (Hughes & Carpenter 2005, Hughes & Carpenter 2006).
Relapse exacts some severe costs, though, and these may undermine recovery. It frequently causes demoralization (Kirchner et al 2012, Shiffman et al 1997) and can lead smokers to disengage from smoking treatment and refuse to engage in renewed quit attempts. Once smokers fail in a quit attempt, they may not make another quit attempt for many months or longer. Also, as noted in the Maintenance section, relapsers face challenges that include severe tobacco dependence (e.g., strong urges), feelings of giving up/demoralization, low self-efficacy, and stress (Shiffman et al 1996a).
Although there is research on relapse prevention, there is very little research on relapse response or relapse recovery interventions. Most tested post-relapse interventions merely recycle smokers through additional rounds of cessation treatment and do not offer a treatment option for smokers not interested in making an immediate quit attempt (e.g., Ellerbeck et al 2009). Only Joseph et al., (Joseph et al 2011) have tested a relapse response intervention consistent with a chronic care model.
Psychosocial Interventions
While many relapsers may be discouraged, there is evidence that recently relapsed smokers will, in fact, re-engage or remain in treatment if approached correctly. For instance, Joseph et al. (Joseph et al 2004) found that about 95% of relapsed smokers expressed willingness to engage in additional treatment with a goal of smoking reduction; however only 50% were willing to make an immediate quit attempt. A related study (Joseph et al 2011) found that a high percentage of relapsed smokers actually did engage in reduction or a new quit attempt. Research also shows that an outreach call from a counselor boosts re-engagement (Carlini et al 2008), as do offers of cessation treatment that are repeated over time (Ellerbeck et al 2009, Joseph et al 2011).
A crucial question that deserves further research is the nature of the treatment goal that recently relapsed smokers should be encouraged to pursue. The traditional goal of most relapse recovery treatments is that the smoker make an immediate new quit attempt. However, this approach may put too much pressure on a smoker whose confidence and self-efficacy have been shaken. The alternative, as manifested in the Joseph study (Joseph et al 2011), is to encourage relapsed smokers to adopt a smoking reduction goal—including using smoking medication and behavioral training to limit the amount they smoke (e.g., substituting smoking medication for cigarettes, eliminating smoking in key contexts). This strategy could be considered either a long-term harm reduction strategy (although the extent to which smoking reduction decreases harm is not known; Hughes & Carpenter 2006); or a transitional stage leading to a new quit attempt at a later point in time. Making a new quit attempt, preferably before the momentum from the former attempt is lost, is certainly the standard treatment approach to relapse (e.g., Asfar et al 2012, Brandon et al 1987). And, there is evidence that this strategy can increase the likelihood that a smoker will return to abstinence (Lichtenstein et al 1996). However, this strategy is unsuccessful with most relapsed smokers, and other approaches should be tested.
Smoking Medications
NRT can help the lapsing smoker re-establish abstinence (Ferguson et al 2012b, Japuntich et al 2011b, Medioni et al 2005, Schnoll et al 2010). It is, however, a somewhat open question whether smoking medications can help smokers who have fully relapsed, return to abstinence. The Joseph et al. study (Joseph et al 2011) mentioned earlier tested a longitudinal care intervention with a mixed group of abstainers seeking to maintain abstinence and smokers who had recently relapsed. In the longitudinal care intervention, smokers who had relapsed were offered medication “readjustment” and could, for example, increase their dose, change smoking medications, or switch to combination NRT, and they could use the medication to make another quit attempt or to help them smoke at a reduced level. This medication readjustment was offered in combination with behavioral phone counseling so it is not possible to disentangle their effects. Longitudinal care was more effective than usual care at promoting prolonged abstinence in the mixed sample of abstainers and smokers who had initially relapsed. However, the effect was modest in size and was significant in a longitudinal analysis but not at 18 months per se (at 18 months after the quit day, 30.2% in longitudinal care vs. 23.5% in usual care reported they had been abstinent for the past 6 months).
The Ellerbeck study (Ellerbeck et al 2009) also suggests that very long-term or repeated medication cycles plus on-going counseling can increase long-term abstinence rates. This study offered patients smoking at the beginning of the study treatment (either medication management alone or medication management plus six counseling calls) every 6 months over the course of 2 years. At the 2 year follow-up, 23.0% of those offered medication management alone versus 27.9% offered medication plus counseling reported 7-day abstinence. This direct comparison was not significant, but related secondary analyses were. However, our ability to make inferences from research in this area is compromised by the fact that this study and other studies do not separate out the effects of treatment of relapsers from overall treatment effects. Progress in this area will require research on recent relapsers examining the effects of relatively specific treatments on smoking reduction, treatment engagement, and long-term abstinence.
RESEARCH NEEDS AND POTENTIAL POLICY AND REGULATORY IMPACTS
The following topics are especially worthy of further investigation:
-
■
A chronic care approach to smoking treatment has been widely recommended (Ellerbeck et al 2009, Fiore et al 2008, Joseph et al 2011) in recognition of smoking’s chronic, relapsing nature, suggesting that effective interventions are needed to motivate quit attempts, maintain abstinence, and promote relapse recovery. Additional research is needed to identify those intervention components that work best together both within and across these different phases of the quitting process.
-
■
Continued research is needed to determine how best to deliver smoking interventions to more smokers, more efficiently. Promising strategies include offering eHealth interventions and using electronic health record (EHR) routes to link smokers with treatments.
-
■
While tremendous progress has been made in identifying effective smoking treatments, to accelerate progress researchers need to take advantage of innovative research methods such as adaptive and factorial designs (e.g., Collins et al 2011). Factorial designs permit efficient screening and comparisons of multiple intervention components on the bases of effect sizes, costs, feasibility, patient adherence, and so on (Collins et al 2011). Research can efficiently screen numerous intervention components in such designs before comparing the most promising combinations of intervention components (i.e., treatment packages) with one another in randomized controlled trials.
-
■
Smokers are increasingly using modified tobacco products (e.g., e-cigarettes). Researchers need to explore how the use of such products affects health, smoking cessation, response to smoking treatments, and whether such products can be harnessed to enhance cessation. While these products are already on the market, their safety is not known. If these products are shown to be safer alternatives to cigarettes, the FDA would have the authority—under the Family Smoking Prevention and Tobacco Control Act of 2009—to allow these products to be advertised as posing less risk than cigarette smoking, which could greatly increase their appeal and use.
-
■
Smoking intervention in the healthcare setting could be significantly affected by the way in which the 2010 Patient Protection and Affordable Care Act is implemented. This act designates tobacco dependence treatment as a core required element in healthcare evaluation. The act also mandates that, by 2014, new insurance plans provide coverage for evidence-based prevention treatments, which includes tobacco cessation treatment. These influences (along with others such as the 2012 Joint Commission Tobacco Cessation Performance Measure-Set for hospitals) suggest that tobacco dependence treatment will become increasingly common in healthcare settings. This underscores the need to develop strategies to integrate smoking intervention with healthcare delivery effectively.
-
■
Researchers need to explore innovative behavioral and psychosocial treatments for smoking. Our major psychosocial treatments are decades old, and we do not understand how they work. We are developing more sophisticated and efficient delivery systems for treatments, but while the bottle is new, the wine is old. We need to do more focused work on understanding how existing treatments work, and use that information to develop new, more effective treatments that are appropriate within and across the different phases of smoking cessation.
CONCLUSIONS
In sum, can we effectively intervene and help smokers initially not interested in quitting to quit successfully? Yes, we can help a small percentage to quit. Half or more of such smokers may be willing to engage in a noncessation treatment, but their quit rates, unsurprisingly, are low. Two treatments (motivational interviewing and smoking reduction instruction combined with NRT) modestly increase cessation success amongst smokers previously unmotivated to quit, but abstinence rates remain on average under 12%. Around 70-90% of smokers say they are not ready to make an immediate quit attempt so finding more effective Motivation Phase treatments is a high priority.
Can we help smokers who are motivated to make a quit attempt quit successfully? Yes, but even with counseling and/or medication, only 10-30% typically achieve long-term abstinence. Still counseling, medication (particularly varenicline and combination NRT), and the combination of counseling plus medication clearly increase abstinence rates. Can we help smokers who quit avoid relapse? Yes, both extended counseling over the course of a year and extended medication seem promising. In one study (Hall et al 2009), smokers received a 12-week cessation treatment comprising both counseling and medication and then some were randomized to close to a year of either extended counseling or extended medication; the 2-year point prevalence abstinence rates were 55% and 40% in the two groups, respectively. While these outcomes are striking, this type of treatment is very intense and unlikely to achieve widespread use. More efficient maintenance treatments are needed.
Can we help smokers who relapse to recover and requit? At this point, we can help some of them. Repeatedly offering retreatment to such smokers appears to increase the likelihood that they will return to abstinence. However, this strategy is unsuccessful with most relapsed smokers. Since the great majority of smokers who attempt to quit relapse, there is a critical need to develop more effective treatments in this area.
Researchers have made substantial progress in developing effective treatments for smoking. But greater progress is needed. Most smokers who try to quit do not receive or use evidence-based smoking treatments, and amongst those who do, the great majority generally fail in the long run. Given that tobacco use, world-wide, claims nearly 6 million lives per year (World Health Organization 2012), the need for progress, and rapid progress, defies description or even comprehension. Preventing as many young people as possible from becoming smokers is critical. But we also need to develop more effective treatments for current smokers, more rapidly, which can be accomplished by creating and using more efficient research methods.
SUMMARY POINTS.
In the U.S., around 19% of adults smoke and tobacco dependence remains the leading avoidable cause of death.
Without treatment only around 5% of smokers attempting to quit achieve long-term abstinence. Even with counseling and/or medication, only 10-30% typically achieve long-term abstinence.
Most smokers need to make repeated quit attempts before quitting permanently, and this has led to chronic care models of smoking intervention. Such models build in contingencies for patients who relapse and offer appropriate ongoing care to smokers regardless of their willingness to quit.
The Phase-Based Model of Cessation depicts pragmatically defined phases that can organize smoking treatment development and evaluation.
Perhaps 70-90% of smokers are unwilling to make a quit attempt at a given time, but half or more of these smokers may be willing to engage in a Motivation Phase intervention with a noncessation goal. Both motivational interviewing and smoking reduction instruction combined with NRT modestly increase cessation success amongst smokers previously unmotivated to quit, but abstinence rates remain on average under 12%.
Cessation Phase interventions are designed to help smokers willing to quit to remain abstinent during their first weeks post-quit. Smoking cessation counseling increases cessation rates over conditions involving very little or no counseling, and multiple studies have demonstrated the cost-effectiveness of smoking cessation treatments, both counseling and medications. There are 7 FDA approved medications for smoking cessation: five nicotine replacement products (including the nicotine patch and lozenge), as well as bupropion and varenicline. In general, based on meta-analyses, the single agents double the likelihood of abstinence at 6-months relative to placebo. Varenicline and combination NRT each close to triple the likelihood of successful cessation. Smoking medication and counseling should be used together whenever possible since neither by itself is as effective as is the combination.
Smokers remain at notable risk of relapse for many months after establishing initial abstinence. Ongoing counseling over the course of a year appears to increase long-term abstinence rates meaningfully, but such counseling may be too intense to achieve widespread use. Smoking medications used for extended periods of time (up to 6 months or even a year) also seem to help smokers maintain long-term abstinence, but effects are modest in size.
Little research has focused on Relapse Recovery interventions. Repeatedly encouraging relapsers to engage in additional courses of cessation treatment can increase the likelihood that they will re-establish abstinence. However, this strategy is unsuccessful with most relapsed smokers, and there is a strong need for new treatment strategies in this area.
Acknowledgments
We are grateful to Linda Kurowski and Wendy Theobald for their help in preparing this manuscript. The writing of this manuscript was supported by grant P50 DA019706 from the National Institutes of Health/National Institute on Drug Abuse (NIH/NIDA); by grant M01 RR03186 from the General Clinical Research Centers Program of the National Center for Research Resources, NIH; by grants 1K05CA139871 from NIH; and by the Wisconsin Partnership Program. This work was carried out while Dr. Schlam was a Primary Care Research Fellow supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine.
ACRONYMS
- CBT
cognitive behavioral therapy.
- EMA
ecological momentary assessment real-time measures sometimes assessed multiple times a day, using an electronic diary or interactive voice response calls.
- FDA
U.S. Food and Drug Administration.
- NRT
nicotine replacement therapy
DEFINITIONS
- Contingency management
a behavioral agreement specifying consequences that will follow if a person engages in a behavior (e.g., smoking or not smoking).
- Lapse
aperiodic isolated incidences of the problematic behavior (e.g., smoking one cigarette) from which some can fairly easily return to abstinence.
- Relapse
a resumption of the problematic behavior (e.g., smoking 7 consecutive days) from which it may prove challenging to resume abstinence.
- Study arm
A group of patients in a clinical trial who are assigned to a particular treatment condition (or to a placebo).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Agboola S, McNeill A, Coleman T, Leonardi Bee J. A systematic review of the effectiveness of smoking relapse prevention interventions for abstinent smokers. Addiction. 2010;105:1362–80. doi: 10.1111/j.1360-0443.2010.02996.x. [DOI] [PubMed] [Google Scholar]
- Asfar T, Ebbert JO, Klesges RC, Klosky JL. Use of smoking reduction strategies among U.S. tobacco quitlines. Addict Behav. 2012;37:583–6. doi: 10.1016/j.addbeh.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Aveyard P, Begh R, Parsons A, West R. Brief opportunistic smoking cessation interventions: a systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction. 2012;107:1066–73. doi: 10.1111/j.1360-0443.2011.03770.x. [DOI] [PubMed] [Google Scholar]
- Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, et al. New methods for tobacco dependence treatment research. Ann Behav Med. 2011;41:192–207. doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, Schlam TR, Cook JW, Smith SS, et al. Are tobacco dependence and withdrawal related amongst heavy smokers? Relevance to conceptions of dependence. J Abnorm Psychol. 2012 doi: 10.1037/a0027889. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmford J, Borland R, Hammond D, Cummings KM. Adherence to and reasons for premature discontinuation from stop-smoking medications: data from the ITC Four-Country Survey. Nicotine Tob Res. 2011;13:94–102. doi: 10.1093/ntr/ntq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittoun R. A combination nicotine replacement therapy (NRT) algorithm for hard-to-treat smokers. J Smoking Cessation. 2006;1:3–6. [Google Scholar]
- Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol. 2012;80:54–65. doi: 10.1037/a0026366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R, Partos TR, Cummings KM. Systematic biases in cross-sectional community studies may underestimate the effectiveness of stop-smoking medications. Nicotine Tob Res. 2012 doi: 10.1093/ntr/nts002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Collins BN, Juliano LM, Lazev AB. Preventing relapse among former smokers: a comparison of minimal interventions through telephone and mail. J Consult Cli Psychol. 2000;68:103–13. doi: 10.1037//0022-006x.68.1.103. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Meade CD, Herzog TA, Chirikos TN, Webb MS, Cantor AB. Efficacy and cost-effectiveness of a minimal intervention to prevent smoking relapse: dismantling the effects of amount of content versus contact. J Consult Clin Psychol. 2004;72:797–808. doi: 10.1037/0022-006X.72.5.797. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–84. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Zelman DC, Baker TB. Effects of maintenance sessions on smoking relapse: delaying the inevitable? J Consult Clin Psychol. 1987;55:780–82. doi: 10.1037//0022-006x.55.5.780. [DOI] [PubMed] [Google Scholar]
- Carlini BH, Zbikowski SM, Javitz HS, Deprey TM, Cummins SE, Zhu SH. Telephone-based tobacco-cessation treatment: re-enrollment among diverse groups. Am J Prev Med. 2008;35:73–6. doi: 10.1016/j.amepre.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72:371–81. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Relapse prevention as a psychosocial treatment: a review of controlled clinical trials. Exp Clin Psychopharmacol. 1996;4:46–54. [Google Scholar]
- Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, et al. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine Tob Res. 2011;13:361–8. doi: 10.1093/ntr/ntr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: current cigarette smoking among adults aged >/=18 years—United States, 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60:1207–12. [PubMed] [Google Scholar]
- Chen L-S, Baker TB, Piper ME, Breslau N, Cannon DS, et al. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. Am J Psychiatry. 2012 doi: 10.1176/appi.ajp.2012.11101545. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Baker TB, Mermelstein RJ, Piper ME, Jorenby DE, et al. The Multiphase Optimization Strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011;41:208–26. doi: 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T. Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research. JAMA. 1997;278:1759–66. [PubMed] [Google Scholar]
- Cummings KM, Fong GT, Borland R. Environmental influences on tobacco use: evidence from societal and community influences on tobacco use and dependence. In: Nolen-Hoeksema S, Cannon TD, Widiger TA, editors. Annual Review of Psychology. Vol. 5. Palo Alto, CA: Annual Reviews; 2009. pp. 433–58. [DOI] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila EP, Zhao W, Byrne M, Webb M, Huang Y, et al. Correlates of smoking quit attempts: Florida Tobacco Callback Survey, 2007. Tob Induced Dis. 2009;5:10. doi: 10.1186/1617-9625-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JR, Glaros AG. Relapse prevention and smoking cessation. Addict Behav. 1986;11:105–14. doi: 10.1016/0306-4603(86)90034-1. [DOI] [PubMed] [Google Scholar]
- Dunn KE, Saulsgiver KA, Sigmon SC. Contingency management for behavior change: applications to promote brief smoking cessation among opioid-maintained patients. Exp Clin Psychopharmacol. 2011;19:20–30. doi: 10.1037/a0022039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbeck EF, Mahnken JD, Cupertino AP, Cox LS, Greiner KA, et al. Effect of varying levels of disease management on smoking cessation: a randomized trial. Ann Intern Med. 2009;150:437–46. doi: 10.7326/0003-4819-150-7-200904070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Perneger TV, Ronchi A. Distributions of smokers by stage: international comparison and association with smoking prevalence. Prev Med. 1997;26:580–5. doi: 10.1006/pmed.1997.0179. [DOI] [PubMed] [Google Scholar]
- Farkas AJ, Gilpin EA, Distefan JM, Pierce JP. The effects of household and workplace smoking restrictions on quitting behaviours. Tob Control. 1999;8:261–5. doi: 10.1136/tc.8.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J, Docherty G, Bauld L, Lewis S, Lorgelly P, et al. Effect of offering different levels of support and free nicotine replacement therapy via an English national telephone quitline: randomised controlled trial. BMJ. 2012a;344:e1696. doi: 10.1136/bmj.e1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Gitchell JG, Shiffman S. Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction. 2012b;107:1349–53. doi: 10.1111/j.1360-0443.2012.03801.x. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. Effect of high-dose nicotine patch on the characteristics of lapse episodes. Health Psychol. 2010;29:358–66. doi: 10.1037/a0019367. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ. Smoking cessation: Clinical practice guideline No 18. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1996. [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. This presents evidence-based recommendations for the clinical treatment of tobacco use and dependence. [Google Scholar]
- Foulds J, Gandhi KK, Steinberg MB, Richardson DL, Williams JM, et al. Factors associated with quitting smoking at a tobacco dependence treatment clinic. Am J Health Behav. 2006;30:400–12. doi: 10.5555/ajhb.2006.30.4.400. [DOI] [PubMed] [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, et al. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behav Ther. 2011;42:700–15. doi: 10.1016/j.beth.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Gifford EV, Shoenberger D. Rapid smoking. In: O’Donohue WT, Fisher JE, editors. General principles and empirically supported techniques of cognitive behavior therapy. Hoboken, NJ: John Wiley & Sons, Inc; 2009. pp. 513–19. [Google Scholar]
- Glasgow RE, Gaglio B, Estabrooks PA, Marcus AC, Ritzwoller DP, et al. Long-term results of a smoking reduction program. Med Care. 2009;47:115–20. doi: 10.1097/MLR.0b013e31817e18d1. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Metrik J, Kahler CW, Shiffman S. Self-efficacy and smoking cessation: a meta-analysis. Psycho Addict Behav. 2009;23:56–66. doi: 10.1037/a0013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AL, Munoz RF, Humfleet GL, Reus VI, Hall SM. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. J Consult Clin Psychol. 2004;72:563–70. doi: 10.1037/0022-006X.72.4.563. [DOI] [PubMed] [Google Scholar]
- Hall SM, Humfleet GL, Munoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction. 2009;104:1043–52. doi: 10.1111/j.1360-0443.2009.02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Humfleet GL, Reus VI, Munoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry. 2004;161:2100–7. doi: 10.1176/appi.ajp.161.11.2100. [DOI] [PubMed] [Google Scholar]
- Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103:1009–18. doi: 10.1111/j.1360-0443.2008.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Delucchi KL, Hall SM. Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug Alcohol Depend. 2010;109:114–9. doi: 10.1016/j.drugalcdep.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology (Berl) 2006;187:385–96. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez M, Luciano MC, Bricker JB, Roales-Nieto JG, Montesinos F. Acceptance and commitment therapy for smoking cessation: a preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychol Addict Behav. 2009;23:723–30. doi: 10.1037/a0017632. [DOI] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes J. An algorithm for choosing among smoking cessation treatments. J Subst Abuse Treat. 2008;34:426–32. doi: 10.1016/j.jsat.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. The hardening hypothesis: is the ability to quit decreasing due to increasing nicotine dependence? A review and commentary. Drug Alcohol Depend. 2011;117:111–117. doi: 10.1016/j.drugalcdep.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Carpenter MJ. The feasibility of smoking reduction: an update. Addiction. 2005;100:1074–89. doi: 10.1111/j.1360-0443.2005.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Carpenter MJ. Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine Tob Res. 2006;8:739–49. doi: 10.1080/14622200600789726. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Fagerstrom KO, Callas PW. Intentions to quit smoking change over short periods of time. Addict Behav. 2005;30:653–62. doi: 10.1016/j.addbeh.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Peters EN, Naud S. Effectiveness of over-the-counter nicotine replacement therapy: a qualitative review of nonrandomized trials. Nicotine Tob Res. 2011;13:512–22. doi: 10.1093/ntr/ntr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A, Borland R, Li Q, Young H-H, McNeill A, et al. Individual-level predictors of cessation behaviours among participants in the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15:iii83–iii94. doi: 10.1136/tc.2005.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip DT, Cohen JE, Bondy SJ, Chaiton MO, Selby P, et al. Do components of current ’hardcore smoker’ definitions predict quitting behaviour? Addiction. 2012;107:434–40. doi: 10.1111/j.1360-0443.2011.03674.x. [DOI] [PubMed] [Google Scholar]
- Irvin JE, Brandon TH. The increasing recalcitrance of smokers in clinical trials. Nicotine Tob Re s. 2000;2:79–84. doi: 10.1080/14622200050011330. [DOI] [PubMed] [Google Scholar]
- Japuntich SJ, Leventhal AM, Piper ME, Bolt DM, Roberts LJ, et al. Smoker characteristics and smoking-cessation milestones. Am J Prev Med. 2011a;40:286–94. doi: 10.1016/j.amepre.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. J Consult Clin Psychol. 2011b;79:34–42. doi: 10.1037/a0022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Fu SS, Lindgren B, Rothman AJ, Kodl M, et al. Chronic disease management for tobacco dependence: a randomized, controlled trial. Arch Intern Med. 2011;171:1894–900. doi: 10.1001/archinternmed.2011.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Rice K, An LC, Mohiuddin A, Lando H. Recent quitters’ interest in recycling and harm reduction. Nicotine Tob Res. 2004;6:1075–7. doi: 10.1080/14622200412331324893. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Borland R, Hyland A, McKee SA, Thompson ME, Cummings KM. Alcohol consumption and quitting smoking in the International Tobacco Control (ITC) Four Country Survey. Drug Alcohol Depend. 2009;100:214–20. doi: 10.1016/j.drugalcdep.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza KA, Hyland AJ, Borland R, McNeill AD, Bansal-Travers M, et al. Effectiveness of stop-smoking medications: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2012 doi: 10.1111/j.1360-0443.2012.04009.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Augustson E, Davis K, Finney Rutten LJ. Awareness and use of tobacco quitlines: evidence from the Health Information National Trends Survey. J Health Commun. 2010;15(Suppl 3):264–78. doi: 10.1080/10810730.2010.526172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271:589–94. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg AF, Arredondo C, Murphy G, et al. Extended cognitive behavior therapy for cigarette smoking cessation. Addiction. 2008;103:1381–90. doi: 10.1111/j.1360-0443.2008.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner TR, Shiffman S, Wileyto EP. Relapse dynamics during smoking cessation: recurrent abstinence violation effects and lapse-relapse progression. J Abnorm Psychol. 2012;121:187–97. doi: 10.1037/a0024451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight C, Howard P, Baker CL, Marton JP. The cost-effectiveness of an extended course (12+12 weeks) of varenicline compared with other available smoking cessation strategies in the United States: an extension and update to the BENESCO model. Value Health. 2010;13:209–14. doi: 10.1111/j.1524-4733.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Duhig AM, McKee SA, McMahon TJ, Liss T, et al. Contingency management for smoking cessation in adolescent smokers. Exp Clin Psychopharmacol. 2006;14:306–10. doi: 10.1037/1064-1297.14.3.306. [DOI] [PubMed] [Google Scholar]
- Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD006936.pub2. CD006936. [DOI] [PubMed] [Google Scholar]
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD001292.pub2. CD001292. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lee CW, Kahende J. Factors associated with successful smoking cessation in the United States, 2000. Am J Public Health. 2007;97:1503–9. doi: 10.2105/AJPH.2005.083527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein E, Glasgow RE, Lando HA, Ossip-Klein DJ, Boles SM. Telephone counseling for smoking cessation: rationales and meta-analytic review of evidence. Health Educ Res. 1996;11:243–57. doi: 10.1093/her/11.2.243. [DOI] [PubMed] [Google Scholar]
- Lichtenstein E, Zhu SH, Tedeschi GJ. Smoking cessation quitlines: an underrecognized intervention success story. Am Psychol. 2010;65:252–61. doi: 10.1037/a0018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindson N, Aveyard P. An updated meta-analysis of nicotine preloading for smoking cessation: investigating mediators of the effect. Psychopharmacology. 2011;214:579–92. doi: 10.1007/s00213-010-2069-3. [DOI] [PubMed] [Google Scholar]
- Loh WY, Piper ME, Schlam TR, Fiore MC, Smith SS, et al. Should all smokers use combination smoking cessation pharmacotherapy? Using novel analytic methods to detect differential treatment effects over 8 weeks of pharmacotherapy. Nicotine Tob Res. 2012;14:131–41. doi: 10.1093/ntr/ntr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psychol. 2010;78:55–61. doi: 10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee TA. Quitlines a tool for research and dissemination of evidence-based cessation practices. Am J Prev Med. 2007;33:S357–67. doi: 10.1016/j.amepre.2007.09.011. [DOI] [PubMed] [Google Scholar]
- McAfee TA, Bush T, Deprey TM, Mahoney LD, Zbikowski SM, et al. Nicotine patches and uninsured quitline callers: a randomized trial of two versus eight weeks. Am J Prev Med. 2008;35:103–10. doi: 10.1016/j.amepre.2008.04.017. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Jorenby DE, Lawrence DL, Shiffman S, Baker TB. A multi-level analysis of non-significant counseling effects in a randomized smoking cessation trial. Addiction. 2010;105:2195–208. doi: 10.1111/j.1360-0443.2010.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medioni J, Berlin I, Mallet A. Increased risk of relapse after stopping nicotine replacement therapies: a mathematical modelling approach. Addiction. 2005;100:247–54. doi: 10.1111/j.1360-0443.2004.00961.x. [DOI] [PubMed] [Google Scholar]
- Mermelstein R, Hedeker D, Wong SC. Extended telephone counseling for smoking cessation: does content matter? J Consult Clin Psychol. 2003;71:565–74. doi: 10.1037/0022-006x.71.3.565. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2. New York: Guilford Press; 2002. [Google Scholar]
- Mills EJ, Ping W, Lockhart I, Kumanan W, Ebbert JO. Adverse event associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 adults. Tob Induced Dis. 2010;8:8. doi: 10.1186/1617-9625-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ. 2009;338:b1024. doi: 10.1136/bmj.b1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute, National Institutes of Health, Department of Health and Human Services. Cancer Trends Progress Report – 2009/2010 Update. 2010 http://progressreport.cancer.gov.
- National Center for Health Statistics. Health, United States, 2011. Hyattsville, MD: 2012. http://www.cdc.gov/nchs/data/hus/hus11.pdf. [PubMed] [Google Scholar]
- Nides M, Glover ED, Reus VI, Christen AG, Make BJ, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. Am J Health Behav. 2008;32:664–75. doi: 10.5555/ajhb.2008.32.6.664. [DOI] [PubMed] [Google Scholar]
- North American Quitline Consortium. Results from the 2010 NAQC Annual Survey of Quitlines. 2011 http://www.naquitline.org/?page=survey2010.
- Orleans CT, Rimer BK, Cristinzio S, Keintz MK, Fleisher L. A national survey of older smokers: treatment needs of a growing population. Health Psychol. 1991;10:343–51. doi: 10.1037//0278-6133.10.5.343. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1610–16. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. J Abnorm Psychol. 2003;112:3–13. [PubMed] [Google Scholar]
- Pierce J, Giovino G, Hatziandreu E, Shopland D. National age and sex differences in quitting smoking. J Psychoactive Drugs. 1989;21:293–8. doi: 10.1080/02791072.1989.10472170. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Gilpin EA. Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA. 2002;288:1260–4. doi: 10.1001/jama.288.10.1260. [DOI] [PubMed] [Google Scholar]
- Piper ME, Baker TB, Mermelstein R, Collins LM, Fraser D, et al. Recruiting and engaging all smokers in treatment in a primary care setting: use of a chronic care model implemented through a modified electronic health record. Transl Behav Med. doi: 10.1007/s13142-012-0178-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction. 2011;106:418–27. doi: 10.1111/j.1360-0443.2010.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010a;12:647–57. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol. 2008;117:94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66:1253–62. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, et al. Psychiatric disorders in smokers seeking treatment for tobacco dependence: relations with tobacco dependence and cessation. J Consult Clin Psychol. 2010b;78:13–23. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med. 2010;152:144–51. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. J Pers. 2005;73:1715–48. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008a;34:102–11. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J Consult Clin Psychol. 1996a;64:993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. The abstinence violation effect following smoking lapses and temptations. Cogn Ther Res. 1997;21:497–523. [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996b;64:366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. 2008b;30:1852–8. doi: 10.1016/j.clinthera.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Smith SS, McCarthy DE, Japuntich SJ, Christiansen B, Piper ME, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–55. doi: 10.1001/archinternmed.2009.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JA, Russell MA, Feyerabend C, Wiseman SM, Gustavsson G, et al. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction. 1995;90:31–42. doi: 10.1046/j.1360-0443.1995.901316.x. [DOI] [PubMed] [Google Scholar]
- Stead L, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD002850.pub2. CD002850. [DOI] [PubMed] [Google Scholar]
- Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD000165.pub3. CD000165. [DOI] [PubMed] [Google Scholar]
- Stead LF, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005231.pub2. CD005231. [DOI] [PubMed] [Google Scholar]
- Tomar SL, Husten CG, Manley MW. Do dentists and physicians advise tobacco users to quit? J Am Dent Assoc. 1996;127:259–65. doi: 10.14219/jada.archive.1996.0179. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- Walsh RA. Over-the-counter nicotine replacement therapy: a methodological review of the evidence supporting its effectiveness. Drug Alcohol Rev. 2008;27:529–47. doi: 10.1080/09595230802245527. [DOI] [PubMed] [Google Scholar]
- Warner KE, Burns DM. Hardening and the hard-core smoker: concepts, evidence, and implications. Nicotine Tob Res. 2003;5:37–48. doi: 10.1080/1462220021000060428. [DOI] [PubMed] [Google Scholar]
- Wennike P, Danielsson T, Landfeldt B, Westin A, Tonnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction. 2003;98:1395–402. doi: 10.1046/j.1360-0443.2003.00489.x. [DOI] [PubMed] [Google Scholar]
- West R, Zatonski W, Cedzynska M, Lewandowska D, Pazik J, et al. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med. 2011;365:1193–200. doi: 10.1056/NEJMoa1102035. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Tobacco. 2012 http://www.who.int/mediacentre/factsheets/fs339/en/index.html.
- Zelman DC, Brandon TH, Jorenby DE, Baker TB. Measures of affect and nicotine dependence predict differential response to smoking cessation treatments. J Consult Clin Psychol. 1992;60:943–52. doi: 10.1037//0022-006x.60.6.943. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, West R. Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addict Behav. 2009;34:365–73. doi: 10.1016/j.addbeh.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Zimovetz EA, Wilson K, Samuel M, Beard SM. A review of cost-effectiveness of varenicline and comparison of cost-effectiveness of treatments for major smoking-related morbidities. J Eval Clin Pract. 2011;17:288–97. doi: 10.1111/j.1365-2753.2010.01439.x. [DOI] [PubMed] [Google Scholar]
RELATED RESOURCES
- Abrams DB, Niaura R, Brown RA, Emmons KM, Goldstein MG, Monti, Linnan LA. The tobacco dependence treatment handbook: A guide to best practices. New York: Guilford Press; 2003. [Google Scholar]
- Fiore MC, Baker TB. Treating smokers in the health care setting. N Engl J Med. 2011;365:1222–31. doi: 10.1056/NEJMcp1101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Conklin CA, Levine MD. Cognitive-behavioral therapy for smoking cessation: A practical guidebook to the most effective treatments. New York: Taylor & Francis Group; 2008. [Google Scholar]


